Introduction
Osteosarcoma (OS) is the most prevalent primary
malignant bone tumor, which predominantly affects children and
adolescents (1). Over the past
decade, the development of multiple therapeutic strategies for OS
has significantly enhanced patient outcomes, and the 5-year
survival rate of patients with OS has improved markedly (2). However, outcomes remain poor, and the
majority of patients eventually succumb to pulmonary
metastases-associated mortality (3). Therefore, there is an urgent
requirement to identify biomarkers and therapeutic targets for the
treatment of patients with OS.
Class A scavenger receptors (SR-As) are type of cell
surface receptors, which bind a range of ligands, including
modified low-density lipoproteins and nucleic acids (4). SR-As are characterized by the
presence of a collagen-like domain and include macrophage scavenger
receptor type A (SR-A; SCARA1) (5), MARCO (SCARA2) (6), CSR1 (SCARA3) (7), SRCL4 (SCARA4) (8) and SCARA5 (9). These receptors are known to make
important contributions to host defense. For example, Suzuki et
al showed that SR-A−/− mice were more
susceptible to Listeria monocytogenes infection, compared
with wild-type control mice (10).
Several previous studies have demonstrated that SCARA5 is involved
in cancer progression. For example, one study showed that
inhibiting the downregulation of SCARA5 significantly attenuates
the epithelial-to-mesenchymal transition-associated migration of
human lung carcinoma A549 cells that is induced by transforming
growth factor-β1 (11). Another
study reported that SCARA5 knockdown markedly enhanced human
hepatocellular carcinoma (HCC) cell growth in vitro, colony
formation in soft agar, and invasiveness, tumorigenicity, and lung
metastasis in vivo (12).
However, its role in the progression and metastasis of OS remains
to be fully elucidated.
Therefore, the present study investigated whether
SCARA5 is involved in OS tumor growth and metastasis, and examined
the potential underlying mechanisms as SCARA5 may be a potential
therapeutic target for the treatment of patients with OS.
Materials and methods
Tissue specimens
Fresh OS tissue specimens were collected from 26
patients who underwent surgery for OS resection at Dongfang
Hospital Affiliated to Beijing University of Chinese Medicine
(Beijing, China) between October 2012 and May 2014. The group of
patients with OS comprised 14 males and 12 females, aged between 13
and 58 years. Without any preoperative treatment, all 26 cases were
pathologically diagnosed as OS postoperatively. In addition, 22
normal bone tissue specimens were collected from the long bones of
22 healthy subjects aged 24–61 years (males, 8; females, 14). The
specimens were immediately preserved in liquid nitrogen (Leshan
Dongya Cryogenic Vessel Co., Ltd, Beijing, China) for subsequent
analyses. All subjects provided written informed consent, and the
specimen collection procedure of the present study was approved by
the Medical Ethics Committee of Dongfang Hospital Affiliated to
Beijing University of Chinese Medicine (Beijing, China).
Cell culture
The U2OS and MG63 human OS cell lines and normal
osteoblast cell line (hFOB1.19) were purchased from the American
Type Culture Collection (Manassas, VA, USA). hFOB1.19 normal
osteoblast cell line was used as the control group when evaluating
the expression levels of SCARA5 in OS cells. All OS cell lines were
cultured in complete Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.), 1% L-glutamine, and 1% penicillin/streptomycin
(both Sigma-Aldrich, St. Louis, MO, USA). The cultures were
incubated at 37°C with 5% CO2 in a humidified
incubator.
Reverse transcription-quantitative
polymerase chain traction (RT-qPCR) analysis
OS tissues were frozen in liquid nitrogen, washed
twice with phosphate-buffered saline and lysed using ice-cold
radioimmunoprecipitation assay buffer and 0.1 PMSF supplemented
with a protease inhibitor cocktail (Sigma-Aldrich). Following
centrifugation at 12,000 × g for 5 min, the supernatant was
collected. When the cells reached 90% confluency, total RNA was
extracted from the OS tissues and cells using TRIzol reagent,
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). Subsequently, 2 µg of total RNA was
reverse transcribed to first-strand cDNA using TaqMan reverse
transcription reagents (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following primers were used: SCARA5, sense
5'-CAGCTGGTTTCTTACCACGTAT-3' and antisense
5'-GCACAAGTTCTCCCACACTTAG-3'; and β-actin, sense
5'-CCGTGAAAAGATGACCCAGATC-3' and antisense
5'-CACAGCCTGGATGGCTACGT-3'. RT-qPCR was performed using 1 µl
cDNA templates, 2 µl forward and reverse primers and 5
µl SYBR Green qPCR Master Mix. Thermal cycling was performed
as follows: 95°C for 10 min, then 40 cycles of 95°C for 15 sec,
59°C for 30 sec, 72°C for 30 sec, followed by an extra extension at
72°C for 5 min. Reactions were performed using a Step One Plus
Real-Time PCR machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.). For analysis, the expression levels of the
target gene were normalized by the gene expression of β-actin.
Based on the ΔΔCq method (13),
the relative quantities of mRNA were expressed as
2−ΔΔCq.
