Introduction
The treatment strategy of trisomy 18 is
controversial as the prognosis is lethal. Approximately 50% of
newborns survive >1 week while >90% do not survive beyond 1
years of age. The medical progression of neonatal intensive care
management in Japan has been previously established and the first
described trisomy 18 infants were reported in 1960 (1,2). The
definition of the trisomy 18 syndrome, also known as common
autosomal aneuploidy chromosomal abnormality due to the presence of
an extra chromosome 18, using the standard G-banded karyotype
allows for confirmation of the clinical diagnosis of multiple
anomalies (3). It is clinically
associated with various major and minor multisystem anomalies,
including cardiovascular, brain with neurological, renal,
gastrointestinal, respiratory, and skeletal malformations (4,5).
Trisomy 18 is clinically associated with ≥50% of anomalies
including craniofacial abnormalities such as prominent occiput,
narrow bifrontal diameter, low-set and malformed auricles and
micrognathia; hand and feet anomalies including clenched hand,
overlapping of index finger and hypoplasia of nails; thorax
deformities; inguinal hernia; small pelvis; male with
cryptorchidism; cardiac and circulatory system, in particular
ventricular septal defect (VSD), patent ductus arteriosus (PDA) and
auricular septal defect. In addition, 10–50% of cases showed other
craniofacial, hand, feet and thorax anomalies, cardiac with
pulmonic stenosis, coarctation of aorta, renal anomaly with
horseshoe defect, double ureter, hydronephrosis and polycystic
kidney, while ≥50% of cases exhibited central nervous system
anomaly with cerebellar hypoplasia, agenesis of corpus callosum,
hydrocephalus, meningomyelocele and Dandy-walker malformation
(6,7). All the cases had severe mental and
psychomotor disorders.
Epidemiologically, previously conducted studies have
focused on trisomy 18 (4,8–23).
Root and Carey (9) reported that
the incidence of trisomy 18 syndrome is as low as 1 in 8,000
births; however, this is the second highest incidence of
chromosomal aberrations in live-born infants following trisomy 21.
Findings of prevalence studies by Goldstein and Nielsen (10) Forrester and Merz (11) focusing on populations in Hawaii and
Denmark, respectively, showed that live-born infants of trisomy 18
had an incidence of 1 in 8,000 to 1 in 10,000. By contrast, Bergin
et al (12) reported the
incidence of trisomy 18 to be 1 of 555 conceptuses, with the most
recently recorded frequencies in live-born infants in Dublin and
Leicester in England being in the range of approximately 1 in
3,000. On the basis of the reported literature, the live birth
prevalence of trisomy 18 ranges from 1/3,000 to 1/10,000 and the
estimated average is approximately 1 in 6,000 (3,9–12).
Gender differences during gestation have been previously
identified, with a 4-fold higher incidence in female subjects
compared to male subjects (5). By
contrast, Kuroki and Kurosawa (24) reported no gender differences in
trisomy 18, as indicated by data obtained from the prefecture birth
defects monitoring program in Japan. An increase in the age of the
parturient female leads to an increased risk of trisomy 21, as well
as the development of trisomy. The recurrence risk of trisomy 18
has been analyzed and estimated at <1% (25,26).
The precise mechanism of this syndrome is known to develop from an
error in maternal meiosis, with the chromosomal abbreviation being
due to maternal origin in ~96% of cases of trisomy 18 (27).
When issues such as the medical treatment of
newborns, genetic counseling and medical ethics arise, management
with treatment strategy for this genetic trisomy syndrome is
clinically essential (6). The life
prognosis of trisomy 18 is controversial as the outcome is usually
lethal. Patients with long-term survival of trisomy 18 exhibited
severe pschyomotor developmental delay. Thus, any approach to the
treatment strategy and policy of a third-trimester fetus and
live-born neonate with trisomy 18 is complicated, resulting in
controversial findings. The primary issue involves the complexity
surrounding the decision-making process regarding the treatment
strategy immediately following birth in newborns diagnosed with
trisomy 18. Rasmussen et al (13), Crider et al (18) and Irving et al (19) reported that the important issues
involved in the parental primary care of live-born infants with
trisomy 18 is associated with a high neonatal and infantile
mortality death rate. When a fetus is known to have trisomy 18,
delivery by cesarean section is avoided due to ethics issues, as
indicated by Norup (28) in a
study conducted in Denmark. The findings of that study showed
consensus for physicians working in the perinatal and neonatal
medical unit until 21 weeks with regard to trisomy 18 (28). Irving et al (19) reported that the prenatal diagnosis
of trisomy 18 leads to a decision in support of pregnancy
termination in 86% of cases (19).
Sociologically, the medical treatment for the neonatal to infantile
periods with disabilities was found to be different for various
countries depending on factors such as culture, religion, human
rights, law and views on bioethics. Traditionally, a
non-intervention approach in the newborn management of trisomy 18
and 13 has been previously utilized. Bos et al (29) summarized these ethical issues,
indicating that early diagnosis was extremely important for the
avoidance of unnecessary surgical treatment. At present, trisomy 18
and 13 are considered to have a high mortality and survivors suffer
from severe mental impairment and fail to thrive. Consequently,
surgical procedures are withheld in the early stages of infancy to
determine the outcome following the first few months (6).
In Japan, the therapeutic policies for poor
long-term life prognosis for trisomy 18 and 13 involve two
controversial concepts: i) the provision of thorough affection and
care and avoidance of excessive intensive treatment; or ii) the
provision of active intensive treatment including resuscitation and
surgery according to the clinical conditions of an infant in
accordance with the wishes of the affected infant's parents
(14,30). However, there was insufficient
evidence regarding the improvement of prognosis of trisomy 18
patients treated at the Neonatal Intensive Care Unit (NICU) who
underwent surgery. Kosho et al (14,31)
studied 24 cases of trisomy 18 treated at the NICU, resulting in
improved life prognosis as compared to previously reported
data.
After written informed consent was obtained from the
family members following genetic counseling, intensive treatment
including resuscitation, intra-tracheal intubation, support of
artificial ventilation, and surgical procedures was performed for
long-term survival of trisomy 18 at the NICU of Dokkyo Medical
University School of Medicine. In addition, active support
management in the form of home nursing care in cooperation with the
family was provided. In this study, in order to certify the
clinical details and survival periods of trisomy 18 patients
managed at the NICU level, we investigated a retrospective
single-center study of 44 patients with trisomy 18, admitted to the
Dokkyo Medical University School of Medicine over a period of
>20 years.
In addition, prior to performing this study,
important factors regarding development of neonatal medicine over a
period of >20 years was considered. Thus, whether medical
progress influenced improvement of the life prognosis of trisomy 18
patients over such a long study period was examined. The patients
were divided into two groups and criteria considered included the
general condition and life prognosis immediately following the
delivery. The results provide useful information concerning genetic
counseling for parents/guardians and life prognosis.
