Introduction
Hepatocellular carcinoma (HCC), the predominant form
of primary liver cancer, is one of the most frequent types of human
malignancy and the third leading cause of cancer-asssociated
mortality worldwide (1). It is
reported that >700,000 cases of HCC are diagnosed annually, with
a 5-year-survival rate of ≤10% (2). Effective treatment of HCC remains a
significant challenge (3). Even in
patients who have received surgical management of HCC, including
resection and liver transplantation, recurrence and metastasis
remain major obstacles in further prolonging the survival rates of
patients with HCC (3). Therefore,
understanding the pathogenesis of HCC and examining more efficient
therapeutic strategies to prevent HCC metastasis and recurrence are
important.
Sustained proliferation, invasion, metastasis, and
angiogenesis are important features of cancer (1). Therefore, preventing the growth and
metastatic spread of HCC is a principal aim of HCC therapy.
Angiogenesis is known as the formation of new blood vessels from
pre-existing vessels, as well as the remodeling of the newly formed
vascular network (4). In addition,
angiogenesis is widely considered as pivotal procedures in removing
metabolic waste products, to nourish the growing tumor (4). Pang and Poon (5) revealed that HCC was a hypervascular
carcinoma characterized by neovascularization, and vascular
endothelial growth factor (VEGF), and has a significant role in the
angiogenesis of HCC. Evidence has shown that angiogenesis is a
critical factor for tumor growth and metastasis, and tumor growth
was not considerable when emerging new capillary blood vessels were
inhibited (6). Targeting
angiogenesis has been validated in several types of solid tumor
(7–9). For example, Zhao et al
reported that inhibition of tumor angiogenesis by suppression of
the Notch signaling pathway may be a potential mechanism underlying
the antitumor activity of total alkaloids of Rubus
alceifolius Poir in a mouse model of HCC (7). Thus, anti-angiogenic strategy has
been shown promising effects for HCC therapy.
Oriental herbal medicine has been used since ancient
times to treat malignancies (10).
Hu et al indicated that Chinese herbal medicine was emerging
as a treatment of choice due to its multi-target, multilevel and
coordinated intervention effects against HCC (11). For example, Han et al
reported that Chinese medicines can exert anti-angiogenic effects
through regulating the expression of VEGF or reducing the
activities of angiogenic factor receptors, or by inhibiting the
proliferation of endothelial cells (12). In addition, blood-activating and
stasis-eliminating herbs, including Salvia miltiorrhiza and
Turmeric rhizome attenuated tumor angiogenic activities
(12). Dong et al revealed
that Cucurbitacin E, which is extracted from Chinese medicine,
inhibited tumor angiogenesis through a specific pathway (13). In addition, Huang et al
reported that the herbal compound extract 'Songyou Yin' inhibited
the growth and invasion of HCC, and decreased microvessel density
and the abundance of VEGF (14).
Buyang Huanwu decotion (BYHWD), a traditional Chinese medicine
functionally characterized by activated energy, Qi,
invigorates the body, enhances blood circulation and meridian
circulation, and has been traditionally used in the treatment of
stroke and paralysis for centuries (15). Cai et al found that BYHWD
improved the recovery of neurological function, stimulated neural
proliferation, reduced infarction volume and modulated the
expression of VEGF and its receptor fetal liver kinase in transient
focal cerebral ischemic rat brains (16). In addition, Jain et al
demonstrated that certain anti-angiogenic agents transiently
normalized the abnormal vasculature structure and function,
improving its efficiency for drug delivery and alleviating hypoxia
(17). Additionally, several
traditional Chinese herbal drugs have exhibited anti-angiogenic
effects and enhanced normalization of tumor vasculature (18). However, the detail and potential
mechanism underlying the effects of BYHWD on HCC remains to be
elucidated.
The established metastatic model of human HCC in
nude mice exhibits the metastatic ability, transplantability and
manifestations reminiscent of tumor behavior in patients with HCC
(19). The present study aimed to
investigate the effect and the potential mechanism of BYHWD on
tumor growth, metastasis and angiogenesis in nude mice bearing
human HCC HCCLM3 xenografts.
Materials and methods
Characterization and preparation of
herbal medicine
The uses of the Chinese medicine formula, BYHWD, and
its disassembled prescriptions, Yiqi decoction (YQD) and Huoxue
decoction (HXD), in the present study were authorized according to
the medical publications; Formulas of Chinese medicine (20) and the Chinese Pharmacopoeia
(21). BYHWD was composed of seven
medicinal components: 120 g milkvetch root, 6 g Chinese angelica, 5
g red peony root, 3 g earth worm, 3 g Szechwan lovage rhizome, 3 g
peach seed and 3 g safflower. YQD consisted of only 120 g milkvetch
root, and HXD consisted of the remaining medicinal components: 6 g
Chinese angelica, 5 g red peony root, 3 g earth worm, 3 g Szechwan
lovage rhizome, 3 g peach seed and 3 g safflower. All the herbal
components were purchased from Shanghai Pharmacy (Shanghai, China),
and authenticated by experts at the Department of Pharmacology,
Shanghai University of Chinese Medicine (Shanghai, China). The
three medicinal herb decoctions were prepared as follows. The
mixture of the crude drugs were soaked in 500 ml distilled water
and decocted by boiling for 35 min. The resulting decoction was
then filtered through a 0.22 µm polytetrafluoroethylene
filter (Whatman; GE Healthcare Life Sciences, Chalfont, UK) and
collected. The remnants were added to 350 ml distilled water, and
decocted by boiling was continued for 20 min, followed by
filtering. The filtered decoctions were combined and condensed to a
specific dose. The doses were calculated according to the formula:
dB = dA * RB/RA*
(WA/WB)1/3 (22), where dA represents the human dose
and dB represents the mouse dose; WA is the average human weight
(60 kg) and WB is the average mouse weight (0.2 kg). R is the build
coefficient (RA=1.00; RB=0.59). The combined filtrates of BYHWD,
YQD and HXD were condensed to 1.03, 0.56 and 0.166 g/ml,
respectively. All the combined filtrates were stored at 4°C until
use.
