Introduction
Psoriasis is a complex inflammatory skin disease
that is characterized by inflammatory cell infiltration, increased
dermal vascularity, and keratinocyte proliferation (1). The HaCaT cell line is an immortalized
line of human epidermal keratinocytes, which has previously been
used in experiments examining the effects of therapeutic drugs on
keratinocytes (2). A series of
clinical studies have indicated that certain Chinese herbs are
effective in psoriasis treatment (3,4).
Shikonin, which is a naphthoquinone isolated from the Chinese
herbal plant Lithospermum erythrorhizon, has long been used
in traditional medicine to treat hemorrhoids, burns, infected
wounds, anal ulcers, external wounds, and psoriasis (5,6).
Shikonin is known to possess several medicinal
properties, including promotion of wound healing, and
antibacterial, anti-inflammatory and antitumor effects (5). In addition, shikonin has been shown
to exert inhibitory effects on tumor necrosis factor-α-induced
angiogenesis, tumor cell-induced angiogenesis, and normal
programmed developmental angiogenesis (7). Shikonin also inhibits angiogenesis in
inflammatory skin diseases, such as psoriasis (8). Shikonin treatment has been reported
to activate the caspase pathway, in order to induce cellular
apoptosis in HL-60 leukemia cells (9) and human colorectal cancer cells
(10). Shikonin induces apoptosis
via the reactive oxygen species (ROS)/extracellular
signal-regulated kinase (Erk) pathway in osteosarcoma cells
(11). Furthermore, it inhibits
cell proliferation by decreasing Erk activities in human epidermoid
carcinoma cells (12), and
triggers apoptosis through the ROS/Akt and nuclear factor-κB
(NF-κB) pathways in hepatocellular carcinoma cells (13). Shikonin also elevates ROS
generation to induce apoptosis in human glioma cells (14), and is able to increase
intracellular ROS generation during the early phase of apoptotic
progression, alongside a disturbance in mitochondrial transmembrane
potential, in SK-Hep-1 hepatoma cells (15).
Two apoptotic pathways have been established: The
cell death receptor pathway and the mitochondria-initiated pathway
(16). Members of the B-cell
lymphoma 2 (Bcl-2) family are key regulators of
mitochondria-initiated apoptosis. When anti-apoptotic members of
the Bcl-2 family are inhibited and/or proapoptotic members are
activated, mitochondrial integrity is disrupted and cytochrome
c is released into the cytosol (17), thus activating caspase 9 and
caspase 3, and subsequently leading to cell apoptosis (18). The present study aimed to
investigate whether shikonin was able to induce
mitochondrial-initiated apoptosis in HaCaT cells, in order to
inhibit cell proliferation.
Materials and methods
Chemicals and reagents
Shikonin was purchased from Shanghai PureOne
Biotechnology Co., Ltd. (Shanghai, China), and its purity was
determined to be ~99.5% using high-performance liquid
chromatography. Cell culture medium (RPMI-1640), trypsin,
3-(4,5-dimethyl-2-thi-azolyl) -2,5-diphenyl-2H-tetrazolium bromide
(MTT), Hoechst 33258 and dimethyl sulfoxide (DMSO) were purchased
from Sigma-Aldrich (St. Louis, MO, USA). RNase, propidium iodide
(PI), Annexin V-fluorescein isothiocyanate (FITC), ROS and
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenz-imidazolylcarbocyanine
iodide (JC-1) were purchased from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, China). Fetal bovine serum (FBS) was purchased from
National Hyclone (Lanzhou) Bio-engineering Co., Ltd. (Lanzhou,
China). Rabbit polyclonal antibodies against caspase 3 (9662), Akt
(9272), phosphorylated (p)-Akt (9271), Erk1/2 (9102) and p-Erk1/2
(9101) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Antibodies against cyclin B1 (55004-1-AP),
cyclin D1 (60186-1-Ig), cyclin E (11554-1-AP), Bcl-2 (12789-1-AP),
Bcl-2-associated X protein (Bax; 50599-2-Ig) and Bcl-2 homologous
antagonist killer (Bak; 14673-1-AP) were purchased from Proteintech
Group, Inc. (Rosemont, IL, USA), and antibodies against
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; sc-365062) and
β-actin (sc-47778) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG (H+L) secondary antibodies (A0208) were
purchased from Beyotime Institute of Biotechnology, Nanjing,
China).
Cell culture
The HaCaT normal human epidermal keratinocyte cell
line was obtained from the Chinese Academy of Sciences (Kunming,
China). The cells were cultured in RPMI-1640 supplemented with 10%
FBS and 1% penicillin-streptomycin (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) at 37°C in an atmosphere containing 5%
CO2.
Cell viability assay
Cell viability was determined using the MTT
colorimetric assay. Exponentially growing cells were seeded in
96-well plates in culture medium at density of 2×104
cells/well. Following a 24 h incubation, the cells were treated
with various concentrations of shikonin between 0–20 µM for
24 and 48 h. Subsequently, the medium was discarded, and 200
µl MTT (0.5 mg/ml) was added to each well and incubated for
4 h at 37°C. The medium was then removed, and the formazan salt was
dissolved in 150 µl DMSO. Optical density of the cells was
determined using a Bio-Rad Model 680 microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a wavelength of 490 nm.
Cell viability was expressed as a percentage of the control. Three
replicate wells were used for each analysis.
Cell cycle analysis using flow
cytometry
Exponentially growing cells were seeded in 6-well
culture plates in culture medium at a density of 4×105
cells/well. Following treatment with 1, 2 and 4 µM shikonin
for 24 h, both adherent and floating cells were collected, washed
in ice-cold phosphate-buffered saline (PBS), and fixed with
ice-cold 70% ethanol overnight. After fixation, the ethanol was
removed via centrifugation, and the cells were suspended in 0.1 ml
RNase solution at 37°C for 30 min. Subsequently, 0.4 ml PI was
added and incubated at 4°C for 30 min in the dark. Stained cells
were analyzed using a FACSCanto flow cytometer (BD Biosciences, San
Jose, CA, USA). Data acquisition and analyses were performed using
WinMDI 2.9 software (BD Biosciences).