Patients and methods
General
The study comprised 6,183 newborn infants who were
admitted to the NICU of Dokkyo Medical University Hospital in
Japan, during the period of 1992–2012. The 21-year period was
divided into two groups: group A constituted the first 11 years
(1992–2002) and group B the second 10 years (2003–2012). Group A
and B comprised 2,928 and 3,255 newborn patients, respectively. The
newborn infants manifested minor and/or major external
malformations or organ malformations that were observed during the
clinical examinations by a neonatologist and pediatrician. The
infants underwent a congenital chromosomal test and peripheral
blood cells obtained were analyzed via the standard method of
G-banding karyotype. A breakdown of the patient numerical autosomal
trisomies identified 96 patients (1.55%), with trisomy 21; 44
patients (0.71%) with trisomy 18 including full trisomy, mosaic
type and patients with an extra chromosome 18; and 17 patients
(0.27%) with trisomy 13 were admitted to the NICU. Other
chromosomal abnormalities exhibited included 2 patients with
trisomy 8 mosaicism, 3 patients with 5p-syndrome and
4p-syndrome.
In this study, a retrospective single-center study
was conducted using NICU medical records from 44 patients with
trisomy 18 obtained at the Dokkyo Medical University Hospital.
Group A comprised 20 patients and group B comprised 22 patients
with trisomy 18. There were no cases of prenatal diagnostic
screening for trisomy 18 prior to birth, thus all 44 cases were
diagnosed after birth.
Statistical analysis
Statistical analysis was performd to determine any
significant differences between groups A and B. The positive effect
of the NICU management and treatment was determined when no
significant differences were observed for the two groups at
delivery but there was a significant difference in the survival
prognosis. The following statistical procedure was performed as
establishment for it.
Continuous data during pregnancy and delivery were
described as mean ± standard deviation, if normally distributed, or
median (interquartile range), if not normally distributed.
Comparisons between groups A and B for continuous variables were
made using the unpaired t-test, if normally distributed, or
Mann-Whitney U test, if not normally distributed. Categorical data
were described as number and percentage, and differences were
analyzed using Fisher's exact test.
The percentage survival was analyzed using
Kaplan-Meier curves after establishing the longest survival time at
1 year. The survival curves were estimated according to the
Kaplan-Meier method. The generalized Wilcoxon and log-rank tests
were used to determine statistically significant differences for
the prognosis of survival time at 180 days, 1 year and the entire
investigated period of patients for groups A and B. The time
periods were analyzed using SPSS version 22.0 for Windows (IBM
Japan, Tokyo, Japan). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical details at pregnancy and
delivery
The statistical analysis results for groups A and B
are shown in Table I. None of the
44 patients with trisomy 18 syndrome had a family history of
chromosomal abnormalities. The karyotypes of chromosomal
abnormalities associated with chromosome 18 were identified in the
44 patients using a G-banding method. The result of karyotypes
included the full type of trisomy 18 in 42 patients (95%), the
mosaic type of trisomy 18 in 1 patient (2%), and the rare
isodicentric chromosome 18, i.e., 46,XX,idic(18)(p11.31) in 1 patient.
 | Table IClinical details at delivery of
newborns with trisomy 18 included in this study (n=44). |
Table I
Clinical details at delivery of
newborns with trisomy 18 included in this study (n=44).
|
Characteristics | No. | Entire
investigation period (1992–2012) | No. | Group A
(1992–2002) | No. | Group B
(2003–2012) | P-value groups A
vs. B |
|---|
| No. of
patients | – | 6,183 | – | 2,928 | – | 3,255 | – |
| Trisomy 18
cases | 44 | 44 | 20 | 20 | 24 | 24 | – |
| Karyotype | 44 | | 20 | | 24 | | 0.493a |
| Full | | 42 | | 20 | | 22 | |
| Others | | 2 | | 0 | | 2 | |
| Gender | 44 | | 20 | | 24 | | >0.999a |
| Male | | 16 | | 7 | | 9 | |
| Female | | 28 | | 13 | | 15 | |
| Term of delivery
(week) | 44 | | 20 | | 24 | | |
| Mean ± SD | | 36.7±3.2 | | 37.3±3.2 | | 36.3±3.2 | 0.309b |
| Median (IQR) | | 37.0 (34.0,
39.0) | | 37.5 (35.0,
39.8) | | 36.5 (34.0,
39.0) | 0.374c |
| Delivery place | 44 | | 20 | | 24 | | 0.436a |
| Our hospital | | 36 | | 15 | | 21 | |
| Other
hospital | | 8 | | 5 | | 3 | |
| Delivery | 38 | | 15 | | 23 | | 0.045a |
| Cesarean
section | | 19 | | 4 | | 15 | |
| Vaginally | | 19 | | 11 | | 8 | |
| Apgar score
(point) | | | | | | | |
| 1 min | 37 | | 14 | | 23 | | |
| Mean ± SD | | 3.4±2.1 | | 3.2±2.2 | | 3.6±2.1 | 0.631b |
| Median (IQR) | | 3.0 (2.0, 5.0) | | 2.5 (1.8, 5.0) | | 3.0 (2.0, 5.0) | 0.546c |
| 5 min | 35 | | 13 | | 22 | | |
| Mean ± SD | | 6.0±2.0 | | 5.7±2.0 | | 6.2±2.0 | 0.492b |
| Median (IQR) | | 6.0 (5.0, 7.0) | | 5.0 (4.0, 6.5) | | 6.0 (5.0, 7.0) | 0.180c |
| Birth weight
(g) | 44 | | 20 | | 24 | | |
| Mean ± SD | | 1585.5±493.6 | | 1654.9±576.8 | | 1527.8±415.9 | 0.402b |
| Median (IQR) | | 1536.0 (1186.5,
1969.5) | 1552.0 (1175.0,
2164.0) | | | 1519.0 (1186.5,
1851.0) | 0.525c |
| Categorical
data | 44 | | 20 | | 24 | | 0.525c |
| <1,000 | | 4 | | 2 | | 2 | |
| 1,000–1,500 | | 17 | | 7 | | 10 | |
| 1,501–2,000 | | 13 | | 5 | | 8 | |
| 2,001–2,500 | | 9 | | 5 | | 4 | |
| <2.500 | | 1 | | 1 | | 0 | |
Of the 44 patients, 16 were males and 28 females.
None of the patients underwent a prenatal diagnosis using the
amniotic fluid marker test for trisomy 18. The term of delivery was
29–42 gestational weeks, with an average of 36.7 gestational weeks.
