Introduction
Kidney transplantation is the best therapeutic
option for patients suffering from end-stage renal disease
(1,2). However, following transplantation
these patients are on a life-long immunosuppressive regiment
consisting of a combination of corticosteroids, calcineurin
inhibitors, mammalian target of rapamycin inhibitors,
antimetabolites and, more recently, co-stimulation blockers.
However, the agents that are currently used are associated with
certain toxicities resulting in increased morbidity and mortality,
for example cardiovascular toxicity, infection and malignancy. In
addition, calcineurin and mammalian target of rapamycin inhibitors
are associated with graft injury (3). Thus, there is a requirement for novel
immunosuppressive agents that are able to diminish the immune
alloreactive response.
Substances able to interfere with T-cell metabolism
are candidates for immunosupressive agent. During T-cell
activation, rapidly proliferating T-cells reprogram their metabolic
pathways from pyruvate oxidation via the Krebs' cycle to the
glycolytic, pentose-phosphate, and glutaminolytic pathways in order
to fulfill the bioenergetic and biosynthetic demands of
proliferation (4). It has also
been confirmed that apart from clonal expansion, aerobic glycolysis
is a prerequisite for T-cell differentiation into effector cell
lineages (5). For instance,
CD4+ effector T-cells (Teff) express high levels of the
glucose transporter GLUT1 and are reliant on glucose metabolism,
whereas regulatory T-cells (Tregs) express low levels of GLUT1 and
are reliant on lipid oxidation (6). Notably, the key immunomodulatory
enzyme indoleamine 2,3-dioxyganase exerts its immunosuppressive
effects at least in part by affecting glucose metabolism in T-cells
(7,8).
Dichloroacetate (DCA) activates pyruvate
dehydrogenase (PDH) through inhibition of the mitochondrial enzyme
pyruvate dehydrogenase kinase (PDK). Since PDH converts pyruvate to
acetyl-CoA, DCA upregulates the influx of pyruvate into the
mitochondria, increasing the ratio of glucose oxidation to
glycolysis (9). It is expected
that by inhibiting aerobic glycolysis, DCA may suppress T-cell
activation. In support of the above hypothesis, DCA was shown to
alleviate the development of collagen-induced arthritis, a
T-cell-mediated disease, in an animal study (10). Additionally, in CD4+
T-cells derived from healthy individuals and stimulated by
anti-CD3/CD28, DCA inhibited proliferation, increased interleukin
(IL)-10 production and decreased IL-17 production, albeit at the
high concentration of 20 mM. However, Treg cell differentiation was
initially observed at a DCA concentration of 2 mM (11).
Compared with other potential immunosuppressive
agents, an advantage of DCA is that it has already been used for
the treatment of patients suffering from congenital lactic
acidosis. Long-term administration of DCA in these patients has an
acceptable toxicity, with the main side effect being reversible
peripheral neuropathy, while no increased incidence of
cardiovascular disease, infection or malignancy has yet been
reported (9,12,13).
Notably, regarding malignancy, DCA is currently under investigation
as a treatment for cancer, since numerous types of cancer rely on
aerobic glycolysis (14). This
safety profile of DCA suggests that it is a good candidate for
clinical trials in the field of transplantation, where the
existence of potent and effective immunosuppressive agents with
well-characterized toxicities renders the introduction of novel
substances with unknown toxicity a challenge.
In this study, the effect of DCA on GLUT1 and
certain enzymes involved in glycolysis, as well as on the
expression of the signature transcription factors of the Teff Th1,
Th2 and Th17 subsets and of the Treg cells, was evaluated. The
two-way mixed lymphocyte reaction (MLR) was used as a model of
alloreactivity (15). A DCA
concentration of 1 mM was selected as it is close to the serum
concentration achieved in patients with congenital lactic acidosis
(9,13), and it has been shown not to be
toxic for human peripheral blood mononuclear cells (PBMCs)
(16).
Materials and methods
Subjects
Blood samples were collected from 5 non-related
healthy volunteers (3 males and 2 females; age, 33–42 years).
Healthy volunteers were individuals from the Nephrology Department
of the Medical School, University of Thessaly (Larissa, Greece). A
state of health was confirmed by checking medical records and
conducting physical examinations and laboratory tests to perform a
routine checkup by an experienced physician. The health state was
confirmed by medical records, physical examination and usual
laboratory tests. Informed consent was obtained from each
individual enrolled into the study and the ethics committee of the
Medical School of the University of Thessaly approved the study
protocol.
Two-way MLR and CD4+ T-cell
isolation
PBMCs were isolated from whole blood by
Ficoll-Hypaque density gradient centrifugation (Histopaque 1077;
Sigma-Aldrich, St. Louis, MO, USA) and counted by optical
microscopy on a Neubauer plaque. Cell viability was assessed by a
Trypan blue assay (Sigma-Aldrich).
For measurement of glucose consumption and lactate
production, eight MLRs were performed in 12-well plates incubated
for 7 days. The MLR cell-based assay is characterized as an ex
vivo cellular immune assay that occurs between two allogenic
lymphocyte populations of the same species, yet genetically
distinct. In the present study, isolated PBMCs from the blood of
each volunteer was paired with the PBMCs from another volunteer,
generating a MLR pair. The number of PBMCs for each member of the
MLR pair was 5×105, summing up to 1×106 PBMCs
in each well. After 7 days of incubation the supernatant from each
MLR was collected.