DNA morphological observation using
Hoechst staining assay
To visualize apoptotic cell death and nuclear
morphology, the cells were stained with Hoechst 33258. Briefly, the
treated cells were collected, washed twice in PBS, and fixed in 4%
formaldehyde for 10 min. The cells were then washed and stained
with Hoechst 33258 for 20 min at room temperature, after which they
were examined under a Nikon Eclipse TE2000-PFS inverted
fluorescence microscope (Nikon Corporation, Tokyo, Japan) at 340
nm. The number of apoptotic cells was measured by calculating the
percentage of cells displaying chromatin condensation compared with
the total number of cells.
Detection of apoptosis using flow
cytometry
Cellular apoptosis was detected using an Annexin
V-FITC/PI Apoptosis Detection kit. Briefly, HaCaT cells were
treated with 1, 2 and 4 µM shikonin for 24 h, and collected
via centrifugation. The cells were then washed in PBS and
resuspended in binding buffer, and the apoptotic cell death rate
was examined using Annexin V-FITC and PI double staining
(incubation with 5 µl Annexin V-FITC and 10 µl PI for
15 min in the dark), according to the manufacturer's protocol.
Subsequently, the cells stained with Annexin V-FITC/PI were
detected using a FACSCanto flow cytometer (BD Biosciences). Data
acquisition and analyses were performed using WinMDI 2.9 software.
All experiments were performed in triplicate.
Measurement of the mitochondrial membrane
potential (Δψm)
The Δψm was assessed as previously described
(19). Briefly, the HaCaT cells
were treated with 1, 2 and 4 µM shikonin for 4 h, and were
harvested and collected via centrifugation. The cells were
resuspended in PBS and were then incubated with 10 µM JC-1
for 15 min at room temperature in the dark. The fluorescently
labeled cells were washed in PBS and analyzed using a BD
FACSCalibur flow cytometry system (excitation, 485 nm; emission,
530/590 nm; BD Biosciences). The 590 nm/530 nm fluorescence ratio
was used to quantify the Δψm. Data acquisition and analyses were
performed using WinMDI 2.9 software.
Intracellular ROS assay
The ROS generation assay was performed as described
in our previous study (20).
Briefly, the HaCaT cells were treated with 1, 2 and 4 µM
shikonin for 24 h, and were harvested and collected via
centrifugation. The cells were resuspended in PBS and were then
incubated with 10 µM H2DCF-DA (Nanjing KeyGen
Biotech Co., Ltd.) for 30 min at room temperature in the dark. The
fluorescently labeled cells were washed in PBS and analyzed using a
BD FACSCalibur flow cytometry system (excitation, 485 nm; emission,
538 nm; BD Biosciences). Data acquisition and analyses were
performed using WinMDI 2.9 software.
Western blot analysis
HaCaT cells, treated with 1, 2 and 4 µM
shikonin for 24 h, were lysed in radioimmunoprecipitation assay
lysis buffer containing a protease and phosphatase inhibitor
cocktail on ice for 30 min. Then lysate was then collected and
centrifuged at 56,000 × g for 15 min at 4°C. Protein concentration
was determined using the Bicinchoninic Acid Protein Quantification
kit (Pierce Biotechnology, Inc., Rockford, IL, USA), according to
the manufacturer's protocol. Protein lysates were then denatured
for 10 min at 95°C and 50 µg protein per lane was separated
by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred to polyvinylidene difluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA). After immunoblotting, the PVDF
membranes were blocked with 5% skimmed milk for at least 2 h,
washed and incubated with anti-caspase 3, anti-Akt, anti-p-Akt,
anti-Erk 1/2, anti-p-Erk 1/2, anti-cyclin B1, anti-cyclin D1,
anti-cyclin E, anti-Bcl-2, anti-Bax, anti-Bak, anti-GAPDH and
anti-β-actin primary antibodies at 4°C overnight (all 1:1,000).
Subsequently, the membranes were washed three times with
Tris-buffered saline with Tween 20 and incubated with the
HRP-conjugated goat anti-rabbit IgG (H+L) secondary antibodies
(1:10,000) at 37°C for 1 h. Chemiluminescence detection was assayed
using an enhanced chemiluminescence detection kit (Pierce
Biotechnology, Inc.). Results were analyzed using Quantity One
software (version 4.4.0.36; Bio-Rad Laboratories, Inc.), in order
to obtain the optical density ratio of the target protein to GAPDH
and β-actin.
