Introduction
Monoacylglycerol lipase (MAGL), which is an enzyme
of the serine hydrolase family, is the key enzyme associated with
the degradation of triacylglycerol (TAG) into free-fatty acids
(FFA) (1). MAGL hydrolyzes
2-arachidonoylglycerol (2-AG) into arachidonic acid and glycerol,
thus regulating the endocannabinoid system (2). A previous study regarding MAGL
focused on the role of 2-AG hydrolysis in endocannabinoid signaling
(3). 2-AG belongs to the
endocannabinoid system, which is associated with the regulation of
inflammation, nociception and the immune response (4), and exerts a neuroprotective effect in
brain injury (5). Long et
al (6) identified a selective
and effective inhibitor of MAGL, JZL184, which significantly
decreased the hydrolytic activity of MAGL and inhibited 2-AG
hydrolysis in the brains of mice. It has previously been
demonstrated that MAGL is highly expressed in certain types of
aggressive cancer, and regulates the fatty acid network to
influence tumor metastasis (7).
Furthermore, Nomura et al (8) demonstrated that in androgen-dependent
prostate cancer, MAGL influenced tumor proliferation and metastasis
via the cannabinoid system and fatty acid network. As a
characteristic of the malignant colorectal cancer phenotype,
metastasis is markedly associated with the epithelial-mesenchymal
transition (EMT) process (9). A
previous study indicated that MAGL is a marker of EMT; therefore,
the effects of JZL184 in colorectal cancer have recently garnered
interest (10). Furthermore, Ye
et al (11) observed that
MAGL was highly expressed in colorectal cancer tissues, as compared
with normal tissue, and MAGL expression was associated with the
body mass index (BMI) of patients. With decreasing MAGL activity,
the protein expression levels of cyclin D1 and B-cell lymphoma 2
(Bcl-2) were reduced, apoptosis was increased, and the cell cycle
was arrested in colorectal cancer cell lines (11). Metastasis and decreased apoptosis
are associated with tumorigenesis, both of which are particularly
associated with Bcl-2/Bcl-2-associated X protein (Bax) and the EMT
process. A previous study demonstrated that Bcl-2/Bax was closely
associated with apoptosis in colorectal cancer pathogenesis
(12,13). Bcl-2 decreases apoptosis in cancer
cells (14); however, Bax inhibits
the function of Bcl-2 in order to induce cell death (15). However, the association between
JZL184 and Bcl-2/Bax in colorectal cancer remains to be
elucidated.
The present study aimed to determine the effects of
JZL184 on apoptosis and migration, and to elucidate the association
between JZL184, Bcl-2/Bax and EMT markers.
Materials and methods
Cell culture
The HCT116, SW480 and LoVo colorectal cancer cell
lines were supplied by the Laboratory of Gastrointestinal Surgery,
Union Hospital (Wuhan, China). The cells were grown in high glucose
Dulbecco's modified Eagle's medium (DMEM; Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). SW620 cells (supplied by the Laboratory of Gastrointestinal
Surgery, Union Hospital) were grown in RPMI-1640 (Hyclone; GE
Healthcare Life Sciences) and supplemented with 10% FBS (16). All cells were maintained in a
humidified 37°C incubator containing 5% CO2. The HCT116,
SW480 and LoVo cell lines were grown in DMEM with varying
concentrations of JZL184 (10, 25 or 50 µM; Sigma-Aldrich,
St. Louis, MO, USA) or dimethyl sulfoxide (DMSO; 0.1%),
representing the control group, for 48 h. These cells were then
used in the following assays, except the migration assay.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells (1×104) of the HCT116, SW480 and
LoVo cell lines were grown in 96-well plates for 48 h, as
described. Medium was then replaced in the cell cultures with DMEM
only (control group) or DMEM supplemented with 10 µM JZL184
(experimental group). Cells were then treated with varying
concentrations of 5-fluorouracil (10, 100, 200 or 500 µM)
for 48 h. Subsequently, 20 µl (5 mg/ml) MTT was added to
each well for 4 h. The MTT formazan precipitate was dissolved in
DMSO to 150 µl. The absorbance of each well was measured at
a wavelength of 490 nm, and the cell proliferation inhibition rate
was calculated using the following formula: Proliferation
inhibition rate = [1 - value of the experimental group/average
value of the control (5-fluorouracil only) group] × 100% (17).