For assessment of various proteins in alloreactive
CD4+ T-cells, eight MLRs were performed in the same
conditions as above and at the end of the 7 day period,
CD4+ T-cells were isolated by negative selection using
the CD4+ T Cell Isolation kit, Human (Miltenyi Biotec
GmbH, Bergisch Gladbach, Germany). More specifically, all
non-CD4+ PBMCs are labeled using a cocktail of
biotin-conjugated antibodies. The cocktail contains antibodies
against CD8, CD14, CD15, CD16, CD19, CD36, CD56, CD123, TCR γ/δ and
CD235a that bind to the non-CD4+ cell populations,
whereas negative selection of highly pure CD4+ T-cells
is achieved by depletion of magnetically labeled cells.
For assessment of cell proliferation, eight MLRs
were performed in 96-well plates for 7 days. The number of PBMCs
from each member of the MLR couple was 5×104, adding up
to 1×105 PBMCs in total in each well.
Cells were cultured in RPMI-1640 medium (Thermo
Fisher Scientific, Inc., Rockford, IL, USA) with L-glutamine and 10
mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich) and
antibiotic-antimycotic solution (Sigma-Aldrich), at 37°C in a
humidified atmosphere containing 5% CO2.
Assessment of glucose consumption and
lactate production in two-way MLR
Glucose uptake was assessed by measuring the
decrease of glucose concentration in the supernatants, calculated
as the glucose concentration in RPMI-1640 supplemented with 10% FBS
at the start of the experiment, where the maximum glucose
concentration is expected, minus the final glucose concentration in
the supernatants at the end of the experimental procedure. Glucose
measurements were obtained using the Element Blood glucose monitor
along with the test strips (Element, Infopia, Titusville, FL,
USA).
Aerobic glycolysis was assessed by measuring the
concentration of lactate, the end product of this pathway. Lactate
was measured by loading 100 µl of each supernatant sample on
to the prove of a Blood Gas Analyzer (Czito Medical, Moscow,
Russia), according to the manufacturer's details.
These experiments were performed in triplicate and
the results refer to the mean of the three measurements.
Cell proliferation in two-way MLR
Cell proliferation was assessed via a Cell
Proliferation ELISA (Roche Diagnostics, Indianapolis, IN, USA)
using bromodeoxyuridine (BrdU) labeling and immunoenzymatic
detection according to the manufacturer's protocol. An ELISA reader
(Microplate Reader PR2100; Sanofi Diagnostics Pasteur Inc.,
Redmond, WA, USA) was used to determine the optical densities (ODs)
of the immunoenzymatic reaction. Proliferation index of DCA-treated
MLRs was calculated by dividing the OD derived from these MLRs to
the ODs derived from the untreated MLRs. Eight MLRs were performed.
These experiments were performed in triplicate and the results
refer to the mean of the three measurements.
Assessment of certain protein levels in
alloreactive CD4+ T-cells
Levels of GLUT1, the enzymes of glycolysis
hexokinase II (HKII), lactate dehydrogenase A (LDH-A),
phosphorylated at serine 293 PDH, total PDH, along with signature
transcription factors T box transcription factor TBX21 (T-bet),
trans-acting T-cell specific transcription factor GATA 3 (GATA-3),
retinoic acid receptor related orphan receptor-γt (RORγt) and
forkhead box P3 (FoxP3) of Th1, Th2, Th17 and Treg, respectively,
and the apoptotic marker cleaved caspase 3, were assessed in MLR
CD4+ T-cells.
Isolated CD4+ T-cells were counted via
optical microscopy using an optical microscope (Axiovert 40C; Carl
Zeiss AG, Oberkochen, Germany) and a Neubauer chamber (Paul
Marienfeld GmbH & Co., KG., Lauda-Königshofen, Germany). Cell
viability was assessed by trypan blue staining (Sigma-Aldrich)
Equal numbers of T-cells (5×105 cells) from each MLR
were lysed using the T-PER tissue protein extraction reagent
(Thermo Fisher Scientific, Inc.) supplemented with protease
inhibitors 4-(2-aminoethyl) benzenesulfonyl fluoride, E-64,
bestatin, leupeptin, aprotinin, phenylmethanesulfonyl fluoride or
phenylmethylsulfonyl fluoride and ethylenediaminetetraacetic acid
and phosphatase inhibitors against acid and alkaline phosphatases,
in addition to serine/threonine (PP1, PP2A, and PP2B) and tyrosine
protein phosphatases (Sigma-Aldrich and Roche Diagnostics,
respectively). Protein was quantified via a Bradford assay
(Sigma-Aldrich) and western blotting was performed. Equal
quantities of protein extracts (10 µg) from each sample were
loaded for electrophoresis in 4–12% sodium dodecyl sulfate
(SDS)-polyacrylamide gels (Thermo Fisher Scientific, Inc.).
Subsequently proteins were transferred to polyvinylidene difluoride
(PVDF) membranes (Thermo Fisher Scientific, Inc.). Blots were
incubated with primary antibody for 16 h, followed by anti-rabbit
IgG, horseradish peroxidase (HRP)-linked secondary antibody (Cell
Signaling Technology, Inc., Danvers, MA, USA) for 30 min. Benchmark
pre-stained protein ladder (Thermo Fisher Scientific Inc.) was used
as a marker. Bands were visualized by enhanced chemiluminescent
detection using the LumiSensor Plus Chemiluminescent HRP Substrate
kit (GenScript, Piscataway, NJ, USA) and analysis was performed
using the Image J software (version 1.49; National Institute of
Health, Bethesda, MD, USA). In case of reprobing PVDF blots, the
previous primary and secondary antibodies were safely removed via
the use of Restore Western Blot Stripping Buffer (Thermo Fisher
Scientific Inc.), according to the manufacturer's instructions. The
PVDF blot was then reused and western blotting resumed as
previously described, using a different primary antibody.
