Introduction
Oxidative stress occurs due to the inability of
various antioxidant mechanisms to scavenge excessive levels of
reactive oxygen species (ROS) and/or a reduction in antioxidant
defense mechanisms. Consequently, degenerative diseases, including
hepatopathies (1) and
nephropathies (2) may occur. The
liver and kidneys are the first tissues to be affected by oxidative
stress produced by infectious agents, alcohol, drugs, toxic
industrial chemicals, food additives, and pollutants in the air and
water. Furthermore, free radicals and ROS have a crucial role in
the initiation and progression of liver disease and cancer
(3).
Carbon tetrachloride (CCl4) is an
industrial solvent, which is extensively used as a xenobiotic to
induce chemical liver injury. CCl4-induced oxidative
stress is commonly used in rodent models to determine the
protective effects of synthetic or natural products against
drug-associated hepatotoxicity and nephrotoxicity (4,5).
CCl4 is metabolized by hepatic microsomal cytochrome
P450 into trichloromethyl free radicals. Trichloromethyl can react
with sulfhydryl groups (glutathione and protein thiols) and
antioxidant enzymes, including catalase (CAT) and superoxide
dismutase (SOD) (4).
Overproduction of trichloromethyl free radicals may initiate
membrane lipid peroxidation, eventually leading to various
pathological alterations (6). ROS
have an important role in the development and progression of human
disease, including liver disorders, lung and kidney damage,
diabetes mellitus, atherosclerosis and aging (7), via free radical-induced lipid
peroxidation and cell membrane damage (8). Furthermore, CCl4 causes
tissue damage during inflammation, cancer and aging (9). Parallel to oxidative stress,
CCl4 may induce alterations in various pathways, which
affect the metabolic and healthy state of subjects, including
changes in the gene expression of acute phase proteins, cytokines
(inflammatory and anti-inflammatory), and genes associated with
lipid metabolism.
It has previously been demonstrated that various
natural products can protect organs against CCl4-induced
oxidative stress by enhancing the activities of antioxidant
enzymes, including CAT, glutathione S-transferase (GST) and SOD
(10). Pomegranate (Punica
granatum; POM) is widely renowned in the Middle East due to its
health benefits (11). POM fruit,
juice and peel possess a marked antioxidant capacity, exert
anti-obesity and antihypertensive effects, and may be used to treat
prostate cancer (12–14). POM contains high levels of
polyphenols, particularly ellagitannins, condensed tannins and
anthocyanins (15). POM juice
consumption has been reported to significantly increase sperm
quality, spermatogenic cell density, antioxidant activity and
testosterone levels in male rats (16). In addition, POM juice has been
proposed to exert chemopreventive, chemotherapeutic,
antiatherosclerotic and anti-inflammatory effects (14,17);
therefore, its consumption has markedly increased (18,19).
It is well known that the liver is the main organ responsible for
detoxification and drug metabolism; therefore, the present study
aimed to investigate the effects of POM on CCl4-induced
hepatotoxicity. In addition, the molecular mechanisms underlying
the effects of POM on CCl4-induced alterations in the
expression of antioxidants, cytokines, inflammatory markers and
genes associated with lipid metabolism, as well as hepatic
histopathology, were examined.
Materials and methods
Materials
CCl4 was purchased from Sigma-Aldrich
(St. Louis, MO, USA). The DNA ladder (100 bp) was purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). QIAzol for RNA
extraction and oligo-dT primers were purchased from Qiagen, Inc.
(Valencia, CA, USA). Rabbit anti-rat heat shock protein 70 (HSP70)
primary antibody was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
Experimental animals
Male Wistar rats, weighing 200–250 g, were purchased
from the Animal House, King Abdulaziz University (Jeddah, Saudi
Arabia). The adult male Wistar rats were divided into four equal
groups (n=6/group) in the present study. The rats were kept under
observation for ~1 week, in order to acclimate to the environment
prior to onset of the experiment. The rats were maintained in
stainless steel cages at normal atmospheric temperature (27±5°C),
under good ventilation and a 12 h/12 h light/dark cycle. The rats
had access to food and water ad libitum. All animal
procedures were approved by the Ethics Committee of the Dean of
Scientific Affairs of Taif University (Taif, Saudi Arabia).
