Introduction
Prostate cancer is the most common cancer diagnosed
and the second leading cause of cancer-associated mortality in
males in the USA, with an estimated 220,800 newly diagnosed cases
and 27,540 cases of cancer-associated mortality predicted to occur
in 2015 in the USA (1). Lycopene,
a carotenoid found in tomatoes, exhibits multiple bioactivities and
has been reported to protect against prostate cancer via inhibition
of cancer cell proliferation and induction of apoptotic cell death
(2–5). A previous study suggested that
lycopene regulates the breast cancer cell cycle and apoptosis via
the suppression of expression of cell cycle regulatory proteins
(6). Additional studies have
reported that high intakes of tomato-based products were associated
with a 10–20% reduction in the risk of prostate cancer (7,8).
However, the molecular mechanisms responsible for the effects of
lycopene on prostate cancer remain to be fully elucidated.
MicroRNAs (miRNAs) are a class of endogenous small
non-coding RNAs that regulate the expression of target genes at
transcriptional and post-transcriptional levels (9,10).
Previous studies have reported that miRNAs may function as
oncogenes or tumor suppressors in various types of cancer,
including lung cancer, breast cancer, hepatocellular carcinoma and
gastric cancer (11–14). Additionally, studies have observed
aberrant expression of numerous miRNAs in prostate cancer (15–17).
In addition, circulating miRNAs in serum or plasma samples have
been demonstrated to be associated with patient survival, and may
be developed as potential biomarkers for prostate cancer diagnosis
and recurrence (18). Let-7f-1 was
reported to inhibit proliferation, migration and in vivo
tumor formation of human glioblastoma cancer cells by
downregulating the expression of the oncogenes pan-RAS, N-RAS and
K-RAS (19). A previous study
demonstrated that miR-let-7f-1 mediated cisplatin resistance via
targeting secreted protein, acidic, cysteine-rich (osteonectin)
(SPARC), a crucial regulator of multiple cellular signal
transduction pathways (20).
Let-7f was reported to be upregulated in primary breast cancer
using miRNA microarray, however was not validated by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis (21). Previous studies
have indicated that lycopene inhibited prostate cancer progression
though multiple growth factor-mediated signaling pathways (22–25).
Therefore, whether specific miRNAs targeting growth factor
signalling pathways are involved in the antitumor activity of
lycopene remains to be further elucidated.
Materials and methods
Cell culture and treatment
Human prostate carcinoma cells PC3 (CRL-1435) were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and were maintained in RPMI 1640 media (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in a 5% CO2 incubator. Lycopene
(Sigma-Aldrich, St. Louis, MO, USA) was dissolved in
tetrahydrofuran (Sigma-Aldrich) as 20 mM stock solution and
maintained at −20°C. For the experiments, PC3 cells were cultured
in serial concentrations of lycopene (10, 20 and 50 µm) and
control cultures were treated with water only.
RNA extraction and RT-qPCR
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. For
miR-let-7f-1 detection, miRNA was polyadenylated and reverse
transcribed into cDNA with the One Step PrimeScript miRNA cDNA
Synthesis kit (Takara Biotechnology, Co., Ltd., Dalian, China) in
triplicate. For AKT2 detection, cDNA (50 ng) was synthesized using
the PrimeScript RT reagent kit (Takara Biotechnology, Co., Ltd.)
according to the manufacturer's protocol. RT-qPCR analysis was
performed with SYBR Green (Takara Biotechnology, Co., Ltd.) in an
ABI 7500 Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The small nuclear RNA U6 and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA were used as
internal controls for miRNA and mRNA detection, respectively. The
forward primers for the miR-let-7f-1 and U6 were synthesized by
Biosune Co., Ltd. (Shanghai, China), and the sequences were as
follows: miR-let-7f-1, 5′ CTATACAATCTATTGCCTTCCC 3′ and U6, 5′
TGCGGGTGCTCGCTTCGGCAGC 3′. The reverse primers for the miR-let-7f-1
and U6 were universal adaptor primers available in a ready-to-go
format in the One Step PrimeScript miRNA cDNA Synthesis kit (D350A;
Takara Biotechnology, Co., Ltd). The primers for the AKT2 and GAPDH
genes were obtained from Primerbank (http://pga.mgh.harvard.edu/primerbank/) and the
sequences were as follows: AKT2, forward 5′-AGGCACGGGCTAAAGTGAC-3′
and reverse 5′-CTGTGTGAGCGACTTCATCCT-3′; and GAPDH, forward
5′-CTGGGCTACACTGAGCACC-3′ and reverse 5′-AAGTGGTCGTTGAGGGCAATG-3′.
The ΔΔCq method (26) was used in
the analysis of the PCR data.
Oligonucleotide transfection
miR-let-7f-1 mimics, miR-let-7f-1 inhibitors
(anti-miR-let-7f-1) and their corresponding controls, scramble and
negative controls (NC), respectively, were synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). Cells were transfected with
50 nM oligonucleotides using Superfect™ Transfection Reagent
(Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's
instructions. Subsequent to 48 h transfection, cells were harvested
and the miR-let-7f-1 expression level was confirmed by RT-qPCR.