The primary antibodies used in western blotting were
specific for rabbit polyclonal GLUT1 (1:200; #sc-7903; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit monoclonal HKII
(1:1,000; #2867; Cell Signaling Technology, Inc.), rabbit
monoclonal LDH-A (1:1,000; #2012; Cell Signaling Technology, Inc.),
rabbit monoclonal PDH (1:1,000; #2784; Cell Signaling Technology
Inc.), rabbit polyclonal phosphorylated (and inactivated) at serine
293 PDH (p-PDH) (1:100; #orb6670; Biorbyt, Cambridge, UK), rabbit
monoclonal c-Myc (1:500; #5605; Cell Signaling Technology, Inc.),
rabbit monoclonal FoxP3 (1:500; #5605; Cell Signaling Technology,
Inc.), rabbit polyclonal RORγt (1:100; #orb6888; Biorbyt), rabbit
monoclonal T-bet (1:500; #5852; Cell Signaling Technology, Inc.),
rabbit monoclonal GATA-3 (1:500; #5852; Cell Signaling Technology,
Inc.), rabbit monoclonal cleaved caspase-3 (1:500; #9664; Cell
Signaling Technology, Inc.) and rabbit monoclonal β-actin (1:2,500;
#4967; Cell Signaling Technology, Inc.).
Statistical analysis
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) was
used to perform statistical analyses. Regarding glucose consumption
and lactate production, a paired-samples t-test was used for
comparison of means. Regarding all the other evaluated variables,
the effect of DCA was estimated after normalization to the values
obtained from untreated cells. The ratios of the results obtained
from DCA-treated cells to untreated cells were estimated first.
Then the normality of each variable was evaluated and confirmed
with the Kolmogorov-Smirnoff test. Finally, a one sample t-test was
performed comparing the values of each case to the test value of
one.
Results are expressed as the mean ± standard
deviation and P<0.05 was considered to indicate a statistically
significant difference. Standard error of the mean was also
calculated.
Results
DCA decreases glucose uptake and aerobic
glycolysis in MLRs
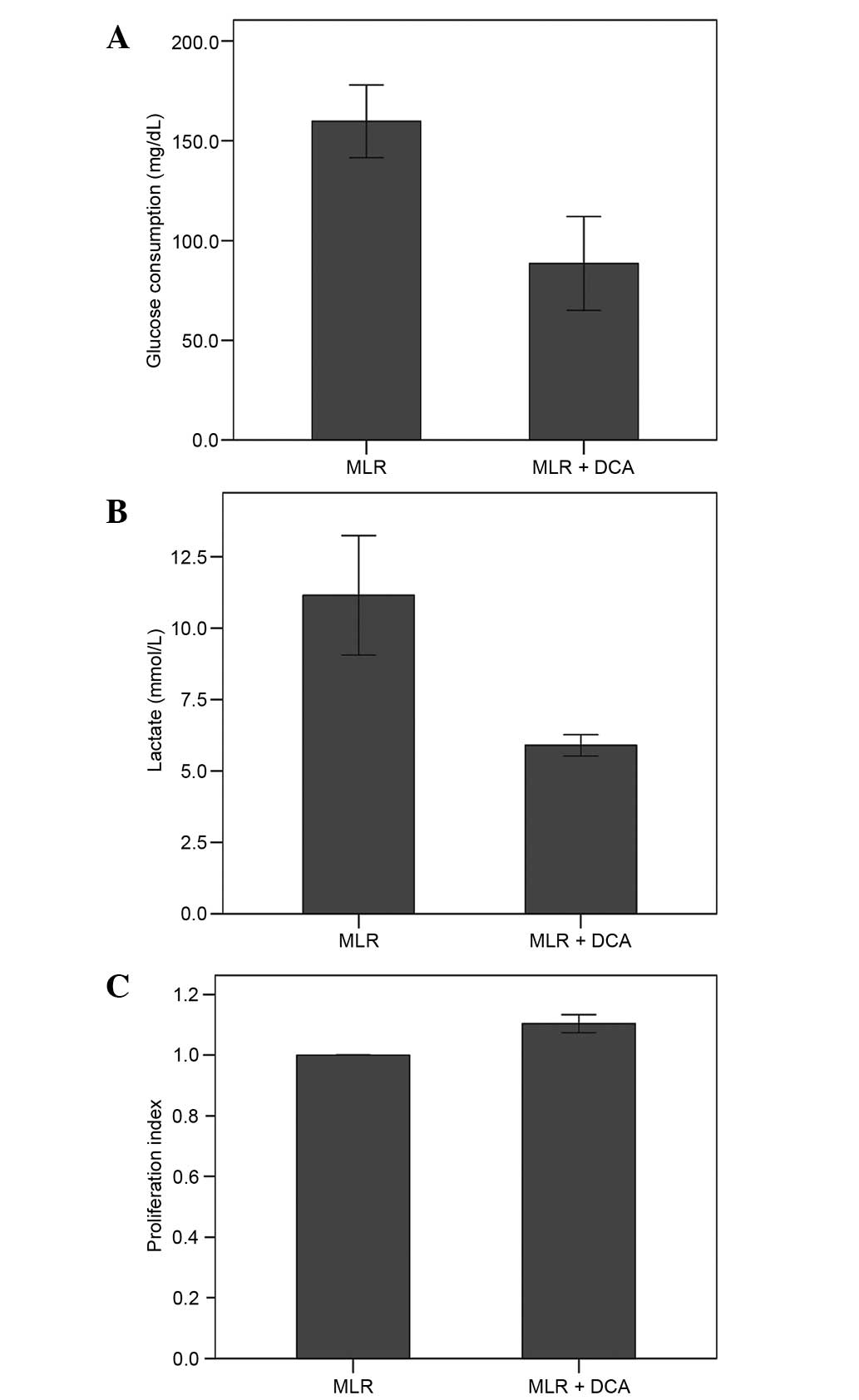
DCA decreased glucose uptake. In MLRs, the glucose
consumption was 159.75±27.77 mg/dl. Treatment with DCA decreased
glucose consumption significantly to 88.50±33.35 mg/dl (P<0.001;
Fig. 1A).
