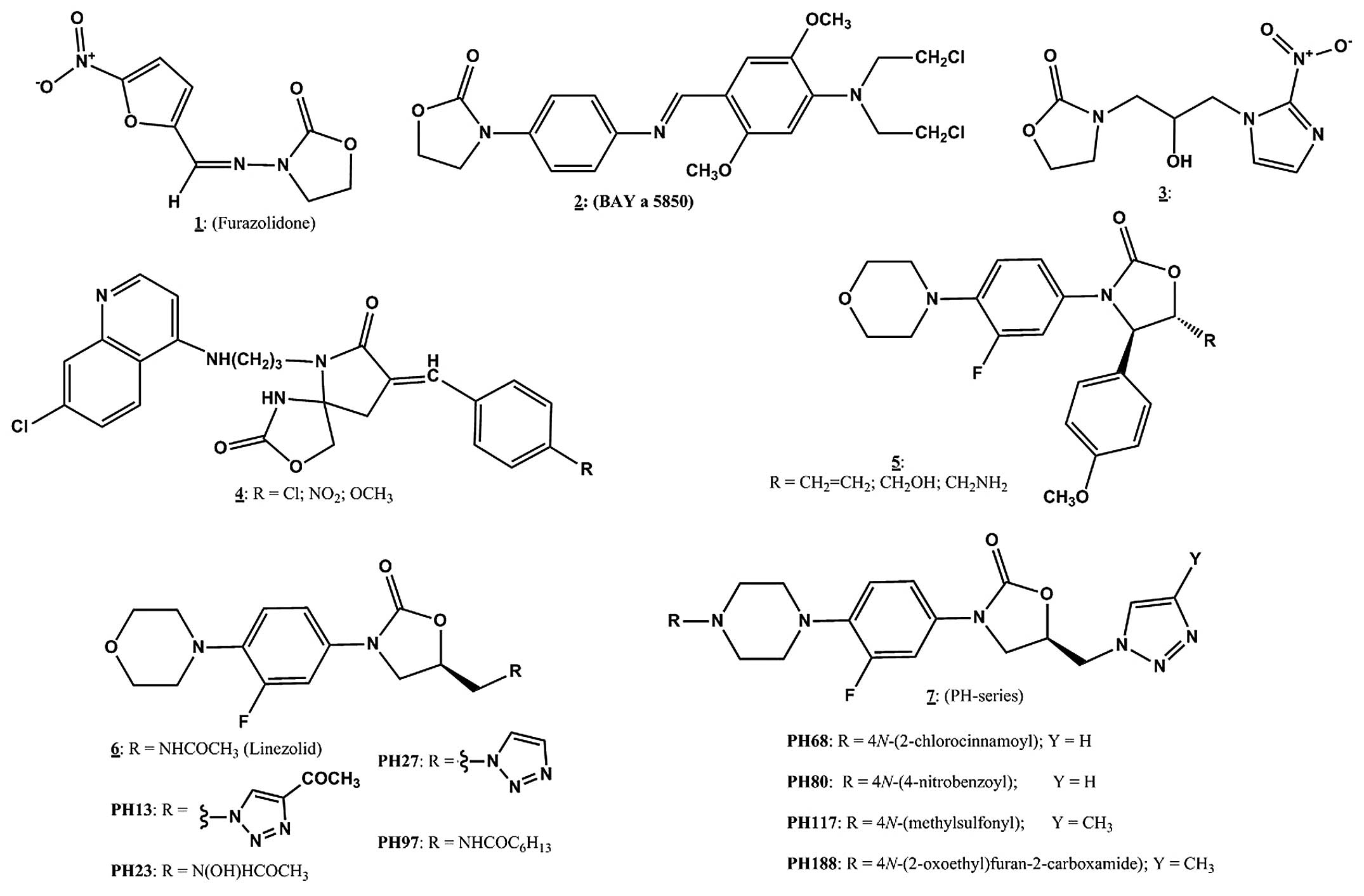
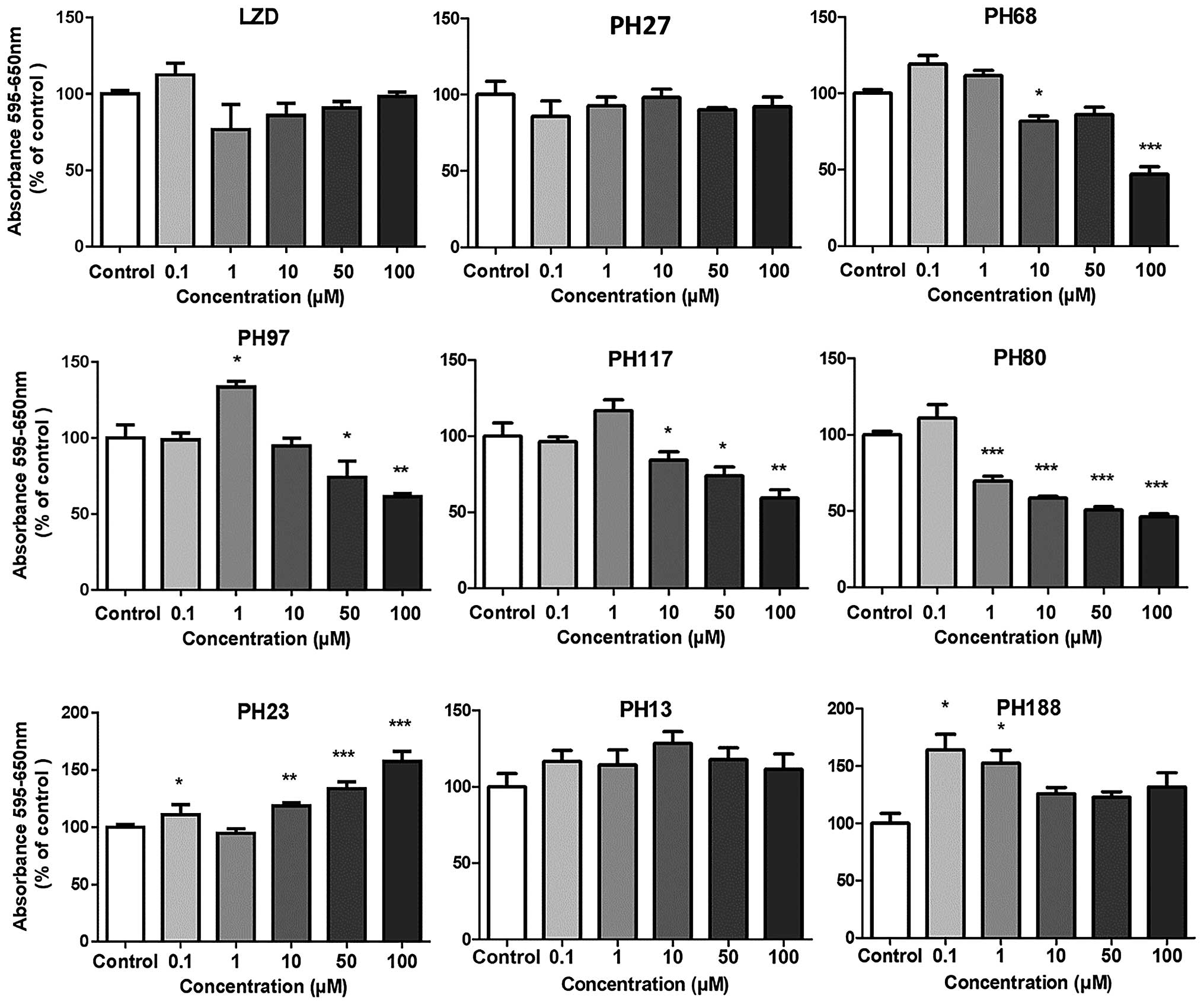
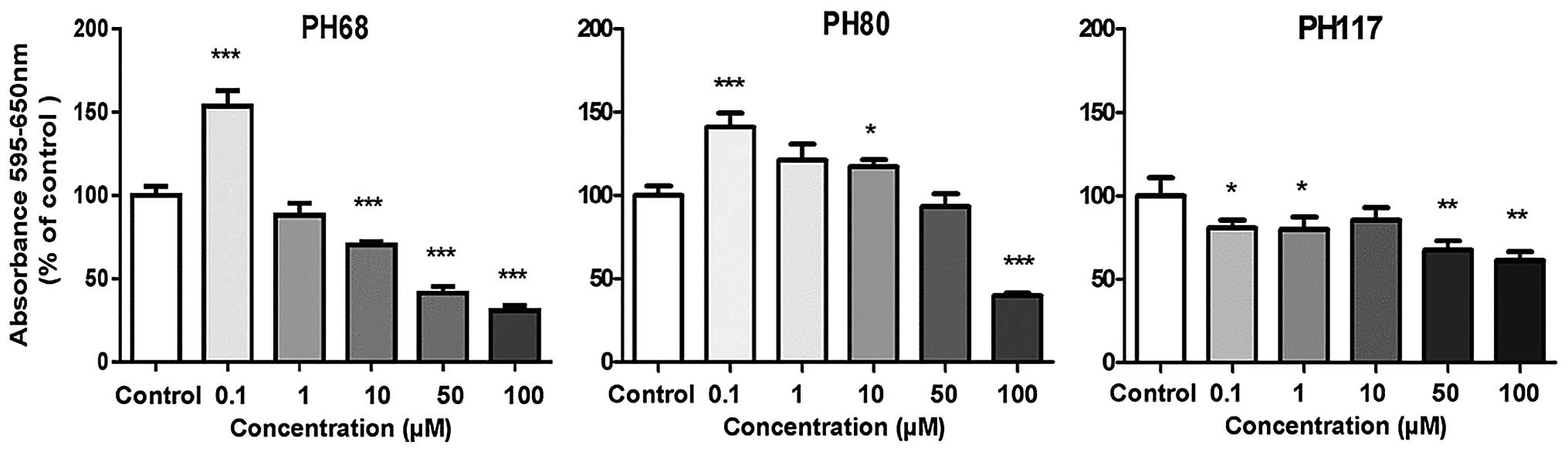
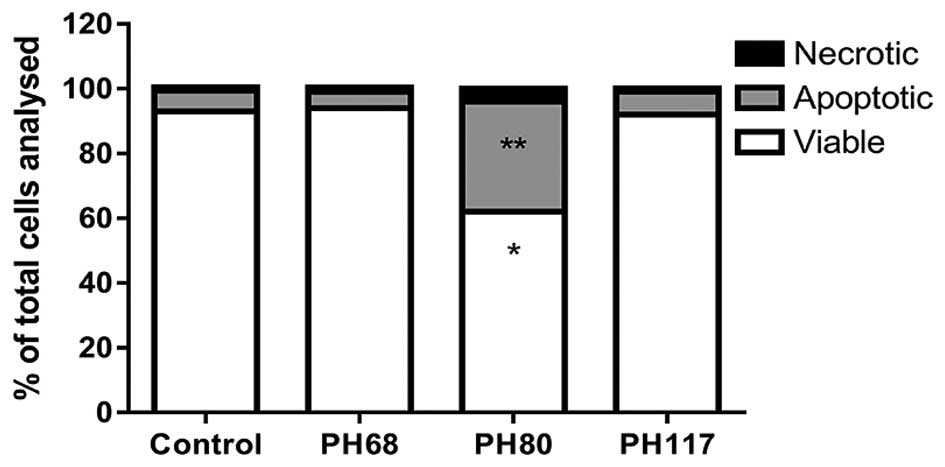
|
1
|
Pandit N, Singla RK and Shrivastava B:
Current updates on oxazolidinone and its significance. Int J Med
Chem. 2012:1592852012.PubMed/NCBI
|
|
2
|
Jiang X, Sun L, Qui JJ, Sun X, Li S, Wang
X, So CW and Dong S: A novel application of furazolidone:
Anti-luekemic activity in acute myeloid leukemia. PLOS ONE.