Animals and metastatic model of human HCC
in nude mice
Male and female athymic BALB/c nu/nu mice (15–50 g;
4–6 weeks old) were purchased from Shanghai Slack Experimental
Animals Co., Ltd. (Shanghai, China) and maintained under specific
pathogen-free conditions. All mice were handled according to the
Guidance Suggestions for the National Institutes for the Care and
Use of Laboratory Animals (23).
The study was approved by the ethics committee of Shanghai
University of Traditional Chinese Medicine.
The HCCLM3 HCC cell line, which was cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), was established at the Liver Cancer Institute of Fudan
University (Fudan, China) (24).
The human HCC tumor models were established using HCCLM3 in nude
mice via the orthotopic implantation of intact metastatic tumor
tissue, as described in previous reports (25,26).
Briefly, following the acquirement of HCCLM3, 5×106 (0.2
ml) cells were injected subcutaneously into four nude mice (male or
female). When the subcutaneous tumor had reached ~1.5 cm in
diameter, the mice were sacrificed by cervical dislocation. The
tumor tissue was removed, cut into small sections (~1
mm3) and implanted into the liver of separate recipient
mice, which were kept in standard facilities. This animal model
showed 100% spread into the liver and metastasis to the lungs.
Abnormal serum α-fetoprotein was excreted, and hepatitis B surface
antigens were also identified in this model.
Mice grouping and treatment
A total of 96 nude mice bearing orthotopic
xenografts were randomly divided into four groups: Model control
group (LM); BYHWD-treated group (LB), YQD-treated group (LY) and
HXD-treated group (LH). Each of these groups was randomized into
three subgroups, which were treated consecutively for 21 days
(LM21, LB21, LY21 and LH21), 28 days (LM28, LB28, LY28 and LH28),
and 35 days (LM35, LB35, LY35, and LH35), respectively. Each
subgroup contained eight mice. Treatment began the day following
that on which xenograft surgery was performed. The groups of
animals (n=8) were orally gavaged with 0.2 ml of the respective
treatment solution twice daily, with the exception of the animals
in the model group, which received 0.2 ml of 0.9% sodium chloride
solution twice daily. Daily general observations and weekly mice
body weights were all recorded.
Parameters observed and grading of lung
metastasis
The mice were sacrificed after 21, 28 and 35 days,
respectively. The tumors were removed, images were captured, and
the tumor tissues were weighed and processed for histology.
Following autopsy, the longest (a) and the smallest (b) diameters
of the tumors were measured using a slide gauge (Control Co.,
Friendswood, TX, USA) under an operating microscope (OPMI Pentero;
Carl Zeiss, Oberkochem, Germany). The tumor volume was calculated
as follows: Tumor volume =ab2/2. Both lungs of each
mouse were removed, and paraffin blocks of 10% buffered formalin
(Tissue Prep-II; Thermo Fisher Scientific, Inc.)-fixed samples of
the lungs were prepared. A total of five coronal sections were
selected from each paraffinized lung sample, with each coronal
section cut consecutively into five slices. Serial sections were
cut at 4 µm, and 20 slices in the group were randomly
selected and stained with hematoxylin and eosin (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) to determine
the presence of lung metastases under a light microscope (Leica
DMIRBl; Leica Microsystems GmbH, Wetlzar, Germany). If at least one
in these 20 slices was found to exhibit lung metastasis, the mice
were confirmed to have lung metastasis. The degree of lung
metastasis was graded by the number (N) of tumor cells counted in
the maximum section of a solitary pulmonary metastatic nodule:
Grade I, N<20; grade II, N=20–50; grade III, N=50–100; grade IV,
N>100.
Immunohistochemical assessment of
microvessel density
Paraffin-embedded tumor tissues were cut into
4-µm-thick sections, dewaxed in xylene (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) and dehydrated in ethanol.
Two-step methods (EnVision™ system; DakoCytomation, Glostrup,
Denmark) were used for CD105 staining. Briefly, the endogenous
peroxidase activity was quenched with 3% H2O2
(Sinopharm Chemical Reagent Co., Ltd.) in methanol for 10 min. The
CD105 antigen was unmasked by microwave oven (Panasonic 1380W;
Panasonic Corporation, Osaka, Japan) pre-treatment in pH 9.0
ethylene diamine tetraacetic acid buffer (Invitrogen; Thermo Fisher
Scientific, Inc.), and then cooled to room temperature. The
sections were then incubated with the following primary monoclonal
antibodies: mouse anti-endoglin anti-CD105 (SN6 h; DAKO; 1:50
dilution) for 2 h at 37°C, followed by incubation with rabbit
anti-mouse horseradish peroxidase (HRP)-conjugated streptavidin
(P0397; DAKO; 1:50 dilution) for 45 min at 37°C. Antibody binding
was visualized with 3,3-diaminobenzidine (DAB; Sigma-Aldrich, St.
Louis, MO, USA), and the sections were counterstained with Mayer's
hematoxylin (Sgma-Aldrich). Prior to each subsequent step, the
sections were washed extensively with phosphate-buffered saline
(PBS) twice for 5 min. Tissues incubated with PBS as the primary
antibody served as negative controls.
The quantification of MVD was performed in
accordance with a previously described method (27). Briefly, the three most vascularized
areas of the tumor tissues were initially identified under a
low-power field (×40). The numbers of microvessels were then
counted in each of these areas under a high-power field (×400)
using a LEICA DMLB light microscope (Leica Microsystems GmbH). Any
brown-stained single endothelial cells, or cell clusters with or
without a discernible lumen and separated from adjacent
microvessels and other connective tissue elements, were considered
to be an individual, countable microvessel. The mean value of three
×400 field (0.40 mm2) counts was recorded as the MVD of
the section. All microvessel counts were performed independently by
two investigators, who were blinded to the clinicopathological
data. The rate of disagreement between the two investigators'
analyses was <10%.
Immunohistochemical (IHC) detection of
the expression levels of VEGF, regulator of G protein signaling 5
(RGS-5) and hypoxia-inducible factor 1α (HIF-1α)
Immunohistochemistry was performed using an
EnVision™ method using the reagents supplied within the kit.