Statistical analysis
All of the data, which were obtained from at least
three independent experiments, were expressed as the mean ±
standard deviation for each group. Statistical analyses, including
Student's t-test, one-way analysis of variance and regression
analysis, were performed using GraphPad Prism 4.0 software
(GraphPad, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Shikonin inhibits the growth of HaCaT
cells
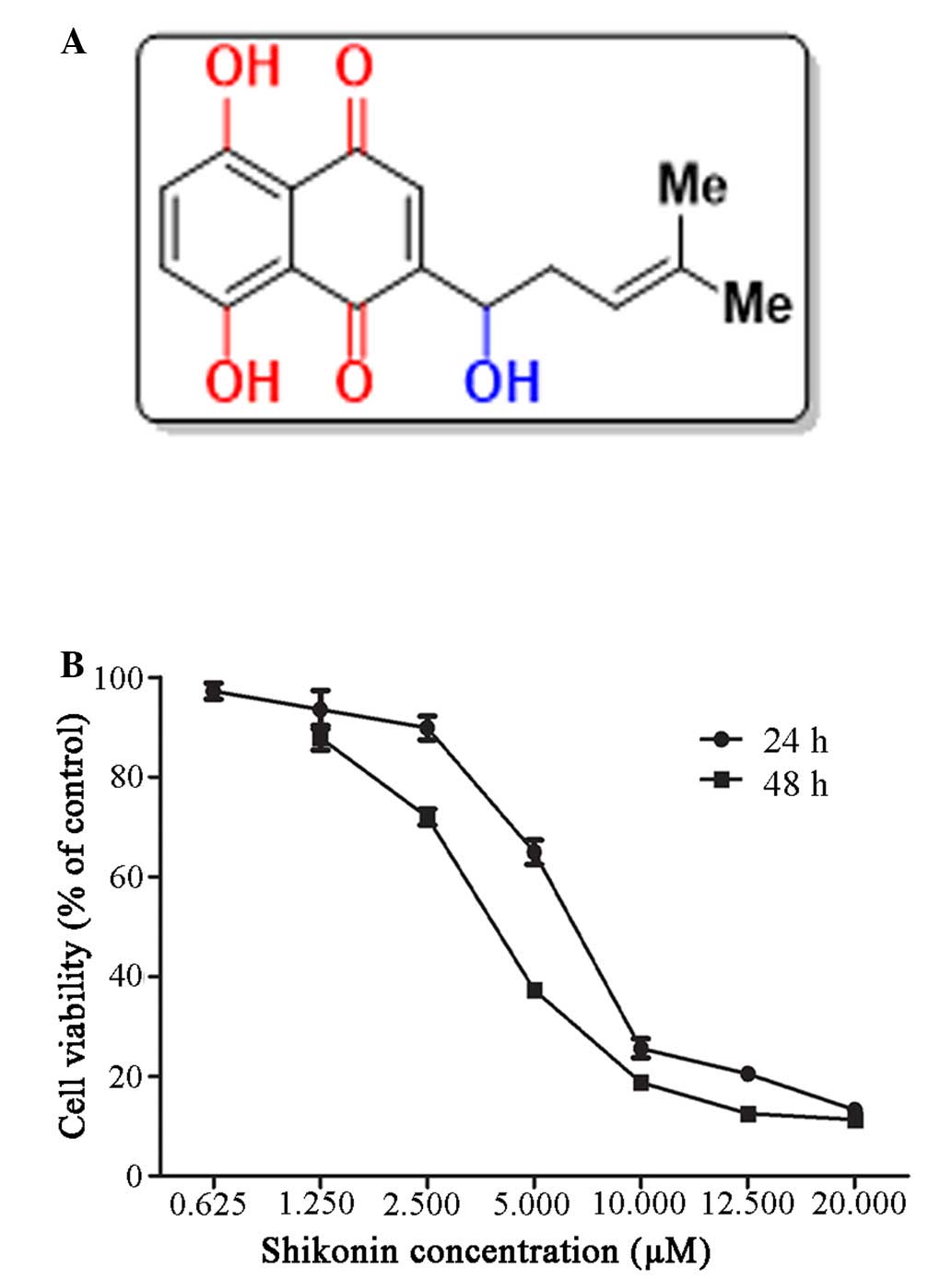
The chemical structure of shikonin is shown in
Fig. 1A (21). The effects of shikonin on the
growth of HaCaT cells were evaluated using the MTT assay. Human
HaCaT cells were treated with various concentrations of shikonin
(0–20 µM) for 24 and 48 h. As shown in Fig. 1B, the proliferation of
shikonin-treated HaCaT cells was markedly suppressed at 24 and 48 h
compared with the control group. These results suggest that
shikonin may exhibit dose- and time-dependent inhibitory effects on
the viability of HaCaT cells. The calculated half maximal
inhibitory concentration (IC50) values for shikonin were
6.34 and 2.43 µM at 24 and 48 h, respectively. Based on
these IC50 values, doses of 1, 2 and 4 µM
shikonin were selected for use in subsequent experiments to assess
HaCaT cell growth inhibition. Notably, concentrations of DMSO,
which was used to dissolve shikonin, were maintained at <0.2%
(v/v).
Shikonin induces a cell cycle arrest at
G0/G1 phase in HaCaT cells
To investigate the mechanisms underlying
shikonin-induced inhibition of cell proliferation, changes in cell
cycle progression were detected after shikonin treatment using flow
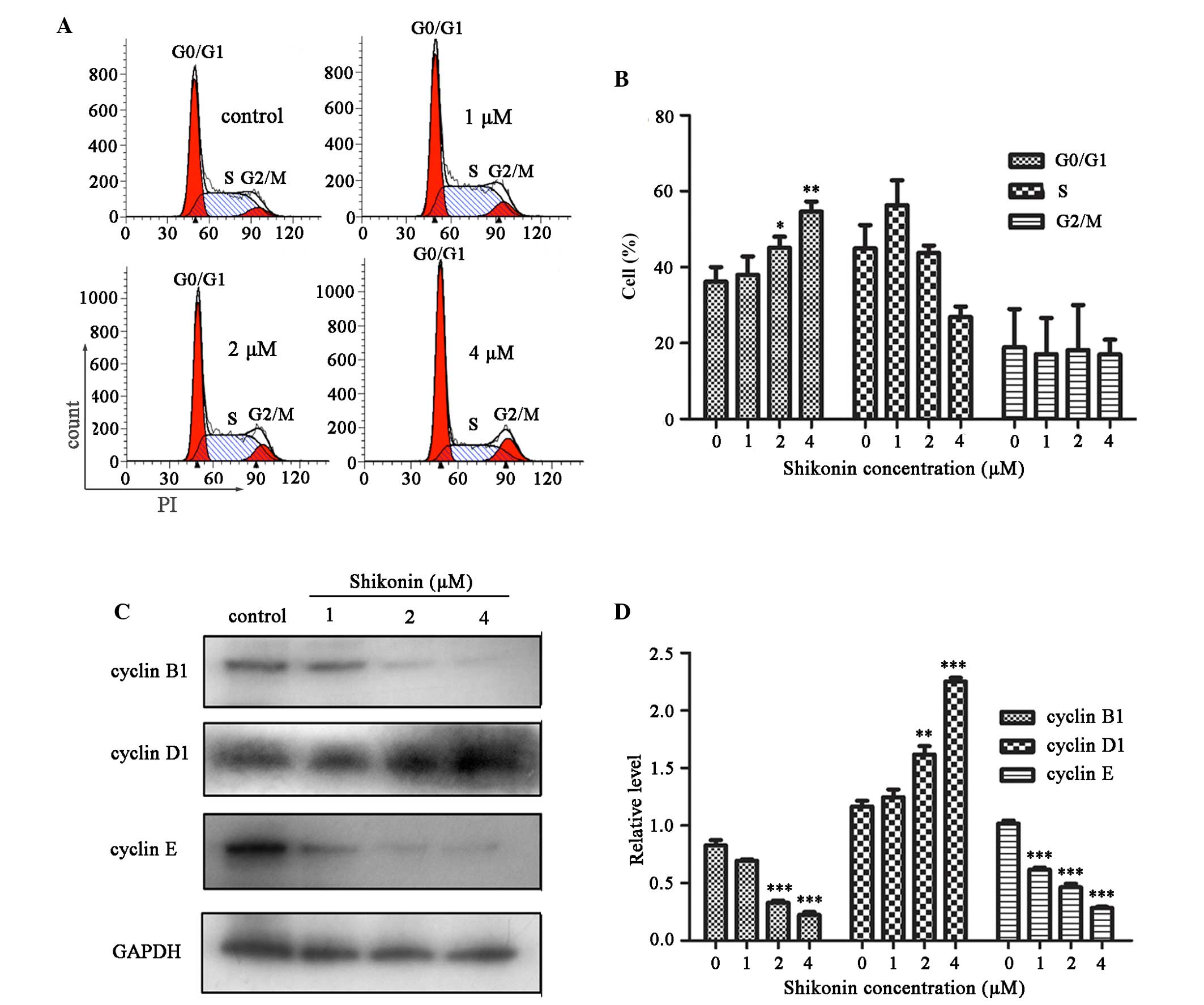
cytometry. The percentage of cells that accumulated in
