Introduction
Rheumatoid arthritis (RA) is a chronic auto-immune
inflammatory disease resulting in an inflammatory response in the
synovium and injuries in cartilage and bone (1,2).
Nuclear factor (NF)-κB, a transcription factor, is a crucial
regulator of inflammation in RA (3). NF-κB controls the expression levels
of the pro-inflammatory cytokines, including interleukin 1β (IL-1β)
and tumor necrosis factor α (TNF-α), which are observed at a high
expression level in the peripheral blood and synovial membrane
(4). IL-1β and TNF-α cytokines are
associated with the pathology of RA and induce the activation of
NF-κB, suggesting that the expression levels of IL-1β and TNF-α are
regulated by NF-κB (5).
Furthermore, NF-κB activation is necessary for the production of
cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase
(iNOS), which catalyze the synthesis of prostanoids and nitric
oxide metabolites (6).
Astragalus membranaceus Bge is a traditional
Chinese tonic herb, widely used as a single herb or a collection of
herbs in a complex prescription (7). Astragaloside IV (AS-IV), an active
component purified from Astragalus membranaceus Bge
(8,9), serves a role in the regulation of
numerous biological behaviors as a modulator of the immune system,
and has been used in traditional Chinese medicine for many years to
treat numerous diseases, such as Parkinson's disease, myocardial
ischemia and RA (10–13). AS-IV suppresses joint inflammation
in rat adjuvant-induced arthritis (AIA) via the inhibition of
IL-1β, TNF-α and iNOS production (14–16).
However, the mechanism of this anti-inflammatory activity remains
to be fully elucidated. Therefore, the mechanisms of AS-IV in the
treatment of RA require further investigation in vivo or
in vitro.
Collagen induced arthritis (CIA) is a chronic
immune-inflammatory model used to elucidate the mechanism of RA
pathogenicity and to evaluate anti-arthritic drugs (17,18).
A previous in vivo study demonstrated the effect of AS-IV on
the splenocytes proliferation in AIA rats (16). Thus, in the present study, the cell
proliferation Cell Counting Kit-8 (CCK-8), enzyme-linked
immunosorbent assay (ELISA) and western blot assays were utilized
to investigate whether or not AS-IV exerts an anti-inflammatory
effect in the lipopolysaccharides (LPS)-stimulated synoviocytes and
CIA rats.
Materials and methods
Animals
All animal care and experimental procedures in the
current study complied with the protocol approved by the
Institutional Animal Care and Use Committee at Qingdao University
(Shandong, China). A total of 30 male Sprague-Dawley rats (weight,
150–180 g; age, 8 weeks) were purchased from the Shanghai BK
Experimental Animal Center (Shanghai, China). Rats were separated
into cages (n=5) and were fed laboratory feed and water, and were
kept under a 12 h light/dark cycle at a constant temperature of
25°C.
Cell culture
The synovial membrane was dissected from the
underlying adipose subintima, transferred to Invitrogen Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% (v/v) heat-inactivated fetal calf
serum (Gibco; Thermo Fisher Scientific, Inc.), 1% (v/v) penicillin
(5,000 U/ml) and streptomycin (5,000 µg/ml) (Invitrogen;
Thermo Fisher Scientific, Inc.), and immediately subjected to
enzymatic digestion for 3 h at 37°C. Newly released synoviocytes
were seeded into 25 cm3 flasks. Subsequent to incubation
for 48 h, the medium was changed, removing any cells not adhered to
the culture flask. Flasks were maintained by changing the medium at
48 h intervals until cell replication was observed; at that time,
synoviocytes were seeded into 24-well plates and grown to 80%
confluence. Synoviocytes were incubated for 48 h, using the
following treatment conditions: Unstimulated (medium only); and
LPS-stimulated (50 mg/ml; Sigma-Aldrich, St. Louis, MO, USA), LPS +
AS-IV (5, 20 or 50 mg/ml; Yuanmu Biotech Co., Ltd., Shanghai,
China).
CCK-8 cell proliferation assay
Cell proliferation was assessed using the CCK-8
assay (Dojindo Molecular Technologies, Inc., Rockville, MD, USA) as
previously described (19). Cells
were plated at a density of 2×104 cells/well onto a
96-well plate, treated with LPS in the presence or absence of AS-IV
(5, 20, and 50 mg/ml) for 72 h and the cell proliferation was
determined according to the manufacturer's instructions. Absorbance
of the supernatant for each well was measured at 450 nm using the
Multiskan EX plate reader (Thermo Fisher Scientific, Inc.).
Collagen-induced arthritis
Sprague-Dawley rats were immunized with 500 mg/kg of
bovine type II collagen emulsified with an equal volume of complete
Freund's adjuvant (Chondrex, Inc., Redmond, WA, USA) as previously
described (20). Following
immunization, arthritic rats were randomly divided into five groups
(n=6), control rats, no treatment; LPS treatment; LPS treatment in
the presence of three different concentrations of AS-IV (5, 20 and
50 mg/ml). To examine the therapeutic effect of AS-IV, arthritic
rats were injected with 5, 20 and 50 mg/kg AS-IV on days 27 and 30.
On day 45, the rats were anesthetized with CO2 and then
a cervical dislocation was performed. Synovial tissues were
harvested from each animal for ELISA and western blot analysis.
ELISA assay
The experimental procedure was performed using an
ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA) according
to the manufacturer's instructions. LPS-stimulated synoviocytes
(2×105 cells per 60-mm dish per 2 ml of serum-free
media) and collagen-induced arthritic rats were treated with AS-IV
for 24 h. Cultured supernatants and serum were collected and
subjected to ELISA for measurement of TNF-α, IL-1β, IL-6, IL-8,
COX-1, COX-2, high mobility group box 1 protein (HMGB1) and
intercellular adhesion molecule 1 (ICAM-1).
