Introduction
One of the greatest challenges in tendon healing is
adhesion formation, caused by tissue scarring around the injured
tendon sheath, resulting in limited finger functions (1). Despite improvements in materials and
surgical techniques, various pharmacological modalities and the
evolution of rehabilitative therapies, the postoperative outcome of
flexor tendon healing remains limited by flexor tendon adhesion. In
particular, peritendinous adhesion formation continues to present a
problem. Tendon healing often causes disorganization of the
extracellular matrix (ECM) and the formation of scar tissue
predominantly composed of dense collagenous fibers. Healed tendons
often have inferior mechanical properties compared with undamaged
tendons. Therefore, restoration of function and structure of
injured tendons is an important problem in hand surgery (2).
Injured tendon healing relies on the levels of
growth factors and cytokines to ensure that cellular responses are
mediated in an appropriate manner. Among the numerous growth
factors involved, transforming growth factor β (TGF-β) is suggested
to be key in tendon healing. TGF-βs are a family of a number of
isoforms, which have multiple roles in tissue morphogenesis and
cell proliferation (3). The TGF-β
isoforms have different effects during wound healing and scarring.
TGF-β1 is found at high levels in the wound microenvironment and
promotes myofibroblast differentiation, production of ECM
components and fibroblast chemotaxis. In addition, TGF-β1 promotes
the formation of scar tissue during adult wound healing. However,
embryonic wound microenvironments contain low levels of TGF-β1 and
high levels of TGF-β3 (4).
Furthermore, the addition of exogenous TGF-β3 to an adult wound
promotes scar-free healing in rats (5), and injuries obtained in utero
heal scar free, possibly due to the relatively high levels of
TGF-β3 compared with TGF-β1. In addition, developmental research in
chickens has shown that the TGF-β3 isoform is existent throughout
the morphogenesis of tendon tissue (6).
The role of TGF-β in wound healing has been well
demonstrated and numerous cellular and molecular mechanisms
underlying the TGF-β/Smad signaling pathway have been identified;
however, the intracellular mechanisms and downstream signals of
TGF-β in wound healing are poorly understood. Targeting the
inhibition of TGF-β signaling pathway using therapeutic agents to
reduce scarring and improve wound healing has been successful in
pre-clinical studies (7,8). Despite these studies, the
intracellular mechanism and downstream signals by which TGF-β3
adjusts these effects in healing tendons are poorly understood. A
recent study has demonstrated that Smad proteins act as key
transcription factors for the TGF-β signaling pathway (9). There are three groups of Smad
proteins: Receptor activated Smads, common mediator Smads and
inhibitory Smads (10). Smad3 is a
receptor activated Smad and is phosphorylated in response to TGF-β
signaling through the TGF-β type I and TGF-β type II transmembrane
receptors (11). Once activated,
Smad3 heterodimerizes with Smad4, which is a common mediator Smad,
and translocates to the nucleus where Smad3 is hypothesized to
modulate the transcription of genes involved in cell growth
(12), inflammatory responses
(13) and the formation of the ECM
(14). Inhibitory Smad proteins,
including Smad6 and Smad7, inhibit the phosphorylation of receptor
Smad proteins via TGF-β3 receptors, and prevent the association of
receptor Smad proteins with Smad4.
The aim of this study was to determine the influence
of TGF-β3 in regulating the expression of Smad proteins in
tenocytes, and to investigate the underlying TGF-β/Smad signaling
pathway.
Materials and methods
Chemicals and reagents
TGF-β3 was purchased from Abcam (Cambridge, UK).
Trypsin-EDTA and Dulbecco's modified Eagle's medium (DMEM) were
obtained from Gibco; Thermo Fisher Scientific Inc. (Waltham, MA,
USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and
fetal bovine serum (FBS) was purchased from HyClone (Logan, UT,
USA). All other analytical grade chemicals were received from
typical commercial sources in China.
Tenocyte culture
Tendon tissue was obtained under sterile conditions
from the flexor tendon of New Zealand white rabbits (n=3; age, 12
weeks; weight, 2 kg) obtained from the Experimental Animal Center
of The Third Military Medical University (Chongqing, China).
Animals were maintained in a room with a constant temperature
(22±1°C), relative humidity (40–60%) with a 12 h light/dark cycle.
The study was approved by the ethics Animal Health Trust Research
Ethics Committee of The Third Military Medical University. Rabbits
were sacrificed by anaesthetic injection of 3% pentobarbital sodium
(1 ml/kg; Merck & Co., Inc., Kenilworth, NJ, USA) and venous
air embolism. Three experimental repeats were performed using one
line of tendon cells from each animal. The tendon tissue was rinsed
with phosphate-buffered saline (PBS) separately 3 times, cut into
1-mm3 sections and then placed into sterile containers.
The sections were transferred into 0.1% trypsin-DMEM. Mixtures were
then placed on a shaker and digested by 0.1% trypsin-DMEM
(Sigma-Aldrich) for 30 min, followed by centrifugation at 44.72 × g
for 5 min at 4°C. The supernatant was then discarded and the
resulting cell pellets were resuspended in 0.1% collagenase II
(Sigma-Aldrich) with DMEM containing 10% FBS for 2 h at 37°C.
Tenocytes were then centrifuged at 44.72 × g for 5 min at 4°C and
washed twice with Hank's Buffer solution (Sigma-Aldrich), and then
filtered through a 70 μm nylon sterile filter to remove
debris. The samples were centrifuged at 44.72 × g for 5 min at 4°C
to obtain tenocyte pellets and resuspended in a sterile culture
bottle in 5 ml DMEM/F12 containing 10% FBS, 100 mg/ml streptomycin
and 100 U/ml penicillin (Gibco; Thermo Fisher Scientific, Inc.).