Thirty-six of the patients were born in the Department of
Gynecology at the Dokkyo Medical University Hospital and 8 patients
were born at other hospital and transferred immediately after
birth. Immediately following the delivery, apgar scores of 1 and 5
min were 3 and 6, respectively. The average birth weight was
1585.5±493.6 g, with a range of 770–2,946 g. The breakdown of birth
weight included 4 patients weighing <1,000 g, 17 patients
weighing 1,001–1,500 g, 13 patients weighing 1,501–2,000 g, 9
patients weighing 2,001–2,500 g, and 1 patient weighing >2,500 g
at delivery. Regarding methods of delivery, 19 patients were
delivered via cesarean section and 19 patients via spontaneous
delivery.
Three types of statistical analyses were performed
for groups A and B. Significant differences were not identified in
any of the general conditions examined with the exception of
caesarean section which was statistically significant (P<0.045).
Thus, there were no differences between the two groups with regard
to the general state of neonate during time of delivery.
Organ malformations, use of mechanical
ventilation, and discharge from NICU
Ventilation and surgical treatment as well as
discharge from the NICU and prognosis are shown in Tables II and III. Disease manifestations including
trisomy 21, 18 and 13, were indicated in the ultrasonography
examination during the course of pregnancy, such as intrauterine
growth impairment, brain malformation, and congenital heart
anomalies. These anomalies were primarily cardiac and brain, and
mechanical ventilation as well as surgical procedures were used
prior to analysis of outcome and survival (Tables II and III).
 | Table IIOrgan malformations, use of
mechanical ventilation, surgical treatments and discharge from NICU
for patients with trisomy 18 (group A, 1992–2002, n=20). |
Table II
Organ malformations, use of
mechanical ventilation, surgical treatments and discharge from NICU
for patients with trisomy 18 (group A, 1992–2002, n=20).
| (Group A)
Patients | Major organ
malformations | Mechanical
ventilation | Surgical
treatment | Discharge from NICU
(D, dead; A, alive; T, transfer) | Survival
(days) |
|---|
| 1 | TOF PDA EA | − | | D | 0 |
| 2 | ECD IAA EA | + | | D | 0 |
| 3 | TGA ECD | − | | D | 0 |
| Anal atresia | | | | |
| Defects of both
thumb | | | | |
| 4 | PDA CoA EA | − | | D | 2 |
| 5 | VSD PDA UH | + | Repair UH | D | 3 |
| Urachal
remnants | | | | |
| 6 | VSD EA | − | | D | 3 |
| 7 | EA | + | Gastric
fistula | D | 8 |
| 8 | VSD ASD PDA EA | + | | D | 17 |
| 9 | VSD EA | − | Gastric
fistula | D | 27 |
| 10 | VSD CoA | + | Colostomy | D | 30 |
| Cleft lip and
palate | | Intestinal
segmentectomy | | |
| 11 | VSD ASD EA | + | | D | 47 |
| 12 | VSD PDA | + | | D | 53 |
| 13 | VSD EA | + | Gastric
fistula | D | 56 |
| 14 | ASD PDA CoA UH | + | | D | 78 |
| 15 | VSD ASD PDA UH | + | | D | 83 |
| 16 | VSD ASD | − | | A | 102 |
| 17 | EA | − | | D | 116 |
| 18 | TA | − | | D | 202 |
| 19 | VSD DWM | + | | D | 414 |
| 20 | VSD PDA | − | | A | 2311 |
| Total | | +:11/−:9 | | D:18/A:2 | |
 | Table IIIOrgan malformations, use of
mechanical ventilation, surgical treatments and discharge from NICU
for patients with trisomy 18 (group B, 2003–2012, n=24). |
Table III
Organ malformations, use of
mechanical ventilation, surgical treatments and discharge from NICU
for patients with trisomy 18 (group B, 2003–2012, n=24).
| Group B
Patients | Major organ
malformations | Mechanical
ventilation | Surgical
treatment | Discharge from NICU
(D, dead; A, alive; T, transfer) | Survival
(days) |
|---|
| 21 | n.d. | + | | D | 0 |
| 22 | VSD ASD PDA IAA
EA | + | | D | 1 |
| Cleft lip and
palate | | | | |
| Spurious
meningocele | | | | |
| 23 | ASD TA | + | | D | 4 |
| 24 | ECD | + | Gastric
fistula | D | 8 |
| 25 | VSD PDA | − | | D | 25 |
| Cleft lip and
palate | | | | |
| 26 | VSD ASD DORV
UH | + | | D | 45 |
| Defects of right
radius | | | | |
| 27 | VSD CoA | + | | A | 51 |
|
Holoprosencephaly | | | | |
| 28 | VSD CoA | + | | D | 60 |
| 29 | VSD ASD PDA EA | + | Gastric
fistula | D | 70 |
| 30 | VSD MS EA | + | Gastric
fistula | T | 92 |
| 31 | VSD ASD PDA | + | | D | 96 |
| 32 | VSD PDA CDH | + | Biliary duct
drainage | D | 108 |
| Biliary duct
dilatation | | | | |
| 33 | VSD ASD EA | + | Gastric
fistula | D | 116 |
| 34 | TOF | − | Blalock-Taussig
operation | T | 127 |
| 35 | VSD ASD CoA | − | | D | 132 |
| 36 | VSD ASD | − | | A | 162 |
| Laryngeal
malacia | | | | |
| 37 | VSD ASD PDA
CoA | + | | D | 250 |
| 38 | VSD PDA | − | | D | 250 |
| Pyloric
stenosis | | | | |
| 39 | PDA DORV EA | − | | A | 263 |
| 40 | DORV MA HLHS | + | | T | 263 |
| 41 | DORV | + | | D | 309 |
| Spurious
meningocele | | | | |
| Defects of
radius | | | | |
| 42 | VSD PDA | + | | D | 420 |
| 43 | VSD DORV | + | | T | 667 |
| 44 | VSD CoA VORV
EA | + | Gastric
fistula | D | 699 |
| Imperforate
anus | | | | |
| Total | | +:18/−:6 | | D:17/T:4/A:3 | |
As for the congenital heart diseases, 42 of 44
patients (95%) had some types of cardiac disorder. The breakdown
include VSD in 37 patients, PDA in 17 patients, atrial septal
defect (ASD) in 14 patients, coarctation of the aorta in 8
patients, double outlet right ventricle in 6 patients, endocardial
cushion defect in 3 patients, tricuspid atresia in 2 patients and
tetralogy of Fallot (TOF) in 2 patients, mitral valve atresia in 2
patients, transposition of great arteries and hypoplastic left
heart syndrome in 1 patient with trisomy 18. Although 42 cases had
a cardiac problem, only one case with TOF underwent surgery
following transfer from another hospital. Diuretic medication was
prescribed for the management of the cardiac anomalies. Among the
major central nervous malformations, 3 patients with trisomy 18 had
complicated brain or spinal anomaly. Two patients had spinal
meningocele, 1 patient had holoprosencephaly and 1 patient had
Dandy-Walker malformation. Sixteen cases of esophageal atresia were
also identified, while 4 cases had umbilical hernia. Surgical
treatment of gastric fistula was performed in 8 cases of trisomy 18
with complicated severe esophageal atresia with cardiac
anomalies.