Cell proliferation assay and apoptosis
assay
Following 24 h cultivation, cells were transfected
with 50 µm lycopene. To measure the effect of miR-let-7f-1
mimics or lycopene on cell proliferation, cells (2×103)
were incubated in 96-well plates in 100 µl medium containing
10% FBS. A total of 10 µl WST-1 (Roche Diagnostics GmbH,
Mannheim, Germany) was added to each well at the indicated time
points and the plate was incubated for another 2 h at 37°C. The
absorbance was measured at 450 nm using a microtiter plate reader
(Spectra Rainbow; Tecan Group Ltd., Männedorf, Switzerland)
according to the manufacturer's instructions. Relative optical
density was calculated using a Spectra Rainbow microplate reader
(Tecan Group, Ltd., Männedorf, Switzerland) in three independent
experiments.
Cell apoptosis assay was determined using the
Annexin V-Fluorescein Isothiocyanate (FITC) Apoptosis Detection kit
(BD Pharmingen, San Diego, CA, USA) according to the manufacturer's
instructions. PC3 cells treated as indicated were harvested and
resuspended in 100 µl annexin V-FITC labeling solution
containing 5 µl annexin V-FITC and 5 µl propidium
iodide (BD Pharmingen) and incubated for 30 min at room temperature
in dark. Subsequent to incubation, the samples were analyzed by the
FC500 Flow Cytometer (Beckman Coulter, Inc., Miami, FL, USA). Each
measurement was performed in quadruplicate and each experiment was
repeated at least three times.
Western blotting
Whole cell protein lysates were extracted using
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) containing 200 ml Na3VO4, 200 mM NaF,
0.5 M ethylenediaminetetraacetic acid and proteinase inhibitors,
for 30 min on ice. The protein concentrations were quantified with
Bio-Rad protein assay reagent (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Equal amounts of proteins (40 µg) were
separated by 10% sodium dodecyl sulfate-polyacrylimide gel
electrophoresis (Bio-Rad Laboratories, Inc.) and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA), which were incubated with primary monoclonal antibodies
against rabbit AKT2 (1:1,000; 2964), mouse phosphorylated AKT2
(Ser474) (1:1,000; 12694; both Cell Signaling Technology, Inc.,
Danvers, MA, USA) and rabbit GAPDH (1:1,000; sc-47724; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight. The
membranes were washed 3 times in Tris-buffered saline with Tween-20
(TBST) and incubated with the corresponding horseradish
peroxidase-conjugated secondary bovine anti-mouse (sc-2371)and
anti-rabbit (sc-2370) IgG antibodies, (both 1:1,000, Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Subsequent to
three washes with TBST, the bound secondary antibody was detected
using an enhanced chemiluminescence system (Pierce Biotechnology,
Inc., Rockford, IL, USA). The signals were detected using a
SuperSignal Protein Detection kit (Pierce Biotechnology, Inc.). The
band density of specific proteins was quantified subsequent to
normalization with the density of GAPDH using ImageJ (version 1.48;
National Institutes of Health, Bethesda, MA, USA.
Bioinformatics and luciferase assay
In silico analyses were performed to
determine the putative miRNAs able to target AKT2. TargetScan 6.2
software (http://www.targetscan.org) was used
and the 3′ untranslated region (UTR) target regions were selected
in order to determine miRNA recognition elements which were
involved in cell proliferation.
The full length 3′UTR of the AKT2 gene was
PCR-amplified from genomic DNA and inserted into the XhoI and NotI
sites of the psi-CHECK2 vector (Promega Corporation, Madison, WI,
USA), downstream of the luciferase gene, to generate the plasmids
AKT2-UTR-WT. The sequences of primers used were: Forward
5′-AAACTCGAGGCAGTCTGCCCACGCAGA-3′ and reverse
5′-AAAGCGGCCGCCAGGACTGCTGGTAGCACCA-3′. AKT2-UTR-MUT plasmids were
generated from AKT2-UTR-WT by mutating the miRNA binding site using
a Quick Change Site-Directed Mutagenesis kit (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA) with the following
primers: 5′-TGGGCACAGGCCTGGCGGGGTCATCTTTTTAGTGCCTCTC-3′ (forward)
and 5′-GAGAGGCACTAAAAAGATGACCCCGCCAGGCCTGTGCCCA-3′ (reverse). All
constructs were verified by sequencing.
For the luciferase reporter assay, PC3 cells were
cultured in 96-well plates and incubated at 37°C for 24 h prior to
transfection. Luciferase reporter constructs and miR-let-7f-1
mimics or miR-let-7f-1 inhibitors were transfected using Superfect™
transfection reagent (Qiagen, Inc.). Luciferase activity was
measured using the Dual Luciferase Reporter Assay System (Promega
Corporation) 48 h post-transfection. Renilla luciferase
activity was normalized to firefly lucif-erase activity. All
transfection experiments were conducted in triplicate and repeated
3 times independently.
Statistical analysis
Data were expressed as the mean ± standard deviation
of at least three independent experiments. Statistical analyses
were analyzed using Student's two-tailed t-test. All analyses were
performed with SPSS software, version 15.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Lycopene downregulated the expression of
AKT2
Previous reports have demonstrated that lycopene was
able to inhibit the growth of human colon cancer cells and breast
cancer cells by altering PI3K/Akt signaling pathways (27–29),
which served a central role in the promotion of cell proliferation
and the inhibition of cell death (30,31).