Briefly, 4 µm-thick sections were cut consecutively from
paraffin-embedded tumor tissue. Following deparaffinization and
dehydration, the tissue sections were repaired for 40 min with 10%
ethylenediamine tetraacetic acid (Invitrogen; Thermo Fisher
Scientific, Inc.). The slides were then incubated with either
primary VEGF monoclonal mouse antibody (clone VG1; M7273; DAKO;
1:100 dilution) for 60 min at 37°C, primary rabbit ant-mouse RGS-5
polyclonal antibody (SAB1411523; Sigma-Aldrich; 1:80 dilution)
overnight at 4°C, or primary rabbit anti-mouse HIF-1α polyclonal
antibody (sc-10790; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA; 1:200 dilution) overnight at 4°C. Following three rinses in
PBS, the slides were incubated with the secondary antibodies (goat
anti-mouse/rabbit unbiotinylated antibody-HRP; EnVision™ System;
K5007; DAKO, 1:100 dilution) for 30 min at 37°C. The tissue
staining was visualized with DAB substrate (DAKO), and the sections
were counterstained with Mayer's hematoxylin. Antibody binding was
visualized with DAB. Prior to each subsequent-step, the sections
were washed extensively with 0.01 M (pH 7.4)
triethanolamine-buffered saline (TBS; Wuhan Boster Biological
Technology, Ltd., Wuhan, China). Tissues incubated with TBS as the
primary antibody served as negative controls.
Positive staining was located in the cytoplasm for
VEGF and RGS-5. Positive HIF-1α expression was located as brown
staining, predominantly in the nucleus. A total of five areas were
counted under ×400 field. The levels of expression were graded, as
follows: Negative (–), <5% of the cancerous cells were
positively stained. Weak positive (+), 5–25% of the cancerous cells
were positively stained and the cytoplasm was light brown. Positive
(++), 25–50% of the cancer cells were positively stained, and
positive cells were light brown with a granule-like appearance in
the cytoplasm. Strongly positive (+++), 50% of the cancer cells
were positively stained and the cytoplasm was dark brown. The level
of expression was represented by the grade: ≥5%=positive,
<50%=low level expression, ≥50% high level expression. The
percentage of positive tumor cells and the mean optical density
(OD) values were calculated.
Statistical analysis
The data are presented as the mean ± standard
deviation, and were analyzed using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA). One-way analysis of variance and Student's
t-test were used for comparisons between groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
BYHWD treatment has no significant effect
on tumor growth
At the point at which the mice were sacrificed,
lumps were grossly visible. The tumors removed from the mice in the
treatment groups and model control group at day 35 are shown in
Fig. 1. The tumor weights and
volumes in each group are presented in Table I. The results revealed that the
changes in tumor weight (g) and tumor volume (mm3) in
the LB35 group were smaller, compared with those in the LM35 group,
but without statistical significance (P>0.05; Table I). No animals experienced weight
loss of >10%.
 | Table IComparison of tumor growth and
metastases between groups 35 days following treatment. |
Table I
Comparison of tumor growth and
metastases between groups 35 days following treatment.
| Group | n | Tumor
weight
(g) | Tumor
volume
(mm3) |
|---|
| LB | 8 | 0.55±0.50 | 440.70±401.15 |
| LY | 8 | 1.08±0.70 | 765.33±534.96 |
| LH | 8 | 0.90±0.45 | 718.41±376.01 |
| LM | 8 | 0.62±0.34 | 533.47±336.82 |
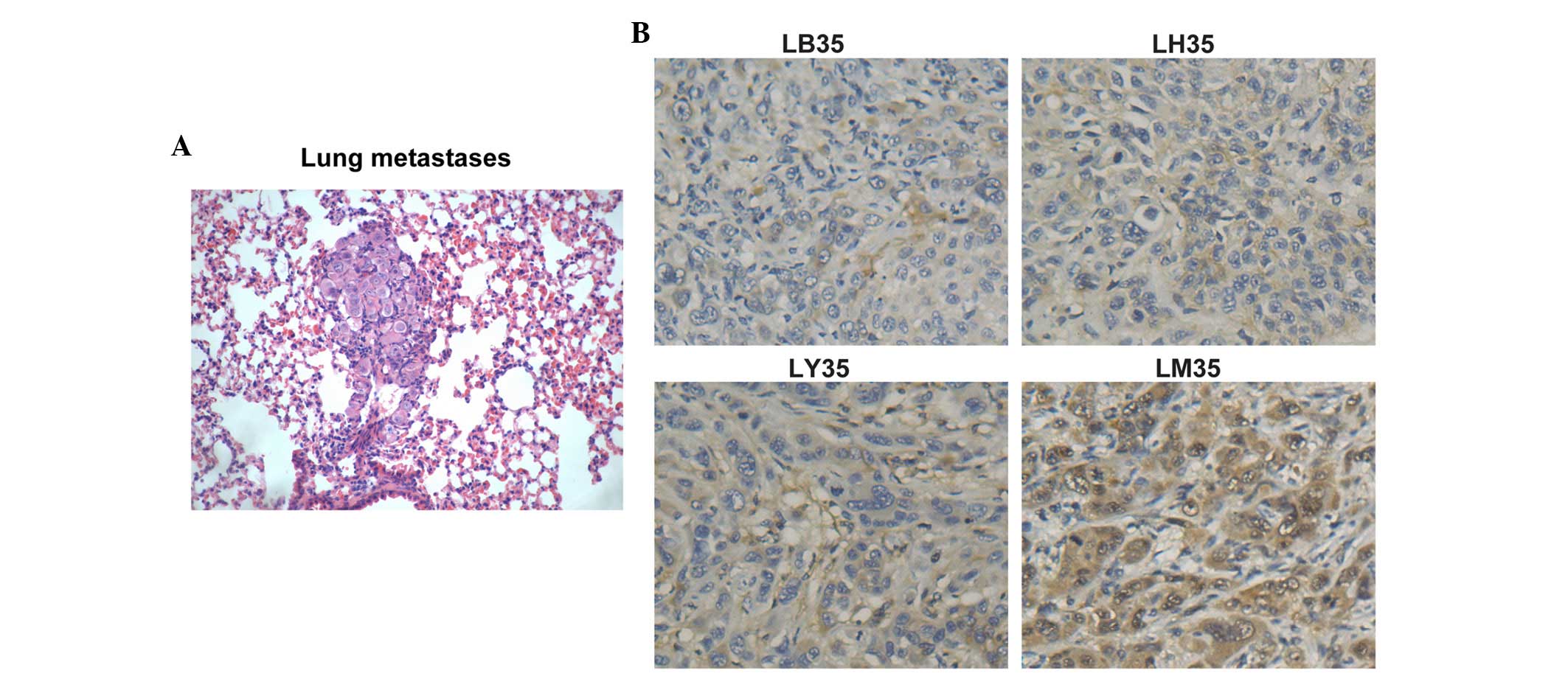
BYHWD treatment decreases the number of
lung metastases in HCC
Visible metastases were observed in all groups, as
shown in Fig. 2A, the number of
which were recorded. At 35 days post-treatment, the rate of
metastasis to the lungs was 100% in all groups, however, the number
of metastases in the lungs of the treatment groups were
significantly lower, compared with the number in the LM group
(P<0.05). In the LB group, the number of lung metastases was the
lowest (P<0.05). The majority of the lung sections in the LM35
group were observed to contain scattered hemorrhagic spots. The
degree of lung metastasis ranged between grade I and grade III,
with 10% of metastases in the LB35 group being grade II and the
others in the group being grade I. In the LH35 group, 40% were
grade I and 60% were grade III. In the LY35 group, 20% were grade
III and the others in the group were grade I.