8:e723352013. View Article : Google Scholar
|
|
3
|
Reck F, Zhou F, Girardot M, Kern G,
Eyermann CJ, Hales NJ, Ramsay RR and Gravestock MB: Identification
of 4-substituted 1,2,3-triazoles as novel oxazolidinone
antibacterial agents with reduced activity against monoamine
oxidase A. J Med Chem. 48:499–506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kombian SB and Phillips OA: In vitro
electrophysiological investigations of the acute effects of
linezolid and novel oxazolidinones on central nervous system
neurons. Neurosci. 180:53–63. 2011. View Article : Google Scholar
|
|
5
|
Sun Y, Tang S, Jin X, Zhang C, Zhao W and
Xiao X: Opposite effects of JNK and p38 MAPK signaling pathways on
furazolidinone-stimulated S phase cell cycle arrest of human
hepatoblastoma cell line. Mutat Res. 775:24–29. 2013. View Article : Google Scholar
|
|
6
|
Auro A, Sumano H, Ocampo L and Barragán A:
Evaluation of the carcinogenic effects of furazolidone and its
metabolites in two fish species. The Pharmacogenomics J. 4:24–28.
2004. View Article : Google Scholar
|
|
7
|
Artico M, De Martino G and Giuliano R:
Research on compounds with antiblastic activity. XL Synthesis of
3-p-(2′, 5′-dimethoxy-4′-(N,
N-bis-(-chloroethyl)-amino)benzylideneamino) phenyl-2-oxazolidinone
(GEA 29; BAY a 5850) and its analogues. Farmaco Sci. 26:771–783.
1971.PubMed/NCBI
|
|
8
|
Devi K, Asmat Y, Agrawal M, Sharma S and
Dwived J: Synthesis and evaluation of some novel precursors of
oxazolidinone analogues of chloroquinoline for their antimicrobial
and cytotoxic potential. J Chem Sci. 125:1093–1101. 2013.
View Article : Google Scholar
|
|
9
|
Naresh A, Venkateswara Rao MV, Kotapalli
SS, Ummanni R and Venkateswara Rao B: Oxazolidinone derivatives:
Cytoxazone-linezolid hybrids induces apoptosis and senescence in
DU145 prostate cancer cell. Eur J Med Chem. 80:295–307. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brickner SJ, Hutchinson DK, Barbachyn MR,
Manninen PR, Ulanowicz DA, Garmon SA, Grega KC, Hendges SK, Toops
DS, Ford CW and Zurenko GE: Synthesis and antibacterial activity of
U-100592 and U-100766, two oxazolidinone antibacterial agents for
the potential treatment of multidrug-resistant gram-positive
bacterial infections. J Med Chem. 39:673–679. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilcox MH: Update on linezolid: The first
oxazolidinone antibiotic. Expert Opin Pharmacother. 6:2315–2326.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eustice DC, Feldman PA, Zajac I and Slee
AM: Mechanism of action of DuP 721: Inhibition of an early event
during initiation of protein synthesis. Antimicrob Agents
Chemother. 32:1218–1222. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalia V, Miglani R, Purnapatre KP, Mathur
T, Singhal S, Khan S, Voleti SR, Upadhyay DJ, Saini KS, Rattan A
and Raj VS: Mode of action of Ranbezolid against staphylococci and
structural modeling studies of its interaction with ribosomes.
Antimicrob Agents Chemother. 53:1427–1433. 2009. View Article : Google Scholar :
|
|
14
|
Zhou CC, Swaney SM, Shinabarger DL and
Stockman BJ: 1H nuclear magnetic resonance study of oxazolidinone
binding to bacterial ribosomes. Antimicrob Agents Chemother.