We investigated whether the expression of AKT2 was reduced by
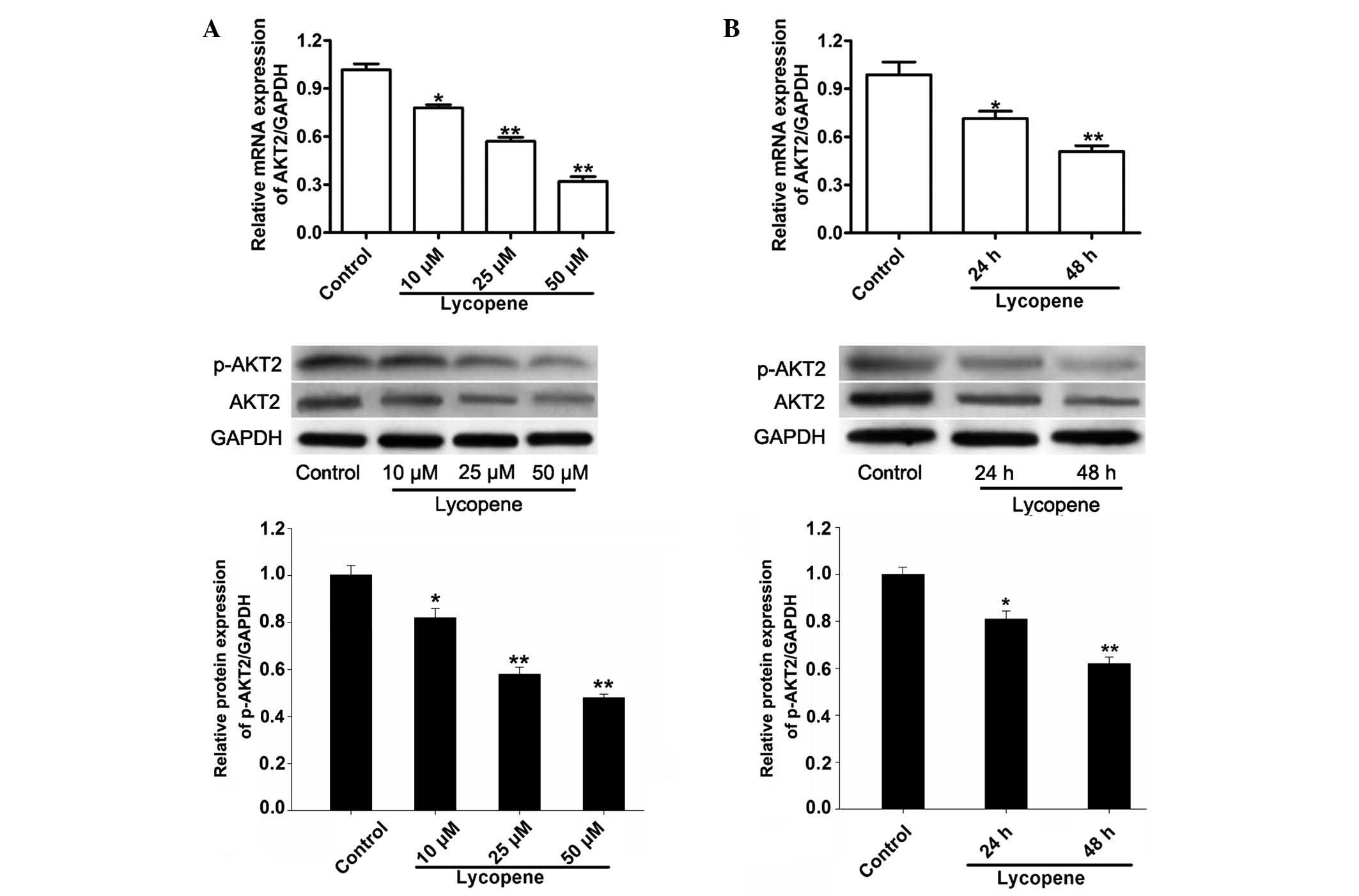
lycopene. As indicated in Fig. 1,
lycopene reduced the expression of AKT2 in a time- and
dose-dependent manner at mRNA and protein levels. The mRNA levels
of AKT2 in the PC3 cells were reduced by 19, 42 and 67% in response
to treatment with 10, 25 and 50 µM lycopene at 48 h compared
with the control (Fig. 1A, upper
panel). Meanwhile the protein levels of AKT2 and phosphorylated
AKT2 in the PC3 cells were significantly reduced by 18, 42 and 52%
following treatment with 10, 25, and 50 µM lycopene at 48 h
(Fig. 1A, lower panel). Treatment
of PC3 cells with 25 µM lycopene for 24 h or 48 h also
significantly reduced AKT2 mRNA expression levels by 29 and 49%
(Fig. 1B, upper panel) and protein
levels by 19 and 38% (Fig. 1B,
lower panel).
miR-let-7f-1 regulates AKT2 expression
through direct binding to its 3′UTR
It is known that that miRNAs exert their biological
functions by binding to the 3′-UTR and inhibiting expression of
their target genes (9). For this
reason, bioinformatics were used to predict the potential miRNAs
targeting AKT2. By searching TargetScan, AKT2 was identified as the
target of miR-let-7f-1 with the highest possibility.
To confirm the negative regulation of miR-let-7f-1
on AKT2, the 3′-UTR of AKT2 was cloned into a luciferase reporter
construct (Fig. 2A). The reporter
assay indicated that overexpression of miR-let-7f-1 triggered a
marked reduction of luciferase activity of the AKT2-UTR-WT plasmid
by 54% in PC3 cells compared with the scramble control, without
alteration in luciferase activity of AKT2-UTR-MUT (Fig. 2B). By contrast, inhibition of
miR-let-7f-1 dramatically led to a marked increase of luciferase
activity of AKT2-UTR-WT by 91%, without alterations in luciferase
activity of AKT2-UTR-MUT (Fig.