Experimental design and treatments
Group 1 served as a control group, in which the rats
were injected intraperitoneally (i.p.) with corn oil (0.5 ml/kg)
once daily. Group 2 were administered water daily for 25 days and
on days 18 and 20 were injected with 1 ml/kg CCl4 (50%
in corn oil; i.p.). Group 3 were administered POM juice daily [30
ml/kg body weight (BW)], and were injected with corn oil (i.p.) on
days 18 and 20. Group 4 were administered POM juice (30 ml/kg BW)
daily for 25 days, and were injected with 1 ml/kg CCl4
(50% in corn oil; i.p.) on days 18 and 20. POM juice was
administered to the rats in the morning, 2 h after water
deprivation, and the rats consumed the given dose within 2 h, in
order to assure no changes due to environmental conditions. The
dose of POM juice was chosen based on a previous study (20).
Sampling
At the end of the experiment, the rats were
sacrificed by cervical dislocation following anesthetization by
diethyl ether (Sigma-Aldrich) inhalation. The liver tissues of all
groups were harvested, homogenized and maintained in either
formalin or QIAzol reagent for histopathology and RNA extraction,
respectively.
Analysis of gene expression
RNA extraction and cDNA synthesis
Total RNA was extracted from 100 mg tissue samples
using QIAzol reagent, according to the manufacturer's instructions.
The integrity of the prepared RNA was determined by
electrophoresis. RNA concentration and purity were determined
spectrophotometrically at 260 and 280 nm (Bio-Rad SmartSpec
Spectrophotometer; Bio-Rab Laboratories, Inc., Hercules, CA, USA).
The 260/280 optical density ratio of all RNA samples was 1.7–1.9. A
total of 2 µg RNA was reverse transcribed using oligo-dT
primers and Moloney murine leukaemia virus (M-MuLV) reverse
transcriptase (SibEnzyme Ltd., Novosibirsk, Russia). For cDNA
synthesis, a mixture of 2 µg total RNA and 0.5 ng oligo dT
primer in a total volume of 11 µl sterilized
diethylpyrocarbonate (DEPC) water was incubated in a PeX 0.5
thermal Cycler (Bio-Rad Laboratories, Inc.) at 65°C for 10 min for
denaturation. Then, 4 µl 5X RT-buffer, 2 µl 10 mM
dNTPs and 100 units M-MuLV Reverse Transcriptase (all purchased
from SibEnzyme Ltd., Novosibirsk, Russia) was added in a total
volume of 20 µl by DEPC water. The mixture was re-incubated
in the thermal cycler at 37°C for 1 h, then at 90°C for 10 min in
order to inactivate the enzyme.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
RNA from hepatic tissues was analyzed by
semi-quantitative RT-PCR using corresponding specific primers for
the indicated genes (Table I). The
primers were designed using Oligo-4 computer program (version 7.0;
Molecular Biology Insights, Cascade, CO, USA) according to the
nucleotide sequences published in GenBank (http://www.ncbi.nlm.nih.gov/genbank/; Table I), and were synthesized by Macrogen
Korea (Seoul, South Korea). PCR was conducted in a final volume of
25 µl consisting of 1 µl cDNA, 1 µl (10
picomoles) of each primer (forward and reverse), and 12.5 µl
PCR Master Mix (Promega Corporation, Madison, WI, USA). The final
volume was brought to 25 µl using sterilized, nuclease-free
deionized water. PCR was carried out using a PeX 0.5 Thermal
Cycler, and the following cycling conditions were used:
Denaturation at 94°C for 5 min for one cycle, followed by 27 cycles
of denaturation at 94°C for 1 min, annealing at the specific
temperature corresponding to each primer (Table I) for 1 min, and extension at 72°C
for 1 min, followed by a final extension step at 72°C for 5 min. As
an internal reference, glyceraldehyde 3-phosphate dehydrogenase
mRNA expression was detected using specific primers (Table I). PCR products subsequently
underwent 1.5% agarose gel electrophoresis (Bio Basic Inc.,
Markham, ON, Canada) in Tris-Borate-ethylenediaminetetraacetic acid
buffer at 100 V for 30 min with ethidium bromide staining
(Sigma-Aldrich). PCR products were visualized under ultraviolet
(UV) light and images were captured using an InGenius 3.0 gel
documentation system (Syngene, Frederick, MD, USA). Band
intensities from the various rats from each group were quantified
densitometrically using ImageJ software version 1.47 (http://imagej.en.softonic.com/).
 | Table ISequences and conditions of
polymerase chain reaction primers. |
Table I
Sequences and conditions of
polymerase chain reaction primers.
| Gene | Product size
(bp) | Annealing temp.