BYHWD treatment decreases the expression
of CD105 and MVD counts
A significant reduction in the number of stained
regions were revealed following herbal medicine treatment. The
expression levels of CD105 in the herbal medicine treatment groups
were weak or even negative, whereas the expression of CD105 was
more marked in the control group (Fig.
2B). Only the results at 35 day post-treatment have been
presented, as the results on days 21 and 28 were similar to those
at day 35. The newborn endothelial cells were stained brown or
yellow, and were sinusoidally distributed in the capillary walls of
the fiber interval and portal area of the liver tissues (Fig. 2B). In the 35 day groups,
microvessel counting revealed that the MVD counts in the LB, LY, LH
and LM groups at a high-power field (×400) were 5.60±3.02,
6.08±1.42, 8.07±2.65 and 12.00±4.45, respectively. The MVD in the
LM group was higher, compared with those in the medicine treatment
groups, and the lowest MVD was found in the LB35 group (P<0.05).
These results showed that BYHWD treatment caused a marginal
decrease in MVD.
BYHWD affects the expression levels of
VEGF, RGS-5 and HIF-1α
To further determine whether BYHWD treatment
inhibits the angiogenesis of HCC by inhibiting VEGF or other
factors, the present study measured the expression levels of VEGF,
RGS-5 and HIF-1α. The expression values of VEGF (positive cell rate
× OD) are shown in Fig. 3B. The
expression values in the LB35, LY35, LH35 and LM35 groups were
285.16±75.80, 228.12±81.45, 231.12±81.84 and 200.00±80.96,
respectively. The results showed that the expression of VEGF was
higher in the LB35 group, compared with LM35 group (P<0.05), and
the IHC results revealed a significant increase in the expression
levels of VEGF in the herbal medicine treatment groups, compared
with LM group (Fig. 3A). The
expression levels of VEGF were also significantly increased in the
LB28 group, compared with the LM28 group (P<0.05; data not
shown).
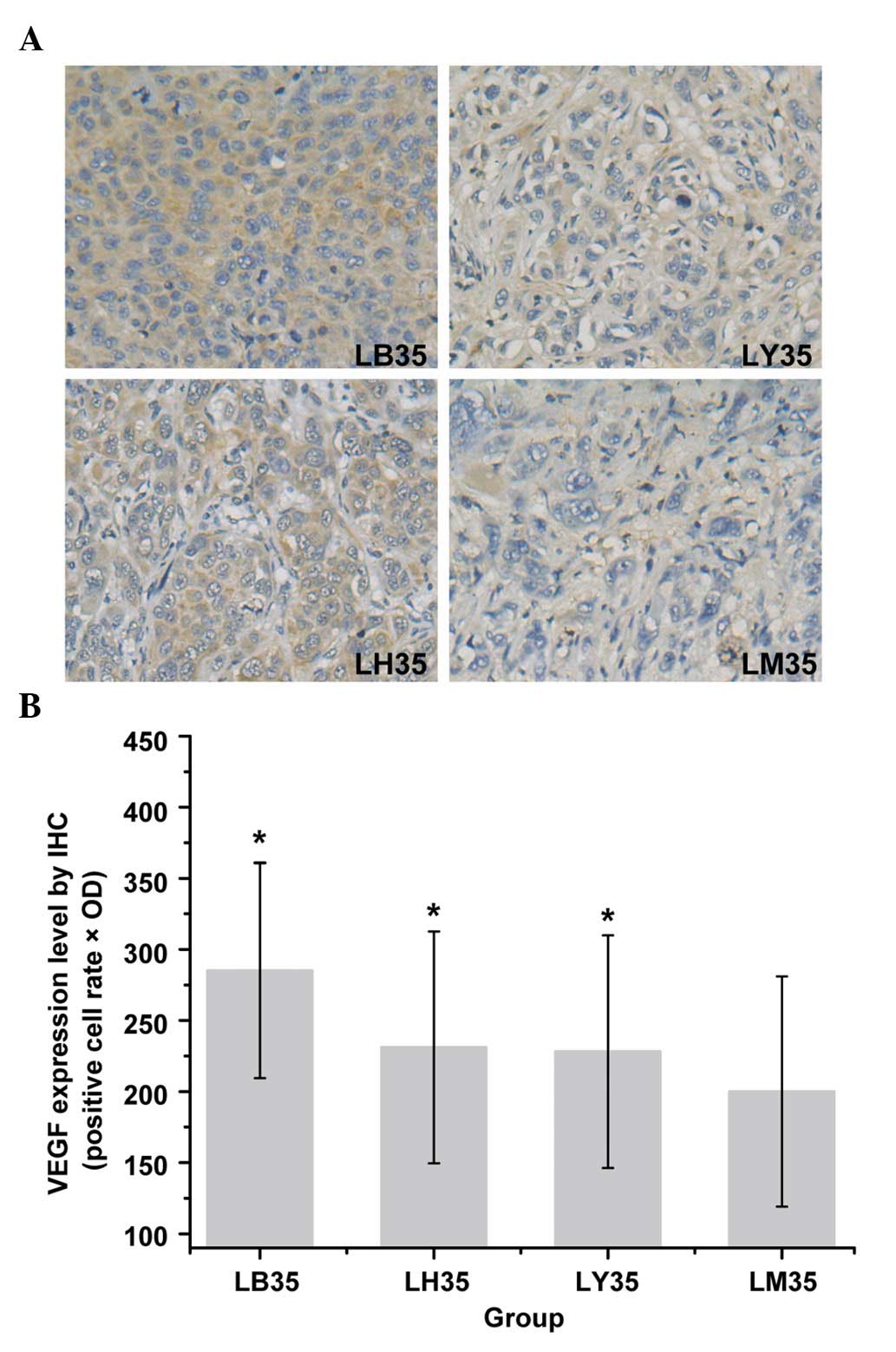 | Figure 3Expression levels of VEGF in HCC
tissues from nude mice of different groups 35 days following
treatment (3,3-diaminobenzidine staining; original magnification,
×400). (A) Quantification of IHC results of the expression of VEGF.