DCA inhibited aerobic glycolysis as determined by
the quantity of the end-product lactate. In the supernatants of the
MLRs lactate concentration was 11.15±2.96 mmol/l. Treatment with
DCA decreased lactate concentration significantly to 5.90±0.53
mmol/l (P<0.001; Fig. 1B).
In MLRs, DCA exerts a negligible effect
on cell proliferation
In MLRs, DCA increased cell proliferation by a
factor of 1.10±0.04 (Fig. 1C).
Although statistically significant (P<0.001), the 10% increase
in cell proliferation could be considered as negligible.
DCA induces apoptosis in alloreactive
CD4+ T-cells
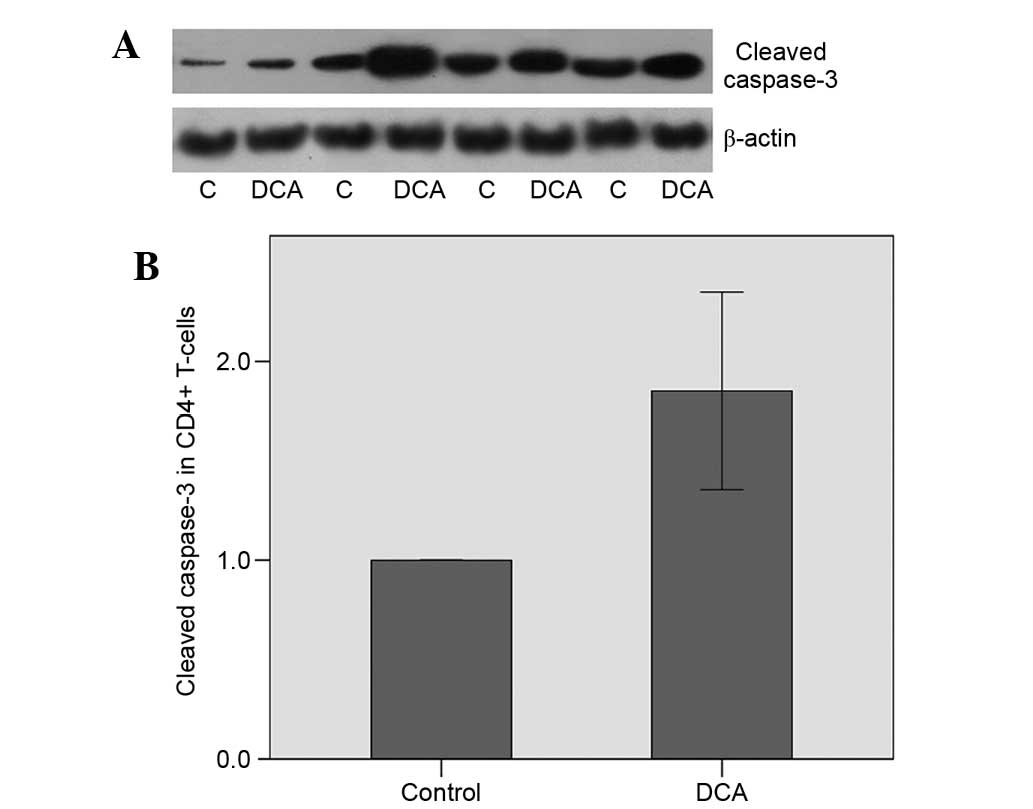
Apoptosis of the CD4+ T-cells was
assessed by the level of the cleaved (and activated) caspase-3,
which is the central caspase in the execution phase of cell
apoptosis where all extrinsic and intrinsic apoptotic pathways
converge (17); consequently, its
expression level may be used as a marker of apoptosis. DCA was
observed to induce apoptosis in alloreactive CD4+
T-cells, which was demonstrated by the increase in the cleaved
caspase-3 level by a factor of 1.85±0.70 (P=0.011; Fig. 2A and B).
Effect of DCA on the levels of certain
proteins in alloreactive CD4+ T-cells
In the cellular context of isolated CD4+
T-cells from MLRs, DCA decreased the expression of GLUT1 by a
factor of 0.55±0.18 (P<0.001), HKII by a factor of 0.66±0.07
(P<0.001) and LDH-A by a factor of 0.64±0.15 (P<0.01)
(Fig. 3A and B).