Mechanical ventilation was performed in 11 cases in
20 samples (55%) obtained from group A and 18 cases in 24 samples
(75%) obtained from group B. Thus, treatment using mechanical
ventilation showed an increasing trend between group A and B. The
primary reason for ventilation therapy was due to respiratory
failure with cardiac failure and management of surgery. In-hospital
death at NICU occurred in 35 cases, with 4 cases being transferred
from another hospital to undergo surgical treatment and 5 cases
that were discharged from the NICU were alive. Alive discharge and
hospital transfer were increased in group B. The longest survival
case was a female patient who survived >6 years and 4
months.
Prognosis
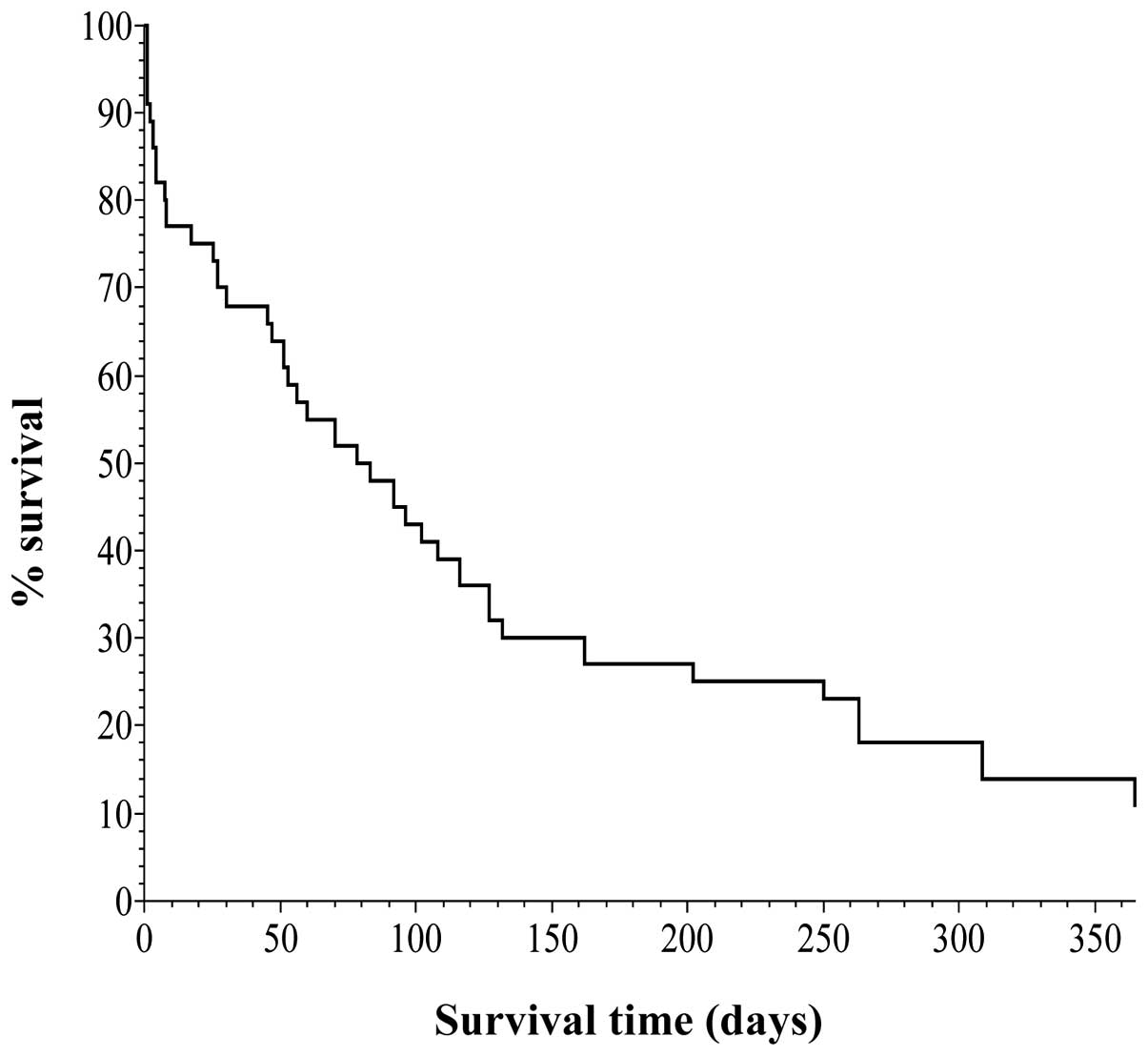
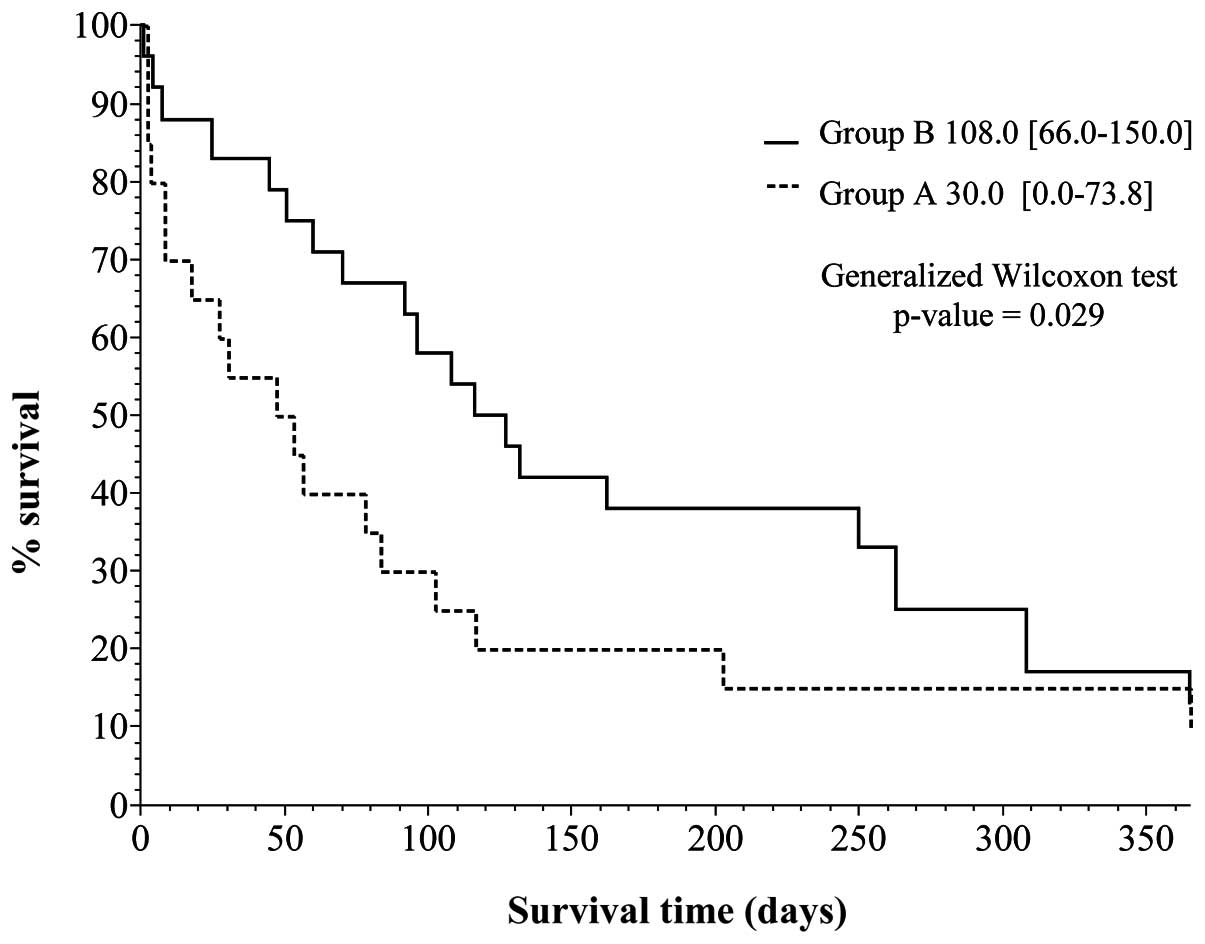
Kaplan-Meier survival curves were created (Figs. 1 and 2), based on the data obtained from
Table IV. Vertical and horizontal
axes of the two figures are the percentage survival and survival
days, respectively, setting the longest survival time at 1
year.
 | Table IVThe analyzed data of the log-rank
test at each observation period (group A vs. B). |
Table IV
The analyzed data of the log-rank
test at each observation period (group A vs. B).
| Survival days | Chi-square
distribution | df | p-value |
|---|
| 180 | 4.381 | 1 | 0.036 |
| 365 | 3.173 | 1 | 0.075 |
| Observation
end | 2.344 | 1 | 0.126 |
Fig. 1 shows the
curve for all 44 cases, indicating that one group survived for 4–6
months and the other group survived for a longer period of
time.
Fig. 2 is a
breakdown of the data shown in Fig.
1, showing the percentage survival curves for groups A and B.
The difference in the curves between groups A and B are evident.
The generalized Wilcoxon test was employed to determine the
differences between the two curves. The results showed a
statistically significant (<0.05) p-value of 0.029 (95%
confidence interval), indicating a better prognosis for group B in
the previous 10 years.
Notably, the two curves showed an increasing
tendency of percentage survival during the first 1- to 6-month
period (Fig. 2). Subsequently,
however, the difference in the percentage survival of the two
groups indicated a decreasing tendency in the 6-month to 1 year
period. We analyzed this tendency using the log-rank test at 180
days and 1 year (Table IV). The
results of the log-rank test indicated a statistically significant
(<0.05) p-value of 0.036 at the survival point of 180 days. At
the point of 1 year as well as the entire investigated period, the
difference was not statistically significant (<0.05) with a
p-value of 0.075 and 0.126.
Discussion
In this study, we have examined the prognosis of 44
cases of trisomy 18 identified in our NICU over the course of 21
years from 1992 to 2013. Depending on the timing of birth, the
subjects with trisomy 18 were divided into two groups, with group A
comprising 20 cases, born during the period of 1992–2002, and group
B comprising 24 cases, born during the period of 1992–2012. The aim
of the present study was to clarify the characteristics and