(B) Expression levels were determined as the positive cell rate ×
OD. data Data are presented as the mean ± standard deviation.
*P<0.05, compared with the LM35 group. HCC,
hepatocellular carcinoma; LM, model control; LB, Buyang Huanwu
decotion-treated; LY, Yiqi decoction-treated; LH, Huoxue
decoction-treated; IHC, immunohistochemistry; VEGF, vascular
endothelial growth factor; OD, optical density. |
The IHC results and the expression values of RGS-5
are presented in Fig. 4 and
Table II. Statistical analysis
indicated that the expression levels of RGS-5 in herbal medicine
treatment groups were significantly different from that in the
model control group. The results showed that the expression value
of RGS-5 on day 28 following treatment in the LB, LY, LH and LM
groups were 262.64±36.33, 280.75±32.46, 281.00±31.64 and
312.87±39.36, respectively. The expression values of RGS-5 in the
LB35, LY35, LH35 and LM35 groups were 294.16±63.70, 303.66±30.02,
310.20±51.23 and 349.25±48.27, respectively. These results showed
that the herbal medicine treatment groups had lower expression
levels of RGS-5 on days 28 and 35, compared with the corresponding
time points in the LM group (P<0.05). BYHWD treatment exerted a
more significant inhibitory effect, compared with the other herbal
medicines. However, no significant difference was found on day 21
in the medicine treatment groups, compared with the model group
(P>0.05; Fig. 4; Table II).
 | Table IIComparison of expression levels of
regulator of G protein signaling 5. |
Table II
Comparison of expression levels of
regulator of G protein signaling 5.
| Group | Day 21 | Day 28 | Day 35 |
|---|
| LB | 241.66±76.90 |
262.64±36.33a |
294.16±63.70b |
| LY | 271.16±46.44 |
280.75±32.46a |
303.66±30.02b |
| LH | 268.12±52.34 |
281.00±31.64a |
310.20±51.23b |
| LM | 285.27±49.69 | 312.87±39.36 | 349.25±48.27 |
The present study also examined the change in
expression of HIF-1α following herbal medicine treatment, compared
with the model control group (Fig.
5). The result showed that the expression of HIF-1α in the
tumor tissue was downregulated by BYHWD and HXD treatment, compared
with the LM group, at day 35 post-treatment (P<0.05). The
expression values (positive cell rate × OD) are shown in Table III. The expression values in the
LB35 and LH35 groups were 250.95±32.08 and 256.29±24.08,
respectively. The expression levels of HIF-1α were lower in the
medicine-treatment groups, compared with the model control groups
at the corresponding time points. However, no significant
differences were found, compared with the control on days 21 and 28
post-treatment (P>0.05). In addition, no significant difference
between the LY group and LM group were identified.
 | Table IIIComparison of expression levels of
hypoxia-inducible factor 1α. |
Table III
Comparison of expression levels of
hypoxia-inducible factor 1α.
| Group | Day 21 | Day 28 | Day 35 |
|---|
| LB | 230.33±30.89 | 243.29±45.10 |
250.95±32.08a |
| LY | 245.33±45.20 | 243.85±31.82 | 277.41±49.28 |
| LH | 259.33±32.40 | 260.00±43.14 |
256.29±24.08a |
| LM | 250.12±46.20 | 269.41±34.35 | 284.66±37.48 |
Discussion
Angiogenesis is critical for the growth, invasion
and metastasis of cancer, including HCC (28). In the present study, a metastatic
HCC nude mice model, which has been successfully used for HCC
therapeutic agent screening, was used to investigate the effect of
BYHWD and its decomposed constituents on the growth, angiogenesis
and metastasis of HCC tumors. The results demonstrated that the
traditional herbal medicine, BYHWD, had no significant growth
inhibitory effect on HCC. However, BYHWD was found to inhibit
metastasis and decrease MVD. In addition, BYHWD increased the
expression of VEGF, and decreased the expression levels of RGS-5
and HIF-1α on day 35 following treatment, compared with the model
control.
Poon et al demonstrated that tumor MVD can be
used as a predictor of recurrence following resection of HCC
(29). In addition, there is
increasing evidence that MVD can be considered as an indirect
marker of neo-angiogenesis (30).