Annexin-V allophycocyanin (APC)/propidium
iodide (PI) double labeling
The cells were collected and washed with
phosphate-buffered saline (PBS). According to the manufacturer's
protocol, samples were stained with Annexin-V APC (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) and PI (Sigma-Aldrich) for 30
min at room temperature in the dark. The cells were then examined
using a BD FACSCanto II flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total mRNA was extracted from the cells using
TRIzol® (Takara Bio, Inc., Otsu, Japan). According to
the manufacturer's protocols the RNA was reverse transcribed to
cDNA using Primescript RT Master mix (Takara Bio, Inc.). RT-qPCR
was used to detect the levels of mRNA in each sample, with
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serving as an
internal control. SYBR Green Master mix (Takara Bio, Inc.) and the
StepOnePlus™ Real-time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) were used to conduct qPCR, according to
the manufacturers' protocols. The qPCR cycling conditions were as
follows: Denaturing the cDNA at 95°C for 30 sec; annealing at 60°C
for 60 sec; and extension at 95°C for 5 sec. The reaction proceeded
for 40 cycles, gathering the data of each sample, and the mRNA
expression levels were evaluated using the 2−ΔΔCq method
(18). The primers were obtained
from Genscript Corporation (Nanjing, China) and the sequences were
as follows: Bcl-2, forward 5′-TTGCCCTCAAACAGAACAGC-3′ and reverse
5′-TGCAGCTCCTCTTGGCTAAA-3′; Bax, forward 5′-GGCCGGGTTGTCGCCCTTTT-3′
and reverse 5′-CCGCTCCCGGAGGAAGTCCA-3′; and GAPDH, forward
5′-GTTCCCACTGTCGATGTCTCA-3′ and reverse
5′-CCCTTCATCTTGCCCTCAGA-3′.
Western blot analysis
Cells were lysed with sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) Sample
Loading buffer (Beyotime Institute of Biotechnology, Haimen, China)
supplemented with proteinase inhibitors (Roche Diagnostics, Basel,
Switzerland), and the concentration of total protein was measured
using a Bradford Protein assay kit (Beyotime Institute of
Biotechnology). Proteins (5 µg/µl) were separated on
8 or 12% SDS-polyacrylamide gels by electrophoresis and were
transferred to polyvinylidene fluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA). The PVDF membranes were blocked
with 5% skimmed milk protein and 0.1% Tween 20 for 1 h. Primary
antibodies were added and incubated overnight at 4°C, prior to the
addition of goat anti-rabbit secondary antibody for 2 h at room
temperature. The primary antibodies used in the present study, from
Cell Signalling Technology Inc. (Danvers, MA, USA) and used at a
dilution of 1:1,000, were: Rabbit anti-E-cadherin (cat. no. 3195),
rabbit anti-vimentin (cat. no. 5741), rabbit anti-zinc finger
protein SNAI1 (Snail; cat. no. 3879), rabbit anti-Bcl-2 (cat. no.
2870), rabbit anti-Bax (cat. no. 2772). Rabbit anti-GAPDH was
supplied by Sigma-Aldrich (cat. no. G9545) and used at a dilution
of 1:5,000. The secondary antibody used was polyclonal horseradish
peroxidase-conjugated goat anti-rabbit IgG (dilution, 1:1,000; cat.
no. BA1054; Boster Biological Technology, Pleasanton, CA, USA).
Background autofluorescence was removed and blots were imaged using
a ChemiDoc XRS+ imaging system and blots were analyzed with Image
Lab software (Bio-Rad Laboratories, Inc., Hercules, CA, USA), with
GAPDH acting as a reference protein. Relative expression of the
relevant protein was calculated using the formula: Relative
expression = (density value of the experimental group − value of
GAPDH) / (density value of the control group − value of GAPDH).
Cell migration assay
SW480 and SW620 cells (1×106) were
treated with 10 µM JZL184 for 48 h and plated onto transwell
cell culture inserts (8 µm microporous filters; BD
Biosciences), which provided an artificial basement membrane, for
12 h. The upper chamber of the transwell system contained DMEM (in
the case of SW480 cells) or RPMI-1640 (for SW620 cells); the lower
chamber contained corresponding medium supplemented with 20% FBS.
The migrating cells were stained with 0.1% crystal violet and five
randomly selected areas were used to count the number of cells
using an Olympus 1X71 inverted microscope (Olympus Corporation,
Tokyo, Japan) (16).
Statistical analysis
Each experiment was performed at least three times
independently. The data are presented as the mean ± standard
deviation. SPSS v. 18.0 software (SPSS, Inc., Chicago, IL, USA) was
used for all statistical analyses. Student's t-test was used to
statistically analyze the data. P<0.05 was considered to
indicate a statistically significant difference.