G0/G1 phase was significantly increased
following treatment with 2 and 4 µM shikonin for 24 h, as
compared with the control group (P<0.05 and P<0.01,
respectively; Fig. 2A and B).
These results indicate that shikonin may partially mediate HaCaT
cell growth inhibition by inducing G0/G1
phase cell cycle arrest.
Following a 24 h treatment with 1, 2 or 4 µM
shikonin, the expression levels of cell cycle regulatory proteins
were examined in HaCaT cells using western blot analysis. As shown
in Fig. 2C, cyclin D1 expression
was significantly increased following treatment with 2 and 4
µM shikonin, as compared with the control group (P<0.01
and P<0.001, respectively); whereas cyclin B1 and cyclin E
expression levels were decreased in a dose-dependent manner
(Fig. 2C and D).
Shikonin induces apoptosis of HaCaT
cells
To assess whether shikonin-induced cell growth
inhibition was associated with cell apoptosis, the effects of
shikonin on apoptosis were evaluated by flow cytometry using
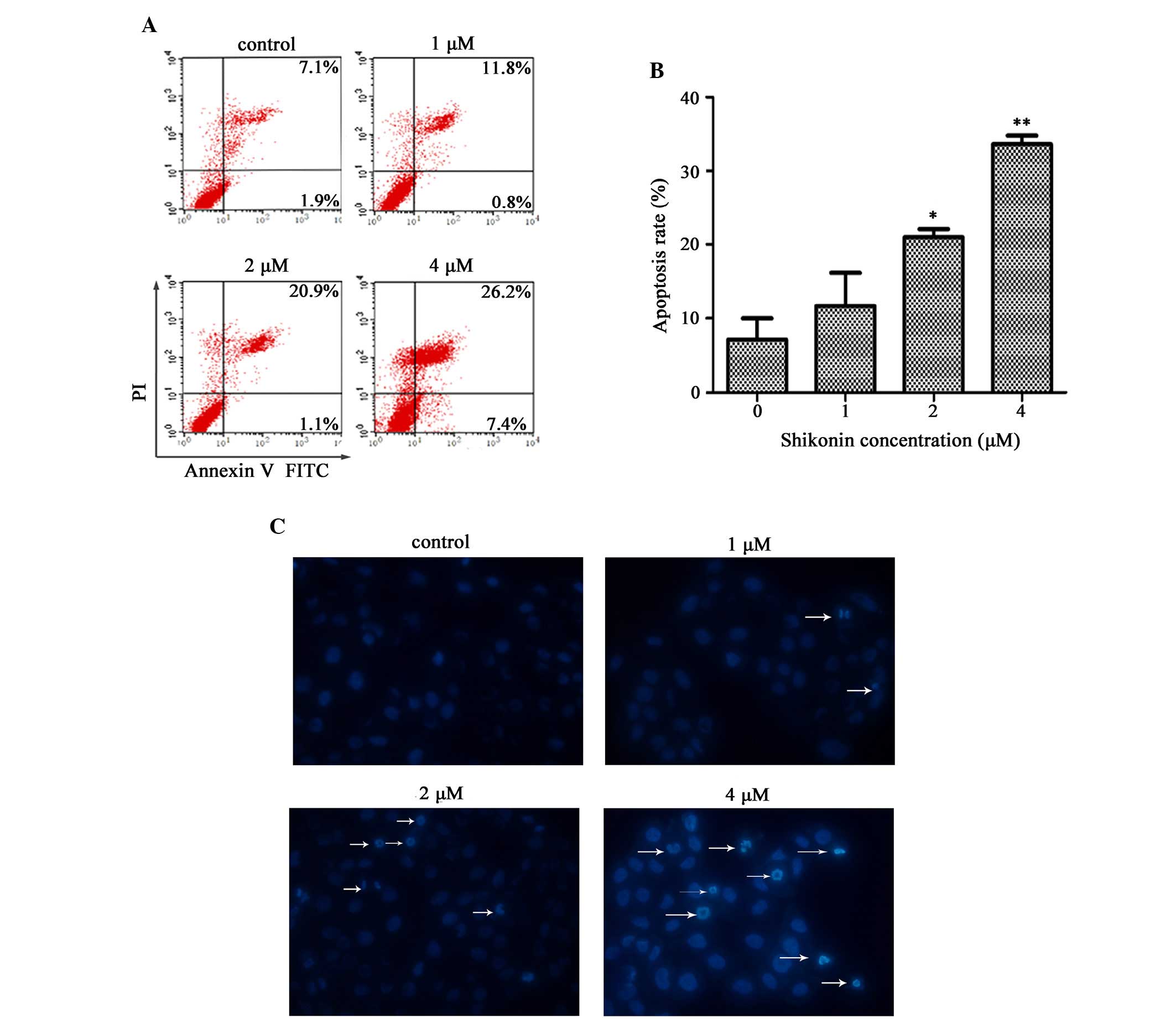
Annexin V-FITC/PI double staining. As shown in Fig. 3A, the percentage of dead cells
(Annexin V-positive, PI-positive) was increased in a dose-dependent
manner. The percentage of apoptotic HaCaT cells following a 24 h
treatment with 2 or 4 µM shikonin was 22.0 and 33.6%,
respectively; therefore, the percentage of apoptotic cells were
significantly increased, as compared with the control group
(P<0.05 and P<0.01, respectively; Fig. 3A and B). These results correspond
to the addition of values that are shown in the higher and lower
right quadrants of each panel, which indicate the early and late
stages of apoptosis, respectively.