Western blot analysis
Western blotting was performed as previously
described (21). LPS-stimulated
synoviocytes and collagen-induced synovial tissues were
serum-starved overnight and then treated with AS-IV for 24 h. Cells
were subjected to sodium dodecyl 10% sulfate-polyacrylimide gel
(Sigma-Aldrich) electrophoresis using a Bio-Rad miniature slab gel
apparatus (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
proteins were electrophoretically transferred onto a nitrocellulose
membrane (EMD Millipore, Billerica, MA, USA). Membranes were
incubated with primary antibodies rabbit pAb against iNOS (cat. no.
ab3523; 1:1,000; Abcam, Cambridge, MA, USA), Rabbit mAb against
COX2 (cat. no. 12282; 1:1,000), c-Jun N-terminal kinase (JNK1/2;
cat. no. 9252; 1:1,000), p38 (cat. no. 8690; 1:1,000), histone H3
(cat. no. 4499; 1:800), β-actin (cat. no. 4970; 1:1,000), and GAPDH
(cat. no. 5174; 1:1,500), mouse mAb against p-JNK1/2 (cat. no.
9255; 1:1,000), p-p38 (cat. no. 9216; 1:800), and NF-κB p65 (cat.
no. 6956; 1:1,000) overnight at 4°C, 9 antibodies purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Membranes were
washed and incubated with goat anti-rabbit and goat anti-mouse
horseradish peroxidase-conjugated secondary antibodies (cat. nos.
A0208 and A0216; 1:1,000; Beyotime Institute of Biotechnology,
Haimen, China) for 1 h at 37°C, and visualized with enhanced
chemiluminescence (Merck Millipore, Beijing, China) according to
the manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software, version 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA). Differences among groups were analyzed using Student's
two-tailed t-test, and all statistical analyses were two-sided.
P<0.05 was considered to indicate a statistically significant
difference.
Results
AS-IV inhibited the proliferation of
LPS-stimulated synoviocytes
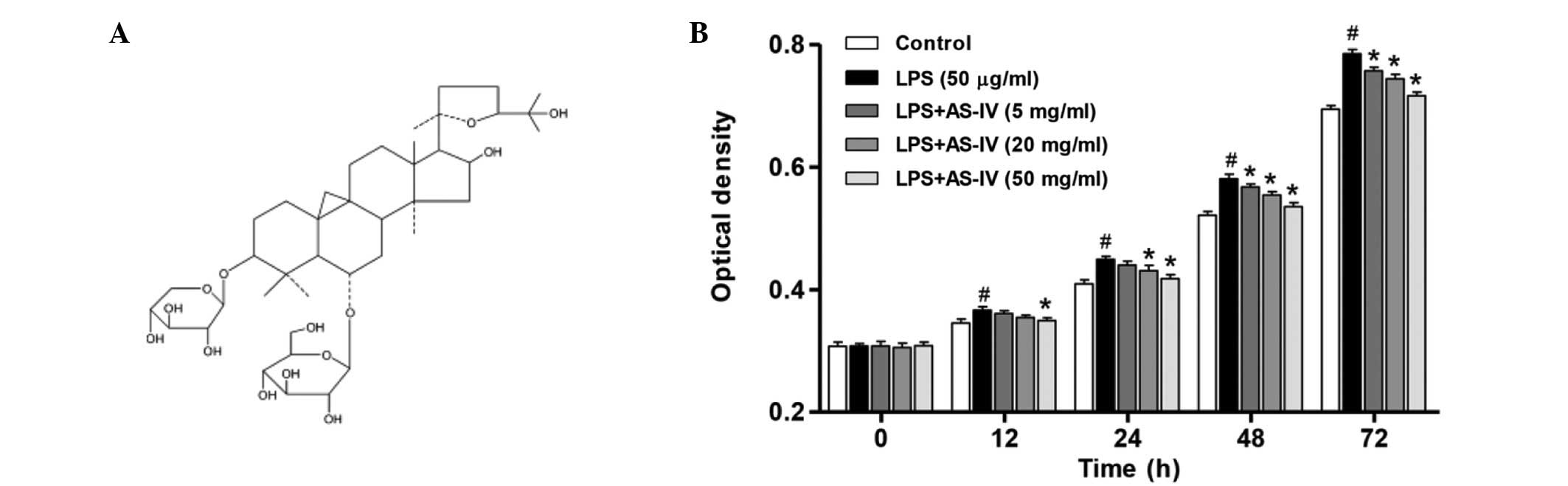
The chemical structure of AS-IV is presented in
Fig. 1A. To determine the effect
of AS-IV on the proliferation of synoviocytes with or without LPS
induction, cell proliferation was determined. As demonstrated in
Fig. 1B, LPS treatment resulted in
a significant increase in the proliferation of synoviocytes
compared with that of the untreated control (P<0.01). In the
presence of LPS, administration of AS-IV (5, 20 or 50 mg/ml)
significantly inhibited synoviocyte proliferation in a dose- and
time-dependent manner compared with the LPS-stimulated synoviocytes
group (Fig. 1B; P<0.01).