Western blot analysis
Proteins were extracted from the OS tissues and
cells, and protein concentrations were measured using the Bradford
method (14). Subsequently, 30
µg of protein was separated by 10% SDS-PAGE and transferred
onto a polyvinylidene fluoride membrane (both Sigma-Aldrich). The
membrane was incubated with 2% nonfat dry milk in Tris-buffered
saline (TBS; Sigma-Aldrich), to block non-specific binding, at room
temperature for 1 h. Subsequently, the membrane was immunoblotted
with the following mouse monoclonal primary antibodies: Anti-SCARA5
(1:1,500; sc-98123), anti-phosphorylated (p)-FAK (Y397; sc-11765),
anti-FAK (both 1:1,000; sc-271195), anti-p-Src (Y416; sc-101802),
anti-Src (both 1:2,000; sc-24621), anti-matrix metalloproteinase 2
(MMP2; sc-13594), anti-MMP9 (sc-21733) and anti-β-actin (all
1:1,000; sc-8432; all Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) overnight at 4°C, followed by three washes in TBS with
0.05% Tween-20 (Sigma-Aldrich) for 10 min. Subsequently, the
membranes were incubated with rat anti-mouse horseradish
peroxidase-conjugated secondary antibody (1:3,000; sc-2370; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. The
expression of the target protein was visualized using enhanced
chemiluminescence (Pierce Biotechnology; Thermo Fisher Scientific,
Inc.). Absorbance values of the target proteins were analyzed using
Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
SCARA5 expression vector construction and
transfection
To construct the SCARA5 recombinant adenovirus
vector, the cDNA encoding SCARA5 was amplified and subcloned into
the adenoviral shuttle vector, pAd-CMV, and green fluorescent
protein (GFP; both Invitrogen; Thermo Fisher Scientific, Inc.) was
used as a non-specific control. The pAd-CMV adenoviral shuttle
vector and pAdEasy-1 (Invitrogen; Thermo Fisher Scientific, Inc.)
adenoviral gene expression vector were homologously recombined in
the Escherichia coli strain, BJ5183 (Type Culture Collection
of the Chinese Academy of Sciences, Shanghai, China) at 4°C for 16
h. The newly recombined plasmid, Ad-SCARA5, was then propagated in
293 cells (Type Culture Collection of the Chinese Academy of
Sciences) at 37°C for 16 h. The recombinant adenoviruses were
harvested, and the titers were determined using a p24 ELISA kit
(Cell Biolabs, Inc., San Diego, CA, USA) prior to use.
For in vitro transfection, the OS cells
(3×103) were seeded in 96-well plates. The cells grown
to 30–50% confluence and were transfected with Ad-SCARA5 at final
concentrations of 50 nM, using Lipofectamine 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol.
Cell proliferation assay
To analyze cell proliferation, an MTT assay was
performed. The transiently transfected cells were seeded in a
96-well plate at a cell density of 2×103 and were
cultured at 24 h intervals for 4 days at room temperature.
Subsequently, the initial culture medium was replaced with fresh
medium containing MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA)
and incubated for an additional 4 h at room temperature. The
formazan was dissolved in dimethylsulfoxide (150 µl/well;
Sigma-Aldrich) for 10 min. The absorbance was measured at 570 nm
using a Spectra Max 190 microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA). All experiments were independently repeated at
least three times.
Colony formation assay
For the soft agar colony formation assay,
2×104 cells were plated in 24-well plates and grown on a
plate containing 1% base agar (Sigma-Aldrich) and 0.5% top agar for
21 days at room temperature, until colonies formed. The colonies
were stained with 1% crystal violet for 30 sec following fixation
with 10% formaldehyde (both Sigma-Aldrich) for 5 min. The numbers
of colonies were counted under a dissecting microscope (BIX-103G,
Luxi Chemical Group Co., Ltd., Beijing, China). All experiments
were independently repeated at least three times.
Cell migration and invasion assays
OS cells (1×104 cells/well) in 200
µl serum-free DMEM were added to the upper chamber of a
Transwell (Invitrogen; Thermo Fisher Scientific, Inc.) with an 8
µm microporous filter (Sigma-Aldrich), following which 500
µl DMEM containing 10% FBS was added to the lower chamber.
Following 24 h incubation at 37°C, the cells on the lower surface
of the filter were fixed in methanol, stained with Giemsa
(Sigma-Aldrich) and examined under a Leica DM5000B microscope
(Leica Microsystems GmbH, Wetzlar, Germany). The average numbers of
migrated cells from five randomly-selected optical fields and
triplicate filters were determined.
For in vitro invasion assays, the cells were
suspended in a volume of 50 µl serum-free medium, which was
then added to the upper chamber of a chemotaxis chamber (Neuro
Probe, Inc., Gaithersburg, MD, USA). Complete medium was added to
the lower chamber. A polycarbonate membrane (Sigma-Aldrich) was
placed between the two chambers, and culture medium supplemented
with 20 µl Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA) was applied. After 24 h at room temperature, the non-invading
cell remaining on the upper surface were removed using
cotton-tipped swabs, following which the filters were fixed in
methanol for 3 min and stained with 0.05% crystal violet in
phosphate-buffered saline for 15 min. The cells on the underside of
the filters were visualized and counted under a Leica DM5000B
microscope (Leica Microsystems GmbH). Each sample was assayed in
triplicate.