Results
JZL184 influences the proliferation of
colorectal cancer cell lines
JZL184 was dissolved in DMSO and administered to the
SW480, LoVo and HCT116 colon cancer cell lines. DMSO was used as a
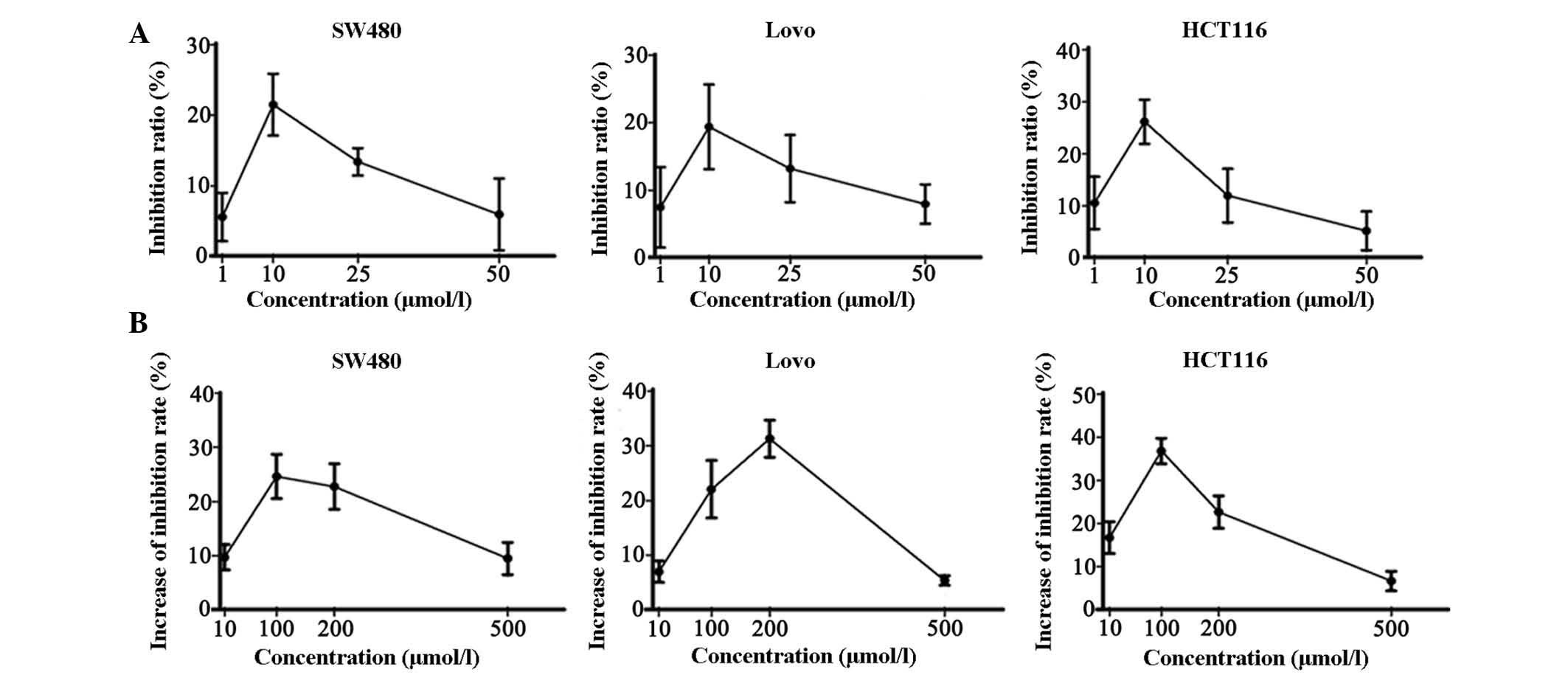
negative control. After a 48 h incubation, the inhibition ratio was
measured by MTT assay. The inhibition ratio was non-significantly
increased (P>0.05) in all three cell lines following treatment
with 10 µM JZL184 (Fig.
1A). In addition, JZL184 (10 µM) was combined with
various concentrations of 5-fluorouracil. In the colorectal cancer
cells treated with JZL184, cell growth was decreased (P>0.05).
The inhibition ratio was increased in the cells treated with both
JZL184 and 5-fluorouracil, as compared with in the cells treated
with 5-fluorouracil alone (Fig.
1B; P>0.05).
JZL184 induces colorectal cancer cell
apoptosis
The present study demonstrated that JZL184 had
marked effects on the proliferation of colorectal cancer cell
lines. Proliferation is closely associated with apoptosis in
cancer; therefore, apoptosis of the colorectal cancer cells treated
with JZL184 was investigated by flow cytometry. Compared with the
control group, the number of cells in early (Q2) and late (Q4)
apoptosis was increased in the treatment group (Fig. 2A). The rate of apoptosis was
increased in the three colorectal cancer cell lines (Fig. 2B). These results indicate that
JZL184 may induce apoptosis via decreasing proliferation in
colorectal cancer cell lines. In addition, the expression levels of
MAGL were significantly decreased following treatment with JZL184
(Fig. 2C and D).