(°C) | Number of PCR
cycles | Direction | Sequence
(5′-3′) |
|---|
| GST | 575 | 55 | 29 | Sense |
GCTGGAGTGGAGTTTGAAGAA |
| | | | Antisense |
GTCCTGACCACGTCAACATAG |
| SOD | 410 | 55 | 28 | Sense |
AGGATTAACTGAAGGCGAGCAT |
| | | | Antisense |
TCTACAGTTAGCAGGCCAGCAG |
| Catalase | 652 | 55.5 | 30 | Sense |
GCGAATGGAGAGGCAGTGTAC |
| | | | Antisense |
GAGTGACGTTGTCTTCATTAGCACTG |
| α-2M | 325 | 56 | 30 | Sense |
GCTCCTGTCTGTTTCCTTAGTT |
| | | | Antisense |
ATTGGCCTTTCGTGGTTTAG |
| IL-6 | 485 | 57 | 32 | Sense |
AGTTGCCTTCTTGGGACTGA |
| | | | Antisense |
GAGCATTGGAAGTTGGGGTA |
| IL-10 | 259 | 57 | 33 | Sense |
ACCAGCTGGACAACATACTGC |
| | | | Antisense |
TCATTCTTCACCTGCTCCACT |
| TGF-β1 | 456 | 58 | 32 | Sense |
TGAGTGGCTGTCTTTTGACG |
| | | | Antisense |
TGGTTGTAGAGGGCAAGGAC |
| SREBP-1c | 191 | 58 | 33 | Sense |
GGAGCCATGGATTGCACATT |
| | | | Antisense |
AGGAAGGCTTCCAGAGAGGA |
| G3PDH | 309 | 52 | 25 | Sense |
AGATCCACAACGGATACATT |
| | | | Antisense |
TCCCTCAAGATTGTCAGCAA |
Immunohistochemical staining of
HSP70
Tissue sections were deparaffinized and were then
treated with 3% H2O2 for 10 min, in order to
inactivate peroxidases. Subsequently, the sections were heated in
10 mM citrate buffer at 121°C for 30 min for antigen retrieval,
blocked in 5% normal serum for 20 min, and were incubated with
primary polyclonal anti-HSP70 [1:100 in phosphate-buffered saline
(PBS); sc-33575; Santa Cruz Biotechnology, Inc.] overnight at 4°C.
After three extensive washes with PBS, sections were incubated with
a biotin-conjugated goat anti-rabbit secondary antibody (1:2,000 in
PBS; sc-2040; Santa Cruz Biotechnology, Inc.) for 20 min at 32°C.
Following a further incubation with horseradish peroxidase-labeled
streptavidin (Santa Cruz Biotechnology, Inc.) according to the
manufacturer's instructions, antibody binding was visualized with
diaminobenzidine (Sigma-Aldrich) and sections were counterstained
with hematoxylin (Sigma-Aldrich). Tissue slides were visualized
using a Wolfe S9-0982 microscope (Carolina Biological Supply Co.,
Burlington, NC, USA) and photos were captured using a Canon Power
Shot SX500 IS digital camera (Canon, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis for the obtained results was
conducted using one-way analysis of variance followed by the least
significant difference test for multiple comparisons among groups.