2C). Consistent with the reporter assay, a significant
reduction of AKT2 mRNA by 50% was observed in
miR-let-7f-1-overexpressed cells compared with the scramble control
in PC3 cells (Fig. 2D).
Furthermore, western blot analysis demonstrated that overexpression
of miR-let-7f-1 following administration of 10, 20 and 50 nM
miR-let-7f-1 mimics was able to downregulate the expression of AKT2
and phosphorylated AKT2 by 35, 45 and 66%, respectively (Fig. 2E). miR-let-7f-1 knockdown at 10, 25
and 50 nm anti-miR-let-7f-1 treatment resulted in a marked increase
in AKT2 and phosphorylated AKT2 expression by 42, 69 and 88%,
respectively, as compared with the NC group (Fig. 2F). These data indicate that AKT2 is
likely to be regulated by miR-let-7f-1 in prostate cancer at
transcriptional and post-transcriptional levels.
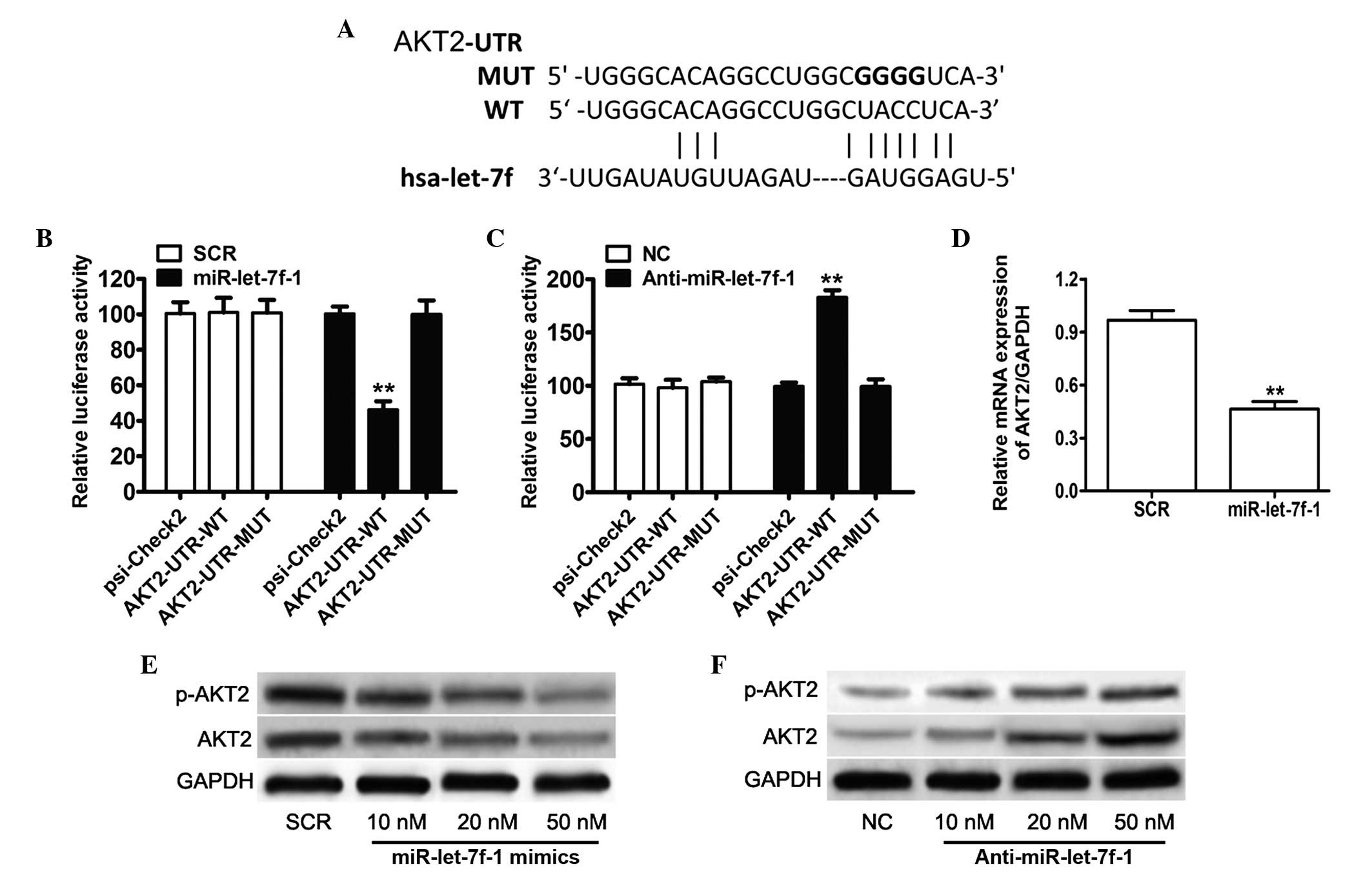 | Figure 2AKT2 was identified as target gene of
miR-let-7f-1. (A) A schematic representation indicating the
putative target site for miR-let-7f-1 in the AKT2 mRNA 3′UTR. (B)
Luciferase assays on PC3 cells transfected with the psi-CHECK2
control, AKT2-UTR-WT or AKT2-UTR-MUT reporters and miR-let-7f-1
mimic oligonucleotides or SCR. (C) Luciferase assays on PC3 cells
transfected with the psi-CHECK2 control, AKT2-UTR-WT or
AKT2-UTR-MUT reporters and miR-let-7f-1 inhibitor oligonucleotides
or NC. (D) The relative mRNA expression levels of AKT2 were
determined by reverse transcription-quantitative polymerase chain