prognosis for each trisomy 18 case, and to compare and consider the
life prognosis of the two groups. A comparison of factors such as
chromosome type and gender, as well as gestational age as at the
time of birth, birth place, vaginal delivery or cesarean section as
method of delivery, and Apgar scores based on the 1- and 5-min
value, and birth weight was made for groups A and B. Statistical
analysis was conducted using three methods to verify the existence
of significant differences for groups A and B regarding trisomy 18
for newborns following birth. The results for the A and B trisomy
18 groups showed that, a significant difference was identified only
for cesarean section, whereas no significant difference was
identified for other factors with regard to the state of the child
at birth. Thus, the two groups were considered uniform. A
comparison of the two groups using the Kaplan-Meier curve and
generalized Wilcoxon test showed an improvement in prognosis in
group B. Thus, the progression of medical intervention during the
two periods prior to and following the study period is evident due
to the life prognosis of patients with trisomy 18 having improved.
However, the results of the log-rank test conducted showed that,
although a significant difference was observed in the trisomy 18
prognosis at the time of 180 days after birth, at 1 year and the
entire survey period after birth, these significant differences
could not be confirmed. Thus, additional studies should be
performed to confirm the life prognosis and medical intervention of
trisomy 18 for neonatal to infantile periods.
Previously, despite medical treatment the prognosis
for patients with trisomy 18 was lethal reason for chromosomal
aberration. Trisomies 21 (Down syndrome), 18 (Edwards syndrome) and
13 (Patau syndrome), as well as other chromosomes are associated
with various types of severe anomalies of the mainly brain and
viscera, and affected neonate usually do not survive infantile
period (4,5). Additionally, complete autosomal
trisomies frequently resulted in non-implantation or miscarriage;
thus, chromosomal abnormality cases that survive following birth
are primarily trisomies 21, 18 and 13 (8). Jenderny (32) conducted a study on a German
population, and revealed that clinically, the majority of trisomies
are unborn due to miscarriage. Pregnancy loss rates ranging from 72
to 87% for trisomy 18 were demonstrated by Sibiude et al
(33) and between 49 and 66% by
Lakovschek et al (34) for
trisomy 13, respectively. It has been previously identified that
trisomy 18 and 13 are the most common trisomies following trisomy
21 (35), with trisomy 18 being
the second common trisomy after trisomy 21. Baird and Sadovnick
(36) and Noble (37) reported that the average life
expectancy of Down syndrome was >50 years old due to medical
advances in medically advanced countries. However, to the best of
our knowledge, the majority of live-born infants with trisomy 18
and 13 survive <1 year and do not survive to adulthood. In our
study, only 5 of the 44 patients survived beyond 1 year of age.