Nuclear fragmentation is an important characteristic
of apoptosis, which is easily distinguished by Hoechst staining. As
shown in Fig. 3C, marked nuclear
condensation or nuclear fragmentation was induced following
treatment of the cells with 2 or 4 µM shikonin for 24 h.
These results clearly indicate that inducing cellular apoptosis is
a primary mechanism underlying the inhibitory effects of shikonin
on HaCaT cell growth. In addition, shikonin-induced apoptosis
occurred in a dose-dependent manner.
Shikonin decreases the Δψm and induces
ROS generation
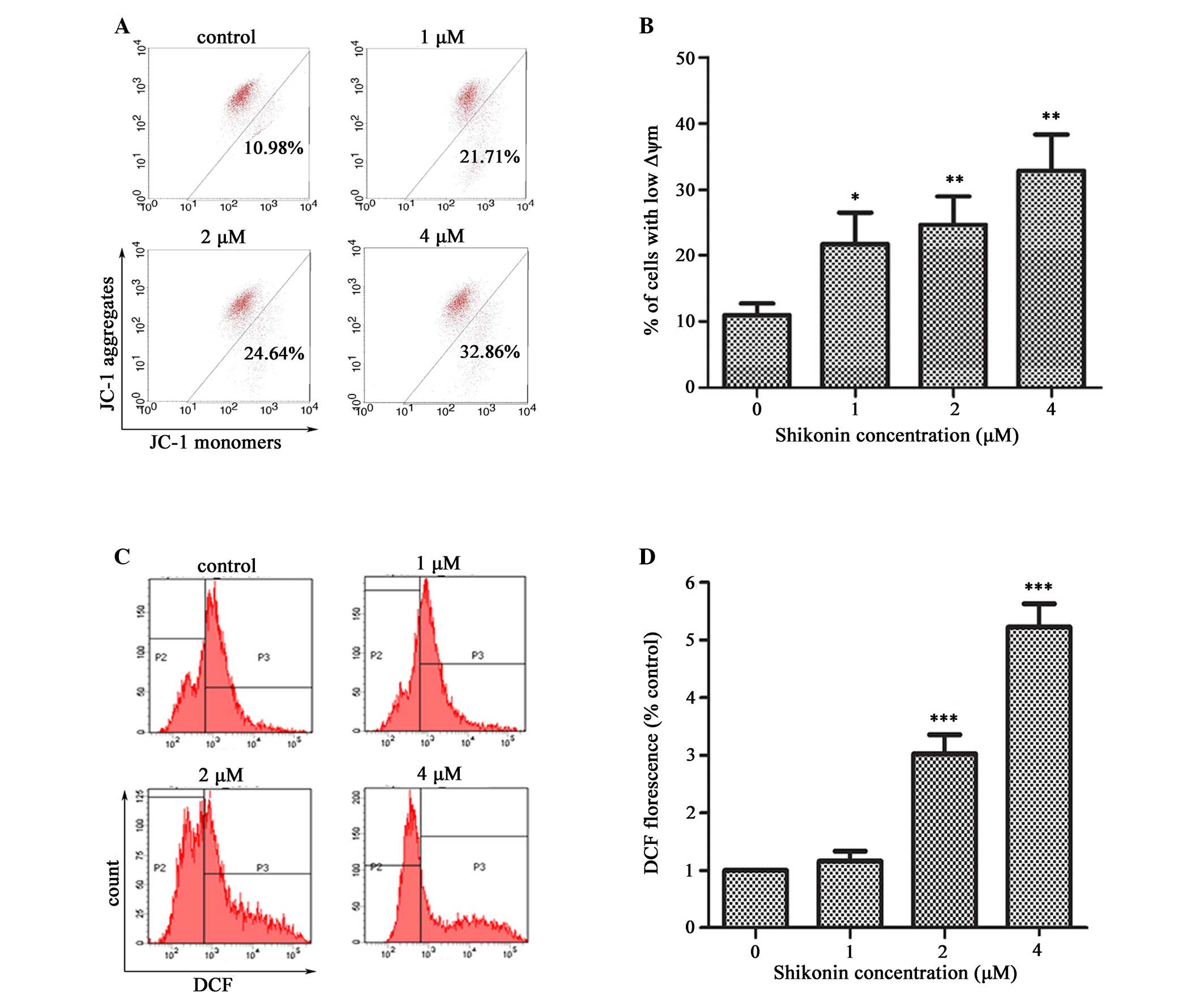
To evaluate whether the mitochondrial pathway was
responsible for shikonin-induced apoptosis, the effects of shikonin
on the Δψm after 4 h were examined using the mitochondria-specific
dye, JC-1. The percentage of cells with depolarized Δψm
significantly increased following treatment of HaCaT cells with 1,
2 or 4 µM shikonin for 4 h, as compared with the untreated
cells, and this trend occurred in a dose-dependent manner
(P<0.05 and P<0.01, respectively; Fig. 4A and B). Depolarization of the Δψm
is a characteristic event of early apoptosis.