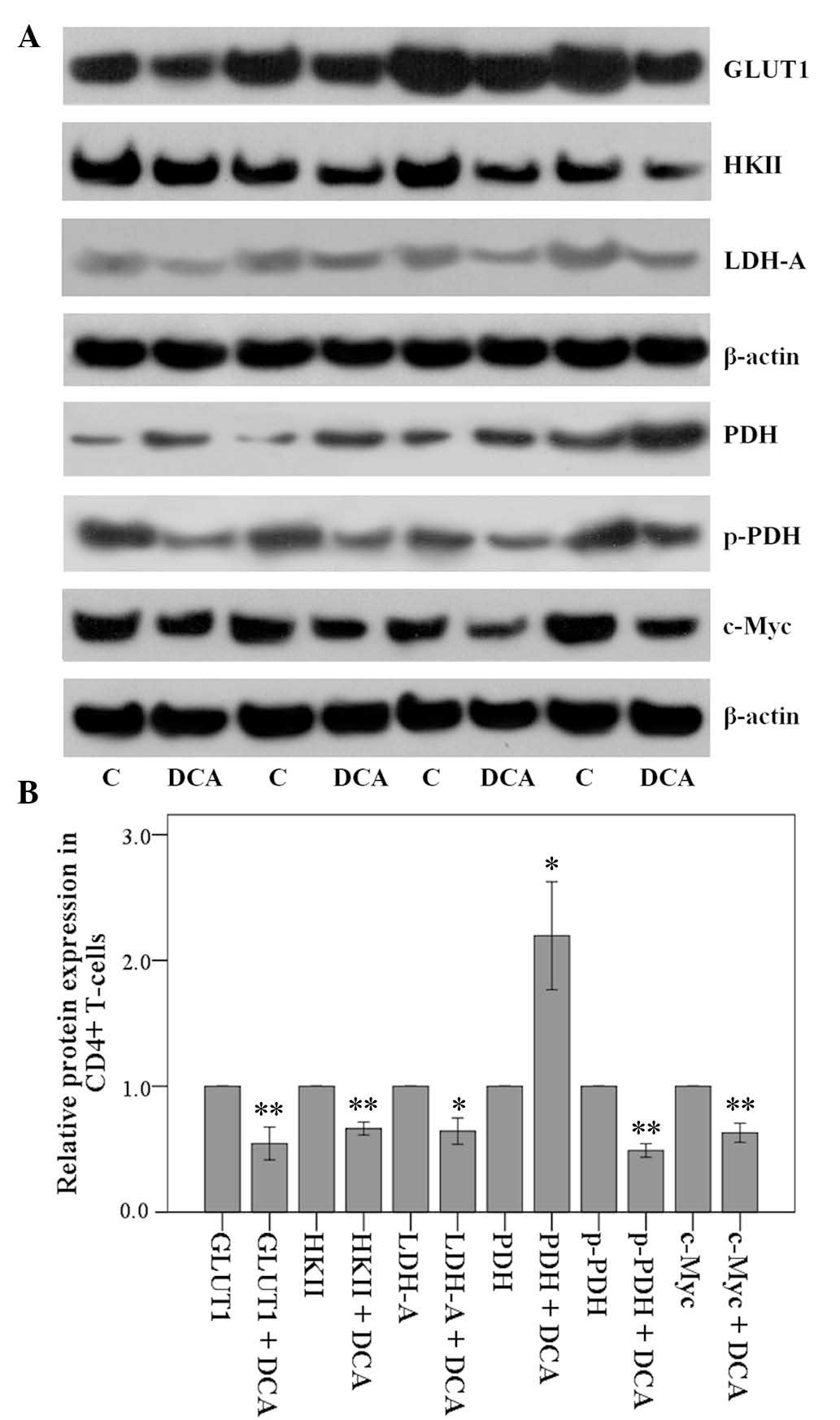 | Figure 3Effect of 1 mM DCA on certain enzymes
involved in glycolysis in isolated CD4+ T-cells. Then,
CD4+ T-cells were isolated from the MLRs and GLUT1,
HKII, LDH-A, PDH, p-PDH and c-Myc were assessed by western blot
analysis. (A) Eight experiments were performed and four of them are
depicted. (B) quantification of the levels demonstrated that DCA
decreased the levels of GLUT1, HKII, LDH-A, p-PDH and c-Myc, while
it increased PDH. These DCA-induced alterations favor a decrease in
glucose consumption and aerobic glycolysis, and diversion of
glucose metabolism towards Krebs' cycle in alloreactive
CD4+ T-cells. Error bars correspond to 2 standard
errors. For reader's convenience bars that correspond to the
control groups are depicted. *P<0.05;
**P<0.001. GLUT1, glucose transporter-1; HKII,
hexokinase II; LDH-A, lactate dehydrogenase-A; PDH, pyruvate
dehydrogenase; p-PDH, phosphorylated PDH; MLR, mixed lymphocyte
reaction; DCA, dichloroacetate. |
Conversely, DCA increased the level of PDH by a
factor of 2.20±0.61 (P=0.001). Concurrently, DCA decreased the
level of the phosphorylated and inactivated p-PDH by a factor of
0.49±0.08 (P<0.001) (Fig.
3).
Notably, DCA decreased the expression of c-Myc, a
transcription factor that controls the expression of a number of
the above proteins in T-cells (18), by a factor of 0.63±0.11
(P<0.001; Fig. 3). Thus, DCA
was observed to alter the protein levels in a manner that favors
decreased glucose consumption and aerobic glycolysis and increased
entry of pyruvate into the Krebs' cycle.