JZL184 regulates Bcl-2 and Bax to induce
apoptosis of colorectal cancer cell lines
JZL184 promoted the apoptosis of colorectal cancer
cells. Bcl-2 and Bax are closely associated cell apoptosis proteins
(19); therefore, the effects of
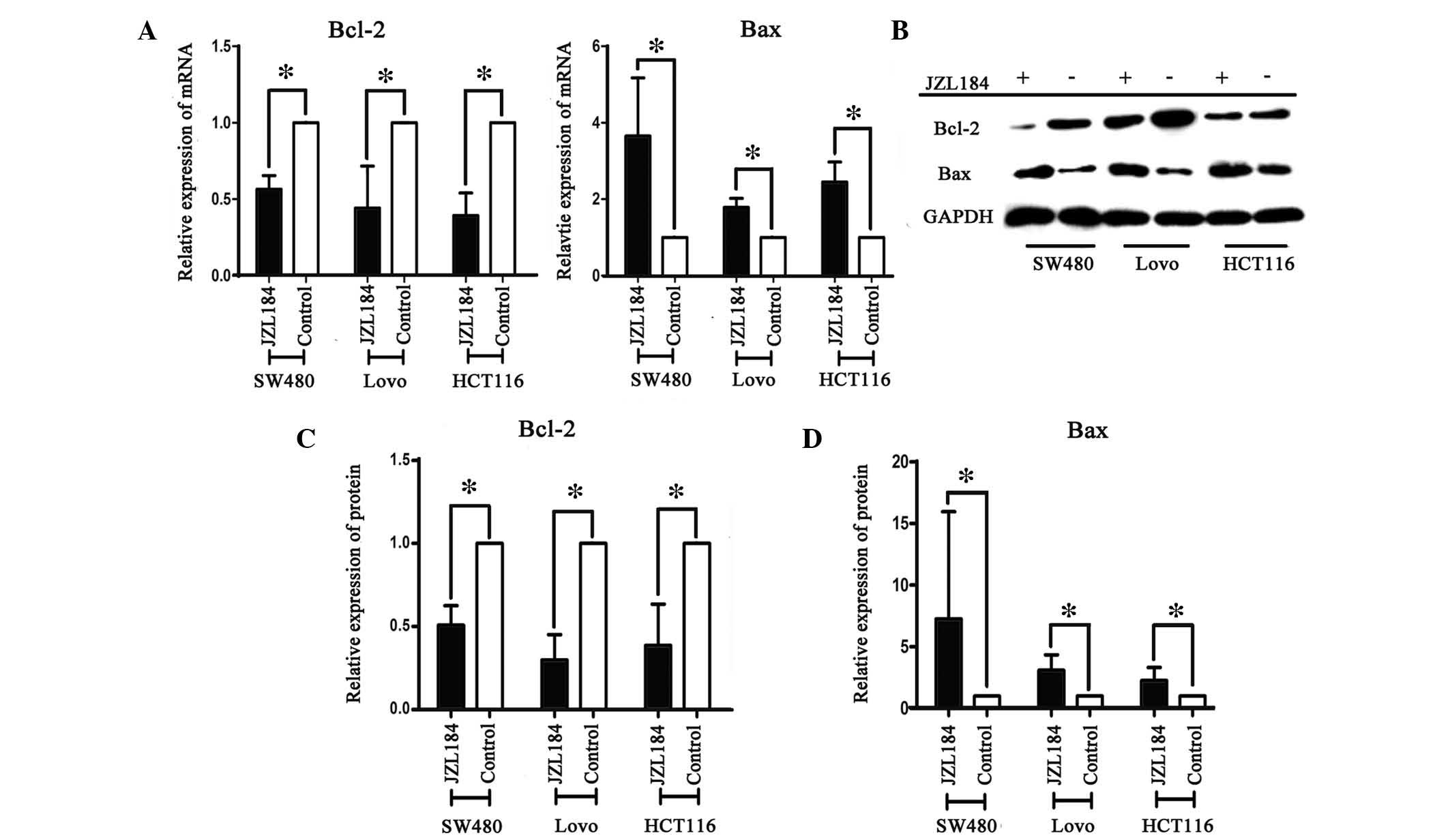
JZL184 on their mRNA expression levels were detected by RT-qPCR.
The mRNA expression levels of Bcl-2 were significantly lower in the
JZL184 intervention groups, as compared with the control group;
however, the expression levels of Bax were significantly higher
following JZL184 administration, as compared with in the control
group in the three colorectal cancer cell lines (Fig. 3A). The protein expression levels of
Bcl-2 and Bax were also detected following JZL184 administration.