The analysis was performed using SPSS version 13.0 (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
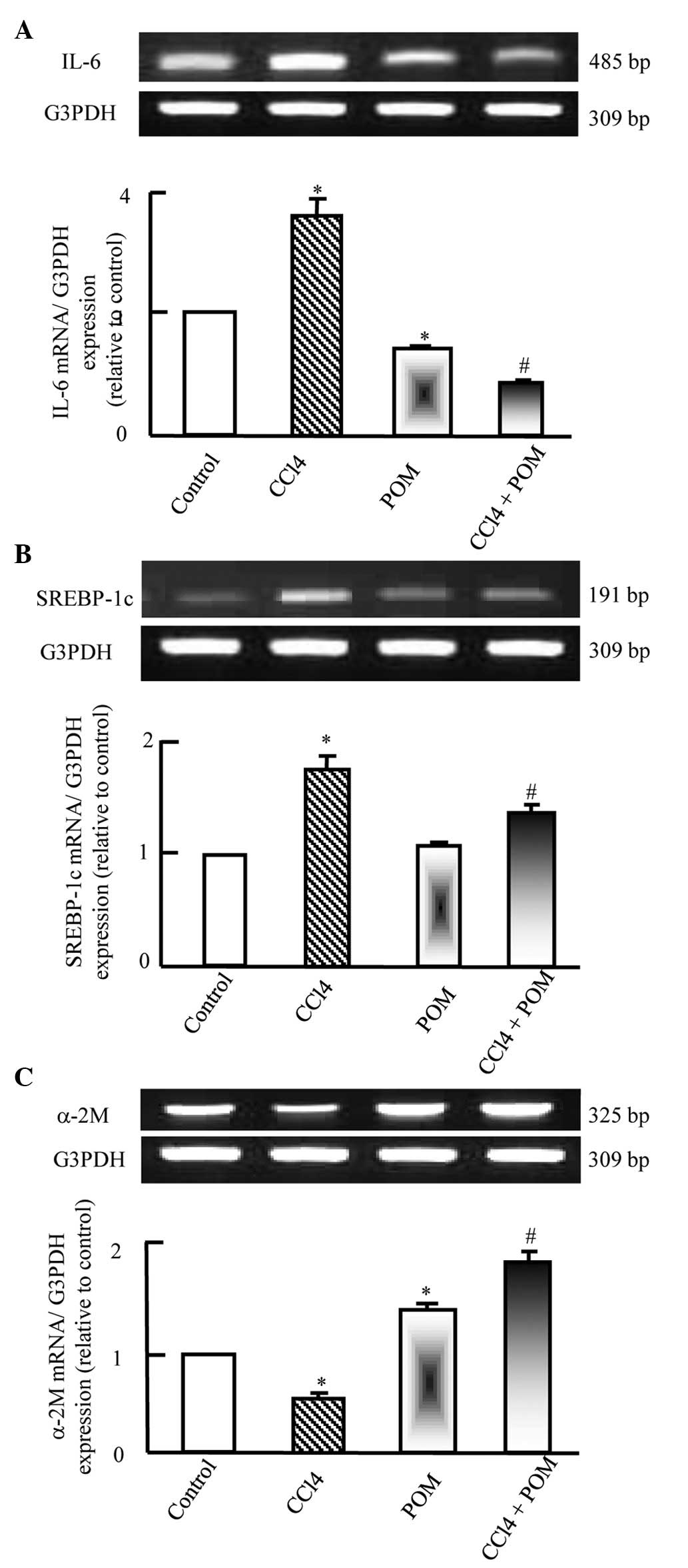
Effects of POM on the hepatic mRNA
expression levels of interleukin (IL)-6, sterol regulatory
element-binding protein 1c (SREBP-1c) and alpha 2-macroglobulin
(α-2M)
To examine whether POM was able to modulate cytokine
expression in order to exert its hepatoprotective effects, the mRNA
expression levels of IL-6 were detected (Fig. 1A). The mRNA expression levels of
IL6 were upregulated following CCl4 injection.
Furthermore, when administered alongside CCl4, POM
normalized the expression of IL6 mRNA. In addition, SREBP-1c, a
lipogenic transcription factor and key regulator of hepatic lipid
metabolism, was detected. The mRNA expression levels of SREBP-1
were significantly upregulated following CCl4 injection
(P<0.05). Furthermore, when administered alongside
CCl4, POM completely normalized SREBP-1c mRNA expression
(Fig. 1B). α-2M is known to
stabilize target protein and enhance its regeneration. In the
present study, α-2M expression was significantly downregulated in
the CCl4 group compared with the control (P<0.05).
However, treatment with POM increased α-2M mRNA expression.
Concurrent POM administration in the CCl4-injected group
resulted in a further upregulation in α-2M expression (Fig. 1C).