AS-IV inhibited inflammatory mediator
production by LPS-stimulated synoviocytes
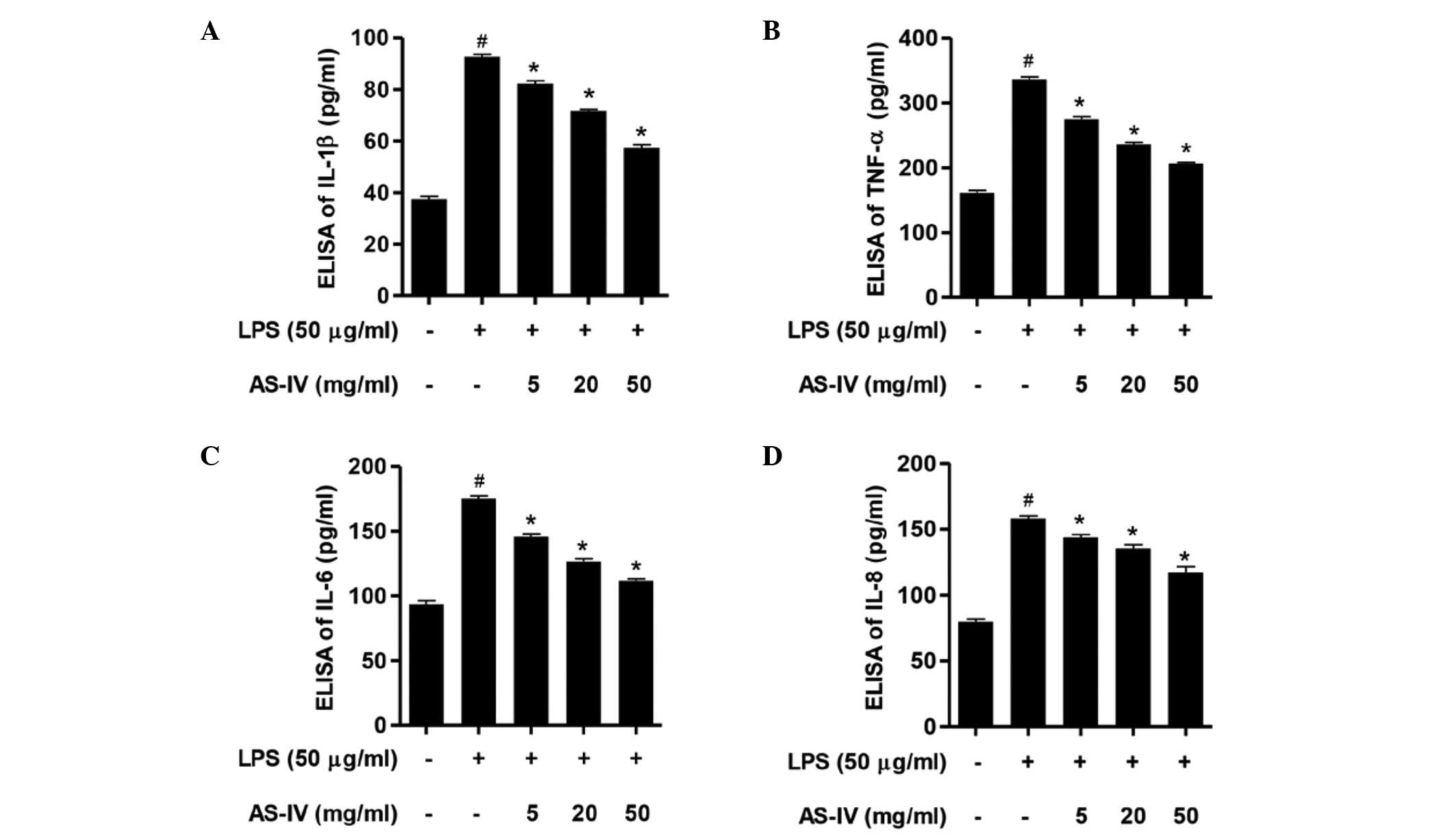
IL-1β, TNF-α, IL-6 and IL-8 expression levels in
response to AS-IV and LPS were determined. Following stimulation of
the synoviocytes with LPS, the expression levels of IL-1β, TNF-α,
IL-6 and IL-8 were significantly increased compared with the
control group (Fig. 2; P<0.01).
Further administration of AS-IV (5, 20 or 50 mg/ml) significantly
inhibited the expression levels of IL-1β, TNF-α, IL-6 and IL-8
compared with the LPS-stimulated synoviocytes (Fig. 2; P<0.01). These results indicate
that AS-IV possesses an anti-inflammatory effect in LPS-stimulated
synoviocytes.
Downregulation of
COX-1/COX-2/HMGB1/ICAM-1 overexpression contributed to the
cytoprotection of AS-IV in LPS-stimulated synoviocytes
Following stimulation of the synoviocytes with LPS,
the ELISA assay demonstrated that the expression levels of COX-1,
COX-2, HMGB1 and ICAM-1 were significantly increased compared with
the control group (Fig. 3;
P<0.01). Further treatment with AS-IV (5, 20 or 50 mg/ml)
significantly attenuated the overexpression of COX-1, COX-2, HMGB1
and ICAM-1 compared with the LPS-stimulated synoviocytes (Fig. 3; P<0.01). These results
demonstrate that COX-1, COX-2, HMGB1 and ICAM-1 mediate
LPS-stimulated cytotoxicity and inflammatory responses, and that
the inhibition of the LPS-stimulated COX-1, COX-2, HMGB1 and ICAM-1