Effect of DCA on alloreactive
CD4+ T-cells expression of certain transcription
factors
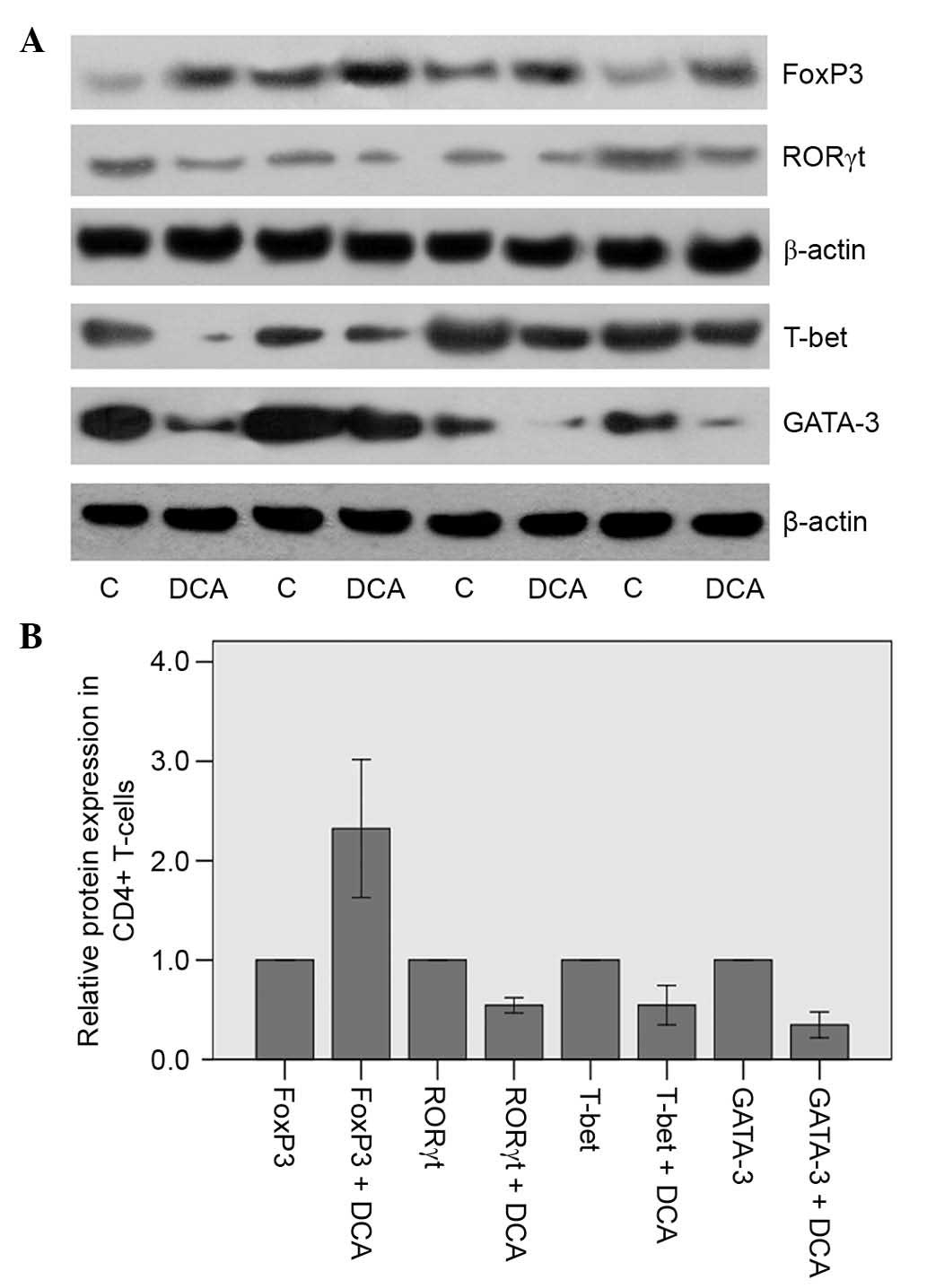
FoxP3, RORγt, T-bet and GATA-3 are the signature
transcription factors for the Treg, Th17, Th1 and Th2 cells,
respectively (19). It is likely
that in isolated CD4+ T-cells from the MLRs, DCA favored
the differentiation into the Treg subset since it increased FoxP3
expression by a factor of 2.32±0.98 (P=0.007) (Fig. 4). Conversely, it appears that DCA
inhibited CD4+ T-cell differentiation into the Teff
subsets Th17, Th1 and Th2 since it decreased the expression of
RORγt by a factor of 0.54±0.11 (P<0.001), T-bet by a factor of
0.55±0.28 (P=0.003) and GATA-3 by a factor of 0.35±0.18
(P<0.001) (Fig. 4).
Discussion
Although kidney transplantation increases survival
and improves quality of life in patients suffering from end-stage
renal disease (1,2), rejection remains a concern, and
currently available immunosuppressive agents contribute to
morbidity and mortality in these patients (3). In this study, the effect of DCA was
evaluated in MLRs, and it was confirmed that DCA alters the level
of certain enzymes of the glycolytic pathway in a manner that
inhibits aerobic glycolysis, a prerequisite for effective
differentiation of CD4+ T-cells into Teffs (4–6).
Then, it was confirmed that DCA decreases the expression of the
signature transcription factors T-bet, GATA-3 and RORγt of the Teff
subsets Th1, Th2 and Th17, respectively, while it increases the
expression of the Treg transcription factor FoxP3 (19).
In MLRs, DCA decreased glucose consumption, which
normally increases following T-cell activation, and lactate
production, which is the end-product of aerobic glycolysis, and
also increases during T-cell activation (4,5).