Samples were kept at 37°C in an incubator (Heal Force Bio-meditech
Holdings Ltd.; Shanghai, China; HF90, CO2 Jacket
Incubator) throughout the digestion process. Culture medium was
checked and changed every third day until tenocytes were confluent
(2×106 cells/cm2). Adherent tenocytes
received short-term treatment with 0.25% trypsin in 0.2% EDTA. The
growth of tenocytes was monitored under a microscope (Olympus 1X71;
Olympus Corporation, Tokyo, Japan). Tenocytes were treated with, or
without, TGF-β3 (10 ng/ml) for 1, 2, 4, 6 and 8 h. To evaluate the
effect of blocking intracellular protein and mRNA synthesis on the
expression of Smad7 and Smad3 induced by TGF-β3, cycloheximide
(CHX; Sigma-Aldrich) or actinomycin D (Sigma-Aldrich) were added to
tenocytes 2 h prior to the addition of TGF-β3 for 4 h.
Immunohistochemistry assessment
Tenocytes were seeded onto sterile cover glass at a
density of 2×106 cells/ml, and then incubated for 3
days. The cover glass was removed and washed with PBS. The
tenocytes were fixed with 4% paraformaldehyde (Aladdin Reagent Co.,
Shanghai, China) for 20 min and washed 3 times for 2 min with PBS.
Cells were then treated with 0.5% Triton X-100 (Aladdin Reagent
Co.) for 20 min and washed with PBS 3 times for 2 min. Then, 3%
H2O2 was applied for 30 min at room
temperature, and washed with PBS 3 times for 2 min. After blocking
for at least 1 h at room temperature, tenocytes were incubated with
1:100 rabbit polyclonal anti-collagen I (bs-10423R) and rabbit
polyclonal anti-collagen III primary antibodies (bs-0549R) (both
purchased from Beijing Biosynthesis Biotechnology Co., Ltd.,
Beijing, China) at 4°C overnight and washed with PBS 3 times for 2
min. Sections were then incubated with 1:100 horseradish peroxidase
(HRP)-conjugated goat polyclonal anti-rabbit immunoglobulin G (IgG;
bs-0295G-HRP; Beijing Biosynthesis Biotechnology Co., Ltd.)
secondary antibody for at least 1 h at room temperature. The cover
glass was washed with PBS three times for 10 min. The signal was
developed using a HRP-DAB detection kit (Beijing Biosynthesis
Biotechnology Co., Ltd.) and washed with water, counterstained with
Harris hematoxylin (Beijing Biosynthesis Biotechnology Co., Ltd.)
and washed again. Immunoreactivity was then observed under a
microscope (Olympus 1X71; Olympus Corporation). The negative
control group was treated with PBS. All the steps were the same as
in the positive control group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cultured tenocytes
using TRIzol reagent (Invitrogen Life Technologies, Grand Island,
NY, USA) and quantified using spectrophotometry at 260 nm
(Eppendorf BioSpectrometer; Eppendorf AG, Hamburg, Germany). After
isolation, 2 μg total RNA was reverse transcribed using the
HiFi-MMLV cDNA kit (Beijing CoWin Biotech Co. Ltd., Beijing, China)
according to the manufacturer's protocol. The primer sequences for
Smad3, Smad7 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
are shown in Table I. Primers were
purchased from Shanghai Generay Biotech Co., Ltd. (Shanghai,
China). According to the manufacturer's protocol, qPCR was
performed with an SYBR Premix Ex Taq [Takara Biotechnology (Dalian)
Co., Ltd., Dalian, China]. All qPCR reactions were performed using
the ABI PRISM 7700 sequence detection system (Applied Biosystems,
Grand Island, NY, USA). In each reaction, 1 μl cDNA, 10
μl SYBR Premix Ex Taq (Takara Biotechnology Inc., Dalian,
China), and 0.4 μM forward and reverse primer in a total
volume of 20 μl were used. The reaction conditions were as
follows: 1 cycle at 95°C for 30 sec followed by 40 cycles at 95°C
for 5 sec, and 60–66°C for 30 sec. qPCR for each sample was run in
triplicate. β-actin was used as an internal control, and all
results were analyzed using the standard 2−ΔΔCq method
described previously (15).
 | Table ISequences of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| Smad3 |
AGGTCTTCGCAGAGTGCCTCA |
GGGTCAACTGGTAGACAGCCTCA |
| Smad7 |
CCATCACCTTAGCCGACTCTG |
CCATCGGGTATCTGG-AGTAAGGA |
| GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
Western blot analysis
At the end, tenocytes culture medium was aspirated
and tenocytes were detached by scrapping in PBS. Detached cells
were centrifuged at 21,000 × g at 4°C for 5 min. Cell pellets were
then lysed in 300 μl lysis buffer (Cytobuster protein
extraction reagent; Beijing Biosynthesis Biotechnology Co., Ltd.)
with 1 mM Na3VO4, 25 mM NaF and 1X protease
inhibitor cocktail (Beijing Biosynthesis Biotechnology Co., Ltd.).