overexpression is involved in the AS-IV-mediated protective effect
in synoviocytes.
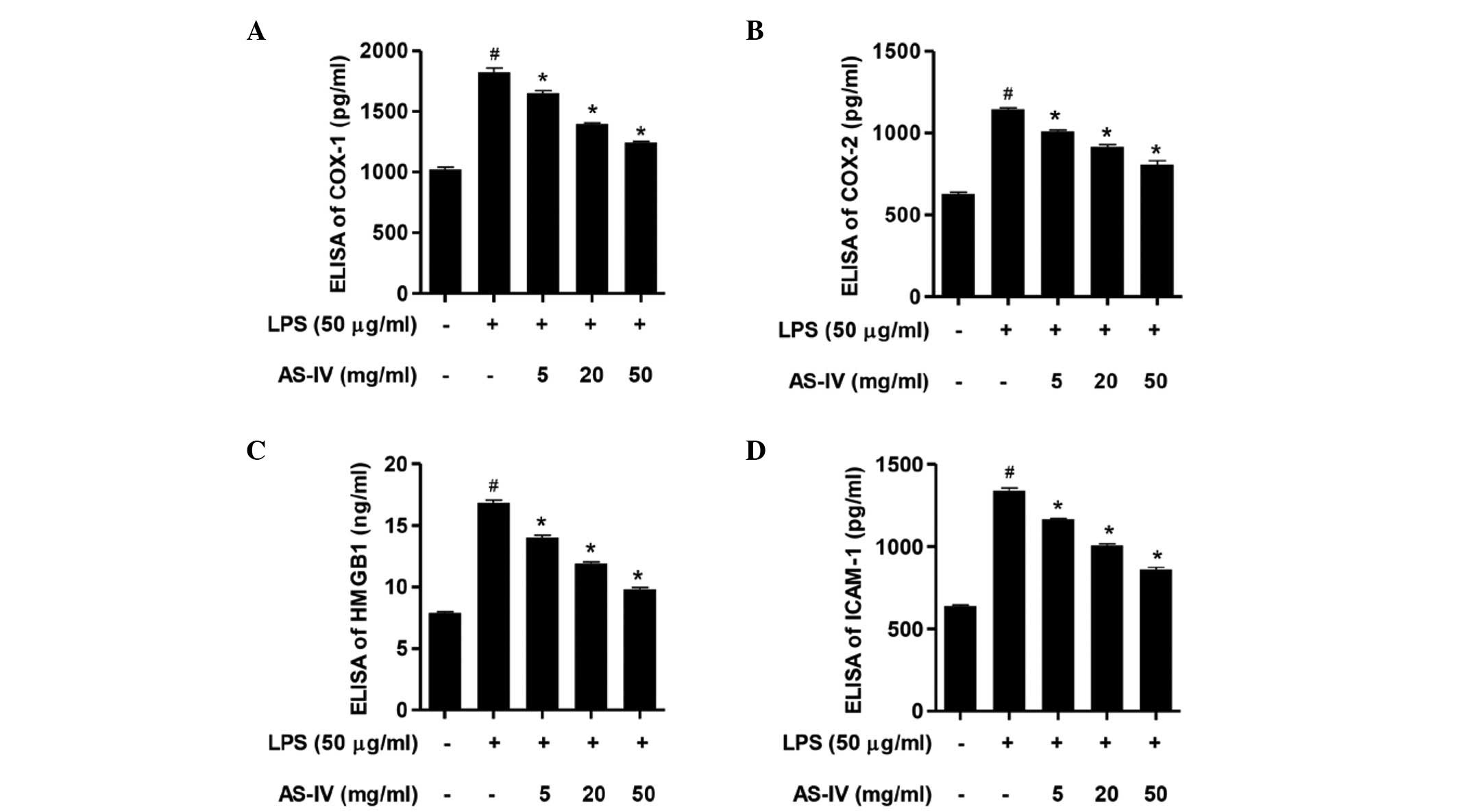 | Figure 3AS-IV inhibited the LPS-stimulated
COX-1, COX-2, HMGB1 and ICAM-1 overexpression. Synoviocytes were
stimulated with LPS in the absence or presence of AS-IV treatment.
ELISA analysis of (A) COX-1, (B) COX-2, (C) HMGB1 and (D) ICAM-1
expression levels in cell supernatants. Data are presented as the
mean ± standard deviation (n=3). #P<0.01 vs. the
untreated control synoviocytes and *P<0.01 vs. the
LPS-stimulated synoviocytes. ELISA, enzyme-linked immunosorbent
assay; COX-1, cyclooxygenase 1; LPS, lipopolysaccharide; AS-IV,
astragaloside IV; HMGB1, high mobility group box 1; ICAM-1,
intercellular adhesion molecule 1. |
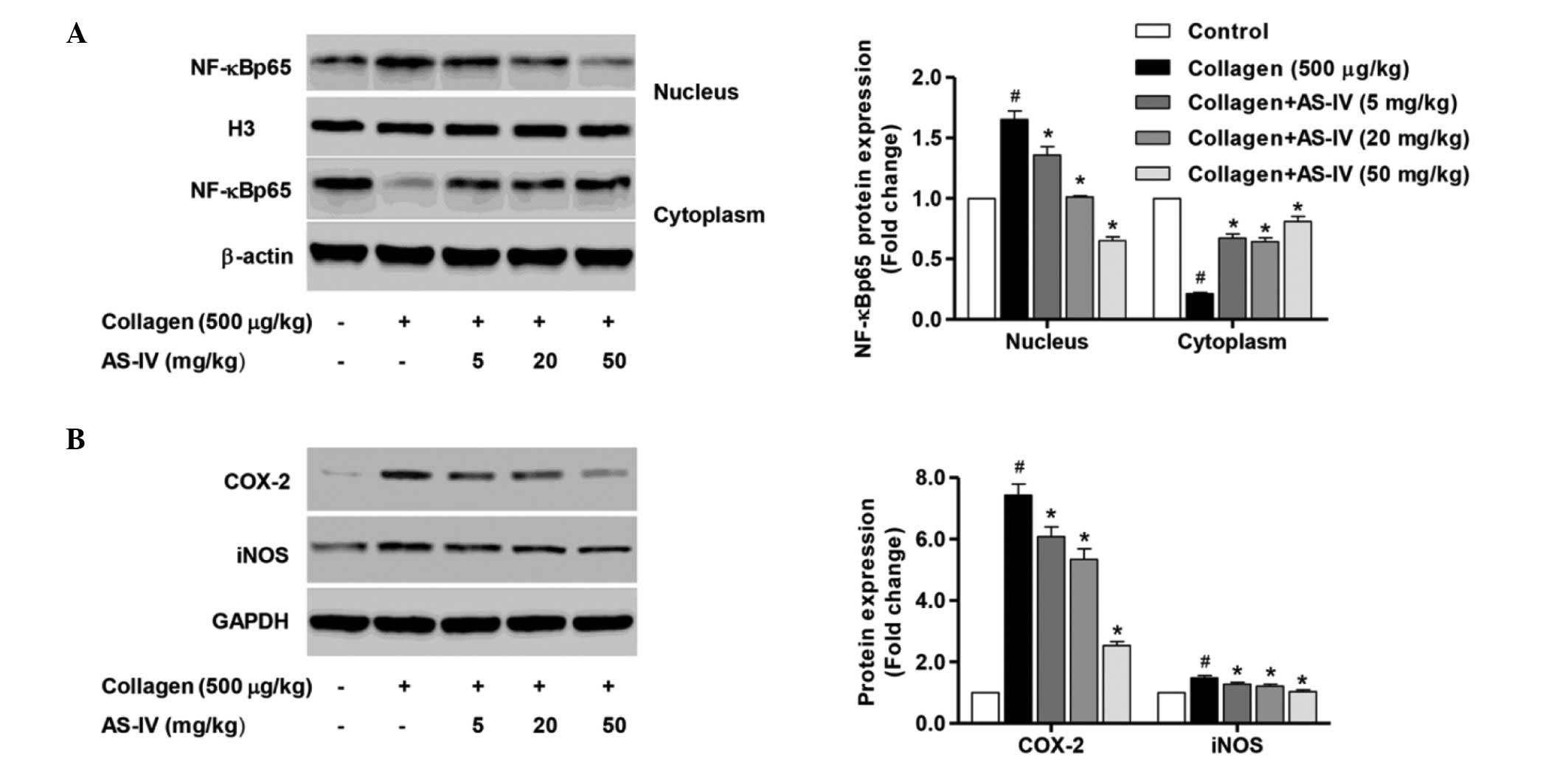
Inhibition of NF-κB, JNK1/2 and p38
activation was implicated in the cytoprotection of AS-IV in
LPS-stimulated synoviocytes
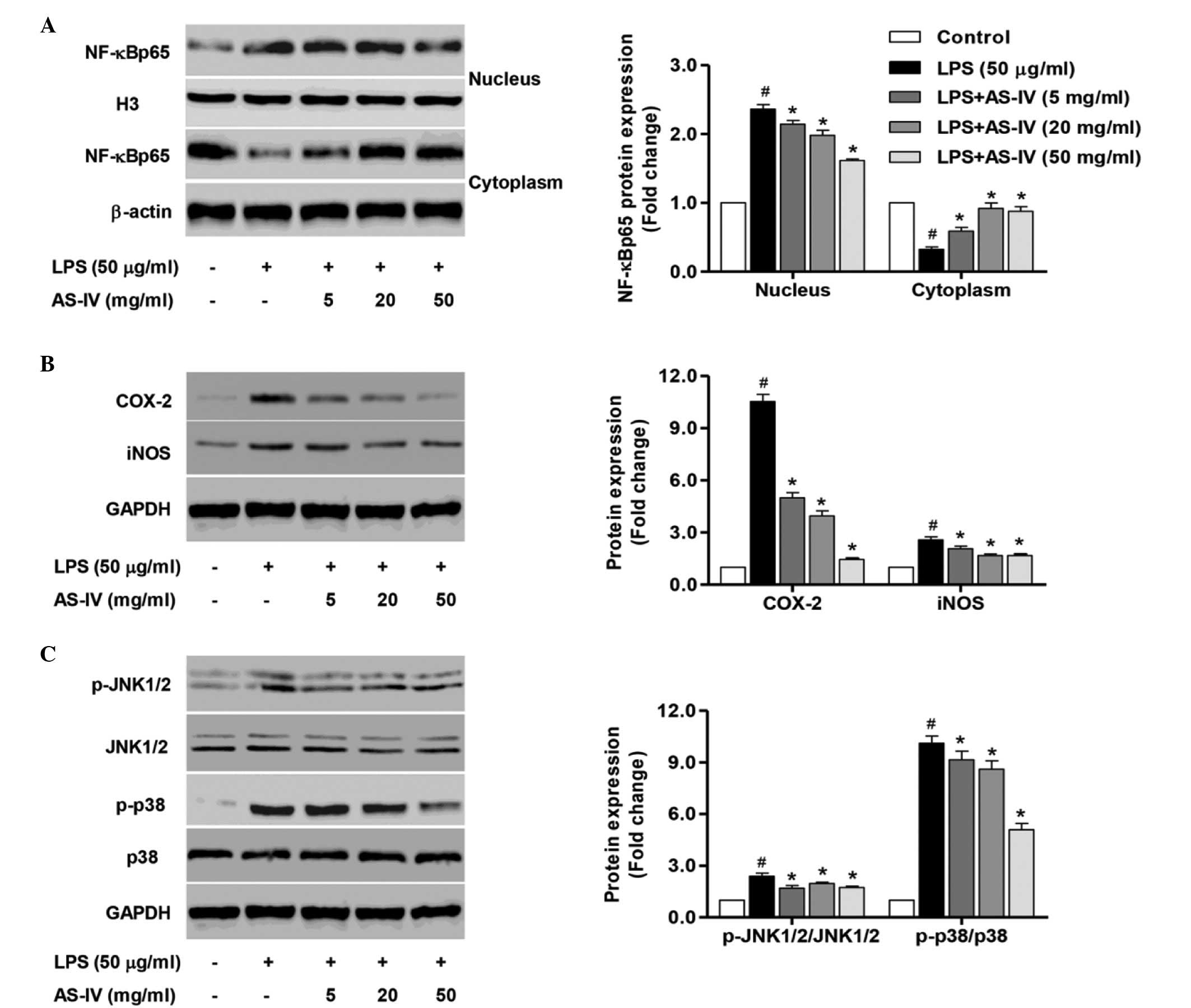
To further clarify the mechanism underlying the
in vitro protective effect of AS-IV, the NF-κB signaling
pathway was investigated in nuclear and cytoplasmic extracts, and
the activity of JNK1/2 and p38 was also measured. Treatment of
synoviocytes with LPS significantly enhanced the expression of the
intranuclear NF-κBp65 subunit, an important step in NF-κB
activation, compared with the control group (Fig. 4A; P<0.0). Furthermore, a
significant reduction in the expression levels of the cytoplasmic