Although statistically significant, the very slight, equal to 10%,
increase in cell proliferation induced by DCA could be considered
as negligible. This is possibly the result of the low DCA
concentration used in the present study in order to simulate the
concentration used clinically for the treatment of congenital
lactic acidosis (9,13). In another model of CD4+
T-cell activation, with anti-CD3/CD28 antibodies, DCA inhibited
cell proliferation, albeit at the suprapharmacological
concentration of 20 mM (11).
Then, it was evaluated whether DCA induces apoptosis
in CD4+ T-cells isolated from MLRs. For this reason, the
level of activated cleaved caspase-3, in which all the apoptotic
pathways converge (17), was
assessed. DCA increased the level of cleaved caspase-3
significantly, and consequently it is likely that it induces
apoptosis in alloreactive T-cells. In cancer cell lines, DCA
induces apoptosis by altering mitochondrial membrane permeability
(20). Regardless of the
responsible mechanism, DCA-induced apoptosis of alloreactive
CD4+ T-cells could be considered to be
immunosuppressive.
In order to evaluate the mechanisms involved in
DCA-induced inhibition of glucose consumption and aerobic
glycolysis, the levels of GLUT1, HKII, LDH-A, PDH and p-PDH were
examined. In CD4+ T-cells from DCA-treated MLRs, the
expression levels of the glucose transporter GLUT1 and of the first
enzyme of the glycolytic pathway, HKII, decreased significantly.
Thus, decreased glucose consumption in DCA-treated MLRs could be
attributed to the decreased glucose influx into the cell and the
deceleration of glycolysis. Notably, GLUT1 is selectively essential
for CD4+ T-cell activation and effector function
(21), and inhibition of HKII by
2-deoxy-D-glucose suppresses effective CD4+ T-cell
activation (11).
In isolated CD4+ T-cells from the MLRs,
DCA was found to decrease LDH-A, which converts pyruvate to
lactate, and to increase PDH, which irreversibly converts pyruvate
to acetyl-CoA and controls its entry into the Krebs' cycle. In
parallel, DCA decreased the level of the phosphorylated and
inactivated p-PDH. Altogether, these DCA-induced alterations favor
the diversion of glucose metabolism from aerobic glycolysis to the
Krebs' cycle, contrary to what is required for CD4+
T-cell proliferation and differentiation into Teffs (4,5).
The DCA-induced decrease in p-PDH is expected since
DCA is a PDK inhibitor (9).
Regarding the DCA-induced increase of PDH level, a study in other
cell types have shown that DCA enhances PDH activity by increasing
its level through inhibiting its turnover (22). However, the mechanism accounting
for stabilization of PDH by DCA remains unknown (22). The transcription factor c-Myc
controls metabolic reprogramming upon T-cell activation, increasing
among others the expression of GLUT1, HKII and LDH-A (18). In order to assess how DCA exerts
its effect on the expression of the above proteins, the c-Myc level
was assessed in CD4+ T-cells isolated from MLRs. Indeed,
treatment of MLRs with DCA significantly decreased c-Myc expression
in CD4+ T-cells. This may be the underlying reason
behind the decreased expression of GLUT1, HKII and LDH-A due to DCA
treatment. However, the mechanisms involved in DCA-induced c-Myc
downregulation remain to be elucidated.
During CD4+ T-cell activation, cytokines
in the T-cell microenvironment govern its differentiation into the
various subsets. IL-12, IL-4 and IL-6 activate signal transducer
and activator of transcription 4 (STAT4), STAT6 and STAT3 leading
to the expression of the transcription factors T-bet, GATA-3 and
RORγt, and ultimately to CD4+ T-cell differentiation
into the Teff subsets Th1, Th2 and Th17, respectively. Transforming
growth factor-β (TGF-β), through SMAD2-SMAD4 induces FoxP3
expression and differentiation of CD4+ T-cells into
Tregs (19). CD4+
effector T-cells express high levels of the glucose transporter
GLUT1 and are reliant on glucose metabolism, whereas Tregs express
low levels of GLUT1 and are reliant on lipid oxidation (6).
Considering that DCA decreases the level of GLUT1
and inhibits aerobic glycolysis, its effect on the signature
transcription factors of Th1, Th2, Th17 and Treg subsets was
evaluated. In DCA-treated MLRs CD4+ T-cells, the levels
of T-bet, GATA-3 and RORγt were significantly decreased indicating
that DCA inhibits CD4+ differentiation into the Teff
subsets Th1, Th2 and Th17, respectively. Conversely, DCA treatment
augmented the expression of FoxP3 indicating that it favors
CD4+ differentiation into Tregs. This feature of DCA to
inhibit the differentiation of alloreactive CD4+ T-cells
into Teff subsets and concurrently to induce their differentiation
into Tregs, renders DCA a promising immunosuppressant agent in the
field of transplantation. Tregs may be significant in the
suppression of graft rejection by Teffs, with the prospect of
developing suitable treatments that will result in kidney allograft
tolerance (23,24).
In conclusion, in alloreactive CD4+
T-cells, DCA inhibits aerobic glycolysis, induces apoptosis and
favors differentiation towards the Treg subset. Considering that
DCA has already been used for a number of years in the treatment of
congenital lactate acidosis with limited toxicity, the effects
shown in this study suggest it may be a promising immunosuppressive
agent in the field of transplantation.