46:625–629. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ippolito JA, Kanyo ZF, Wang D, Franceschi
FJ, Moore PB, Steitz TA and Duffy EM: Crystal structure of the
oxazolidinone antibiotic linezolid bound to the 50S ribosomal
subunit. J Med Chem. 51:3353–3356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McKee EE, Ferguson M, Bentley AT and Marks
TA: Inhibition of mammalian mitochondrial protein synthesis by
oxazolidinones. Antimicrob Agents Chemother. 50:2042–2049. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Vriese AS, Coster RV, Smet J, Seneca S,
Lovering A, Van Haute LL, Vanopdenbosch LJ, Martin JJ, Groote CC,
Vandecasteele S and Boelaert JR: Linezolid-Induced Inhibition of
Mitochondrial Protein Synthesis. Clin Infect Dis. 42:1111–1117.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuter DJ and Tillotson GS: Hematologic
effects of antimicrobials: Focus on the oxazolidinone linezolid.
Pharmacotherapy. 21:1010–1013. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerson SL, Kaplan SL, Bruss JB, Le V,
Arellano FM, Hafkin B and Kuter DJ: Hematologic effects of
linezolid: Summary of clinical experience. Antimicrob Agents
Chemother. 46:2723–2726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leach KL, Brickner SJ, Noe MC and Miller
PF: Linezolid, the first oxazolidinone antibacterial agent. Ann N Y