NF-κBp65 subunit expression compared with that of the untreated
control synoviocytes was demonstrated (Fig. 4A; P<0.01), indicating that LPS
stimulation may evoke NF-κB activation. Treatment of synoviocytes
with AS-IV (5, 20 and 50 mg/ml) significantly inhibited
intranuclear NF-κBp65 subunit expression and enhanced the
cytoplasmic NF-κBp65 subunit expression compared with the
LPS-stimulated synoviocytes (Fig.
4A; P<0.01). In addition, treatment with AS-IV significantly
reduced the LPS-stimulated COX-2 and iNOS overexpression compared
with the LPS-stimulated synoviocytes (Fig. 4B, P<0.01).
The results from the ELISA assay demonstrated a
significant overexpression of HMGB1 in the LPS-stimulated
synoviocytes, thus the JNK1/2 and p38 signaling pathways activated
by HMGB1 were investigated in the LPS-stimulated synoviocytes in
the presence or absence of AS-IV. As demonstrated in Fig. 4C, the phosphorylation of JNK1/2
(p-JNK1/2) and p38 (p-p38) was significantly increased in the
LPS-stimulated synoviocytes compared with the control group
(P<0.01). Further treatment with AS-IV (5, 20 or 50 mg/ml)
significantly inhibited the activation of JNK1/2 and p38 compared
with the LPS-stimulated synoviocytes (Fig. 4C, P<0.01). The results suggest
that the protection of AS-IV against inflammation and cytotoxicity
resulted by LPS is partially associated with the inhibition of
NF-κB, JNK1/2 and p38 activation in synoviocytes.