CD105 (endoglin) is a transforming growth factor-β (TGF-β) binding
protein, which is expressed on the surface of endothelial cells
(31). Small and likely immature
tumor blood vessels are predominantly stained by anti-CD105
monoclonal antibodies, as demonstrated previously in breast and
prostate cancer (32,33). Of note, several studies have shown
that high MVD values, evaluated using anti-CD105 monoclonal
antibodies, are significantly associated with neovascularization
and prognosis in solid tumors (34). Previous data have shown that CD105
is superior to CD31 and CD34 in the evaluation of angiogenesis in
certain types of cancer, as CD105 has a higher affinity for
activated endothelial cells, whereas CD31 and CD34 react with
normal and activated vessels (35). CD105 was identified as a
significant marker of the presence and degree of neoangiogenesis.
In the present study, the MVD vasculature marker was assessed by
the expression of CD105. From the results, it was found that YQD or
HXD treatment alone did not suppress HCC tumor growth, and no
inhibition of tumor growth was induced by BYHWD until 35 days
post-treatment. The present study demonstrated that the changes in
tumor weight and volume in the BYHWD treatment group at 35 day were
smaller than those in model group, but without statistical
significance (P>0.05). However, BYHWD treatment significantly
reduced the MVD, indicating BYHWD treatment may affect
neovascularization. In addition, the nude mice model in the present
study showed 100% metastasis to the lung, indicating the HCCLM3
model in nude mice is an effective tool for investigating
metastasis in HCC. However, BYHWD decreased lung metastases
compared with the model groups, despite its poor inhibitory effect
on tumor growth. Although increased tumor angiogenesis is usually
associated with increased metastasis, it has been demonstrated that
vascularization is not a marker of tumor metastasis. The present
study hypothesized that the lung metastasis of HCC may have
different predominant angiogenic factors or different vascular
pathways, which is consistent with the results of studies by
Stephens et al (36) and
Zhang et al (37). Taken
together, it was suggested that BYHWD had no significant inhibitory
effect on HCC tumor growth, but BYHWD significantly inhibited the
metastasis of HCC to the lungs and decreased tumor MVD.
Blood vessels are composed of two distinct cell
types, mural cells and endothelial cells. Depending on their
density, location, morphology and marker expression, the mural
cells forming the outer layers of the vascular wall can be
subdivided into pericytes and vascular smooth muscle cells
(38). Masood et al
demonstrated that the inhibition of endothelial cell mitogen VEGF
signaling inhibited tumor angiogenesis and tumor cell growth, and
reduced viability when expression of the VEGF was observed in the
tumor cells (39). In addition,
elevated levels of VEGF are reported to be associated with tumor
metastasis (40). Huang et
al revealed that the herbal extract, Songyou Yin, inhibited HCC
invasion and inhibited the expression of VEGF in the MHCC97H HCC
cells in vitro (14).
However, in the present study, the expression levels of VEGF
increased in the LB28 and LB35 groups, compared with the other
groups, which was consistent with the earlier studies of Zhang
et al (41) and Cai et
al (16). Zhang et al
found that BYHWD combined with bone marrow mesenchymal stem cell
transplantation repaired injured blood vessels and lesion tissues
via the upregulation of VEGF (41). Data has also revealed no survival
benefit following trials of anti-VEGF monotherapy in combination
with chemotherapy for the treatment of patients with metastatic
cancer patients, possibly due to the complexity of tumor
angiogenesis regulation (42).
Thus, the present study hypothesized that BYHWD upregulated the
expression of VEGF in HCC in nude mice.
By contrast, angiogenesis is driven by several other
angiogenic factors, and several studies have suggested that
pericytes may be important in the regulation of angiogenesis in
certain tumor model systems (43).
The marker profile of pericytes differs depending on the tissue of
origin, whereas RGS-5 is one of the most common markers (44). RGS-5 is a signaling protein that
modulates the function of G proteins (45). Bahrami et al found that
RGS-5 was a marker of hepatic stellate cells, and its expression
can mediate responses to liver injury (46). In addition, Chen et al
demonstrated that the expression of RGS-5 was high in HCC (47). Furthermore, Berger et al
demonstrated that RGS-5 was upregulated in pericytes during
neovascularization and antitumor therapy, which reversed the tumor
vasculature to an almost normal morphology and resulted in the
down-regulation of RGS-5 transcription (48). In the present study, whether BYHWD
treatment inhibited the angiogenesis of HCC by inhibiting the
expression of RGS-5 was investigated. The results revealed that the
herbal medicine treatment groups on days 28 and 35 had lower
expression levels of RGS-5, compared with the LM28 and LM35 groups.
Preclinical and initial clinical evidence have revealed that the
normalization of abnormal vasculature is emerging as a
complementary therapeutic application for the treatment of cancer
(49). Therefore, in accordance
with the previous studies, the present study hypothesized that
BYHWD may assist in normalization of vasculature in HCC by
inhibiting the expression of RGS-5.
Intratumoral hypoxia leads to the expression and
stabilization of the pro-tumorigenic HIF-1 transcription factor,
which is a heterodimer composed of HIF-1α and HIF-1β (50). Kaur et al demonstrated that
HIF was an essential regulatory factor in the tumor
microenvironment due to its central role in promoting invasive and
angiogenic properties through its upregulation of target genes
containing hypoxia-responsive elements, including VEGF (50). Under hypoxic conditions, pericytes
are able to secrete VEGF, and the quiescent vascular network is
activated (51). Wu et al
revealed that hypoxia stimulated angiogenesis to support HCC tumor
growth, along with increasing the expression levels of HIF-1α and
VEGF (52). The survival rates of
the patients with HCC exhibiting high expression levels of HIF-1α
were shorter, compared with those exhibiting low expression levels.
In the present study, the expression levels of HIF-1α in the LB35
group and LH35 group were lower, compared with that in the LM
group. In accordance with a study by Fukumura et al
(53), these results indicated
that BYHWD treatment decreased the expression of HIF-1α and
contributed to the HCC tumor microenvironment.