reaction. (E) Endogenous protein levels of AKT2 and phosphorylated
AKT2 in PC3 cells subsequent to transfection with miR-let-7f-1 and
scramble control oligonucleotides were detected by western
blotting. (F) Western blot assay indicating the expression levels
of AKT2 and p-AKT2 in PC3 cells following transfection with the
miR-let-7f-1 inhibitor and NC. **P<0.01. miR,
microRNA; UTR, untranslated region; WT, wild-type; MUT, mutant; NC,
negative control; p-AKT2, phosphorylated AKT2; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; SCR, scramble. |
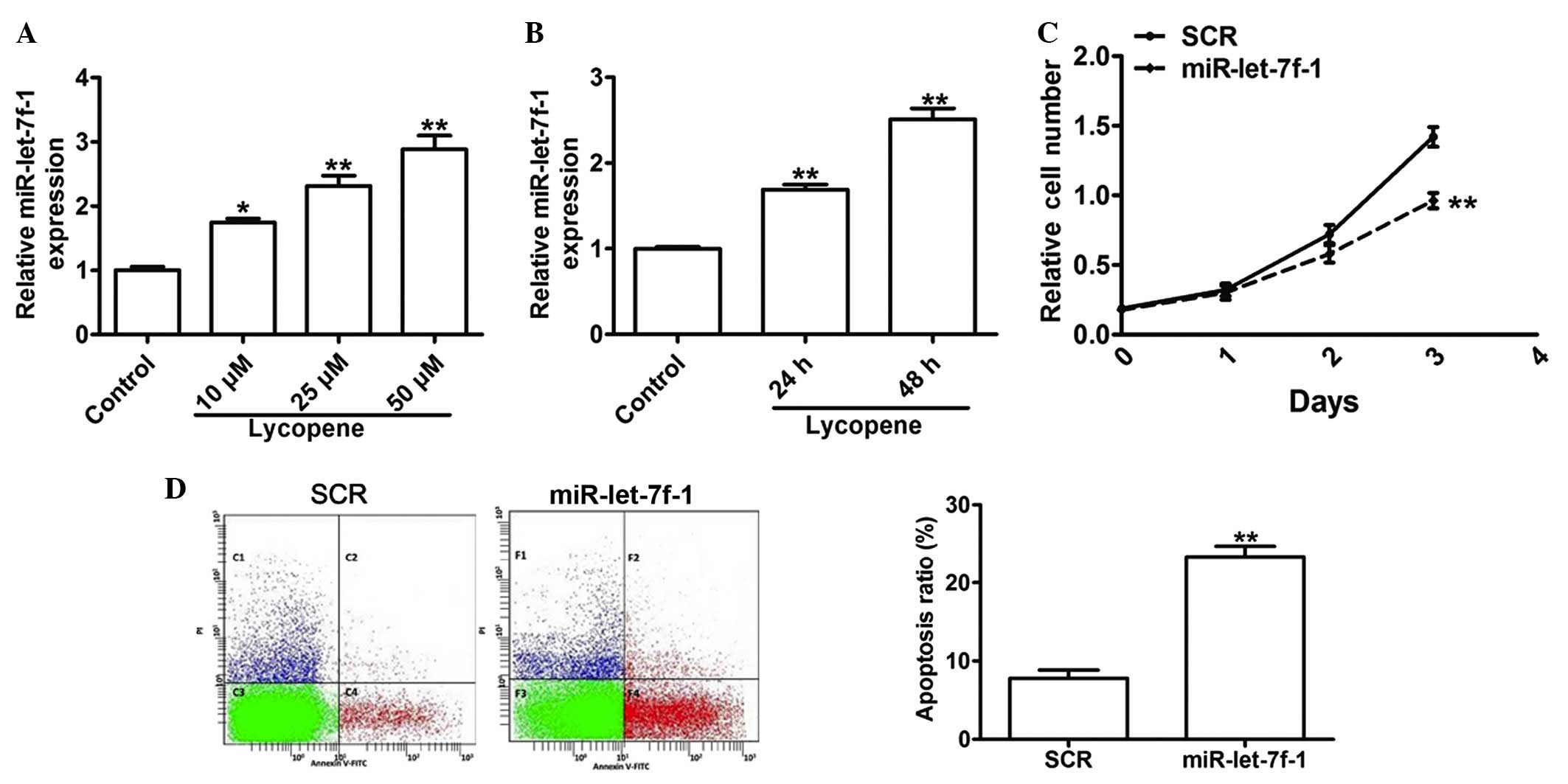
miR-let-7f-1 inhibited the proliferative
abilities and induced apoptosis in vitro
Previous studies have demonstrated that miR-let-7f-1
was involved in the suppression of cell proliferation (19,20),
thus it is hypothesized that the expression of miR-let-7f-1 is
augmented by lycopene in PC3 cells. In order to test whether
lycopene induced the upregulation of miR-let-7f-1, miR-let-7f-1
expression was detected by RT-qPCR in PC3 cells treated with
lycopene. The RT-qPCR assay indicated that the expression of
miR-let-7f-1 was enhanced by 74, 131 and 188% in response to
lycopene treatment at 10, 25 and 50 µM, in a dose-dependent
manner (Fig. 3A). A significant
increase of 69 and 151% in miR-let-7f-1 expression was also
observed in response to 25 µm lycopene treatment at 24 and
48 h, in a time-dependent manner (Fig.