Acad Sci. 1222:49–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Corallo CE and Paull AE: Linezolid-induced
neuropathy. Med J Aust. 177:3322002.PubMed/NCBI
|
|
22
|
Rucker JC, Hamilton SR, Bardenstein D,
Isada CM and Lee MS: Linezolid-associated toxic optic neuropathy.
Neurology. 66:595–598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kraleti S and Soultanova I: Pancytopenia
and lactic acidosis associated with linezolid use in a patient with
empyema. J Ark Med Soc. 110:62–63. 2013.PubMed/NCBI
|
|
24
|
Narita M, Tsuji BT and Yu VL:
Linezolid-associated peripheral and optic neuropathy, lactic
acidosis and serotonin syndrome. Pharmacotherapy. 27:1189–1197.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su E, Crowley K, Carcillo JA and Michaels
MG: Linezolid and lactic acidosis: A role for lactate monitoring
with long-term linezolid use in children. Pediatr Infect Dis J.
30:804–806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Phillips OA, Udo EE, Ali AA and Al-Hassawi
N: Synthesis and antibacterial activity of 5-substituted
oxazolidinones. Bioorg Med Chem. 11:35–41. 2003. View Article : Google Scholar
|
|
27
|
Phillips OA, Udo EE, Ali AAM and Samuel
SM: Structure-antibacterial activity of arylcarbonyl- and
arylsulfonyl-piperazine 5-triazolylmethyl oxazolidinones. Eur J Med