Statistical analysis
All experiments were performed independently, at
least three times. Differences between groups were analyzed via
Student's t-test using SPSS 13.0 software (IBM Corp., Armonk, NY,
USA). Data are expressed as the mean ± standard deviation from
three independent experiments performed in triplicate. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of SCARA5 at low levels in OS
tissues and cell lines
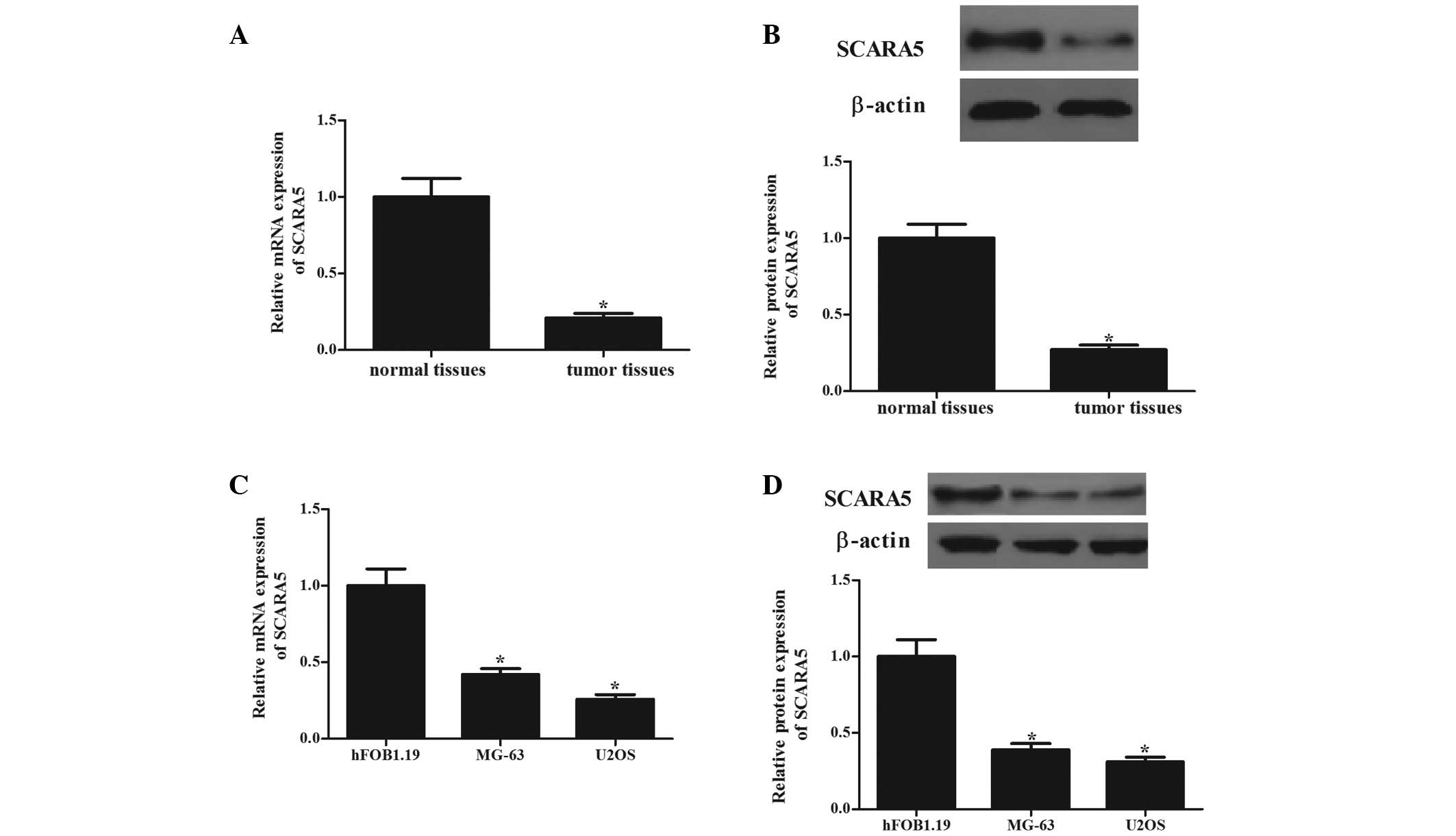
The present study first determined the mRNA and
protein levels of SCARA5 in human OS tissues. As shown in Fig. 1, the mRNA (Fig. 1A) and protein (Fig. 1B) expression levels of SCARA5 in
the OS tissues were significantly lower, compared to those in the
normal bone tissues (P<0.05). The expression levels of SCARA5 in
human OS cells (MG-63 and U2OS) were also analyzed. Consistent with
the observation from the tissue samples, the expression levels of
SCARA5 were significantly decreased in the two cell lines, compared
with those in the normal human primary osteoblasts (Fig. 1C and D). These results indicated