Generally, the prognosis of trisomy 18 and 13 are lethal, but
long-term survival cases of such trisomies have been sporadically
observed. Kelly et al (38)
described 20-year-old women and Torres Hinojal et al
(39) reported a 14-year-old
patient with trisomy 18. Another case report of long survival of
cases of trisomy 18 was conducted by Petek et al (40) who reported on a 19-year-old female
and by Smith et al (41)
who reported on an 11-year-old female patient and a 21-year-old
female patient (42). A search of
the literature yielded ≥10 previously reported cases of affected
children with trisomy 18, aged >10 years (43–45).
In our patient, the oldest individual with trisomy 18 was a 6-year
and 4-month-old female patient with severe intellectual and
psychomotor disorder.
Patients with trisomy 18 who show long-term survival
present severe psychomotor delay (46). For such cases, aggressive medical
treatment in the intensive care unit is controversial as life
prognosis is lethal. Only a few studies of patients being managed
at NICU have been previously reported. Of these, Kosho et al
(14,31) demonstrated that restricted and
intensive medical treatments in NICU are valuable in the form of
genetic counseling. Since the 1980's, resuscitation and surgical
treatments for trisomy 18 have been considered from the standpoints
of medical ethics and consideration of quality of life for patients
as well as parents and their families, medical economic issues, and
medical environment in each countries. In Japan, Nishida and
Sakamoto (30) suggested a policy
of 'no treatments beyond current treatments' for treatment of
newborn infants in 1992. Paris et al (47) also indicated ambiguous ethical
issues in the use of life-prolonging interventions for an infant
with trisomy 18 in 1992. However, long survival of individuals with
chromosomal abnormalities including trisomy 18 and 13, owing to
medical progression has since been identified. Notably, treatment
policy has also led to gradually changes in medical, sociological,
and technical progression. The 4th edition of Smith's textbook in
1988 supports the 'limitation of all medical means for prolongation
of life for trisomy 18' (48).
However, the 7th edition of the same textbook in 2013, supports
that 'limitation of extraordinary medical means should be seriously
considered'. In addition, it advocates that the personal feelings
of the parents and the individual circumstances of each infant be
taken into consideration (49).
The survival of patients with trisomy 18 and the
survival rate from several countries for the period 1967–2010 have
been reported in the present study. A long survival period of over
6 months is difficult for patients with trisomy 18, as indicated by
Lin et al in 2006 (52) and
Weber et al (50) in 1967.
On the other hand, the recent findings of Kosho et al
(14) and those of our group
(8) for populations of Japan,
revealed improved life outcome of trisomy 18. Studies have been
recently conducted to investigate treatment procedures and outcome
for trisomy 18 (53). Mechanical
ventilation treatment was performed in 29 of 44 patients (65.9%) by
in the present study and the percentage was increased in group B.
Paris et al (47) suggested
that physicians should be attentive with regard to concentration
treatment including ventilation therapy and life-prolonging
intervention. Bos et al (29) also report avoidance of emergency
surgery in newborns with trisomy 18. In the present study, patients
underwent ventilation therapy owing to respiratory failure and
general management. Fenton (54)
suggested factors such as quality of life for determining treatment
for trisomy 18. On the other hand, surgical treatment is preferred
following informed consent with counseling from the family support
meeting (55). Kosho (31) emphasized care of children with
trisomy 18 in Japan. Information such as cesarean section (56), general condition (57), health care (58), family (59) and neonatal experience (60) care were important in the treatment
of cases with trisomy 18. However, no clear guideline regarding
treatment policy and strategy has been established. In Japan, the
surgical treatment of a variety of congenital cardiac diseases
(61–65) and esophageal atresia (66) have been performed in patients with
trisomy 18. In addition, treatment for apnea and epilepsy (67–69)
were also provided. Graham et al (70) summarized 35 cases of cardiac
surgery with trisomy 18 and 13, with increased discharge from the
hospital. However, prognosis and quality of life of those patients
remain unclear. Janvier et al (71) discussed the issue of ethics
regarding an infant-associated VSD. Kaneko et al (62) reported 17 cases of trisomy 18 with
cardiac surgery and 14 cases (82%) were discharged from NICU
leading to a conclusion that it was effective in decreasing
mortality in NICU due to cardiac failure. NICU management with
ventilation and surgical procedures has potential to improve the
short-term outcome of <1 year in trisomy 18. In the present
study, an improvement in the outcome of 6 months from delivery by
various treatments including mechanical ventilation and surgical
procedure was observed. However, additional studies on a larger
number of cases should be performed concerning treatment strategy
and genetic counseling to the family with a newborn with trisomy
18.
Although the present study had a small sample size,
our data provide one of the larger single-center studies currently
available. The results of the present study provide information
concerning genetic counseling for parents and their families and
life prognosis, prior to applying intensive management to newborns
with trisomy 18
Acknowledgments
We would like to express our gratitude and
appreciation to all the NICU medical staff, Dokkyo Medical
University Hospital, Tochigi, Japan.
References
|
1
|
Edwards JH, Harnden DG, Cameron AH, Crosse
VM and Wolff OH: A new trisomic syndrome. Lancet. 1:787–790. 1960.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith DW, Patau K, Therman E and Inhorn
SL: A new autosomal trisomy syndrome: multiple congenital anomalies
caused by an extra chromosome. J Pediatr. 57:338–345. 1960.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cereda A and Carey JC: The trisomy 18
syndrome. Orphanet J Rare Dis. 7:812012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carey JC: Trisomy 18 and trisomy 13
syndrome. Management of genetic syndromes. Allanson J and Cassidy
S: 2nd edition. Wiley; Hoboken, NJ: pp. 555–568. 2005
|
|
5
|
Menkes JH and Falk RE: Chapter 3
Chromosomal anomalies and contiguous gene syndrome. Child
Neurology. Menkes JH and Sarnat HB: 6th edition. Lippincott
Williams and Wilkins; Philaderphia, PA: pp. 241–275. 2000
|
|
6
|
Jones KL: Tirsomy 18 syndrome. Smith's
recognized patterns of human malformation. 6th edition. Elsevier
Saunders; Philaderphia, PA: pp. 18–21. 2006
|
|
7
|
Imataka G, Yamanouchi H and Arisaka O:
Dandy-Walker syndrome and chromosomal abnormalities. Congenit Anom
(Kyoto). 47:113–118. 2007. View Article : Google Scholar
|
|
8
|
Imataka G, Nitta A, Suzumura H, Watanabe
H, Yamanouchi H and Arisaka O: Survival of trisomy 18 cases in
Japan. Genet Couns. 18:303–308. 2007.PubMed/NCBI
|
|
9
|
Root S and Carey JC: Survival in trisomy
18. Am J Med Genet. 49:170–174. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldstein H and Nielsen KG: Rates and
survival of individuals with trisomy 13 and 18. Data from a 10-year
period in Denmark. Clin Genet. 34:366–372. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forrester MB and Merz RD: Trisomies 13 and
18: prenatal diagnosis and epidemiologic studies in Hawaii,
1986–1997. Genet Test. 3:335–340. 1999. View Article : Google Scholar
|
|
12
|
Bergin A, McManus SP, Clarke TA and
Moloney M: Trisomy 18: a nine year review. Ir J Med Sci. 157:5–7.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rasmussen SAA, Wong LYC, Yang Q, May KM
and Friedman JM: Population-based analyses of mortality in trisomy
13 and trisomy 18. Pediatrics. 111:777–784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kosho T, Nakamura T, Kawame H, Baba A,
Tamura M and Fukushima Y: Neonatal management of trisomy 18:
clinical details of 24 patients receiving intensive treatment. Am J
Med Genet A. 140:937–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baty BJ, Blackburn BL and Carey JC:
Natural history of trisomy 18 and trisomy 13: I. Growth, physical
assessment, medical histories, survival, and recurrence risk. Am J
Med Genet. 49:175–188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brewer CM, Holloway SH, Stone DH,
Carothers AD and FitzPatrick DR: Survival in trisomy 13 and trisomy
18 cases ascertained from population based registers. J Med Genet.