Protein concentrations were quantified by spectrophotometry
(Eppendorf BioSpectrometer, Eppendorf, Hamburg, Germany). For
western blot analysis, equal quantities of protein were loaded onto
12% SDS-PAGE gels (Beijing Biosynthesis Biotechnology Co., Ltd.)
and electrotransferred onto polyvinylidne difluoride (PVDF)
membranes (Millipore, Bedford, MA, USA). The membranes were then
blocked with 5% (w/v) bovine serum albumin in TBST [10 mM Tris, 150
mM NaCl, and 0.1% (v/v) Tween 20, pH=7.5] for 1 h at room
temperature, and incubated with 1:500 rabbit polyclonal anti-Smad3
(sc-8332), anti-Smad7 (sc-11392), anti-p-Smad3 (sc-130218) and
anti-GAPDH (sc-59540) primary antibodies overnight at 4°C. The
membranes were then incubated with 1:500 HRP-conjugated goat
polyclonal anti-rabbit IgG (sc-2004) secondary antibody at room
temperature for 2 h (Santa Cruz Biotechnology, Inc. Santa Cruz, CA,
USA). Enhanced chemiluminescence (Beyotime Institute of
Biotechnology, Shanghai, China) was used to observe immunoreactive
protein signals. Protein signals were then visualized on films
(Beijing Biosynthesis Biotechnology Co., Ltd.), and scanned and
quantified using the Image J software (version 1.48; National
Institutes of Health, Bethesda, MA, USA). For re-probing, PVDF
membranes were stripped with 0.2 M NaOH for 10 min prior to
blocking with another primary antibody. The expression of molecules
of interest was determined relative to β-actin.
CHX and actinomycin D
CHX was added to block intracellular protein
synthesis. In each group, 2 ml CHX (10 μg/ml) was added to
pre-process tenocytes 2 h prior to the addition of TGF-β3.
Actinomycin D was added to block intracellular mRNA synthesis. In
each group, 2 ml actinomycin D (5 pg/ml) was added to pre-process
tenocytes 2 h prior to the addition of TGF-β3. Total RNA was
isolated for RT-qPCR and statistical analysis as mentioned
above.
Statistical analysis
Data are expressed as the mean ± standard deviation
for three or more independent experiments. Significant differences
were determined using factorial analysis of variance. Statistical
analysis was performed using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Tenocyte culture and immunohistochemistry
assessment
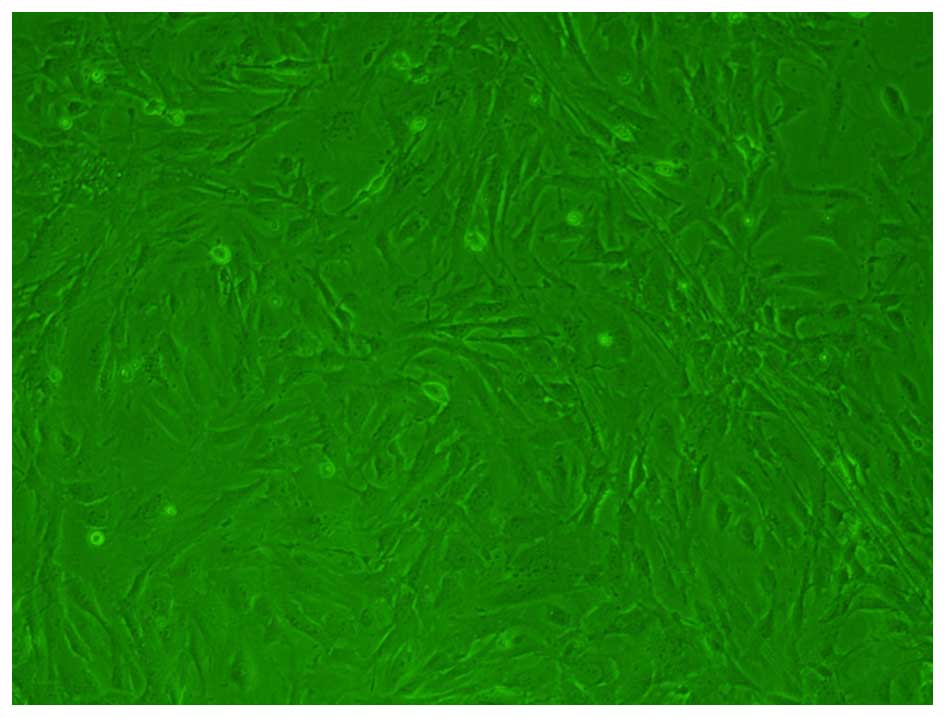
Tenocytes were isolated and cultured, and following
microscopic analysis were observed to be multi-angle shaped or
spindle-shaped (Fig. 1).
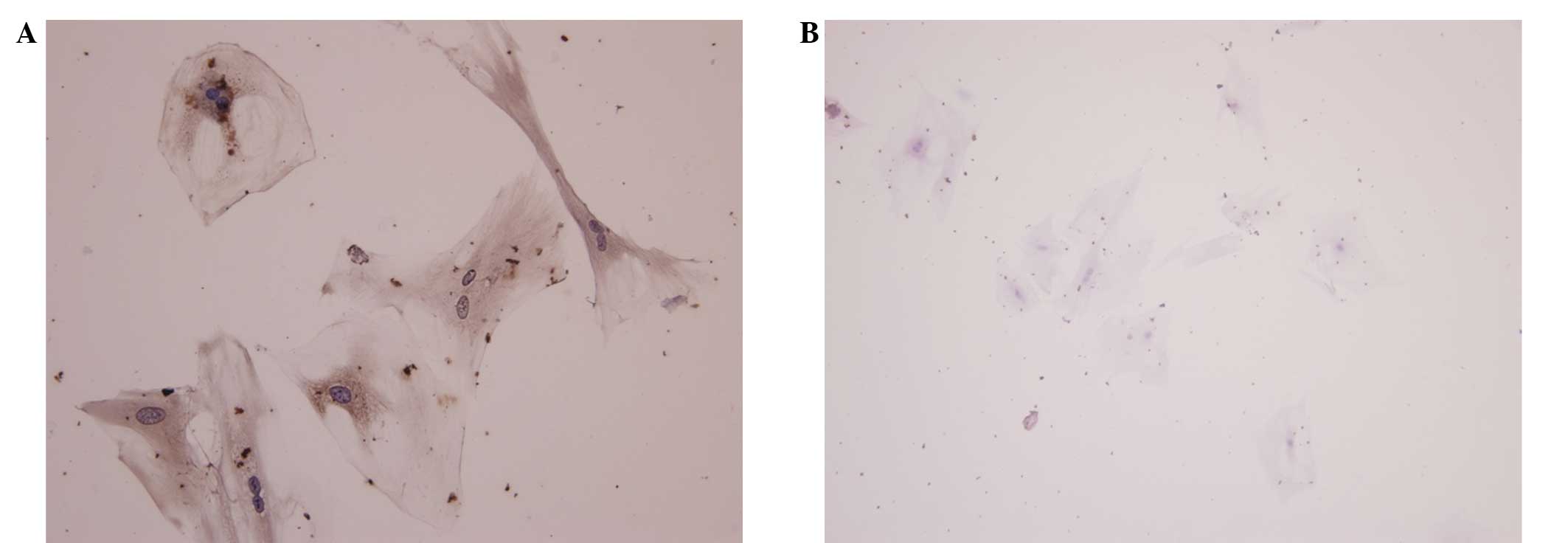
Immunohistochemical analysis was performed to distinguish between
tenocytes and fibroblasts. Immunohistochemical assessment
demonstrated that fibroblasts positively stained for collagen I,
and stained negaitvely for collagen III (Fig. 2). This demonstrated that the cells
were tenocytes.