that SCARA5 may function as a tumor suppressor in OS.
Overexpression of SCARA5 inhibits cell
proliferation and colony formation
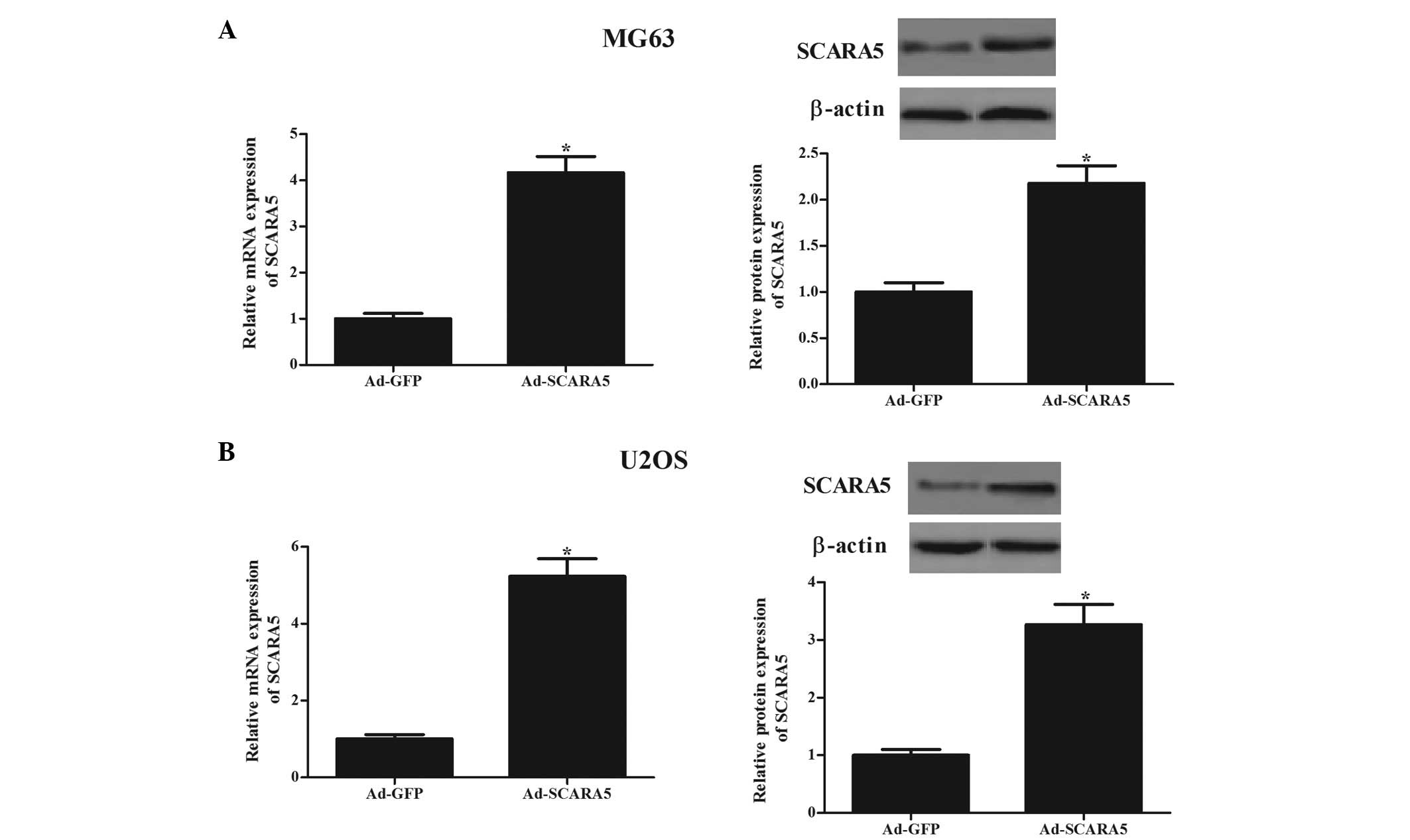
To examine the biological significance of SCARA5 in
OS tumorigenesis, the present study generated SCARA5-overexpressing
MG-63 and U2OS OS cell lines. The transfection efficiency was
confirmed using RT-qPCR and western blot analyses. Following SCARA5
transfection, the mRNA and protein levels of SCARA5 were
significantly increased in the MG-63 (Fig. 2A) and U2OS (Fig. 2B) cells, respectively. The present
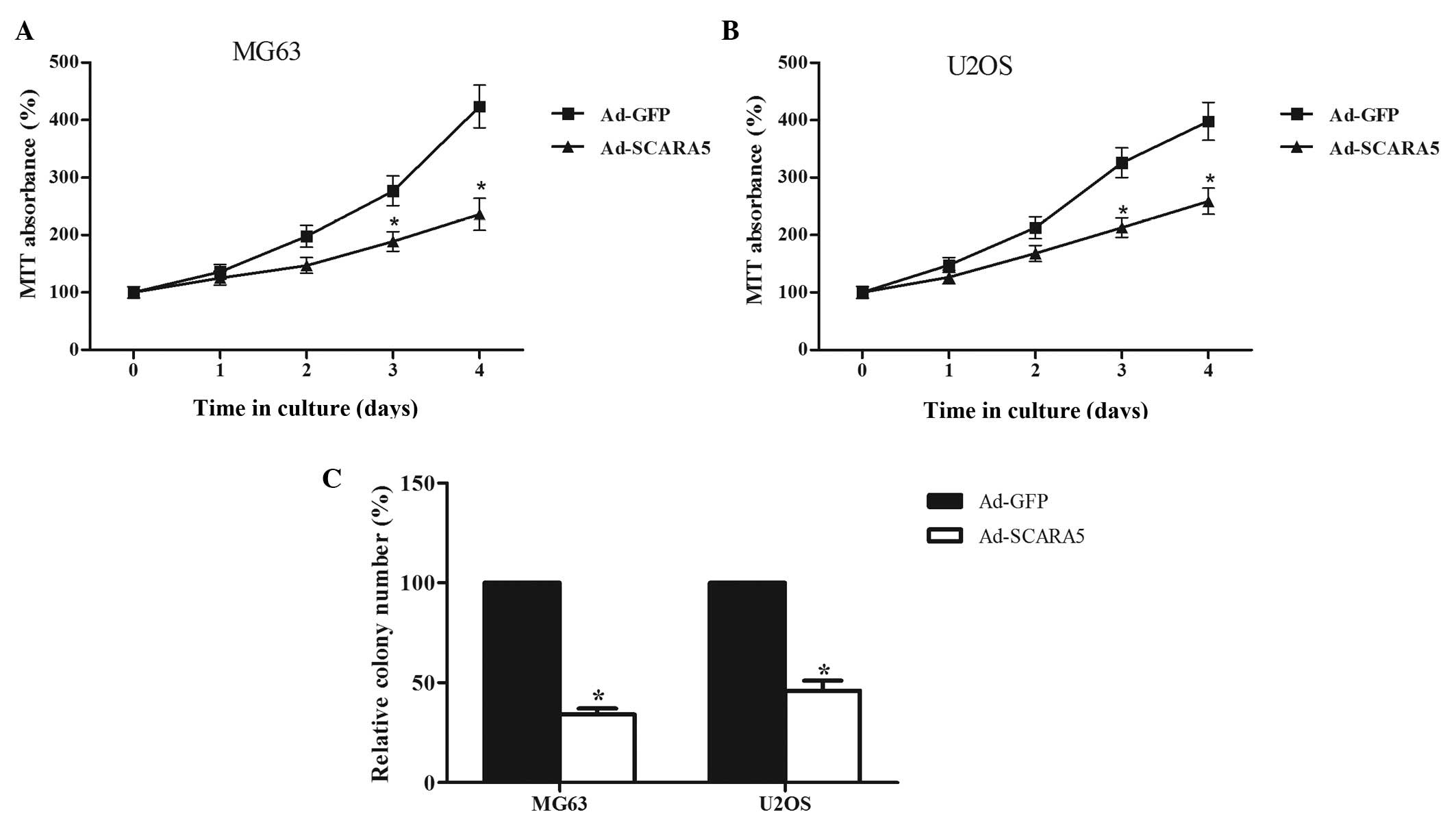
study also examined the effect of SCARA5 overexpression on cell
proliferation and colony formation. As shown in Fig. 3, transfection of the cells with
Ad-SCARA5 significantly inhibited the growth of the MG-63 cells
(Fig. 3A). It also significantly
suppressed colony formation in the MG-63 cells (Fig. 3C). Similarly, the overexpression of
SCARA5 suppressed growth (Fig. 3B)
and colony formation (Fig. 3C) in
the U2OS cells.