Chem. 42:214–225. 2007. View Article : Google Scholar
|
|
28
|
Phillips OA, Udo EE, Abdel-Hamid ME and
Varghese R: Synthesis and antibacterial activities of
N-substituted-gylcinyl 1H-1,2,3-triazolyl oxazolidinones. Eur J Med
Chem. 66:246–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
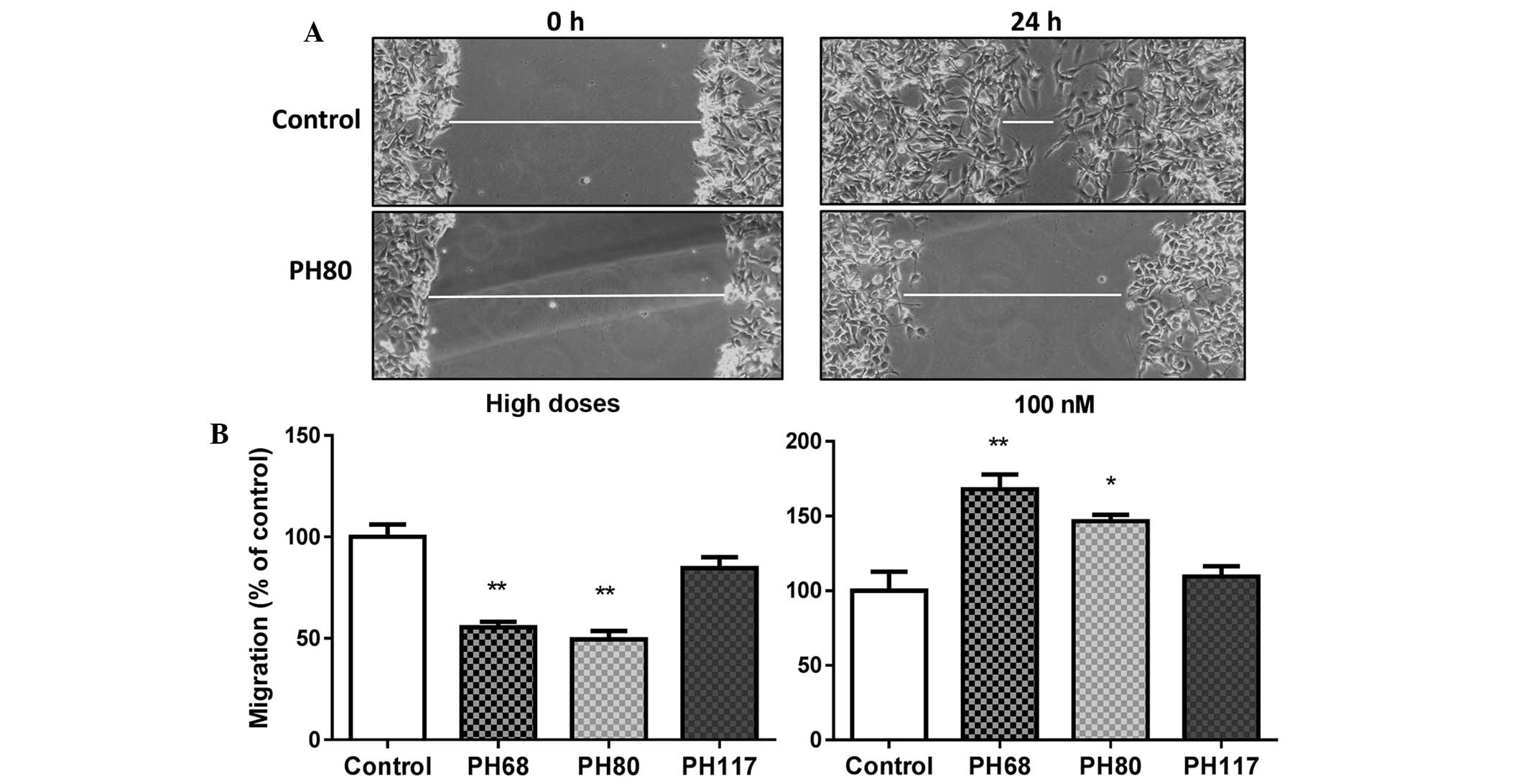
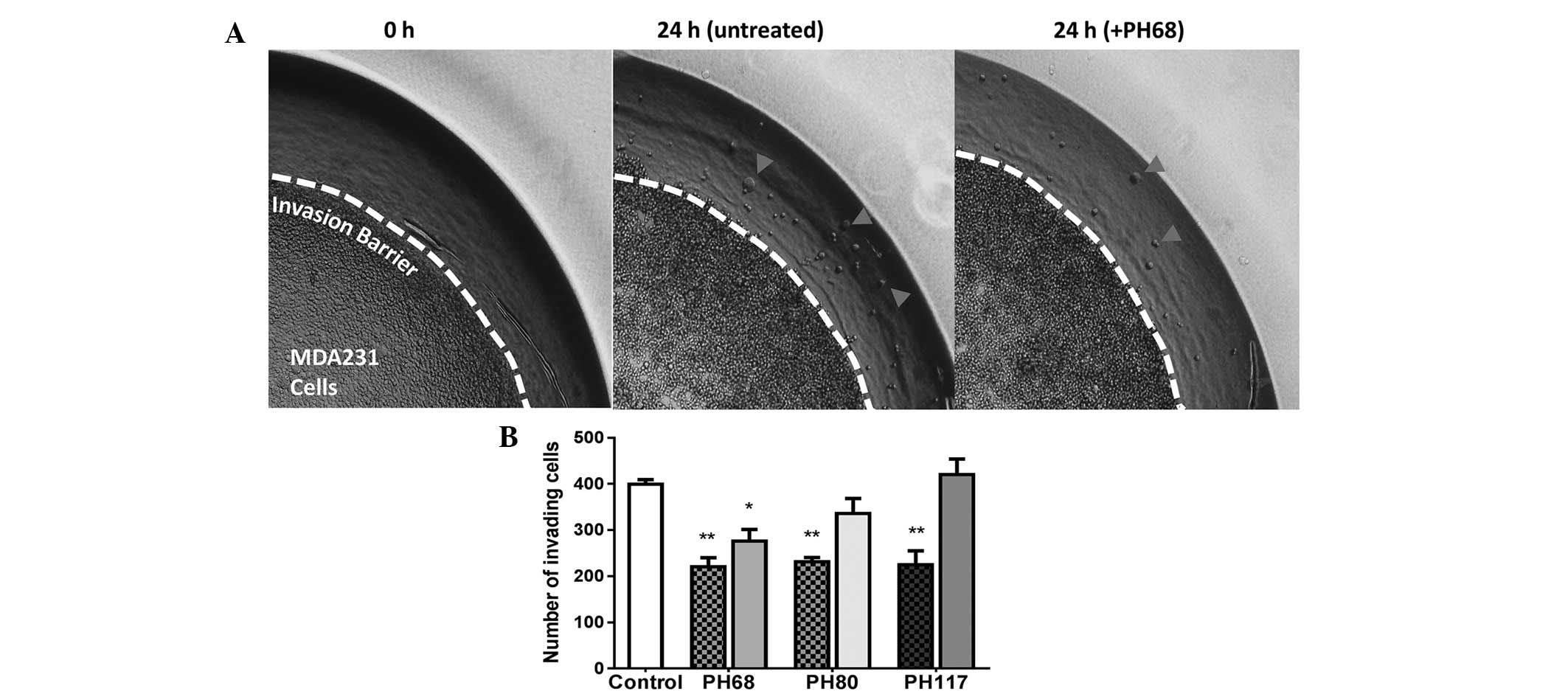
Khajah MA, Al Saleh S, Mathew PM and
Luqmani YA: Differential Effect of Growth Factors on Invasion and
Proliferation of endocrine resistant breast cancer cells. PLoS One.
7:e418472012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang C, Park A and Guan J: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoco. 2:329–333. 2007. View Article : Google Scholar
|
|
31
|
Calabrese EJ and Blain R: The occurrence
of hormetic dose responses in the toxicological literature, the
hormesis database: An overview. Toxicol Appl Pharmacol.
202:289–301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nascarella M, Stanek E, Hoffmann GR and
Calabrese EJ: Quantification of hormesis in anticancer-agent
dose-responses. Dose-Response. 72:160–171. 2009.
|
|
33
|
Wang Y, Gray JP, Mishin V, Heck DE, Laskin
DL and Laskin JD: Role of cytochrome P450 reductase in
nitrofurantoin-induced redox cycling and cytotoxicity. Free Radical
Bio Med. 44:1169–1179. 2008. View Article : Google Scholar
|