3B). In order to investigate the role of miR-let-7f-1 in
prostate cancer cells, miR-let-7f-1 mimics were transfected into
PC3 cells. A proliferation assay was performed to evaluate cell
growth and the data demonstrated that the increased expression of
miR-let-7f-1 induced significant inhibition on cell proliferation
by 7, 20 and 32% at days 1, 2 and 3, respectively (Fig. 3C). Apoptosis assays demonstrated
that PC3 cells that were transfected with miR-let-7f-1 mimics
exhibited an increase in the apoptotic rate by 200%, as compared
with those of the scramble controls (Fig. 3D). These data indicated that
miR-let-7f-1 induced by lycopene may effectively inhibit the growth
and enhance apoptotic levels of PC3 cells in vitro.
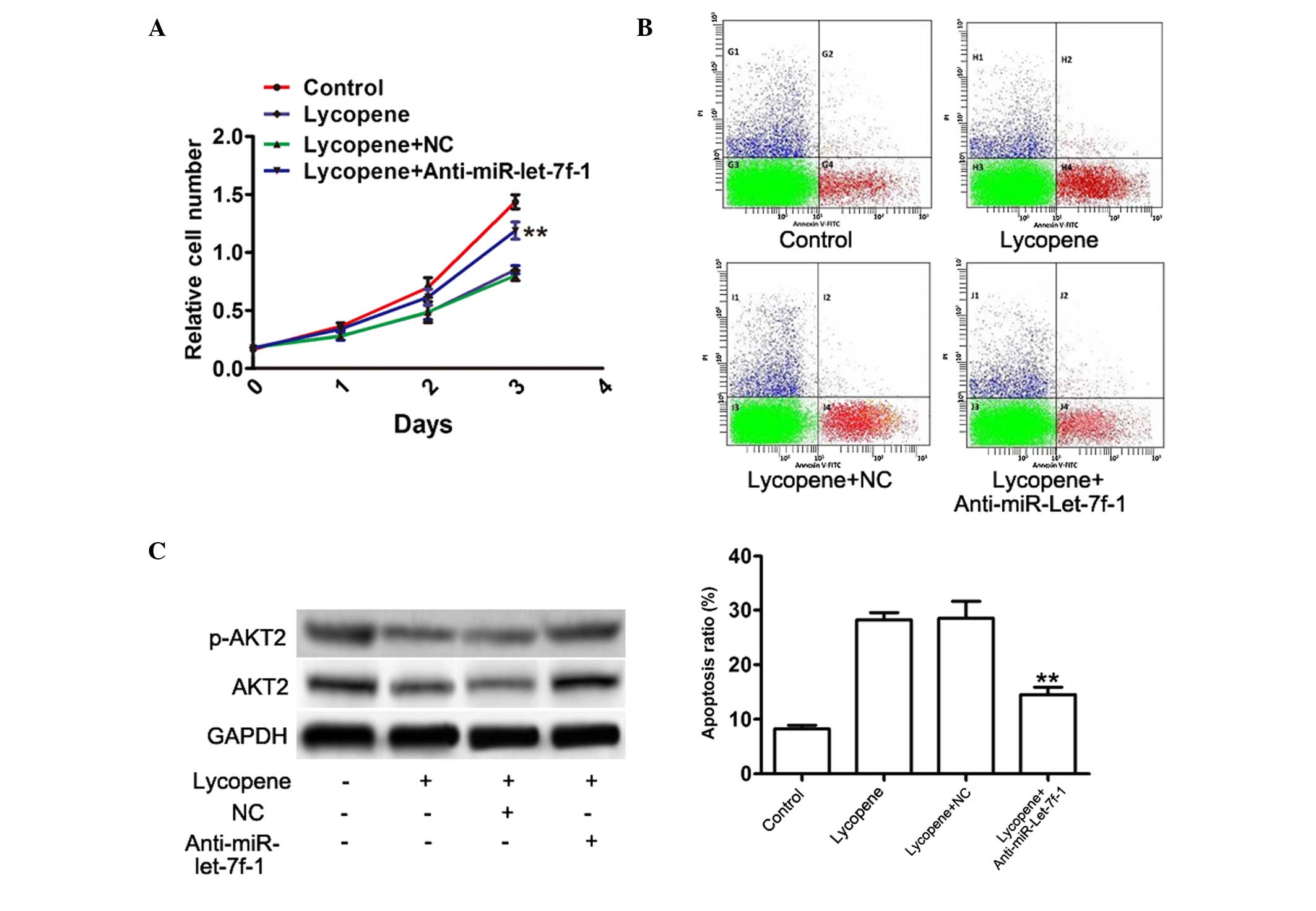
Anti-miR-let-7f-1 oligonucleotides partly
reversed the downregulation of AKT2 induced by lycopene in prostate
cancer PC3 cells
To further confirm that lycopene downregulated the
expression of AKT2 by upregulation of miR-let-7f-1, PC3 cells were
treated 50 µM lycopene, followed by transfection with 50 nM
anti-miR-let-7f-1 oligonucleotides for 48 h. Using the WST-1 assay,
it was demonstrated that anti-miR-let-7f-1 attenuated the
inhibition of cell proliferation caused by lycopene in PC3 cells by
18, 21 and 28% by days 1, 2 and 3, respectively (Fig. 4A). In addition, knockdown of
miR-let-7f-1 in PC3 cells incubated with lycopene resulted in
significantly reduced cell apoptosis (47%; Fig. 4B), indicating reversal of the
increased effects of lycopene on PC3 cell apoptosis. In addition,
anti-miR-let-7f-1 oligonucleotides partly abolished the inhibitory
effect of lycopene on AKT2 and phosphorylated AKT2 protein
expression (96%; Fig. 4C). Thus,
it was confirmed that miR-let-7f-1 was a key mediator of growth
inhibition and apoptotic enhancement of lycopene in prostate
cancer.