39:e542002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Embleton ND, Wyllie JP, Wright MJ, Burn J
and Hunter S: Natural history of trisomy 18. Arch Dis Child Fetal
Neonatal Ed. 75:F38–F41. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crider KS, Olney RS and Cragan JD:
Trisomies 13 and 18: Population prevalences, characteristics, and
prenatal diagnosis, metropolitan Atlanta, 1994–2003. Am J Med
Genet. A146A:820–826. 2008. View Article : Google Scholar
|
|
19
|
Irving C, Richmond S, Wren C, Longster C
and Embleton ND: Changes in fetal prevalence and outcome for
trisomies 13 and 18: a population-based study over 23 years. J
Matern Fetal Neonatal Med. 24:137–141. 2011. View Article : Google Scholar
|
|
20
|
Van Dyke DC and Allen M: Clinical
management considerations in long-term survivors with trisomy 18.
Pediatrics. 85:753–759. 1990.PubMed/NCBI
|
|
21
|
Niedrist D, Riegel M, Achermann J and
Schinzel A: Survival with trisomy 18 - data from Switzerland. Am J
Med Genet A. 140:952–959. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vendola C, Canfield M, Daiger SP, Gambello
M, Hashmi SS, King T, Noblin SJ, Waller DK and Hecht JT: Survival
of Texas infants born with trisomies 21, 18, and 13. Am J Med Genet
A. 152A:360–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naguib KK, Al-Awadi SA, Moussa MAA,
Bastaki L, Gouda S, Redha MA, Mustafa F, Tayel SM, Abulhassan SA
and Murthy DSK: Trisomy 18 in Kuwait. Int J Epidemiol. 28:711–716.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuroki Y and Kurosawa K: No sex
differences in 18 trisomy births in the Kanagawa Birth Defects
Monitoring Program. Congenit Anom (Kyoto). 44:97–98. 2004.
View Article : Google Scholar
|
|
25
|
Jones KL: Tirsomy 18 syndrome. Smith's
recognized patterns of human malformation. 5th edition. Elsevier
Saunders; Philaderphia, PA: pp. 14–15. 1997
|
|
26
|
De Souza E, Halliday J, Chan A, Bower C
and Morris JK: Recurrence risks for trisomies 13, 18, and 21. Am J
Med Genet A. 149A:2716–2722. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fisher JM, Harvey JF, Lindenbaum RH, Boyd
PA and Jacobs PA: Molecular studies of trisomy 18. Am J Hum Genet.
52:1139–1144. 1993.PubMed/NCBI
|
|
28
|
Norup M: Attitudes towards abortion among
physicians working at obstetrical and paediatric departments in
Denmark. Prenat Diagn. 18:273–280. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bos AP, Broers CJ, Hazebroek FW, van Hemel
JO, Tibboel D, Wesby-van Swaay E and Molenaar JC: Avoidance of
emergency surgery in newborn infants with trisomy 18. Lancet.
339:913–915. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishida H and Sakamoto S: Ethical problems
in neonatal intensive care unit - medical decision making on the
neonate with poor prognosis. Early Hum Dev. 29:403–406. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kosho T: Care of children with trisomy 18
in Japan. Am J Med Genet A. 146A:1369–1371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jenderny J: Chromosome aberrations in a
large series of spontaneous miscarriages in the German population
and review of the literature. Mol Cytogenet. 7:382014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sibiude J, Gavard L, Floch-Tudal C and
Mandelbrot L: Perinatal care and outcome of fetuses with trisomies
13 and 18 following a parental decision not to terminate the
pregnancy. Fetal Diagn Ther. 29:233–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lakovschek IC, Streubel B and Ulm B:
Natural outcome of trisomy 13, trisomy 18, and triploidy after
prenatal diagnosis. Am J Med Genet A. 155A:2626–2633. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Imataka G, Suzumura H and Arisaka O:
Diagnosis of sex chromosomal abnormalities in neonatal intensive
care units. Genet Couns. 24:399–403. 2013.
|
|
36
|
Baird PA and Sadovnick AD: Life expectancy
in Down syndrome adults. Lancet. 2:1354–1356. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Noble J: Natural history of Down's
syndrome: a brief review for those involved in antenatal screening.
J Med Screen. 5:172–177. 1998. View Article : Google Scholar
|
|
38
|
Kelly M, Robinson BW and Moore JW: Trisomy
18 in a 20-year-old woman. Am J Med Genet. 112:397–399. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Torres Hinojal MC, Marugán de Miguelsanz
JM and Rodríguez Fernández LM: Fourteen-year survival in a patient
with Edwards syndrome. An Pediatr (Barc). 63:458–459. 2005.In
Spanish. View Article : Google Scholar
|
|
40
|
Petek E, Pertl B, Tschernigg M, Bauer M,
Mayr J, Wagner K and Kroisel PM: Characterisation of a 19-year-old
'long-term survivor' with Edwards syndrome. Genet Couns.
14:239–244. 2003.
|
|
41
|
Smith A, Silink M and Ruxton T: Trisomy 18
in an 11 year old girl. J Ment Defic Res. 22:277–286.