Consistent with the mRNA expression levels, following JZL184
intervention Bcl-2 protein expression levels were significantly
decreased, as compared with the control group, whereas Bax protein
expression levels were increased (Fig.
3B and C). These results indicate that JZL184 is able to
regulate apoptosis via altering the mRNA and protein expression of
Bcl-2 and Bax.
JZL184 decreases migration in colorectal
cancer cell lines
Migration is required for metastasis in colorectal
cancer. To investigate whether JZL184 affected the migratory
ability of colorectal cancer cells, SW480 and SW620 cells were
selected. SW480 cells were initially derived from colon cancer
tissue from a patient with Duke's type B colon cancer, whereas
SW620 cells were initially derived from the metastatic lymph nodes
of a patient with Duke's type C colon cancer, as listed by the
American Type Culture Collection (www.lgcstandards-atcc.org/). The metastatic potential
of these cells from two stages of colorectal cancer differs
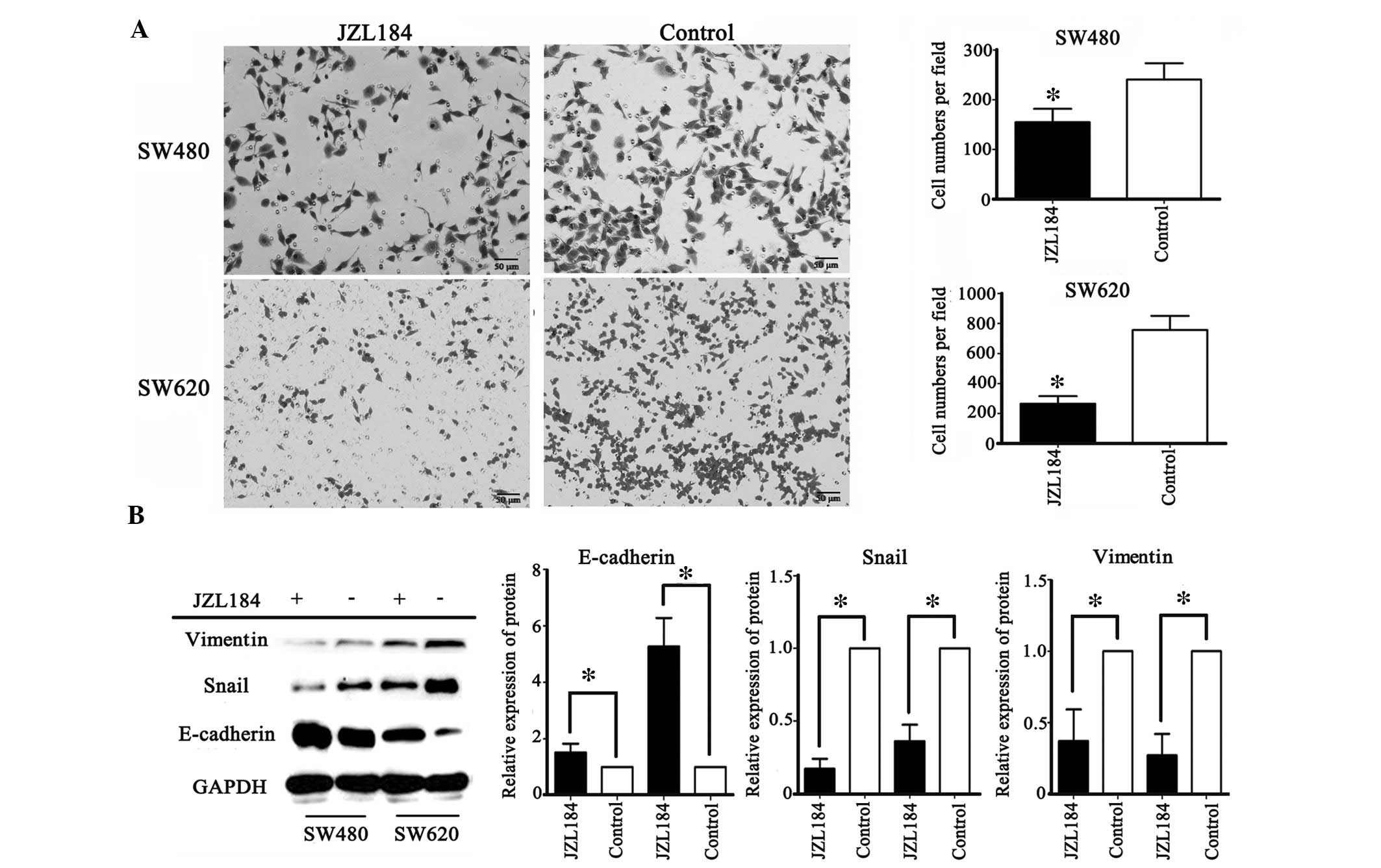
(20). Following administration of
JZL184, the migration of SW480 and SW620 cells was significantly
decreased. Compared with the SW480 cells, the suppression of SW620
cell migration was markedly increased (Fig. 4A).