Since intracellular ROS generation is considered to
be associated with mitochondrial dysfunction, the present study
further examined whether shikonin could stimulate ROS generation in
HaCaT cells. A significant increase in ROS generation was observed
in the HaCaT cells treated with shikonin compared with the
untreated cells (P<0.001; Fig. 4C
and D).
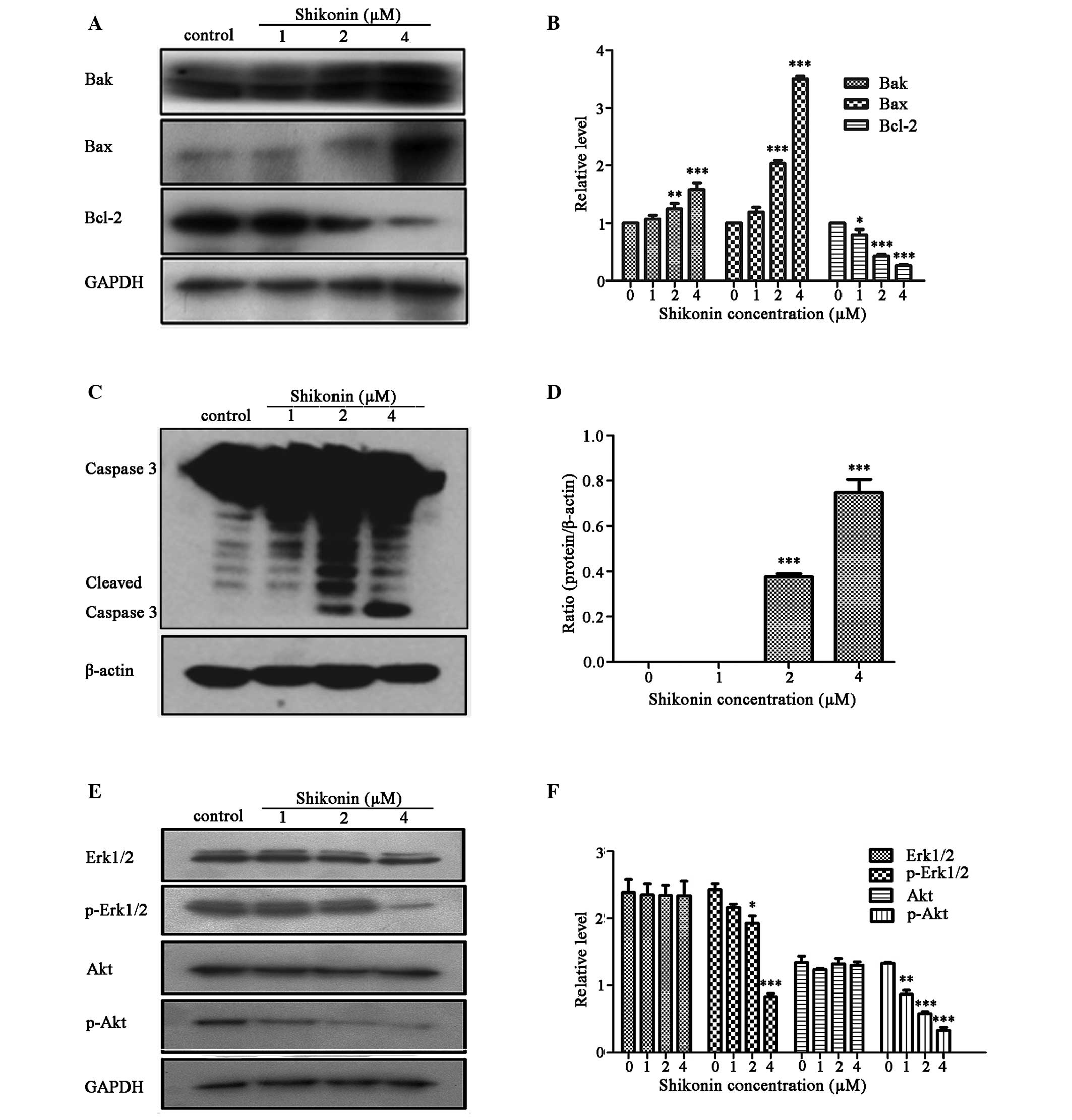
Shikonin regulates Bcl-2 family members
and activates caspase family proteins
Bcl-2 family proteins are known to be involved in
the apoptotic process, due to their ability to form membrane
channels in mitochondria. The Bcl-2 proteins control cytochrome
c release into the cytosol, which activates caspase 3,
subsequently leading to cell apoptosis (22). Therefore, the present study
examined the expression levels of Bcl-2 family proteins (Bcl-2, Bax
and Bak), and caspase 3 in HaCaT cells treated with 1, 2 or 4
µM shikonin for 24 h using western blotting. The effects of
shikonin on the expression levels of Bcl-2 family proteins are
presented in Fig. 5A and B.
Shikonin significantly decreased the expression levels of the
anti-apoptotic Bcl-2 protein, and increased the expression levels
of the proapoptotic Bax and Bak proteins in HaCaT cells, as
compared with the untreated control cells. A dose-dependent
significant increase in the Bax/Bcl-2 ratio was observed
(P<0.001). The present study also verified the induction of
apoptosis via caspase 3 activation using western blotting. The
results revealed that treatment with shikonin significantly
increased caspase 3 cleavage, as compared with the untreated cells
(P<0.001; Fig. 5C and D). These
results indicate that shikonin-induced HaCaT cell apoptosis is
mediated via a mitochondria-dependent pathway.