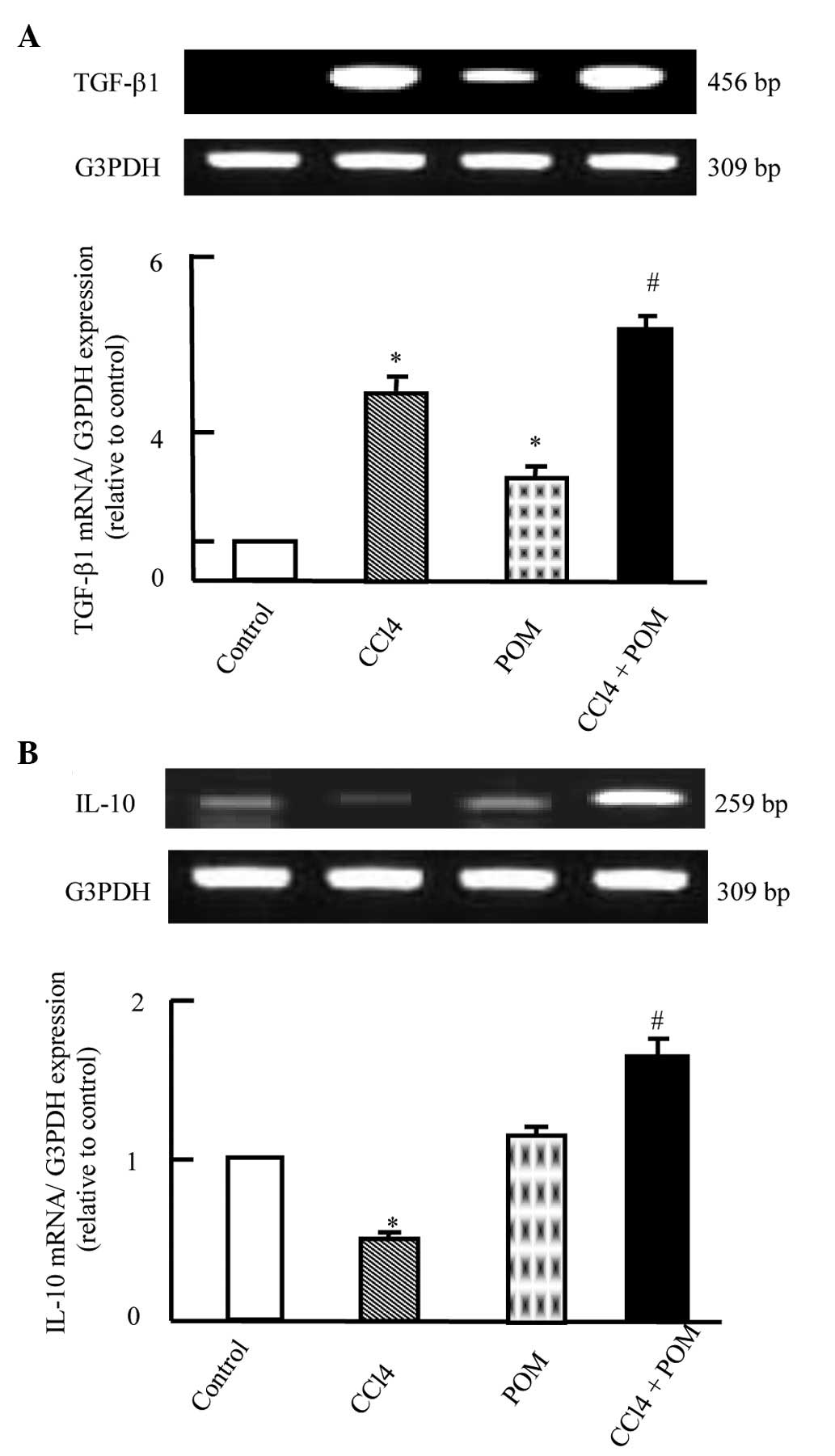
Effects of POM on the mRNA expression
levels of transforming growth factor (TGF)-β1 and IL-10
The mRNA expression levels of TGF-β1 were
significantly upregulated in the CCl4-treated group
compared with in the control group (P<0.05). POM treatment alone
only slightly upregulated TGF-β1 mRNA expression, as compared with
the control group. However, when the rats were co-treated with
CCl4 and POM the mRNA expression levels of TGF-β1 were
further significantly upregulated compared with in the
CCl4 group (P<0.05; Fig.
2A). Treatment of the rats with CCl4 significantly
suppressed the mRNA expression levels of the anti-inflammatory
cytokine IL-10, as compared with the control (P<0.05). However,
co-treatment with POM and CCl4 significantly upregulated
interleukin-10 mRNA expression compared with the control and
CCl4-treated groups (P<0.05; Fig. 2B).