Discussion
Lycopene, which is naturally present in tomato
carotenoids, has been suggested to exhibit potential anticancer
activity in several types of human cancer, including prostate,
colon and breast cancer (4,5,27).
In addition, lycopene has been reported to inhibit the development
of certain cases of chemically induced carcinogenesis (32,33).
Epidemiological and clinical studies have suggested that increased
consumption of tomato products and greater blood concentrations of
lycopene are associated with a reduced risk of prostate cancer
(34,35). However, the inhibitory effect and
possible molecular mechanisms of lycopene, including cell
proliferation arrest and/or apoptosis induction, remain poorly
understood.
In the current study, a novel molecular mechanism of
lycopene in cancer control was proposed. For the first time, to the
best of our knowledge, it was demonstrated that lycopene may
inhibit cellular proliferation progression and induce apoptosis in
PC3 cells, via upregulating miR-let-7f-1 expression and inhibiting
the expression of AKT2. It has been previously reported that AKT2,
an important member of the PI3K signaling pathway, is activated in
prostate cancer (30,36). Subsequently, the expression of AKT2
was investigated. Upon treatment with lycopene, a time- and
dose-dependent reduction in AKT2 expression was observed, with the
greatest inhibition observed when PC3 cells were treated with 50
µm for 48 h, indicating that the growth-inhibitory activity
of lycopene likely reduced the mRNA and protein levels of AKT2.
However, the mechanism by which lycopene downregulates AKT2
expression remains unclear. Previous studies have indicated that
miRNAs act as fine-tuning regulators of protein expression
(9,10). With an aim to identify miRNAs
regulating AKT2 expression in prostate cancer, it was identified
that miR-let-7f-1 could significantly downregulate AKT2 expression,
which was supported by the luciferase reporter assay.
Therefore, it is notable to examine whether
miR-let-7f-1 had an effect on cell proliferation and cell
apoptosis. It was identified that the upregulation of miR-let-7f-1
was able to significantly inhibit the growth of PC3 cells.
Upregulation of miR-let-7f-1 could also enhance apoptosis of PC3
cells. To the best of our knowledge, the current study was the
first to investigate the roles and possible mechanisms of
miR-let-7f-1 upregulation in PC3 cells induced by lycopene. In
addition, it remains unclear whether miR-let-7f-1 is required to
reduce the level of phosphorylated Akt in PC3 cells pretreated with
lycopene. The data of the current study indicated that knockdown of
miR-let-7f-1 could partly reverse the inhibitory effects of
lycopene on cell proliferation and AKT2 expression in PC3 cells. In
addition, lycopene-induced apoptotic induction was abrogated by
knockdown of miR-let-7f-1 in PC3 cells. Taken together, it is
suggested that lycopene-mediated growth inhibition and apoptosis is
mediated through downregulating AKT2 and at both the mRNA and
protein levels.
In conclusion, the data of the current study
indicate that lycopene downregulates AKT2 expression via an miRNA
pathway. These observations suggest that lycopene may be a
potential anticancer compound with therapeutic applications.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zu K, Mucci L, Rosner BA, Clinton SK, Loda
M, Stampfer MJ and Giovannucci E: Dietary lycopene, angiogenesis
and prostate cancer: A prospective study in the prostate-specific
antigen era. J Natl Cancer Inst. 106:djt4302014. View Article : Google Scholar
|
|
3
|
Wei MY and Giovannucci EL: Lycopene,
tomato products and prostate cancer incidence: A review and
reassessment in the PSA screening era. J Oncol. 2012:2710632012.
View Article : Google Scholar
|
|
4
|
Kristal AR, Till C, Platz EA, Song X, King
IB, Neuhouser ML, Ambrosone CB and Thompson IM: Serum lycopene
concentration and prostate cancer risk: Results from the prostate
cancer prevention trial. Cancer Epidemiol Biomarkers Prev.
20:638–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teodoro AJ, Oliveira FL, Martins NB, Maia
GA, Martucci RB and Borojevic R: Effect of lycopene on cell
viability and cell cycle progression in human cancer cell lines.
Cancer Cell Int. 12:362012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwang ES and Bowen PE: Cell cycle arrest
and induction of apoptosis by lycopene in LNCaP human prostate
cancer cells. J Med Food. 7:284–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Venkateswaran V and Klotz LH: Diet and
prostate cancer: Mechanisms of action and implications for
chemoprevention. Nat Rev Urol. 7:442–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Syed DN, Suh Y, Afaq F and Mukhtar H:
Dietary agents for chemoprevention of prostate cancer. Cancer Lett.
265:167–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu XC, Dong QZ, Zhang XF, Deng B, Jia HL,
Ye QH, Qin LX and Wu XZ: microRNA-29a suppresses cell proliferation
by targeting SPARC in hepatocellular carcinoma. Int J Mol Med.
30:1321–1326. 2012.PubMed/NCBI
|
|
14
|
Huang B, Luo W, Sun L, Zhang Q, Jiang L,
Chang J, Qiu X and Wang E: MiRNA-125a-3p is a negative regulator of
the RhoA-actomyosin pathway in A549 cells. Int J Oncol.
42:1734–1742. 2013.PubMed/NCBI
|
|
15
|
Coppola V, De Maria R and Bonci D:
MicroRNAs and prostate cancer. Endocr Relat Cancer. 17:F1–F17.