Suppression of inflammatory mediator
production and NF-κB activation involved in the pathophysiology of
CIA by treatment with AS-IV
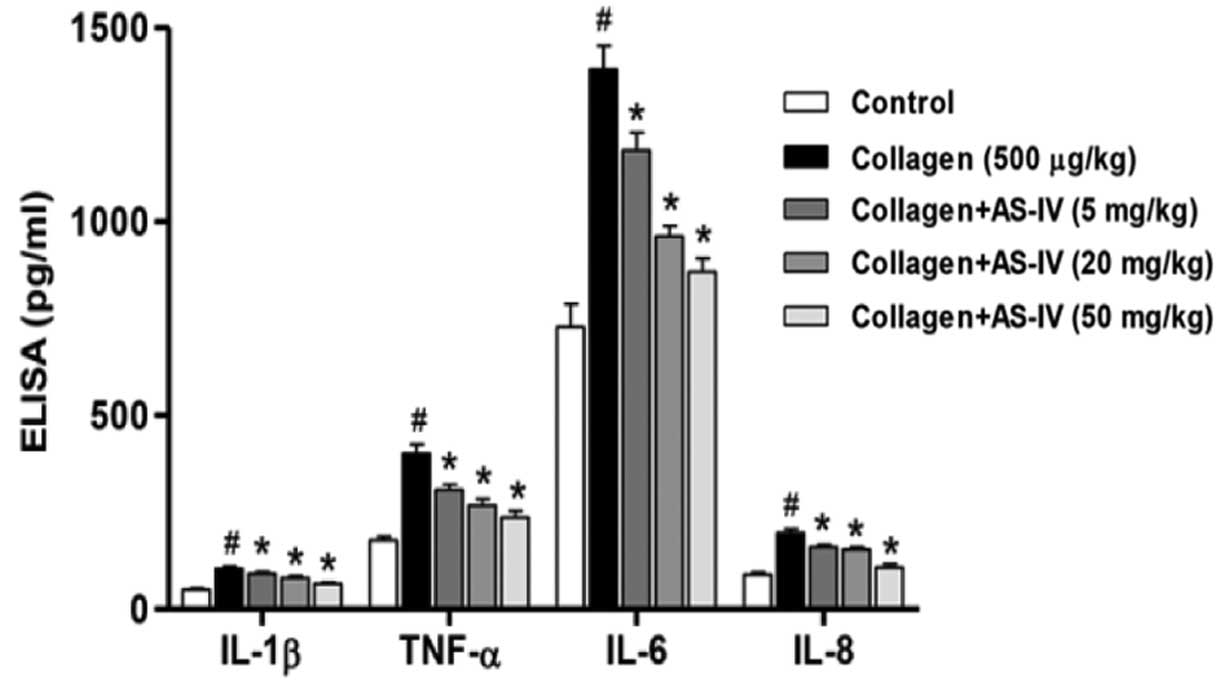
It is well known that inflammatory mediators serve
key roles in the pathogenesis of RA (22). Therefore, the expression levels of
inflammatory mediators obtained from the serum of rats on day 45
were assessed with the ELISA assay. Compared with the CIA rats, a
significant reduction in IL-1β, TNF-α, IL-6 and IL-8 expression
levels was observed in the serum of AS-IV-treated rats (Fig. 5).
To further clarify the mechanism underlying the
in vivo protective effect of AS-IV, the NF-κB signaling
pathway was investigated in nuclear and cytoplasmic extracts.
Treatment of rats with collagen significantly enhanced the
intranuclear NF-κBp65 subunit expression and suppressed cytoplasmic
NF-κBp65 subunit expression, compared with that of the untreated
control rats (Fig. 6A; P<0.01),
indicating that collagen induction may evoke NF-κB activation.
However, treatment of CIA rats with AS-IV (5, 20 or 50 mg/ml)
significantly inhibited intranuclear NF-κBp65 subunit expression
and enhanced cytoplasmic NF-κBp65 subunit expression (Fig. 6A; P<0.01). Furthermore,
treatment with AS-IV (5, 20 and 50 mg/ml) significantly reduced the
collagen-induced COX-2 and iNOS overexpression compared with the
collagen-induced arthritic rats (Fig.
6B; P0<0.01). These observations suggest that AS-IV induced
a protective effect in rats with CIA through downregulating the
synthesis of numerous inflammatory mediators and suppression of
NF-κB activation.
Discussion
Anti-rheumatic drugs including methotrexate,
celecoxib and TNF inhibitors have been used to reduce systemic
inflammation. However, the efficacies of these agents are limited
due to the side effects, such as vomiting and liver damage
(23,24). Thus, novel therapeutic agents with
high efficiency and limited side effects are required for RA
therapy.
The balance between pro-inflammatory mediators and
anti-inflammatory mediators contributes to the development and
progression of joint damage in RA (25). The present study investigated
whether AS-IV exerts anti-inflammatory effects in synoviocytes. The
cells were stimulated with LPS in the absence and presence of
AS-IV, demonstrating that AS-IV inhibited the proliferation of
synoviocytes in the presence of LPS in a dose- and time-dependent
manner. The synoviocytes from patient with RA are pathological
inflammatory cells independent of LPS treatment, thus the
inhibitory effects of AS-IV on the inflammatory responses may not
result from the cytotoxicity of AS-IV alone. In addition,
administration of AS-IV significantly reduced the LPS-induced
production of inflammatory mediators inducing IL-1β, TNF-α, IL-6
and IL-8 in a dose-dependent manner.