RT-qPCR and western blot analysis
results
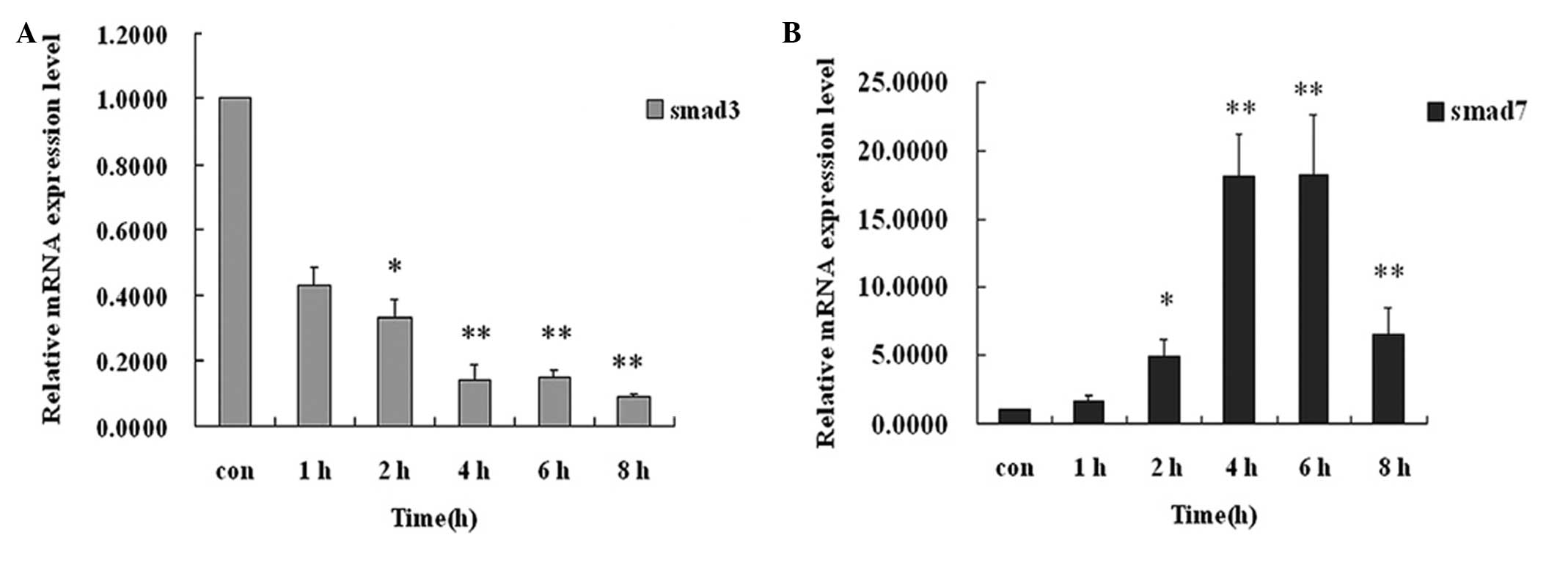
Tenocytes were treated with, or without TGF-β3 (10
ng/ml) for 1, 2, 4, 6 and 8 h (mRNA was detected with each 0.5 h).