JZL184 inhibits EMT in colorectal cancer
cell lines
EMT in epithelial tumor cells is closely associated
with the process of tumor cell metastasis. In the present study,
migration was observed to be inhibited by JZL184 in colorectal
cancer cell lines; therefore, the protein expression levels of EMT
markers were detected by western blotting. Following JZL184
administration, the SW480 and SW620 colorectal cancer cell lines
exhibited significantly increased E-cadherin protein expression and
decreased vimentin and Snail protein expression (Fig. 4B), thus indicating that MAGL may
regulate migration via EMT.
Discussion
In a tumor-bearing state, endogenous fatty acid
hydrolysis and oxidation are increased, triglyceride conversion and
plasma FFA levels are increased, and utilization of exogenous fat
is reduced (21). Previous studies
regarding MAGL have focused on the role of the nervous system
endocannabinoid signals in peripheral tissues and the central
nervous system. These have demonstrated that cannabinoids inhibit
cancer cell proliferation, induce tumor cell apoptosis and
influence tumor angiogenesis (22–24).
Nomura et al (7)
demonstrated that MAGL may regulate the fatty acid metabolism
network via the MAGL-FFA pathway, resulting in an increase in
various lipid signaling molecules, including
lysophosphatidylcholine and lysophosphatidic acid ethanolamine, and
particularly lysophosphatidic acid and prostaglandin E2
(PGE2), which have been indicated to be closely
associated with tumor proliferation and metastasis. Nomura et
al (8) also demonstrated that
in androgen-dependent prostate cancer, MAGL influences tumor
proliferation and metastasis via the cannabinoid system and fatty
acid network.
The present study demonstrated that tumor cell
proliferation was reduced and apoptosis increased in response to
MAGL inhibition. Furthermore, treatment with JZL184 and
5-fluorouracil induced greater cell apoptosis than 5-fluorouracil
alone in colorectal cancer cell lines. These results suggested that
JZL184 may decrease tumor growth and enhance sensitivity to
chemotherapy. In addition, Bcl-2 and Bax mRNA and protein
expression levels were altered by JZL184 administration. This may
suggest that MAGL regulates Bcl-2 and Bax protein expression levels
to affect cancer cell activity. Ye et al (11) demonstrated that MAGL expression was
positively correlated with BMI values, and individuals with a BMI
>30 had higher levels of MAGL. Inhibition of MAGL activity with
corresponding small interfering RNA was able to reduce the protein
expression levels of cyclin D1 and Bcl-2, decrease tumor
proliferation, induce apoptosis of tumor cells, and result in cell
cycle arrest. The Bcl-2 protein family comprises key regulators of
apoptosis, and alterations to protein expression affects the
apoptosis of cells with DNA damage or cell cycle abnormalities,
thus influencing the apoptosis of tumor cells. Bcl-2 and Bax exert
an inhibitory and promoting effect on apoptosis, respectively
(25). Increasing Bax protein
expression increases cell sensitivity and apoptosis. A decrease in
Bax protein expression reduces its preventative effects on Bcl-2,
thus promoting cell survival (26,27).
By inhibiting MAGL with JZL184, the present study demonstrated that
MAGL may regulate Bcl-2 protein expression, thus promoting the
effects of Bcl-2 and reducing Bax protein expression levels,
resulting in anti-apoptotic effects in colorectal cancer cells.