References
|
1
|
Arend SM, Mallat MJ, Westendorp RJ, van
der Woude FJ and van Es LA: Patient survival after renal
transplantation; more than 25 years follow-up. Nephrol Dial
Transplant. 12:1672–1679. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Russell JD, Beecroft ML, Ludwin D and
Churchill DN: The quality of life in renal transplantation-a
prospective study. Transplantation. 54:656–660. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalluri HV and Hardinger KL: Current state
of renal transplant immunosuppression: Present and future. World J
Transplant. 2:51–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fox CJ, Hammerman PS and Thompson CB: Fuel
feeds function: Energy metabolism and the T-cell response. Nat Rev
Immunol. 5:844–852. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pollizzi KN and Powell JD: Integrating
canonical and metabolic signalling programmes in the regulation of
T cell responses. Nat Rev Immunol. 14:435–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Michalek RD, Gerriets VA, Jacobs SR,
Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG and
Rathmell JC: Cutting edge: Distinct glycolytic and lipid oxidative
metabolic programs are essential for effector and regulatory
CD4+ T cell subsets. J Immunol. 186:3299–3303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eleftheriadis T, Pissas G, Yiannaki E,
Markala D, Arampatzis S, Antoniadi G, Liakopoulos V and Stefanidis
I: Inhibition of indoleamine 2,3-dioxygenase in mixed lymphocyte
reaction affects glucose influx and enzymes involved in aerobic
glycolysis and glutaminolysis in alloreactive T-cells. Hum Immunol.
74:1501–1509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eleftheriadis T, Pissas G, Antoniadi G,
Spanoulis A, Liakopoulos V and Stefanidis I: Indoleamine
2,3-dioxygenase increases p53 levels in alloreactive human T cells
and both indoleamine 2,3-dioxygenase and p53 suppress glucose
uptake, glycolysis and proliferation. Int Immunol. 26:673–684.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stacpoole PW: The pharmacology of
dichloroacetate. Metabolism. 38:1124–1144. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bian L, Josefsson E, Jonsson IM, Verdrengh
M, Ohlsson C, Bokarewa M, Tarkowski A and Magnusson M:
Dichloroacetate alleviates development of collagen II-induced
arthritis in female DBA/1 mice. Arthritis Res Ther. 11:R1322009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ostroukhova M, Goplen N, Karim MZ,
Michalec L, Guo L, Liang Q and Alam R: The role of low-level
lactate production in airway inflammation in asthma. Am J Physiol
Lung Cell Mol Physiol. 302:L300–L307. 2012. View Article : Google Scholar :
|
|
12
|
Abdelmalak M, Lew A, Ramezani R, Shroads
AL, Coats BS, Langaee T, Shankar MN, Neiberger RE, Subramony SH and
Stacpoole PW: Long-term safety of dichloroacetate in congenital
lactic acidosis. Mol Genet Metab. 109:139–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stacpoole PW, Kurtz TL, Han Z and Langaee
T: Role of dichloroacetate in the treatment of genetic
mitochondrial diseases. Adv Drug Deliv Rev. 60:1478–1487. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kankotia S and Stacpoole PW:
Dichloroacetate and cancer: New home for an orphan drug? Biochim
Biophys Acta. 1846:617–629. 2014.PubMed/NCBI
|
|
15
|
Sato T, Deiwick A, Raddatz G, Koyama K and
Schlitt HJ: Interactions of allogeneic human mononuclear cells in
the two-way mixed leucocyte culture (MLC): Influence of cell
numbers, subpopulations and cyclosporin. Clin Exp Immunol.
115:301–308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eleftheriadis T, Pissas G, Karioti A,
Antoniadi G, Antoniadis N, Liakopoulos V and Stefanidis I:
Dichloroacetate at therapeutic concentration alters glucose
metabolism and induces regulatory T-cell differentiation in
alloreactive human lymphocytes. J Basic Clin Physiol Pharmacol.
24:271–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar
|
|
18
|
Wang R, Dillon CP, Shi LZ, Milasta S,
Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger
J and Green DR: The transcription factor Myc controls metabolic
reprogramming upon T lymphocyte activation. Immunity. 35:871–882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Shea JJ and Paul WE: Mechanisms
underlying lineage commitment and plasticity of helper
CD4+ T cells. Science. 327:1098–1102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heshe D, Hoogestraat S, Brauckmann C,
Karst U, Boos J and Lanvers-Kaminsky C: Dichloroacetate
metabolically targeted therapy defeats cytotoxicity of standard
anticancer drugs. Cancer Chemother Pharmacol. 67:647–655. 2011.
View Article : Google Scholar
|
|
21
|
Macintyre AN, Gerriets VA, Nichols AG,
Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen
BJ, Hale LP and Rathmell JC: The glucose transporter Glut1 is
selectively essential for CD4 T cell activation and effector
function. Cell Metab. 20:61–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Evans OB and Stacpoole PW: Prolonged
hypolactatemia and increased total pyruvate dehydrogenase activity
by dichloroacetate. Biochem Pharmacol. 31:1295–1300. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dummer CD, Carpio VN, Goncalves LF, Manfro
RC and Veronese FV: FOXP3+ regulatory T cells: From
suppression of rejection to induction of renal allograft tolerance.
Transpl Immunol. 26:1–10. 2012. View Article : Google Scholar
|
|
24
|
Edozie FC, Nova-Lamperti EA, Povoleri GA,
Scottà C, John S, Lombardi G and Afzali B: Regulatory T-cell
therapy in the induction of transplant tolerance: The issue of
subpopulations. Transplantation. 98:370–379. 2014. View Article : Google Scholar : PubMed/NCBI
|