Addition of TGF-β3 (10 ng/ml) can significantly downregulate the
expression of Smad3 mRNA and upregulate the expression of Smad7
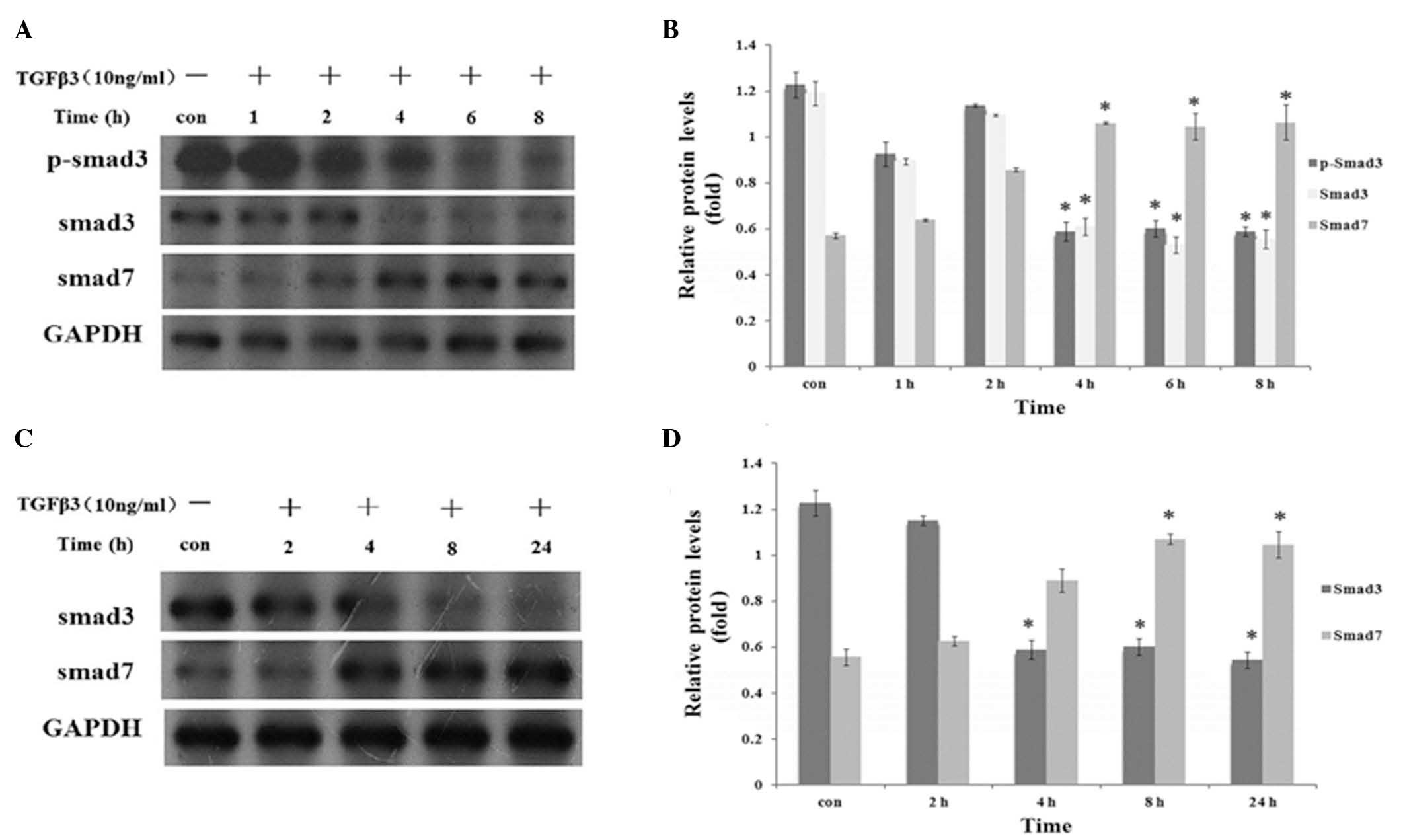
mRNA (P<0.01; Fig. 3). After 4
h, treatment with TGF-β3 (10 ng/ml) was observed to significantly
downregulate the expression of phosphorylated and
non-phosphorylated Smad3 protein and upregulate the expression of
Smad7 protein (P<0.01; Fig.
4).
Addition of CHX and actinomycin D
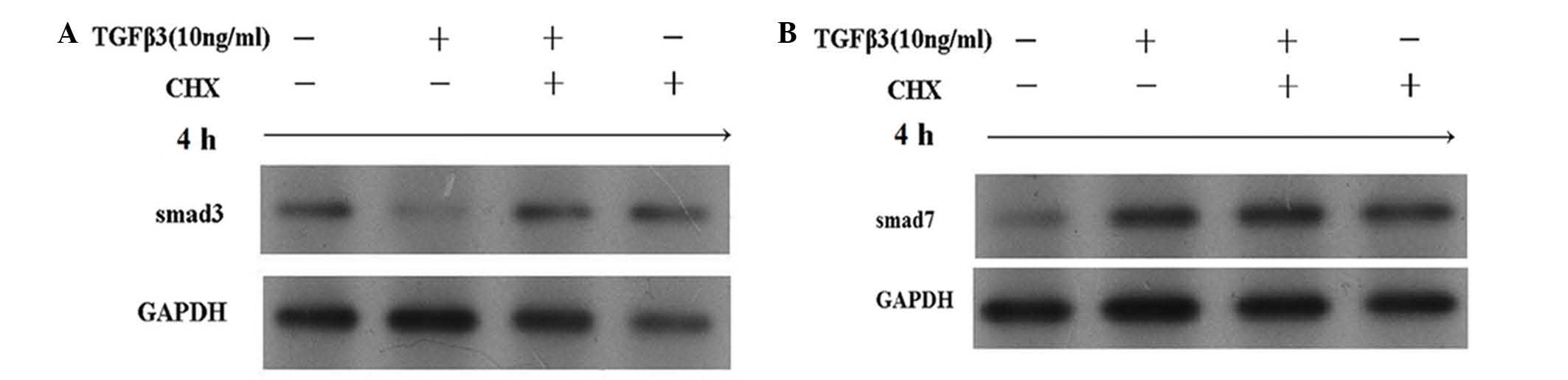
Addition of CHX inhibited the downregulation of
Smad3 protein induced by TGF-β3; however, it was not able to block