Overexpression of SCARA5 inhibits cell
migration and invasion
The present study used Transwell assays to assess
the effects of the expression of SCARA5 on cell migration and
invasion. The results showed that cell migration was significantly
inhibited by SCARA5 overexpression in the MG-63 (Fig. 4A, left) and U2OS cells (Fig. 4B, left), respectively. In addition,
compared with the cells transfected with the empty vector, SCARA5
overexpression significantly inhibited the invasion of the MG-63
(Fig. 4A, right) and U2OS cells
(Fig. 4B, right).
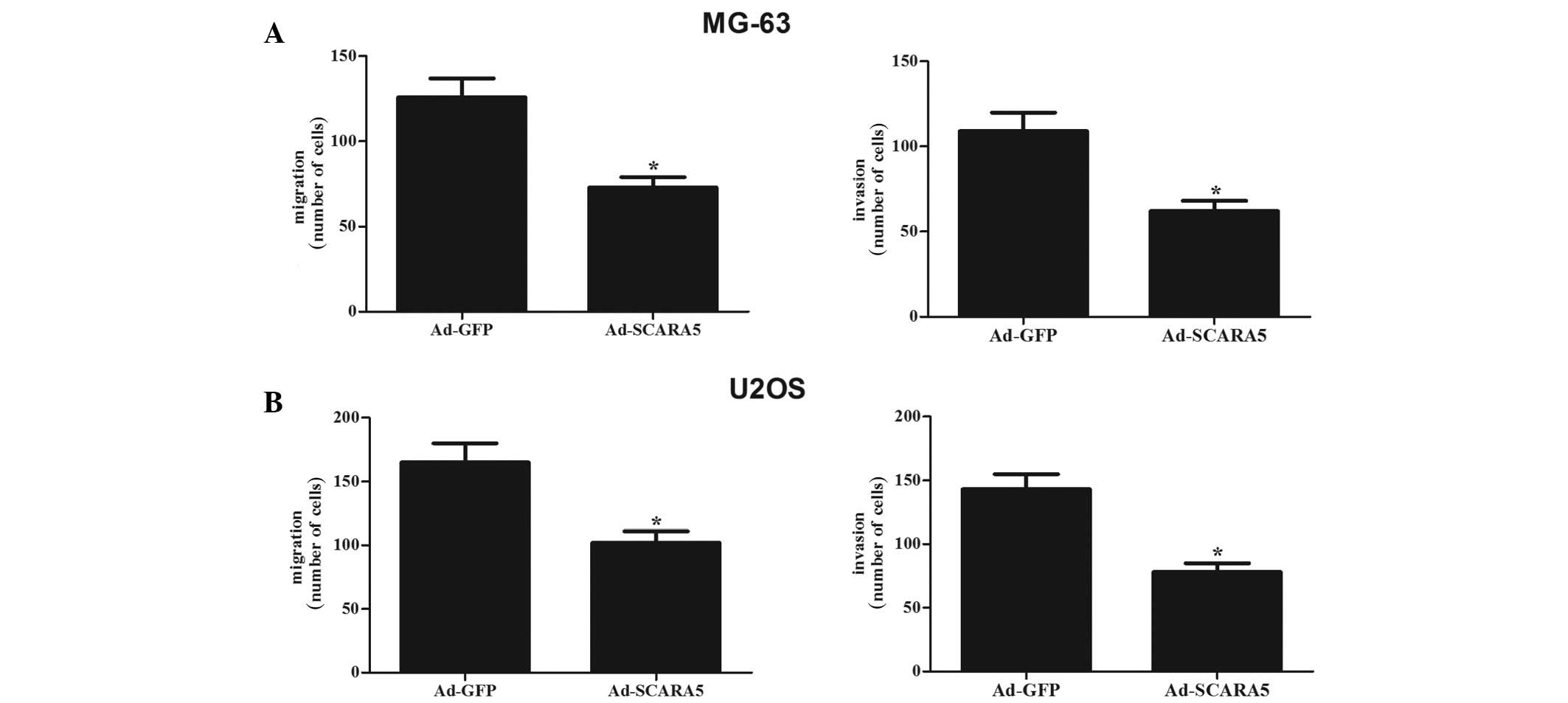 | Figure 4Overexpression of SCARA5 decreases
osteosarcoma cell migration and invasion. A Transwell assay
revealed that SCARA5 decreased migration in the MG63 (A, left) and
U2OS (B, left) cells, compared with the Ad-GFP control cells. A
Matrigel invasion assay revealed that SCARA5 decreased invasion in
the MG63 (A, right) and U2OS (B, right) cells, compared with the
Ad-GFP control cells. Data are expressed as the mean ± standard
deviation from experiments performed in triplicate.