EMT-associated proteins are important in the process
of EMT (28). EMT results in tumor
cell migration and increased invasive ability. The epithelial
marker, E-cadherin, is decreased during EMT; however, vimentin and
Snail are increased (29). It has
previously been demonstrated that vimentin (22) is associated with the degree of
tumor malignancy. EMT regulates the behavior of tumor cells
(29), and the abnormal expression
of E-cadherin is a key marker of the EMT process. Joyce et
al (30) reported that MAGL
may act as an EMT gene expression marker. Hu et al (31) investigated the association between
nasopharyngeal carcinoma (NPC) and MAGL, and observed that MAGL
promoted the metastasis of NPC by affecting EMT. These previous
studies suggested that MAGL may be closely associated with
colorectal cancer and the EMT process. In order to investigate the
role of MAGL in the EMT of colorectal cancer cell lines, cells with
various metastatic potentials were selected. The migration of
colorectal cancer cells was significantly suppressed by JZL184
intervention, particularly in SW620 cell, as compared with SW480
cells. These results suggested that JZL184 interrupted cell
migration, and as the degree of malignancy increased, the tumor
cells were more sensitive to the effects of JZL184. To elucidate a
possible mechanism underlying the effects of JZL184 on the
colorectal cancer cell lines, the expression levels of EMT markers
were detected. The present study observed that JZL184 increased the
protein expression levels of E-cadherin, and decreased the protein
expression levels of Snail and vimentin. These results indicated
that the MAGL inhibitor, JZL184, decreased migration of colorectal
cancer lines via influencing EMT, and the inhibitory effects was
enhanced depending on the metastatic potential of the colorectal
cancer cell line. MAGL, as a colorectal EMT marker, increases the
invasive and metastatic potential of tumor cells, possibly via
regulation of tumor cell EMT. In addition, metabolic products in
the regulation of MAGL, including PGE2 (32), have been demonstrated to be
associated with tumor cell EMT, and the endocannabinoid system is
also associated with the process of EMT (5). However, whether MAGL regulates the
EMT of colorectal cancer via the fatty acid network or
endocannabinoids remains to be elucidated.
In conclusion, the MAGL inhibitor, JZL184, induced
apoptosis by regulating the expression of Bcl-2 and Bax in
colorectal cancer cells. In addition, JZL184 increased the
sensitivity of colorectal cancer cells to 5-fluorouracil, decreased
migration of the tumor cells, and regulated the expression of EMT
markers. These results suggested that MAGL may be considered a
potential therapeutic target, and JZL184 may be a possible
therapeutic agent for the treatment of colorectal cancer. Further
research regarding the role of MAGL in colorectal cancer is
required, which may elucidate the mechanisms underlying how JZL184
and MALG regulate proliferation and metastasis of colorectal cancer
cells.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172294).
References
|
1
|
King AR, Lodola A, Carmi C, Fu J, Mor M
and Piomelli D: A critical cysteine residue in monoacylglycerol
lipase is targeted by a new class of isothiazolinone-based enzyme
inhibitors. Br J Pharmacol. 157:974–983. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blankman JL, Simon GM and Cravatt BF: A
comprehensive profile of brain enzymes that hydrolyze the
endocannabinoid 2-arachidonoylglycerol. Chem Biol. 14:1347–1356.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pisanti S, Picardi P, D'Alessandro A,
Laezza C and Bifulco M: The endocannabinoid signaling system in
cancer. Trends Pharmacol Sci. 34:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hermanson DJ and Marnett LJ: Cannabinoids,
endocannabinoids, and cancer. Cancer Metastasis Rev. 30:599–612.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panikashvili D, Simeonidou C, Ben-Shabat
S, Hanus L, Breuer A, Mechoulam R and Shohami E: An endogenous
cannabinoid (2-AG) is neuroprotective after brain injury. Nature.
413:527–531. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Long JZ, Li W, Booker L, Burston JJ,
Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons
LH, et al: Selective blockade of 2-arachidonoylglycerol hydrolysis
produces cannabinoid behavioral effects. Nat Chem Biol. 5:37–44.
2009. View Article : Google Scholar :
|
|
7
|
Nomura DK, Long JZ, Niessen S, Hoover HS,
Ng SW and Cravatt BF: Monoacylglycerol lipase regulates a fatty
acid network that promotes cancer pathogenesis. Cell. 140:49–61.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nomura DK, Lombardi DP, Chang JW, Niessen
S, Ward AM, Long JZ, Hoover HH and Cravatt BF: Monoacylglycerol
lipase exerts dual control over endocannabinoid and fatty acid
pathways to support prostate cancer. Chem Biol. 18:846–856. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zlobec I and Lugli A: Epithelial
mesenchymal transition and tumor budding in aggressive colorectal
cancer: Tumor budding as oncotarget. Oncotarget. 1:651–661. 2010.
View Article : Google Scholar
|
|
10
|
Joyce T, Cantarella D, Isella C, Medico E
and Pintzas A: A molecular signature for epithelial to mesenchymal
transition in a human colon cancer cell system is revealed by
large-scale microarray analysis. Clin Exp Metastasis. 26:569–587.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye L, Zhang B, Seviour EG, Tao KX, Liu XH,
Ling Y, Chen JY and Wang GB: Monoacylglycerol lipase (MAGL)
knockdown inhibits tumor cells growth in colorectal cancer. Cancer
Lett. 307:6–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hector S and Prehn JH: Apoptosis signaling
proteins as prognostic biomarkers in colorectal cancer: A review.