the TGF-β3-induced upregulation of Smad7 (Fig. 5). Furthermore, the addition of
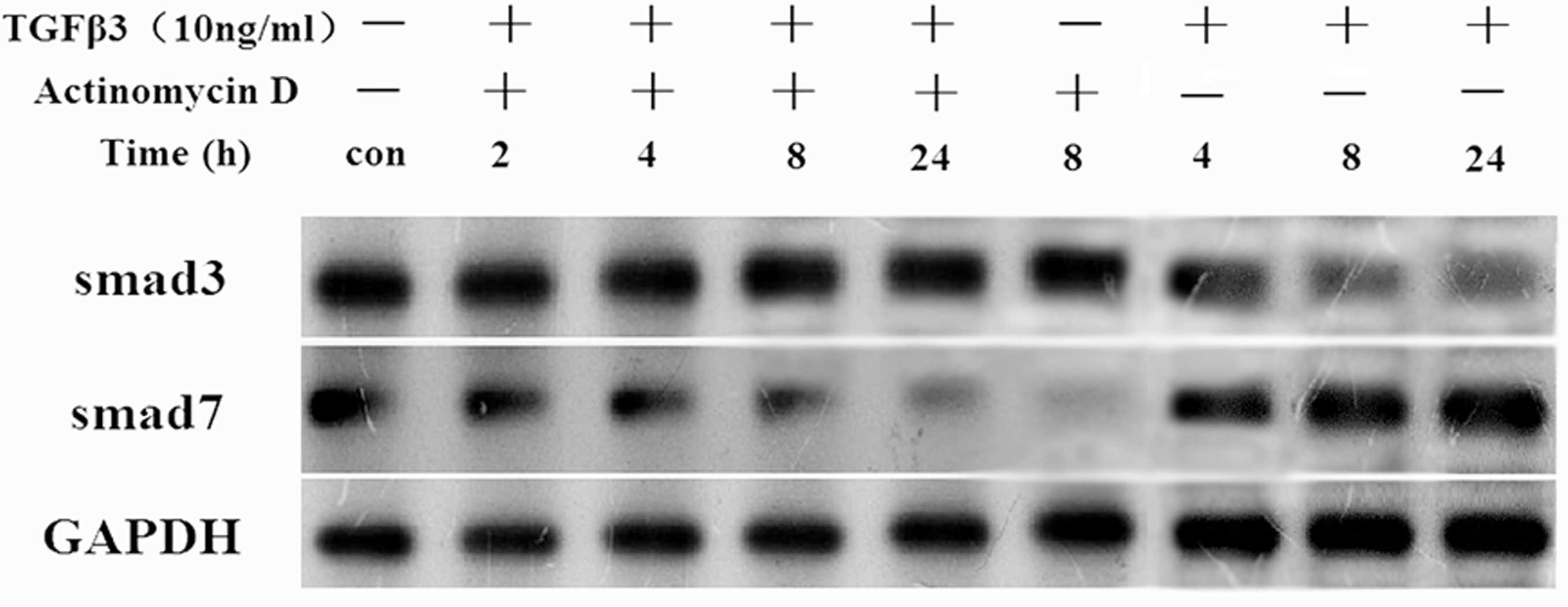
actinomycin D inhibited the TGFβ3-induced downregulation of Smad3
protein, and inhibited the TGFβ3-induced upregulation of Smad7 in
tenocytes (Fig. 6). The results
show that addition of TGF-β3 can significantly downregulate the
expression of Smad3 protein and upregulate the expression of Smad7
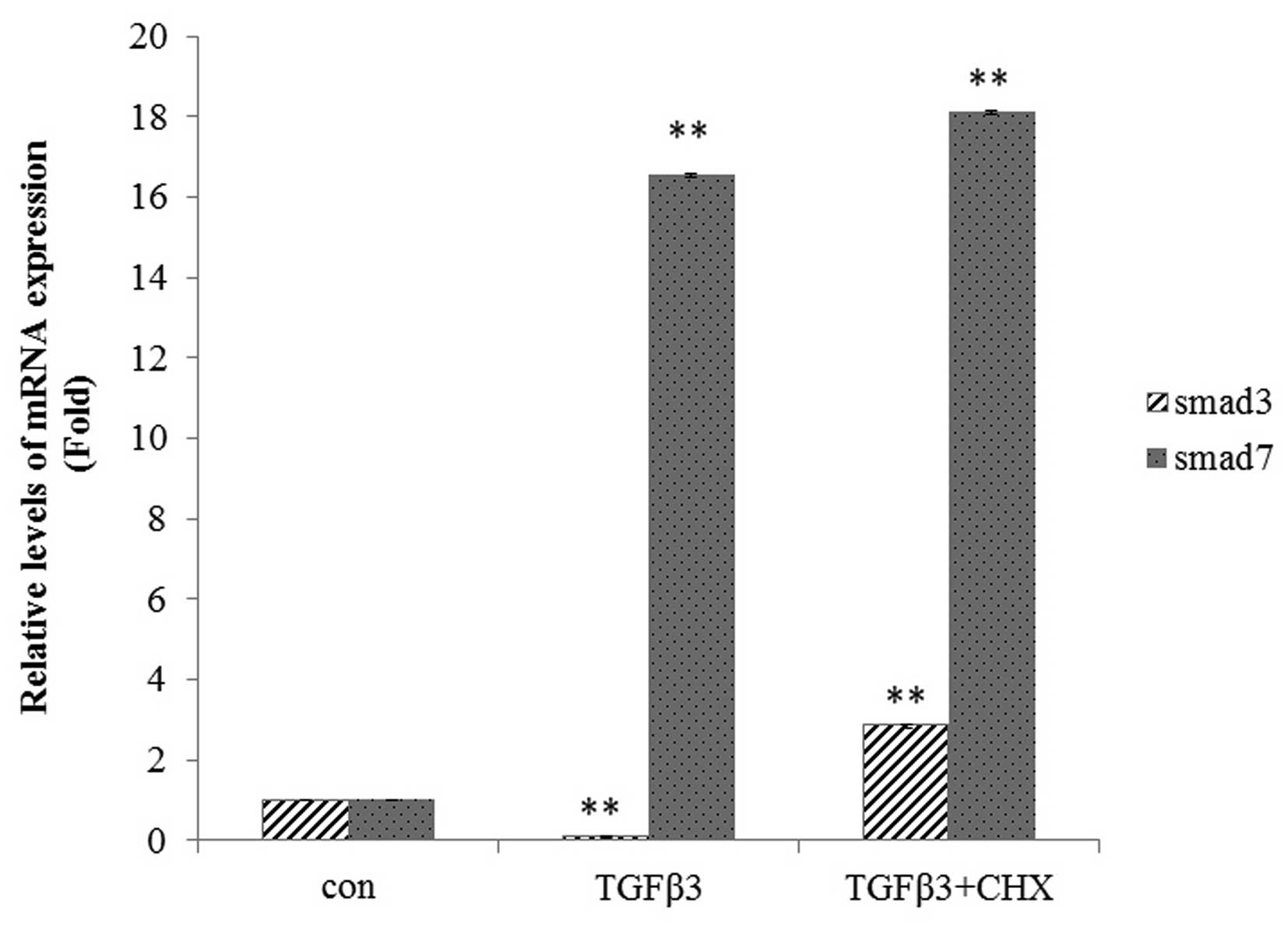
protein. The addition of CHX was shown to inhibit the
downregulation of Smad3 mRNA and protein expression in tenocytes
(P<0.01; Fig. 7), but not
affect Smad7 mRNA or protein expression (Fig. 7).