*P<0.05, vs. Ad-GFP group. SCARA5, scavenger receptor
class A, member 5; GFP, green fluorescent protein. |
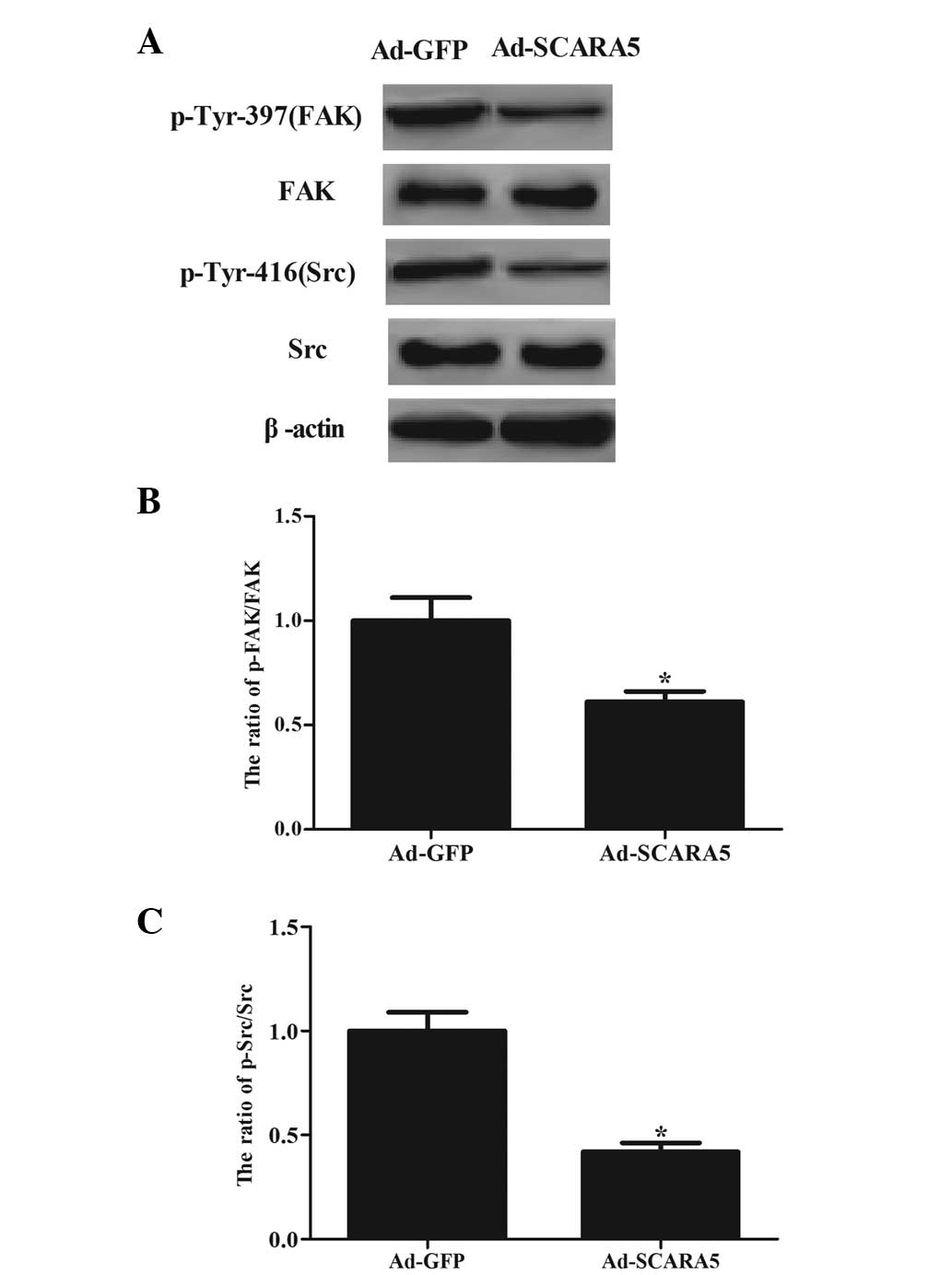
SCARA5 regulates activation of the FAK
signaling pathway by affecting tyrosine phosphorylation
The FAK signaling pathway is important in cancer
cell growth and invasion (15). To
examine the molecular mechanisms by which SCARA5 contributes to
these malignant features, the present study examined the effect of
SCARA5 on the levels of tyrosine phosphorylation at specific sites
of certain molecules involved in the FAK signaling pathway. The
results of the Western blot analysis revealed that the
overexpression of SCARA5 significantly inhibited the
phosphorylation of the FAK residue (Tyr-397; Fig. 5A and B) and Src residue (Tyr-416;
Fig. 5A and C) in the MG63
cells.
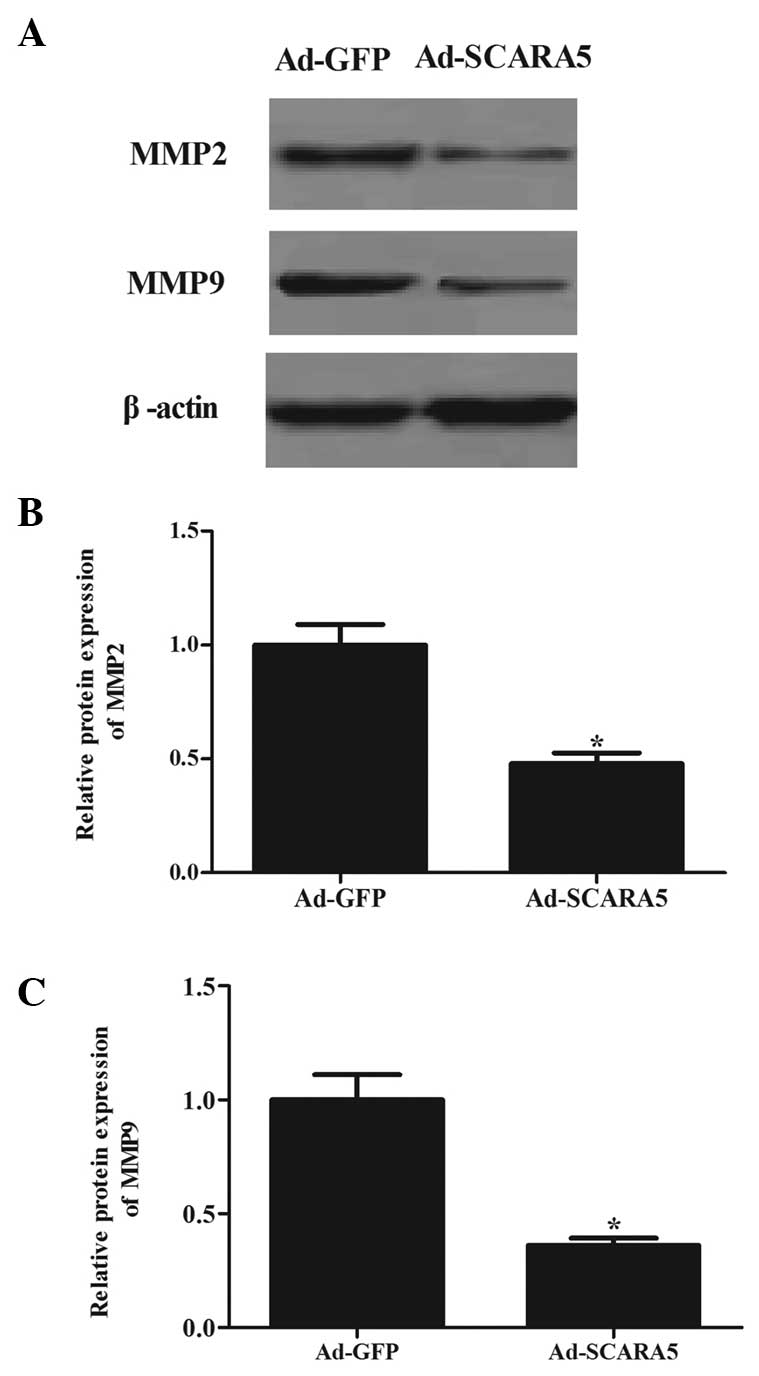
The present study also assessed the expression
levels of MMP-2 and MMP-9, which are crucial downstream molecules
in the FAK signaling pathway (16). As shown in Fig. 6, the overexpression of SCARA5
significantly inhibited the expression levels of MMP-2 (Fig. 6A and B) and MMP-9 (Fig. 6A and C) in the MG63 cells, compared
with the cells in the Ad-GFP group.
Discussion
In the present study, SCARA5 was found to be
expressed at low levels in human OS tissues and cell lines, and
provided the first evidence, to the best of our knowledge, that
SCARA5 overexpression significantly inhibits the proliferation and
reduces the metastatic activity of OS. Furthermore, the
overexpression of SCARA5 inhibited the phosphorylation of FAK and
downstream molecules in human OS cells.
SCARA3 was initially identified as a macrophage
scavenger receptor homolog (17).
A previous study showed that the expression of SCARA3 is
downregulated in prostate cancer, and the downregulation of the
expression of SCARA3 in prostate cancer cell lines is caused by
methylation of its promoter (18).
In the present study, SCARA5 was found to be expressed at low
levels in OS tissues and cell lines, suggesting that SCARA5 may be
a novel candidate tumor suppressor gene, which is downregulated in
OS as a result of promoter hypermethylation.
On obtaining the above results, the present study
subsequently investigated the role of SCARA5 in OS cell growth. It
was found that the overexpression of SCARA5 markedly reduced cell
proliferation and colony formation, suggesting that SCARA5 was
involved in abnormal cell proliferation in OS cells.
The ability of tumor cells to migrate and invade is
considered an important indicator of cell aggressiveness and
metastatic ability. Therefore, reducing cell migration and/or
invasion is essential to inhibit tumor progression (19). A previous report showed that the