COX-2, a pro-inflammatory mediator, promotes a
number of inflammatory factors in numerous cells and tissues
(26). Tsutakawa et al
(27) demonstrated upregulation of
COX-2 mRNA expression in rat skin undergoing ischemia/reperfusion
(I/R) lesions, and that NS-398, an inhibitor of COX-2, abrogates
nicotine induced-skin necrosis and apoptosis resulting from I/R.
The present study indicated that AS-IV reduced the protein
expression levels of COX-1, COX-2, HMGB1 and ICAM-1, suggesting
inhibitory effects of AS-IV on the production of inflammatory
mediators and molecules. Furthermore, in RA rat models induced by
collagen, a daily dose of 500 mg/kg of type II collagen
significantly increased IL-1β, TNF-α, IL-6 and IL-8 production, and
the protein expression of COX-2 and iNOS, whereas treatment of
AS-IV had a negative effect on the productions of these
inflammatory mediators and genes.
NF-κB has an important role in regulating the
expression of numerous genes involved in inflammatory responses,
including the cytokines IL-1β and TNF-α, and the chemokines CXCL5,
CCL5, matrix metalloproteinase-1 (MMP-1) and MMP-3, which are
associated with the development and progression of RA (28,29).
The production of these mediators contributes to the enhancement of
inflammatory reactions leading to further activation of NF-κB. This
continuous NF-κB activation has been observed in human and animal
models of RA (30). In the present
study, the western blot assays demonstrated that AS-IV suppressed
NF-κB activation in the LPS-stimulated synoviocytes and RA rat
models in a dose-dependent manner.
The transcriptional activity of NF-κB is regulated
in the nucleus by three mitogen-activated protein kinase (MAPK)
pathways, including p42/p44 MAPK, p38 MAPK and JNK, all of which
has been reported to be activated by HMGB1 (31). Concomitant with activation of the
NF-κB pathway, HMGB1 results in phosphorylation of extracellular
signal-related kinase and MMP expression (32). HMGB1 promotes protein kinase B
phosphorylation via IL-1β, a signaling pathway associated with
fibroblast survival and proliferation in the RA synovium (33). Therefore, a foundation is provided
to communicate with other signaling pathways via an additional
level of regulation. Based on this, the present study investigated
whether AS-IV had an effect on the MAPK pathways in addition to the
activation of NF-κB. The results of the current study demonstrated
that HMGB1-stimulated p38 and JNK1/2 activation were attenuated by
AS-IV treatment. Thus, p38 and JNK1/2 pathways may have mediated
the suppressive effects of AS-IV.
Various targets are investigated for the development
of novel agents for RA therapy, including pro-inflammatory
mediators, MMPs and osteoclastogenesis. The NF-κB pathway regulates
numerous target molecules and acts as an effective therapeutic
target. The results of the present study demonstrated that AS-IV
significantly reduced the severity of inflammation in the
LPS-stimulated synoviocytes and in the collagen-induced RA rats via
inhibition of the HMGB1-dependent JNK1/2- and p38-activated
NF-κB/COX-2 pathway. In conclusion, the current study suggests that
AS-IV may be used as a therapeutic agent for the treatment of
inflammatory responses in arthritis.
Acknowledgments
The current study was supported by the National
Natural Science Foundation of China (grant no. NSFC 81272056).
References
|
1
|
Davignon JL, Hayder M, Baron M, Boyer JF,
Constantin A, Apparailly F, Poupot R and Cantagrel A: Targeting
monocytes/macrophages in the treatment of rheumatoid arthritis.
Rheumatology (Oxford). 52:590–598. 2013. View Article : Google Scholar
|
|
2
|
Walter GJ, Evans HG, Menon B, Gullick NJ,
Kirkham BW, Cope AP, Geissmann F and Taams LS: Interaction with
activated monocytes enhances cytokine expression and suppressive
activity of human CD4 CD45RO CD25 CD127low regulatory T cells.
Arthritis Rheum. 65:627–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bianchi R, Giambanco I and Donato R:
S100B/RAGE-dependent activation of microglia via NF-κB and AP-1:
Co-regulation of COX-2 expression by S100B, IL-1β and TNF-α.
Neurobiol Aging. 31:665–677. 2010. View Article : Google Scholar
|
|
4
|
Salminen A, Huuskonen J, Ojala J,
Kauppinen A, Kaarniranta K and Suuronen T: Activation of innate
immunity system during aging: NF-κB signaling is the molecular
culprit of inflamm-aging. Ageing Res Rev. 7:83–105. 2008.
View Article : Google Scholar
|
|
5
|
Sheeba M and Asha V: Cardiospermum
halicacabum ethanol extract inhibits LPS induced COX-2, TNF-α and
iNOS expression, which is mediated by NF-κB regulation, in RAW264.7
cells. J Ethnopharmacol. 124:39–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar S, Singhal V, Roshan R, Sharma A,
Rembhotkar GW and Ghosh B: Piperine inhibits TNF-α induced adhesion
of neutrophils to endothelial monolayer through suppression of
NF-κB and IκB kinase activation. Eur J Pharmacol. 575:177–186.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang B: Anti-arthritic effect of
astragaloside IV and its molecular mechanism. ICS.
12014.Review.
|
|
8
|
Ma XQ, Shi Q, Duan J, Dong TT and Tsim KW:
Chemical analysis of Radix Astragali (Huangqi) in China: A
comparison with its adulterants and seasonal variations. J Agric
Food Chem. 50:4861–4866. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei H, Wang B, Li WP, Yang Y, Zhou AW and
Chen MZ: Anti-aging effect of astragalosides and its mechanism of
action. Acta Pharmacol Sin. 24:230–234. 2003.PubMed/NCBI
|
|
10
|
Yu QT, Qi LW, Li P, Yi L, Zhao J and Bi Z:
Determination of seventeen main flavonoids and saponins in the
medicinal plant Huang-qi (Radix Astragali) by HPLC-DAD-ELSD. J Sep
Sci. 30:1292–1299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho WCS and Leung KN: In vitro and in vivo
immunomodulating and immunorestorative effects of Astragalus
membranaceus. J Ethnopharmacol. 113:132–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan WS, Durairajan SSK, Lu JH, Wang Y,
Xie LX, Kum WF, Koo I, Yung KKL and Li M: Neuroprotective effects
of Astragaloside IV in 6-hydroxydopamine-treated primary nigral
cell culture. Neurochem Int. 55:414–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang WD, Chen H, Zhang C, Liu RH, Li HL
and Chen HZ: Astragaloside IV from Astragalus membranaceus shows
cardioprotection during myocardial ischemia in vivo and in vitro.