In conclusion, the results of the present study
showed that BYHWD had no significant growth inhibitory effect on
HCC, however, BYHWD treatment elevated the expression level of VEGF
in HCC. BYHWD may significantly inhibit the angiogenesis and
metastasis of HCC in nude mice via inhibition of the expression
levels of RGS-5 and HIF-1α, and affecting tumor vasculature
structure and function. These results suggested that BYHWD may have
beneficial effects on the tumor microenvironment, vasculature
normalization, and inhibition of metastasis in HCC. However,
further detailed experiments and investigations are required to
assess the role of BYHWD therapy in the treatment of HCC.
Acknowledgments
This study was supported by the Science and
Technology Foundation of Ren Ji Hospital, School of Medicine,
Shanghai Jiao Tong University, Shanghai, China (grant no.
12XJ10051).
References
|
1
|
Liu M, Jiang L and Guan XY: The genetic
and epigenetic alterations in human hepatocellular carcinoma: A
recent update. Protein Cell. 5:673–691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pang R and Poon RT: Angiogenesis and
antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett.
242:151–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Folkman J: Fundamental concepts of the
angiogenic process. Curr Mol Med. 3:643–651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao J, Lin W, Cao Z, Zhuang Q, Zheng L,
Peng J and Hong Z: Total alkaloids of Rubus alceifolius Poir
inhibit tumor angiogenesis through suppression of the Notch
signaling pathway in a mouse model of hepatocellular carcinoma. Mol
Med Rep. 11:357–361. 2015.
|
|
8
|
Casazza A, Laoui D, Wenes M, Rizzolio S,
Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA,
Tamagnone L and Mazzone M: Impeding macrophage entry into hypoxic
tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis
and restores antitumor immunity. Cancer Cell. 24:695–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee E, Koskimaki JE, Pandey NB and Popel
AS: Inhibition of lymphangiogenesis and angiogenesis in breast
tumor xenografts and lymph nodes by a peptide derived from
transmembrane protein 45A. Neoplasia. 15:112–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Konkimalla VB and Efferth T:
Evidence-based Chinese medicine for cancer therapy. J
Ethnopharmacol. 116:207–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Y, Wang S, Wu X, Zhang J, Chen R, Chen
M and Wang Y: Chinese herbal medicine-derived compounds for cancer
therapy: A focus on hepatocellular carcinoma. J Ethnopharmacol.
149:601–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han SY and Li PP: Progress of research in
antitumor mechanisms with Chinese medicine. Chin J Integr Med.
15:316–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong Y, Lu B, Zhang X, Zhang J, Lai L, Li
D, Wu Y, Song Y, Luo J, Pang X, et al: Cucurbitacin E, a
tetracyclic triterpenes compound from Chinese medicine, inhibits
tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling
pathway. Carcinogenesis. 31:2097–2104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang XY, Wang L, Huang ZL, Zheng Q, Li QS
and Tang ZY: Herbal extract 'Songyou Yin' inhibits tumor growth and
prolongs survival in nude mice bearing human hepatocellular
carcinoma xenograft with high metastatic potential. J Cancer Res
Clin Oncol. 135:1245–1255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao LD, Wang JH, Jin GR, Zhao Y and Zhang
HJ: Neuroprotective effect of Buyang Huanwu decoction against focal
cerebral ischemia/reperfusion injury in rats-time window and
mechanism. J Ethnopharmacol. 140:339–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai G, Liu B, Liu W, Tan X, Rong J, Chen
X, Tong L and Shen J: Buyang Huanwu decoction can improve recovery
of neurological function, reduce infarction volume, stimulate
neural proliferation and modulate VEGF and Flk1 expressions in
transient focal cerebral ischaemic rat brains. J Ethnopharmacol.
113:292–299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shang B, Cao Z and Zhou Q: Progress in
tumor vascular normalization for anticancer therapy: Challenges and
perspectives. Front Med. 6:67–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye
QH, Wang L, Zhou J, Qiu SJ, Li Y, et al: A decade's studies on
metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol.
130:187–196. 2004. View Article : Google Scholar
|
|
20
|
Xu JQ: Formulas of Chinese Medicine.
Shanghai Scientific & Technical Publishers; Shanghai: pp.
1–298. 1985
|
|
21
|
Chinese Pharmacopoeia Commission: Chinese
pharmacopoeia. Chemical Industry Press; Beijing China: pp.
5472005
|
|
22
|
Kang M, Ou H, Wang R, Liu W, Mao Y and
Tang A: Effect of trichosanthin on apoptosis and telomerase
activity of nasopharyngeal carcinomas in nude mice. J BUON.
18:675–682. 2013.PubMed/NCBI
|
|
23
|
Institute of Laboratory Animal Resources
(US): Committee on Care, Use of Laboratory Animals, and National
Institutes of Health (US). Division of Research Resources: Guide
for the care and use of laboratory animals. 1st edition. National
Academies Press; Washington, DC: 1996
|
|
24
|
Li Y, Tang Z, Ye L, Liu B, Liu K, Chen J
and Xue Q: Establishment of a hepatocellular carcinoma cell line
with unique metastatic characteristics through in vivo selection
and screening for metastasis-related genes through cDNA microarray.
J Cancer Res Clin Oncol. 129:43–51. 2003.PubMed/NCBI
|
|
25
|
Zhang T, Sun HC, Xu Y, Zhang KZ, Wang L,
Qin LX, Wu WZ, Liu YK, Ye SL and Tang ZY: Overexpression of
platelet-derived growth factor receptor alpha in endothelial cells
of hepatocellular carcinoma associated with high metastatic
potential. Clin Cancer Res. 11:8557–8563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye QH, Qin LX, Forgues M, He P, Kim JW,
Peng AC, Simon R, Li Y, Robles AI, Chen Y, et al: Predicting
hepatitis B virus-positive metastatic hepatocellular carcinomas
using gene expression profiling and supervised machine learning.
Nat Med. 9:416–423. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang ZL, Liu ZS and Sun Q: Effects of
thalidomide on angiogenesis and tumor growth and metastasis of
human hepatocellular carcinoma in nude mice. World J Gastroenterol.