Discussion
The postoperative outcome of injured flexor tendon
healing remains limited by tendon adhesion. Despite improvements in
materials and surgical technique, various pharmacological
modalities and the evolution of rehabilitative therapies, tendon
adhesion remains one of the most unpredictable results of flexor
tendon injury. In particular, peritendinous adhesion formation
continues to present a problem. The aims of tendon healing after
repair are to promote intrinsic tendon healing and to optimize the
tendon range of motion (16). In
recent years, studies have shown TGF-βs is regulated after tendon
injury (17–19). Addition of TGF-β3 induces tenogenic
differentiation of in vitro cultures of mesenchymal stem
cells (20,21), equine embryo-derived stem cells
(17) and micro mass cultures of
undifferentiated limb mesenchyme (22). This suggests that TGF-β signaling
is important in triggering the initiation of tenocyte
differentiation from tendon progenitors. Although the role of TGF-β
in fibrosis has been determined, little is known regarding the
circulating levels of this cytokine in the injured tendon. In
addition, the role of the TGF-β/Smad signaling pathway in tenocyte
differentiation remains largely unknown.
TGF-β3, an isoform of the TGF-β superfamily,
initiates cellular actions by binding to specific cell-surface
receptor complexes typically composed of TGF-β type I (TβRI) and II
receptors. Binding of TGF-β to TβRII activates the intrinsic
serine/threonine kinase activity of TβRI, which phosphorylates
transcription factors Smad2 and Smad3. p-Smad2 or p-Smad3 combine
with Smad4, then the Smad2/Smad4 and/or Smad3/Smad4 complexes
translocate into the nucleus, where they function to regulate the
transcription of specific genes that possess TGF-β response
elements in their promoters (23,24).
Smad3 knockout mice have been shown to exhibit reduced collagen
levels and decreased scarring, as well as increased MMP9 gene and
protein expression (7). TGF-β3 is
antagonized by Smad7, which interacts stably with TβRI to prevent
phosphorylation and activation of receptor-regulated Smad2/3,
therefore inhibiting TGF-β signaling (25).
Previous studies have established a role for TGF-β
signaling in tendon healing by demonstrating that inhibition of
neutralization of TGF-β1 and β2, or the addition of TGF-β3 can
decrease adhesion formation (8,26,27).
A novel TGF-β3 controlled-released chitosan scaffold has been
developed for tissue engineering of the synovial sheath (28). The present study focused on the
effect of TGF-β3, and the underlying the TGF-β/Smad signaling
pathway on wound healing.
In the present study, Smad3 increased collagen
levels and decreased MMP9 gene and protein expression. Smad7
inhibited the phosphorylation of receptor Smads via the TGF-β3
receptors and prevented the association of receptor Smads with
Smad4. If TGF-β3 is able to dowregulate the expression of Smad3
protein expression and upregulate the expression of Smad7 protein,
it may be used to promote intrinsic tendon healing and reduce
adhesion. RT-qPCR and western blot analysis revealed that TGF-β3
downregulated the expression of Smad3 mRNA and upregulated the
expression of Smad7 mRNA. Adding CHX and actinomycin D demonstrated
that TGF-β3 can significantly downregulate the expression of Smad3
protein and upregulate the expression of Smad7 protein. These
results supported the above speculation. Thus, the present study
provides important information regarding the specific role of the
canonical TGF-β signaling pathway in the process of reducing
adhesion formation. The results revealed that adding TGF-β3 is an
effective way to promote intrinsic tendon healing, and result in
optimized a tendons range of motion.
In conclusion, the present study demonstrates that
the addition of TGF-β3 to tenocytes can significantly downregulate
the expression of Smad3, and upregulate the expression of Smad7, at
the gene and protein level. The results suggest that TGF-β3 may
regulate Smad3 and Smad7 protein expression through the TGF-β/Smad
signaling pathway to minimize extrinsic scarring. Thus, it may
provide a novel approach to decrease tendon adhesion and promote
tendon healing. In vivo studies are required in order to
provide further evidence of the potential benefits in clinical
practice. In the future, the delivery of TGF-β3 may be a novel
application in the field of hand flexor tendon surgery.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81000807).