Erk and Akt pathways are associated with
shikonin-induced HaCaT cell apoptosis
The mitogen-activated protein kinases (MAPKs) and
phosphatidylinositol 3-kinase (PI3K)/Akt pathways have essential
roles in regulating cell proliferation, cell survival and
apoptosis. To evaluate whether shikonin is capable of modulating
the PI3K/Akt and MAPK signaling pathways in order to induce the
inhibition of HaCaT cell proliferation, the effects of shikonin on
the phosphorylation of signaling molecules in these two pathways
were detected. As shown in Fig. 5E and
F, treatment of HaCaT cells with 1, 2 or 4 µM shikonin
for 24 h led to a significant reduction in the expression levels of
p-Erk1/2 and p-Akt, as compared with the total protein expression
levels of Erk1/2 and Akt (P<0.001). These results suggest that
shikonin may downregulate the phosphorylation of these proteins in
a dose-dependent manner.
Discussion
HaCaT cells have been used extensively as in
vitro models of psoriasis (2,23,24).
Apoptotic inhibition occurs in psoriatic lesional keratinocytes
(25), resulting in keratinocyte
hyperproliferation, which induces psoriasis. Therefore, the present
study hypothesized that effective therapeutic agents for the
treatment of psoriasis should inhibit keratinocyte
hyperproliferation and induce apoptosis.
The results of the present study revealed that
shikonin significantly decreased HaCaT cell viability and induced a
G0/G1 phase cell cycle arrest. These results
indicated that cell cycle arrest may be partially responsible for
shikonin-induced HaCaT cell growth inhibition. Phosphatidylserine
is translocated from the inner to the outer leaflet of the plasma
membrane in apoptotic cells. In the present study, Annexin
V-FITC/PI staining was used to determine whether apoptosis had
occurred. Compared with untreated cells, the fluorescence intensity
of HaCaT cells treated with shikonin was significantly increased in
a dose-dependent manner, which was indicative of apoptosis. These
findings are similar to those from a previous report, which
demonstrated that apoptosis of HaCaT human keratinocytes can be
induced by celastrol, which is a triterpenoid isolated from
Celastrus orbiculatus, via inhibition of NF-κB activity
(24).
Apoptosis is a highly regulated process leading to
programmed cell death, which is regulated by several signaling
pathways, including the caspase and MAPK pathways (26). The Bcl-2 protein family has an
important role in the mitochondrial apoptotic pathway, which
results in the release of mitochondrial cytochrome c,
leading to caspase 9 activation and subsequent caspase 3 activation
(27). The present study examined
the effects of shikonin on mitochondrial function. The results
demonstrated that the Δψm was significantly decreased, which was
accompanied by an increase in ROS generation, indicating that
mitochondrial dysfunction had occurred. Subsequently, an increase
in caspase 3, Bax and Bak protein expression, and a decrease in
Bcl-2 protein expression was observed in the shikonin-treated HaCaT
cells, thus suggesting that shikonin-induced apoptosis occurred via
the mitochondrial apoptotic pathway.
Previous studies have demonstrated that shikonin
potently inhibits cell growth and induces cell apoptosis in various
types of cells via its effects on several molecular targets,
including members of the MAPK family (28), Akt/apoptosis signal-regulating
kinase 1/p38 (29) and NF-κB
(30). The present study evaluated
the effects of shikonin on the phosphorylation of signaling
molecules in these pathways in HaCaT cells. Treatment with shikonin
resulted in marked reductions in the expression levels of p-Erk1/2
and p-Akt in a dose-dependent manner, thus suggesting that shikonin
may dose-dependently downregulate the phosphorylation of these
proteins.
In conclusion, in HaCaT human epidermal keratinocyte
cells, shikonin is able to exert its anti-proliferative activity by
inducing cellular apoptosis via the mitochondrial apoptotic
pathway. The mechanism underlying these effects is associated with
inactivation of the Akt and Erk pathways. Therefore, the present
study suggested that shikonin may have potential as a component of
therapeutic strategies for the treatment of skin diseases.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81371732 and
81201231), and was partially supported by the Fundamental Research
Funds for the Central Universities, the Key Fund and Medical
Technology of Second Hospital of Xi'an Jiaotong University, and the
Program for Changjiang Scholars and Innovative Research Team in
University (grant no. PCSIRT:1171). The study was also supported by
the Fundamental Research Funds of Xi'an Bureau of Public Health
(grant no. J2014025) and the Xi'an Hospital of Traditional Chinese
Medicine (grant no. 2014G01). The present study was performed in
the laboratory of Professor Langchong He (School of Pharmacy, Xi'an
Jiaotong University), and the authors thank him for his help and
support.
References
|
1
|
Bhalerao J and Bowcock AM: The genetics of
psoriasis: A complex disorder of the skin and immune system. Hum
Mol Genet. 7:1537–1545. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farkas A, Kemény L, Szöny BJ, Bata-Csörgö
Z, Pivarcsi A, Kiss M, Széll M, Koreck A and Dobozy A: Dithranol
upregulates IL-10 receptors on the cultured human keratinocyte cell
line HaCaT. Inflamm Res. 50:44–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koo J and Arain S: Traditional Chinese
medicine for the treatment of dermatologic disorders. Arch
Dermatol. 134:1388–1393. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tse TW: Use of common Chinese herbs in the
treatment of psoriasis. Clin Exp Dermatol. 28:469–475. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Yang L, Oppenheim JJ and Howard
MZ: Cellular pharmacology studies of shikonin derivatives.
Phytother Res. 16:199–209. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kourounakis AP, Assimopoulou AN,
Papageorgiou VP, Gavalas A and Kourounakis PN: Alkannin and
shikonin: Effect on free radical processes and on inflammation - a
preliminary pharmacochemical investigation. Arch Pharm (Weinheim).