2010. View Article : Google Scholar
|
|
16
|
Rajendiran S, Parwani AV, Hare RJ,
Dasgupta S, Roby RK and Vishwanatha JK: MicroRNA-940 suppresses
prostate cancer migration and invasion by regulating MIEN1. Mol
Cancer. 13:2502014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goto Y, Kurozumi A, Enokida H, Ichikawa T
and Seki N: Functional significance of aberrantly expressed
microRNAs in prostate cancer. Int J Urol. 22:242–252. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jackson BL, Grabowska A and Ratan HL:
MicroRNA in prostate cancer: Functional importance and potential as
circulating biomarkers. BMC Cancer. 14:9302014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong Q, Meng P, Wang T, Qin W, Qin W, Wang
F, Yuan J, Chen Z, Yang A and Wang H: MicroRNA let-7a inhibits
proliferation of human prostate cancer cells in vitro and in vivo
by targeting E2F2 and CCND2. PLoS One. 5:e101472010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pannuru P, Dontula R, Khan AA, Herbert E,
Ozer H, Chetty C and Lakka SS: miR-let-7f-1 regulates SPARC
mediated cisplatin resistance in medulloblastoma cells. Cell
Signal. 26:2193–2201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trejo-Solís C, Pedraza-Chaverrí J,
Torres-Ramos M, Jiménez-Farfán D, Cruz Salgado A, Serrano-García N,
Osorio-Rico L and Sotelo J: Multiple molecular and cellular
mechanisms of action of lycopene in cancer inhibition. Evid Based
Complement Alternat Med. 2013:7051212013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Y, Parmakhtiar B, Simoneau AR, Xie J,
Fruehauf J, Lilly M and Zi X: Lycopene enhances docetaxel's effect
in castration-resistant prostate cancer associated with
insulin-like growth factor I receptor levels. Neoplasia.
13:108–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lo HM, Hung CF, Tseng YL, Chen BH, Jian JS
and Wu WB: Lycopene binds PDGF-BB and inhibits PDGFBB-induced
intracellular signaling transduction pathway in rat smooth muscle
cells. Biochemical Pharmacology. 74:54–63. 2007. View Article : Google Scholar
|
|
25
|
Chen ML, Lin YH, Yang CM and Hu ML:
Lycopene inhibits angiogenesis both in vitro and in vivo by
inhibiting MMP-2/uPA system through VEGFR2-mediated PI3K-AKT and
ERK/p38 signaling pathways. Molecular Nutrition & Food
Research. 56:889–899. 2012. View Article : Google Scholar
|
|
26
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
27
|
Tang FY, Shih CJ, Cheng LH, Ho HJ and Chen
HJ: Lycopene inhibits growth of human colon cancer cells via
suppression of the Akt signaling pathway. Mol Nutr Food Res.
52:646–654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeshima M, Ono M, Higuchi T, Chen C,
Hara T and Nakano S: Anti-proliferative and apoptosis-inducing
activity of lycopene against three subtypes of human breast cancer
cell lines. Cancer Sci. 105:252–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Palozza P, Colangelo M, Simone R, Catalano
A, Boninsegna A, Lanza P, Monego G and Ranelletti FO: Lycopene
induces cell growth inhibition by altering mevalonate pathway and
Ras signaling in cancer cell lines. Carcinogenesis. 31:1813–1821.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carver BS, Chapinski C, Wongvipat J,
Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J,
Scher H, et al: Reciprocal feedback regulation of PI3K and androgen
receptor signaling in PTEN-deficient prostate cancer. Cancer Cell.
19:575–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsu AL, Ching TT, Wang DS, Song X,
Rangnekar VM and Chen CS: The cyclooxygenase-2 inhibitor celecoxib
induces apoptosis by blocking Akt activation in human prostate
cancer cells independently of Bcl-2. J Biol Chem. 275:11397–11403.
2000. View Article : Google Scholar
|
|
32
|
Kim DJ, Takasuka N, Kim JM, Sekine K, Ota
T, Asamoto M, Murakoshi M, Nishino H, Nir Z and Tsuda H:
Chemoprevention by lycopene of mouse lung neoplasia after combined
initiation treatment with DEN, MNU and DMH. Cancer Lett. 120:15–22.
1997. View Article : Google Scholar
|
|
33
|
Narisawa T, Fukaura Y, Hasebe M, Nomura S,
Oshima S, Sakamoto H, Inakuma T, Ishiguro Y, Takayasu J and Nishino
H: Prevention of N-methylnitrosourea-induced colon carcinogenesis
in F344 rats by lycopene and tomato juice rich in lycopene. Jpn J
Cancer Res. 89:1003–1008. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giovannucci E: A review of epidemiologic
studies of tomatoes, lycopene and prostate cancer. Exp Biol Med
(Maywood). 227:852–859. 2002.
|
|
35
|
Pohar KS, Gong MC, Bahnson R, Miller EC
and Clinton SK: Tomatoes, lycopene and prostate cancer: A
clinician's guide for counseling those at risk for prostate cancer.
World J Urol. 21:9–14. 2003.PubMed/NCBI
|
|
36
|
Zhong H, Chiles K, Feldser D, Laughner E,
Hanrahan C and Georgescu MM: Modulation of hypoxia-inducible factor
1alpha expression by the epidermal growth
factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human
prostate cancer cells: Implications for tumor angiogenesis and
therapeutics. Cancer Res. 60:1541–1545. 2000.PubMed/NCBI
|