References
|
1
|
Wong JK, Lui YH, Kapacee Z, Kadler KE,
Ferguson MW and McGrouther DA: The cellular biology of flexor
tendon adhesion formation: An old problem in a new paradigm. Am J
Pathol. 175:1938–1951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Renstrom P, Woo SL-Y and Arnoczky SP:
Tendinopathy: A major medical problem in sport. Tendinopathy in
Athletes. Blackwell Publishing; Hoboken, NJ: pp. 1–9. 2007,
View Article : Google Scholar
|
|
3
|
Cox DA: Transforming growth factor-beta 3.
Cell Biol Int. 19:357–371. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferguson MW and O'Kane S: Scar-free
healing: From embryonic mechanisms to adult therapeutic
intervention. Philos Trans R Soc Lond B Biol Sci. 359:839–850.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shah M, Foreman DM and Ferguson MW:
Neutralisation of TGF-beta1 and TGF-beta2 or exogenous addition of
TGF-b3 to cutaneous rat wounds reduces scarring. J Cell Sci.
108:985–1002. 1995.
|
|
6
|
Kuo CK, Petersen BC and Tuan RS:
Spatiotemporal protein distribution of TGF-betas, their receptors
and extracellular matrix molecules during embryonic tendon
development. Dev Dyn. 237:1477–1489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katzel EB, Wolenski M, Loiselle AE, Basile
P, Flick LM, Langstein HN, Hilton MJ, Awad HA, Hammert WC and
O'Keefe RJ: Impact of Smad3 loss of function on scarring and
adhesion formation during tendon healing. J Orthop Res. 29:684–693.
2011. View Article : Google Scholar :
|
|
8
|
Bates SJ, Morrow E, Zhang AY, Pham H,
Longaker MT and Chang J: Mannose-6-phosphate, an inhibitor of
transforming growth factor-beta, improves range of motion after
flexor tendon repair. J Bone Joint Surg Am. 88:2465–2472. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kretzschmar M and Massagué J: Smads:
Mediators and regulators of TGF-beta signaling. Curr Opin Genet
Dev. 8:103–111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown KA, Pietenpol JA and Moses HL: A
tale of two proteins: Differential roles and regulation of Smad2
and Smad3 in TGF-beta signaling. J Cell Biochem. 101:9–33. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moustakas A, Pardali K, Gaal A and Heldin
CH: Mechanisms of TGF-beta signaling in regulation of cell growth
and differentiation. Immunol Lett. 82:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dennler S, Goumans MJ and ten Dijke P:
Transforming growth factor beta signal transduction. J Leukoc Biol.
71:731–740. 2002.PubMed/NCBI
|
|
14
|
Arany PR, Flanders KC, Kobayashi T, Kuo
CK, Stuelten C, Desai KV, Tuan R, Rennard SI and Roberts AB: Smad3
deficiency alters key structural elements of the extracellular
matrix and mechanotransduction of wound closure. Proc Natl Acad Sci
USA. 103:9250–9255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Elliot D, Barbieri CH, Evans RB, Mass D
and Tang JB: IFSSH flexor tendon committee report 2007. J Hand Surg
Eur Vol. 32:346–356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barsby T and Guest D: Transforming growth
factor beta3 promotes tendon differentiation of equine
embryo-derived stem cells. Tissue Eng Part A. 19:2156–2165. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan KM, Fu SC, Wong YP, Hui WC, Cheuk YC
and Wong MW: Expression of transforming growth factor beta isoforms
and their roles in tendon healing. Wound Repair Regen. 16:399–407.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CH, Cao Y, Wu YF, Bais AJ, Gao JS and
Tang JB: Tendon healing in vivo: Gene expression and production of
multiple growth factors in early tendon healing period. J Hand Surg
Am. 33:1834–1842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kapacee Z, Yeung CY, Lu Y, Crabtree D,
Holmes DF and Kadler KE: Synthesis of embryonic tendon-like tissue
by human marrow stromal/mesenchymal stem cells requires a
three-dimensional environment and transforming growth factor β3.
Matrix Biol. 29:668–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barsby T, Bavin EP and Guest DJ:
Three-dimensional culture and transforming growth factor beta3
synergistically promote tenogenic differentiation of equine
embryo-derived stem cells. Tissue Eng Part A. 20:2604–2613. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lorda-Diez CI, Montero JA, Martinez-Cue C,
Garcia-Porrero JA and Hurle JM: Transforming growth factors beta
coordinate cartilage and tendon differentiation in the developing
limb mesenchyme. J Biol Chem. 284:29988–29986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wrana JL, Attisano L, Wieser R, Ventura F
and Massagué J: Mechanism of activation of the TGF-beta receptor.
Nature. 370:341–347. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Finnson KW, McLean S, Di Guglielmo GM and
Philip A: Dynamics of transforming growth factor beta signaling in
wound healing and scarring. Adv Wound Care (New Rochelle).
2:195–214. 2013. View Article : Google Scholar
|
|
25
|
Wang W, Huang XR, Li AG, Liu F, Li JH,
Truong LD, Wang XJ and Lan HY: Signaling mechanism of TGF-beta1 in
prevention of renal inflammation: Role of Smad7. J Am Soc Nephrol.
16:1371–1383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang J, Thunder R, Most D, Longaker MT
and Lineaweaver WC: Studies in flexor tendon wound healing:
Neutralizing antibody to TGF-beta1 increases postoperative range of
motion. Plast Reconstr Surg. 105:148–155. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jorgensen HG, McLellan SD, Crossan JF and
Curtis AS: Neutralisation of TGF beta or binding of VLA-4 to
fibronectin prevents rat tendon adhesion following transection.
Cytokine. 30:195–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang K, Wang Z, Du Q, Yu J, Wang A and
Xiong Y: A new TGF-β3 controlled released chitosan scaffold for
tissue engineering synovial sheath. J Biomed Mater Res A.
102:801–807. 2014. View Article : Google Scholar
|