335:262–266. 2002. View Article : Google Scholar
|
|
7
|
Hisa T, Kimura Y, Takada K, Suzuki F and
Takigawa M: Shikonin, an ingredient of Lithospermum erythrorhizon,
inhibits angiogenesis in vivo and in vitro. Anticancer Res.
18:783–790. 1998.PubMed/NCBI
|
|
8
|
Xu Y, Xu X, Gao X, Chen H and Geng L:
Shikonin suppresses IL-17-induced VEGF expression via blockage of
JAK2/STAT3 pathway. Int Immunopharmacol. 19:327–333. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoon Y, Kim YO, Lim NY, Jeon WK and Sung
HJ: Shikonin, an ingredient of Lithospermum erythrorhizon induced
apoptosis in HL60 human premyelocytic leukemia cell line. Planta
Med. 65:532–535. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu PC, Huang YT, Tsai ML, Wang YJ, Lin JK
and Pan MH: Induction of apoptosis by shikonin through coordinative
modulation of the Bcl-2 family, p27 and p53, release of cytochrome
c, and sequential activation of caspases in human colorectal
carcinoma cells. J Agric Food Chem. 52:6330–6337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang IC, Huang YJ, Chiang TI, Yeh CW and
Hsu LS: Shikonin induces apoptosis through reactive oxygen
species/extracellular signal-regulated kinase pathway in
osteosarcoma cells. Biol Pharm Bull. 33:816–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh F, Gao D, Lebwohl MG and Wei H:
Shikonin modulates cell proliferation by inhibiting epidermal
growth factor receptor signaling in human epidermoid carcinoma
cells. Cancer Lett. 200:115–121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong K and Li W: Shikonin, a Chinese
plant-derived naphthoquinone, induces apoptosis in hepatocellular
carcinoma cells through reactive oxygen species: A potential new
treatment for hepatocellular carcinoma. Free Radic Biol Med.
51:2259–2271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CH, Lin ML, Ong PL and Yang JT: Novel
multiple apoptotic mechanism of shikonin in human glioma cells. Ann
Surg Oncol. 19:3097–3106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CH, Chern CL, Lin CC, Lu FJ, Shih MK,
Hsieh PY and Liu TZ: Involvement of reactive oxygen species, but
not mitochondrial permeability transition in the apoptotic
induction of human SK-Hep-1 hepatoma cells by shikonin. Planta Med.
69:1119–1124. 2003. View Article : Google Scholar
|
|
16
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kinnally KW, Martinez-Caballero S and
Dejean LM: Detection of the mitochondrial apoptosis-induced channel
(MAC) and its regulation by Bcl-2 family proteins. Curr Protoc
Toxicol Unit. 2:122006.
|
|
19
|
Sun W, Zheng Y, Lu Z, Wang H, Feng Z, Wang
J, Xiao S, Liu F and Liu J: LL-37 attenuates inflammatory
impairment via mTOR signaling-dependent mitochondrial protection.
Int J Biochem Cell Biol. 54:26–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun W, Zheng Y, Lu Z, Cui Y, Tian Q, Xiao
S, Liu F and Liu J: Overexpression of S100A7 protects LPS-induced
mitochondrial dysfunction and stimulates IL-6 and IL-8 in HaCaT
cells. PLoS One. 9:e929272014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Qian RQ and Li PP: Shikonin, an
ingredient of Lithospermum erythrorhizon, down-regulates the
expression of steroid sulfatase genes in breast cancer cells.
Cancer Lett. 284:47–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dejean LM, Martinez-Caballero S, Manon S
and Kinnally KW: Regulation of the mitochondrial apoptosis-induced
channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta.
1762:191–201. 2006. View Article : Google Scholar
|
|
23
|
Belsõ N, Széll M, Pivarcsi A, Kis K,
Kormos B, Kenderessy AS, Dobozy A, Kemény L and Bata-Csörgõ Z:
Differential expression of D-type cyclins in HaCaT keratinocytes
and in psoriasis. J Invest Dermatol. 128:634–642. 2008. View Article : Google Scholar
|
|
24
|
Zhou LL, Lin ZX, Fung KP, Cheng CH, Che
CT, Zhao M, Wu SH and Zuo Z: Celastrol-induced apoptosis in human
HaCaT keratinocytes involves the inhibition of NF-κB activity. Eur
J Pharmacol. 670:399–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marija K and Larisa PM: The pole of
apoptosis in psoriasis and lichen planus. Focus on Cell Apoptosis
Research. Cho DW: Nova Science Publishers, Inc; New York: pp.
233–240. 2007
|
|
26
|
Okada H and Mak TW: Pathways of apoptotic
and non-apoptotic death in tumour cells. Nat Rev Cancer. 4:592–603.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gross A, Jockel J, Wei MC and Korsmeyer
SJ: Enforced dimerization of BAX results in its translocation,
mitochondrial dysfunction and apoptosis. EMBO J. 17:3878–3885.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mao X, Yu CR, Li WH and Li WX: Induction
of apoptosis by shikonin through a ROS/JNK-mediated process in
Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell
Res. 18:879–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn J, Won M, Choi JH, Kim YS, Jung CR, Im
DS, Kyun ML, Lee K, Song KB and Chung KS: Reactive oxygen
species-mediated activation of the Akt/ASK1/p38 signaling cascade
and p21(Cip1) downregulation are required for shikonin-induced
apoptosis. Apoptosis. 18:870–881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Min R, Tong J, Wenjun Y, Wenhu D, Xiaojian
Z, Jiacai H, Jian Z, Wantao C and Chenping Z: Growth inhibition and
induction of apoptosis in human oral squamous cell carcinoma
Tca-8113 cell lines by Shikonin was partly through the inactivation
of NF-kappaB pathway. Phytother Res. 22:407–415. 2008. View Article : Google Scholar : PubMed/NCBI
|