1978.PubMed/NCBI
|
|
42
|
Smith A, Field B and Learoyd BM: Trisomy
18 at age 21 years. Am J Med Genet. 34:338–339. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hook EB, Lehrke R, Roesner A and Yunis JJ:
Trisomy-18 in a 15-year-old female. Lancet. 2:910–911. 1965.
View Article : Google Scholar
|
|
44
|
Surana RB, Bain HW and Conen PE:
18-Trisomy in a 15-year-old girl. Am J Dis Child. 123:75–77.
1972.PubMed/NCBI
|
|
45
|
Houlihan OA and O'Donoghue K: The natural
history of pregnancies with a diagnosis of trisomy 18 or trisomy
13; a retrospective case series. BMC Pregnancy Childbirth.
13:2092013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baty BJ, Jorde LB, Blackburn BL and Carey
JC: Natural history of trisomy 18 and trisomy 13: II. Psychomotor
development. Am J Med Genet. 49:189–194. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Paris JJ, Weiss AH and Soifer S: Ethical
issues in the use of life-prolonging interventions for an infant
with trisomy 18. J Perinatol. 12:366–368. 1992.PubMed/NCBI
|
|
48
|
Jones KL: Tirsomy 18 syndrome. Smith's
recognized patterns of human malformation. 4th edition. Elsevier
Saunders; Philaderphia, PA: pp. 16–17. 1988
|
|
49
|
Jones KL: Tirsomy 18 syndrome. Smith's
recognized patterns of human malformation. 7th edition. Elsevier
Saunders; Philaderphia, PA: pp. 14–17. 2013
|
|
50
|
Weber WW: Survival and the sex ratio in
trisomy 17–18. Am J Hum Genet. 19:369–377. 1967.PubMed/NCBI
|
|
51
|
Carter PE, Pearn JH, Bell J, Martin N and
Anderson NG: Survival in trisomy 18. Life tables for use in genetic
counselling and clinical paediatrics. Clin Genet. 27:59–61. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin HY, Lin SP, Chen YJ, Hung HY, Kao HA,
Hsu CH, Chen MR, Chang JH, Ho CS and Huang FY: Clinical
characteristics and survival of trisomy 18 in a medical center in
Taipei, 1988–2004. Am J Med Genet A. 140:945–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ishitsuka K, Matsui H, Michihata N,
Fushimi K, Nakamura T and Yasunaga H: Medical procedures and
outcomes of Japanese patients with trisomy 18 or trisomy 13:
Analysis of a nationwide administrative database of hospitalized
patients. Am J Med Genet A. 167A:1816–1821. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fenton LJ: Trisomy 13 and 18 and quality
of life: Treading 'softly'. Am J Med Genet A. 155A:1527–1528. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kosho T, Kuniba H, Tanikawa Y, Hashimoto Y
and Sakurai H: Natural history and parental experience of children
with trisomy 18 based on a questionnaire given to a Japanese
trisomy 18 parental support group. Am J Med Genet A.
161A:1531–1542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schneider AS, Mennuti MT and Zackai EH:
High cesarean section rate in trisomy 18 births: a potential
indication for late prenatal diagnosis. Am J Obstet Gynecol.
140:367–370. 1981.PubMed/NCBI
|
|
57
|
Imai K, Uchiyama A, Okamura T, Ago M,
Suenaga H, Sugita E, Ono H, Shuri K, Masumoto K, Totsu S, et al:
Differences in mortality and morbidity according to gestational
ages and birth weights in infants with trisomy 18. Am J Med Genet
A. 167:2610–2617. 2015. View Article : Google Scholar
|
|
58
|
Walker LV, Miller VJ and Dalton VK: The
health-care experiences of families given the prenatal diagnosis of
trisomy 18. J Perinatol. 28:12–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Janvier A, Farlow B and Wilfond BS: The
experience of families with children with trisomy 13 and 18 in
social networks. Pediatrics. 130:293–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bruns DA: Neonatal experiences of newborns
with full trisomy 18. Adv Neonatal Care. 10:25–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kaneko Y, Kobayashi J, Yamamoto Y, Yoda H,
Kanetaka Y, Nakajima Y, Endo D, Tsuchiya K, Sato H and Kawakami T:
Intensive cardiac management in patients with trisomy 13 or trisomy
18. Am J Med Genet A. 146A:1372–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kaneko Y, Kobayashi J, Achiwa I, Yoda H,
Tsuchiya K, Nakajima Y, Endo D, Sato H and Kawakami T: Cardiac
surgery in patients with trisomy 18. Pediatr Cardiol. 30:729–734.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kobayashi J, Kaneko Y, Yamamoto Y, Yoda H
and Tsuchiya K: Radical surgery for a ventricular septal defect
associated with trisomy 18. Gen Thorac Cardiovasc Surg. 58:223–227.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Maeda J, Yamagishi H, Furutani Y, Kamisago
M, Waragai T, Oana S, Kajino H, Matsuura H, Mori K, Matsuoka R, et
al: The impact of cardiac surgery in patients with trisomy 18 and
trisomy 13 in Japan. Am J Med Genet A. 155A:2641–2646. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Muneuchi J, Yamamoto J, Takahashi Y,
Watanabe M, Yuge T, Ohno T, Imoto Y, Sese A and Joo K: Outcomes of
cardiac surgery in trisomy 18 patients. Cardiol Young. 21:209–215.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nishi E, Takamizawa S, Iio K, Yamada Y,
Yoshizawa K, Hatata T, Hiroma T, Mizuno S, Kawame H and Fukushima
Y: Surgical intervention for esophageal atresia in patients with
trisomy 18. Am J Med Genet A. 164A:324–330. 2014. View Article : Google Scholar
|
|
67
|
Kumada T, Nishii R, Higashi T, Oda N and
Fujii T: Epileptic apnea in a trisomy 18 infant. Pediatr Neurol.
42:61–64. 2010. View Article : Google Scholar
|
|
68
|
Kumada T, Maihara T, Higuchi Y, Nishida Y,
Taniguchi Y and Fujii T: Epilepsy in children with trisomy 18. Am J
Med Genet A. 161A:696–701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fukasawa T, Kubota T, Tanaka M, Asada H,
Matsusawa K, Hattori T, Kato Y and Negoro T: Apneas observed in
trisomy 18 neonates should be differentiated from epileptic apneas.
Am J Med Genet A. 167A:602–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Graham EM, Bradley SM, Shirali GS, Hills
CB and Atz AM; Pediatric Cardiac Care Consortium: Effectiveness of
cardiac surgery in trisomies 13 and 18 (from the Pediatric Cardiac
Care Consortium). Am J Cardiol. 93:801–803. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Janvier A, Okah F, Farlow B and Lantos JD:
An infant with trisomy 18 and a ventricular septal defect.
Pediatrics. 127:754–759. 2011. View Article : Google Scholar : PubMed/NCBI
|