Planta Med. 72:4–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagy G, Koncz A, Telarico T, Fernandez D,
Érsek B, Buzás E and Perl A: Central role of nitric oxide in the
pathogenesis of rheumatoid arthritis and sysemic lupus
erythematosus. Arthritis Res Ther. 12:2102010. View Article : Google Scholar :
|
|
15
|
Schett G, Coates LC, Ash ZR, Finzel S and
Conaghan PG: Structural damage in rheumatoid arthritis, psoriatic
arthritis, and ankylosing spondylitis: Traditional views, novel
insights gained from TNF blockade, and concepts for the future.
Arthritis Res Ther. 13:S42011.PubMed/NCBI
|
|
16
|
Wang B and Chen MZ: Astragaloside IV
possesses antiarthritic effect by preventing interleukin 1β-induced
joint inflammation and cartilage damage. Arch Pharm Res.
37:793–802. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu L, Wei W, Zheng YQ and Jia XY: Effects
and mechanisms of total glucosides of paeony on joint damage in rat
collagen-induced arthritis. Inflamm Res. 54:211–220. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takaishi S, Okumura T, Tu S, Wang SSW,
Shibata W, Vigneshwaran R, Gordon SAK, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee Y, Hwang J, Lee H, Cheon YJ, Ryu JH,
Lee SI, Kwak HB, Lee SM, Kim JS, Park JW, et al: SPA0355, a
thiourea analogue, inhibits inflammatory responses and joint
destruction in fibroblast-like synoviocytes and mice with
collagen-induced arthritis. Br J Pharmacol. 164:794–806. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han I, Jeong SJ, Lee HJ, Koh W, Lee HJ,
Lee EO, Kim HS, Lee SJ, Chen CY, Jung MH and Kim SH: Proteomic
analysis of mesenchymal stem-like cells derived from ovarian
teratoma: Potential role of glutathione S-transferase M2 in ovarian
teratoma. Proteomics. 11:352–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paramalingam SS, Thumboo J, Vasoo S, Thio
ST, Tse C and Fong K-Y: In vivo pro-and anti-inflammatory cytokines
in normal and patients with rheumatoid arthritis. Ann Acad Med
Singap. 36:962007.
|
|
23
|
Katchamart W, Trudeau J, Phumethum V and
Bombardier C: The efficacy and toxicity of methotrexate (MTX)
monotherapy versus MTX combination therapy with non-biological
disease-modifying anti-rheumatic drugs in rheumatoid arthritis: A
systematic review and meta-analysis. Ann Rheum Dis. 68:1105–1112.
2009. View Article : Google Scholar
|
|
24
|
Simon L and Yocum D: New and future drug
therapies for rheumatoid arthritis. Rheumatology (Oxford). 39(Suppl
1): 36–42. 2000. View Article : Google Scholar
|
|
25
|
Xin W, Huang C, Zhang X, Xin S, Zhou Y, Ma
X, Zhang D, Li Y, Zhou S, Zhang D, et al: Methyl salicylate
lactoside inhibits inflammatory response of fibroblast-like
synoviocytes and joint destruction in collagen-induced arthritis in
mice. Br J Pharmacol. 171:3526–3538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang C, Yang Z, Zhang M, Dong Q, Wang X,
Lan A, Zeng F, Chen P, Wang C and Feng J: Hydrogen sulfide protects
against chemical hypoxia-induced cytotoxicity and inflammation in
HaCaT cells through inhibition of ROS/NF-κB/COX-2 pathway. PLoS
One. 6:e219712011. View Article : Google Scholar
|
|
27
|
Tsutakawa S, Kobayashi D, Kusama M, Moriya
T and Nakahata N: Nicotine enhances skin necrosis and expression of
inflammatory mediators in a rat pressure ulcer model. Br J
Dermatol. 161:1020–1027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ha MK, Song YH, Jeong SJ, Lee HJ, Jung JH,
Kim B, Song HS, Huh JE and Kim SH: Emodin inhibits proinflammatory
responses and inactivates histone deacetylase 1 in hypoxic
rheumatoid synoviocytes. Biol Pharm Bull. 34:1432–1437. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng C, Perera PK, Li YM, Fang WR, Liu LF
and Li FW: Anti-inflammatory effects of Clematis chinensis Osbeck
extract (AR-6) may be associated with NF-κB, TNF-α, and COX-2 in
collagen-induced arthritis in rat. Rheumatol Int. 32:3119–3125.
2012. View Article : Google Scholar
|
|
30
|
Hah YS, Lee YR, Jun JS, Lim H-S, Kim HO,
Jeong YG, Hur GM, Lee SY, Chung MJ, Park JW, et al: A20 suppresses
inflammatory responses and bone destruction in human
fibroblast-like synoviocytes and in mice with collagen-induced
arthritis. Arthritis Rheum. 62:2313–2321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
García-Arnandis I, Guillén MI, Gomar F,
Pelletier JP, Martel-Pelletier J and Alcaraz MJ: High mobility
group box 1 potentiates the pro-inflammatory effects of
interleukin-1β in osteoarthritic synoviocytes. Arthritis Res Ther.
12:R1652010. View
Article : Google Scholar
|
|
32
|
Loeser RF, Yammani RR, Carlson CS, Chen H,
Cole A, Im HJ, Bursch LS and Yan SD: Articular chondrocytes express
the receptor for advanced glycation end products: Potential role in
osteoarthritis. Arthritis Rheum. 52:2376–2385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang HG, Wang Y, Xie JF, Liang X, Liu D,
Yang P, Hsu HC, Ray RB and Mountz JD: Regulation of tumor necrosis
factor alpha-mediated apoptosis of rheumatoid arthritis synovial
fibroblasts by the protein kinase Akt. Arthritis Rheum.
44:1555–1567. 2001. View Article : Google Scholar : PubMed/NCBI
|