overexpression of SCARA5 markedly enhanced HCC cell invasion in
vitro and tumor metastasis in vivo (12). In the present study, the
overexpression of SCARA5 was found to significantly inhibit the
migration and invasion abilities of MG63 cells. These data
indicated that SCARA5 is crucial in OS metastasis.
FAK, a non-receptor tyrosine kinase, localizes at
focal adhesions, and is important in relaying extracellular signals
between integrins and intracellular compartments (20). FAK is activated in a range of tumor
cells, and the activated FAK forms a complex with Src and p130Cas,
which leads to tumor growth and metastasis by promoting cell
survival, cell cycle progression, motility and invasion (21,22).
The concept of targeting FAK as a therapeutic strategy for cancer
treatment is promising (20). A
previous report study by Seong et al demonstrated that short
hairpin RNA-mediated knockdown of SATB2 decreases the migration and
invasion of OS cells by suppressing the phosphorylation of FAK
(23). Hu et al (24) showed that the overexpression of
phosphatase and tensin homolog also inhibits migration and invasion
through down-regulating the expression of p-FAK. The silencing of
galectin-3 also represses OS cell migration and invasion through
inhibition of FAK/Src/Lyn activation (25). Notably, Huang et al also
verified that SCARA5 inhibits HCC progression via the FAK signaling
pathway (12). Similarly, in the
present study, it was demonstrated that the overexpression of
SCARA5n in MG-63 cells markedly inhibited the phosphorylation of
FAK.
MMPs are important roles in the matrix degradation
required for tumor growth and invasion (26). It has been reported that MMP-9 is
directly associated with metastatic processes in OS (27,28).
MMP2 is also a prominent member of the MMP family, and patients
with OS exhibiting high expression levels of MMP2 have a poor
prognosis (29). In addition,
MMP-2 and MMP-9 are crucial downstream molecules in the FAK
signaling pathway. In the present study, the overexpression of
SCARA5 was observed to significantly inhibit the expression levels
of MMP-2 and MMP-9 in MG63 cells, compared with cells in the
Ad-GFP-transfected group. These results suggested that the
overexpression of SCARA5 may contribute to the downregulation of
p-FAK and downstream molecules in MG63 cells, which may lead to
reduced tumor cell growth and invasion.
In conclusion, the present study showed that SCARA5
may be important in tumor growth and metastasis, and that SCARA5
may be a potential therapeutic target for the treatment of OS.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amankwah EK, Conley AP and Reed DR:
Epidemiology and therapies for metastatic sarcoma. Clin Epidemiol.