11:216–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan
ST and Wong J: Tumor microvessel density as a predictor of
recurrence after resection of hepatocellular carcinoma: A
prospective study. J Clin Oncol. 20:1775–1785. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ng IO, Poon RT, Lee JM, Fan ST, Ng M and
Tso WK: Microvessel density, vascular endothelial growth factor and
its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J
Clin Pathol. 116:838–845. 2001. View Article : Google Scholar
|
|
31
|
Yao Y, Kubota T, Takeuchi H and Sato K:
Prognostic significance of microvessel density determined by an
anti-CD105/endoglin monoclonal antibody in astrocytic tumors:
Comparison with an anti-CD31 monoclonal antibody. Neuropathology.
25:201–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wikström P, Lissbrant IF, Stattin P,
Egevad L and Bergh A: Endoglin (CD105) is expressed on immature
blood vessels and is a marker for survival in prostate cancer.
Prostate. 51:268–275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fonsatti E and Maio M: Highlights on
endoglin (CD105): From basic findings towards clinical applications
in human cancer. J Transl Med. 2:182004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Imura S, Miyake H, Izumi K, Tashiro S and
Uehara H: Correlation of vascular endothelial cell proliferation
with microvessel density and expression of vascular endothelial
growth factor and basic fibroblast growth factor in hepatocellular
carcinoma. J Med Invest. 51:202–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka F, Otake Y, Yanagihara K, Kawano Y,
Miyahara R, Li M, Yamada T, Hanaoka N, Inui K and Wada H:
Evaluation of angiogenesis in non-small cell lung cancer comparison
between anti-CD34 antibody and anti-CD105 antibody. Clin Cancer
Res. 7:3410–3415. 2001.PubMed/NCBI
|
|
36
|
Stephens TD, Bunde CJ and Fillmore BJ:
Mechanism of action in thalidomide teratogenesis. Biochem
Pharmacol. 59:1489–1499. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang ZL, Liu ZS and Sun Q: Anti-tumor
effect of thalidomide and paclitaxel on hepatocellular carcinoma in
nude mice. Chin Med J (Engl). 118:1688–1694. 2005.
|
|
38
|
Zhou L, Sohet F and Daneman R:
Purification and culture of central nervous system pericytes. Cold
Spring Harb Protoc. 2014:581–583. 2014.PubMed/NCBI
|
|
39
|
Masood R, Cai J, Zheng T, Smith DL, Hinton
DR and Gill PS: Vascular endothelial growth factor (VEGF) is an
autocrine growth factor for VEGF receptor-positive human tumors.
Blood. 98:1904–1913. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hirakawa S, Brown LF, Kodama S, Paavonen
K, Alitalo K and Detmar M: VEGF-C-induced lymphangiogenesis in
sentinel lymph nodes promotes tumor metastasis to distant sites.
Blood. 109:1010–1017. 2007. View Article : Google Scholar
|
|
41
|
Zhang YK, Han XY and Che ZY: Effects of
buyang huanwu tang combined with bone marrow mesenchymal stem cell
transplantation on the expression of VEGF and Ki-67 in the brain
tissue of the cerebral ischemia-reperfusion model rat. J Tradit
Chin Med. 30:278–282. 2010. View Article : Google Scholar
|
|
42
|
Quesada AR, Medina MA and Alba E: Playing
only one instrument may be not enough: Limitations and future of
the antiangiogenic treatment of cancer. Bioessays. 29:1159–1168.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shaheen RM, Tseng WW, Davis DW, Liu W,
Reinmuth N, Vellagas R, Wieczorek AA, Ogura Y, McConkey DJ, Drazan
KE, et al: Tyrosine kinase inhibition of multiple angiogenic growth
factor receptors improves survival in mice bearing colon cancer
liver metastases by inhibition of endothelial cell survival
mechanisms. Cancer Res. 61:1464–1468. 2001.PubMed/NCBI
|
|
44
|
Bondjers C, Kalén M, Hellström M, Scheidl
SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J and Betsholtz
C: Transcription profiling of PDGF-B deficient embryos identifies
RGS5 as a novel marker for pericytes and vascular smooth muscle
cells. Am J Pathol. 162:721–729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou J, Moroi K, Nishiyama M, Usui H, Seki
N, Ishida J, Fukamizu A and Kimura S: Characterization of RGS5 in
regulation of G protein-coupled receptor signaling. Life Sci.
68:1457–1469. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bahrami AJ, Gunaje JJ, Hayes BJ, Riehle
KJ, Kenerson HL, Yeung RS, Stempien-Otero AS, Campbell JS and
Mahoney WM Jr: Regulator of G-protein signaling-5 is a marker of
hepatic stellate cells and expression mediates response to liver
injury. PLoS One. 9:e1085052014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen X, Higgins J, Cheung ST, Li R, Mason
V, Montgomery K, Fan ST, van de Rijn M and So S: Novel endothelial
cell markers in hepatocellular carcinoma. Mod Pathol. 17:1198–1210.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Berger M, Bergers G, Arnold B, Hämmerling
GJ and Ganss R: Regulator of G-protein signaling-5 induction in
pericytes coincides with active vessel remodeling during
neovascularization. Blood. 105:1094–1101. 2005. View Article : Google Scholar
|
|
49
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kaur B, Khwaja FW, Severson EA, Matheny
SL, Brat DJ and Van Meir EG: Hypoxia and the
hypoxia-inducible-factor pathway in glioma growth and angiogenesis.
Neuro Oncol. 7:134–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Qing G and Simon MC: Hypoxia inducible
factor-2alpha: A critical mediator of aggressive tumor phenotypes.
Curr Opin Genet Dev. 19:60–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu XZ, Xie GR and Chen D: Hypoxia and
hepatocellular carcinoma: The therapeutic target for hepatocellular
carcinoma. J Gastroen Hepatol. 22:1178–1182. 2007. View Article : Google Scholar
|
|
53
|
Fukumura D and Jain RK: Tumor
microvasculature and micro-environment: Targets for
anti-angiogenesis and normalization. Microvasc Res. 74:72–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|