Biochim Biophys Acta. 1795:117–129. 2009.PubMed/NCBI
|
|
13
|
Prabhudesai SG, Rekhraj S, Roberts G,
Darzi AW and Ziprin P: Apoptosis and chemo-resistance in colorectal
cancer. J Surg Oncol. 96:77–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Leary DP, Bhatt L, Woolley JF, Gough DR,
Wang JH, Cotter TG and Redmond HP: TLR-4 signalling accelerates
colon cancer cell adhesion via NF-κB mediated transcriptional
up-regulation of Nox-1. PLoS One. 7:e441762012. View Article : Google Scholar
|
|
16
|
Bai J, Chen J, Ma M, Cai M, Xu F, Wang G,
Tao K and Shuai X: Inhibiting enhancer of zeste homolog 2 promotes
cellular senescence in gastric cancer cells SGC-7901 by activation
of p21 and p16. DNA Cell Biol. 33:337–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leng Z, Tao K, Xia Q, Tan J, Yue Z, Chen
J, Xi H, Li J and Zheng H: Krüppel-like factor 4 acts as an
oncogene in colon cancer stem cell-enriched spheroid cells. PLoS
One. 8:e560822013. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Croker BA, O'Donnell JA, Nowell CJ,
Metcalf D, Dewson G, Campbell KJ, Rogers KL, Hu Y, Smyth GK, Zhang
JG, et al: Fas-mediated neutrophil apoptosis is accelerated by Bid,
Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci
USA. 108:13135–13140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JG, McKinney KQ, Pavlopoulos AJ, Park
JH and Hwang S: Identification of anti-metastatic drug and natural
compound targets in isogenic colorectal cancer cells. J Proteomics.
113:326–336. 2015. View Article : Google Scholar
|
|
21
|
Schlosburg JE, Blankman JL, Long JZ,
Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston
JJ, et al: Chronic monoacylglycerol lipase blockade causes
functional antagonism of the endocannabinoid system. Nat Neurosci.
13:1113–1119. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gustafsson SB, Palmqvist R, Henriksson ML,
Dahlin AM, Edin S, Jacobsson SO, Öberg Å and Fowler CJ: High tumour
cannabinoid CB1 receptor immunoreactivity negatively impacts
disease-specific survival in stage II microsatellite stable
colorectal cancer. PLoS One. 6:e230032011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guzmán M: Cannabinoids: Potential
anticancer agents. Nat Rev Cancer. 3:745–755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeestraten EC, Benard A, Reimers MS,
Schouten PC, Liefers GJ, van de Velde CJ and Kuppen PJ: The
prognostic value of the apoptosis pathway in colorectal cancer: A
review of the literature on biomarkers identified by
immunohistochemistry. Biomark Cancer. 5:13–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moreno A, Figueras A, Lloveras B, Escobedo
A, Griera E, Sierra A and Fabra A: Apoptosis in ductal carcinoma in
situ of the breast. Breast J. 7:245–248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi BH, Kim W, Wang QC, Kim DC, Tan SN,
Yong JW, Kim KT and Yoon HS: Kinetin riboside preferentially
induces apoptosis by modulating Bcl-2 family proteins and caspase-3
in cancer cells. Cancer Lett. 261:37–45. 2008. View Article : Google Scholar
|
|
27
|
Zhao S, Konopleva M, Cabreira-Hansen M,
Xie Z, Hu W, Milella M, Estrov Z, Mills GB and Andreeff M:
Inhibition of phosphatidylinositol 3-kinase dephosphorylates BAD
and promotes apoptosis in myeloid leukemias. Leukemia. 18:267–275.
2004. View Article : Google Scholar
|
|
28
|
Kalluri R and Weinberg RA: The basics of
epithelialmesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joyce T, Cantarella D, Isella C, Medico E
and Pintzas A: A molecular signature for Epithelial to Mesenchymal
transition in a human colon cancer cell system is revealed by
large-scale microarray analysis. Clin Exp Metastasis. 26:569–587.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu WR, Lian YF, Peng LX, Lei JJ, Deng CC,
Xu M, Feng QS, Chen LZ, Bei JX and Zeng YX: Monoacylglycerol lipase
promotes metastases in nasopharyngeal carcinoma. Int J Clin Exp
Pathol. 7:3704–3713. 2014.PubMed/NCBI
|
|
32
|
Neil JR, Johnson KM, Nemenoff RA and
Schiemann WP: Cox-2 inactivates Smad signaling and enhances EMT
stimulated by TGF-beta through a PGE2-dependent mechanism.
Carcinogenesis. 29:2227–2235. 2008. View Article : Google Scholar : PubMed/NCBI
|