5:147–162. 2013.PubMed/NCBI
|
|
3
|
Friedman MA and Carter SK: The therapy of
osteogenic sarcoma: Current status and thoughts for the future. J
Surg Oncol. 4:482–510. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peiser L and Gordon S: The function of
scavenger receptorsex-pressed by macrophages and their role in the
regulation of inflammation. Microbes Infect. 3:149–159. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Platt N, Haworth R, Darley L and Gordon S:
The many roles of the class A macrophage scavenger receptor. Int
Rev Cytol. 212:1–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elomaa O, Sankala M, Pikkarainen T,
Bergmann U, Tuuttila A, Raatikainen-Ahokas A, Sariola H and
Tryggvason K: Structure of the human macrophage MARCO receptor and
characterization of its bacteria-binding region. J Biol Chem.
273:4530–4538. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han HJ, Tokino T and Nakamura Y: CSR, a
scavenger receptor-like protein with a protective role against
cellular damage caused by UV irradiation and oxidative stress. Hum
Mol Genet. 7:1039–1046. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohtani K, Suzuki Y, Eda S, Kawai T, Kase
T, Keshi H, Sakai Y, Fukuoh A, Sakamoto T, Itabe H, et al: The
membrane-type collectin CL-P1 is a scavenger receptor on vascular
endothelial cells. J Biol Chem. 276:44222–44228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Y, Oliver P, Davies KE and Platt N:
Identification and characterization of murine SCARA5, a novel class
A scavenger receptor that is expressed by populations of epithelial
cells. J Biol Chem. 281:11834–11845. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki H, Kurihara Y, Takeya M, Kamada N,
Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, et
al: A role for macrophage scavenger receptors in atherosclerosis
and susceptibility to infection. Nature. 386:292–296. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Hu G, Chen D, Gong AY, Soori GS,
Dobleman TJ and Chen XM: Suppression of SCARA5 by Snail1 is
essential for EMT-associated cell migration of A549 cells.
Oncogenesis. 2:e732013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang J, Zheng DL, Qin FS, Cheng N, Chen
H, Wan BB, Wang YP, Xiao HS and Han ZG: Genetic and epigenetic
silencing of SCARA5 may contribute to human hepatocellular
carcinoma by activating FAK signaling. J Clin Invest. 120:223–241.
2010. View
Article : Google Scholar :
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
14
|
Kruger NJ: The Bradford method for protein
quantitation. Methods Mol Biol. 32:9–15. 1994.PubMed/NCBI
|
|
15
|
Sulzmaier FJ, Jean C and Schlaepfer DD:
FAK in cancer: Mechanistic findings and clinical applications. Nat
Rev Cancer. 14:598–610. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fingleton B: Matrix metalloproteinases:
Roles in cancer and metastasis. Front Biosci. 11:479–491. 2006.
View Article : Google Scholar
|
|
17
|
Yu G, Tseng GC, Yu YP, Gavel T, Nelson J,
Wells A, Michalopoulos G, Kokkinakis D and Luo JH: CSR1 suppresses
tumor growth and metastasis of prostate cancer. Am J Pathol.
168:597–607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kurayoshi M, Oue N, Yamamoto H, Kishida M,
Inoue A, Asahara T, Yasui W and Kikuchi A: Expression of Wnt-5a is
correlated with aggressiveness of gastric cancer by stimulating
cell migration and invasion. Cancer Res. 66:10439–10448. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bock AJ, Nymoen DA, Brenne K, Kærn J and
Davidson B: SCARA3 mRNA is overexpressed in ovarian carcinoma
compared with breast carcinoma effusions. Hum Pathol. 43:669–674.
2012. View Article : Google Scholar
|
|
20
|
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK,
Schaefer E, Damsky CH and Schlaepfer DD: FAK integrates
growth-factor and integrin signals to promote cell migration. Nat
Cell Biol. 2:249–256. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tilghman RW and Parsons JT: Focal adhesion
kinase as a regulator of cell tension in the progression of cancer.
Semin Cancer Biol. 18:45–52. 2008. View Article : Google Scholar
|
|
22
|
Schlaepfer DD, Mitra SK and Ilic D:
Control of motile and invasive cell phenotypes by focal adhesion
kinase. Biochim Biophys Acta. 1692:77–102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seong BK, Lau J, Adderley T, Kee L,
Chaukos D, Pienkowska M, Malkin D, Thorner P and Irwin MS: SATB2
enhances migration and invasion in osteosarcoma by regulating genes
involved in cytoskeletal organization. Oncogene. 34:3582–3592.
2015. View Article : Google Scholar
|
|
24
|
Hu Y, Xu S, Jin W, Yi Q and Wei W: Effect
of the PTEN gene on adhesion, invasion and metastasis of
osteosarcoma cells. Oncol Rep. 32:1741–1747. 2014.PubMed/NCBI
|
|
25
|
Park GB, Kim DJ, Kim YS, Lee HK, Kim CW
and Hur DY: Silencing of galectin-3 represses osteosarcoma cell
migration and invasion through inhibition of FAK/Src/Lyn activation
and β-catenin expression and increases susceptibility to
chemotherapeutic agents. Int J Oncol. 46:185–194. 2015.
|
|
26
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kido A, Tsutsumi M, Iki K, Takahama M,
Tsujiuchi T, Morishita T, Tamai S and Konishi Y: Overexpression of
matrix metalloproteinase (MMP)-9 correlates with metastatic potency
of spontaneous and 4-hydroxyaminoquinoline 1-oxide (4-HAQO)-induced
transplantable osteosarcomas in rats. Cancer lett. 137:209–216.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Himelstein BP, Asada N, Carlton MR and
Collins MH: Matrix metalloproteinase·9 (MMP·9) expression in
childhood osseous osteosarcoma. Med Pediatr Oncol. 31:471–474.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uchibori M, Nishida Y, Nagasaka T, Yamada
Y, Nakanishi K and Ishiguro N: Increased expression of
membrane-type matrix metalloproteinase-1 is correlated with poor
prognosis in patients with osteosarcoma. Int J Oncol. 28:33–42.
2006.
|