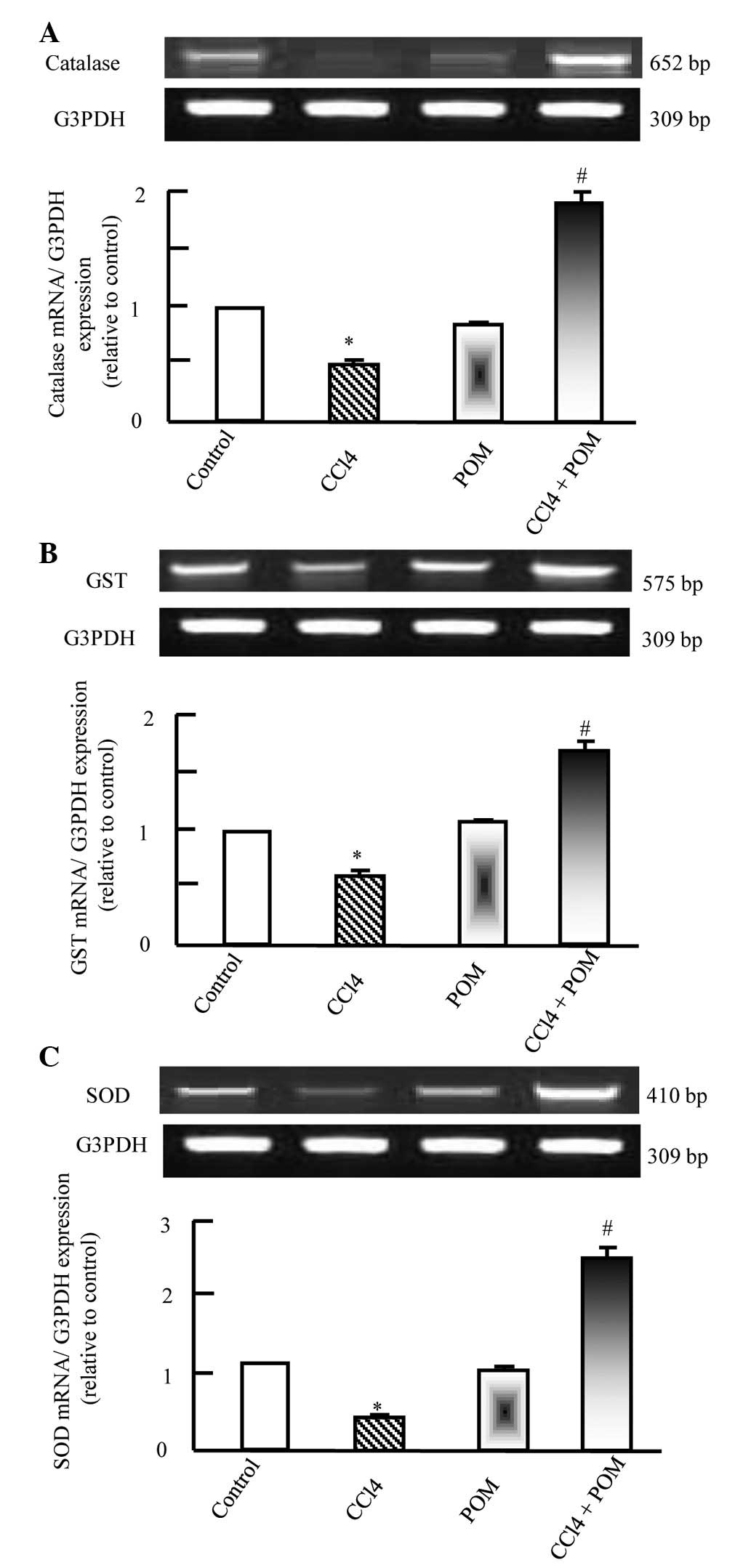
Effects of POM on hepatic antioxidant
enzyme expression
CCl4 induced the downregulation of the
expression of CAT, GST and SOD, which were normalized following
co-administration of POM with CCl4. Following
co-treatment with CCl4 and POM, the mRNA expression
levels of the antioxidant enzymes were completely restored,
compared with in the CCl4 group (Fig. 3A–C).
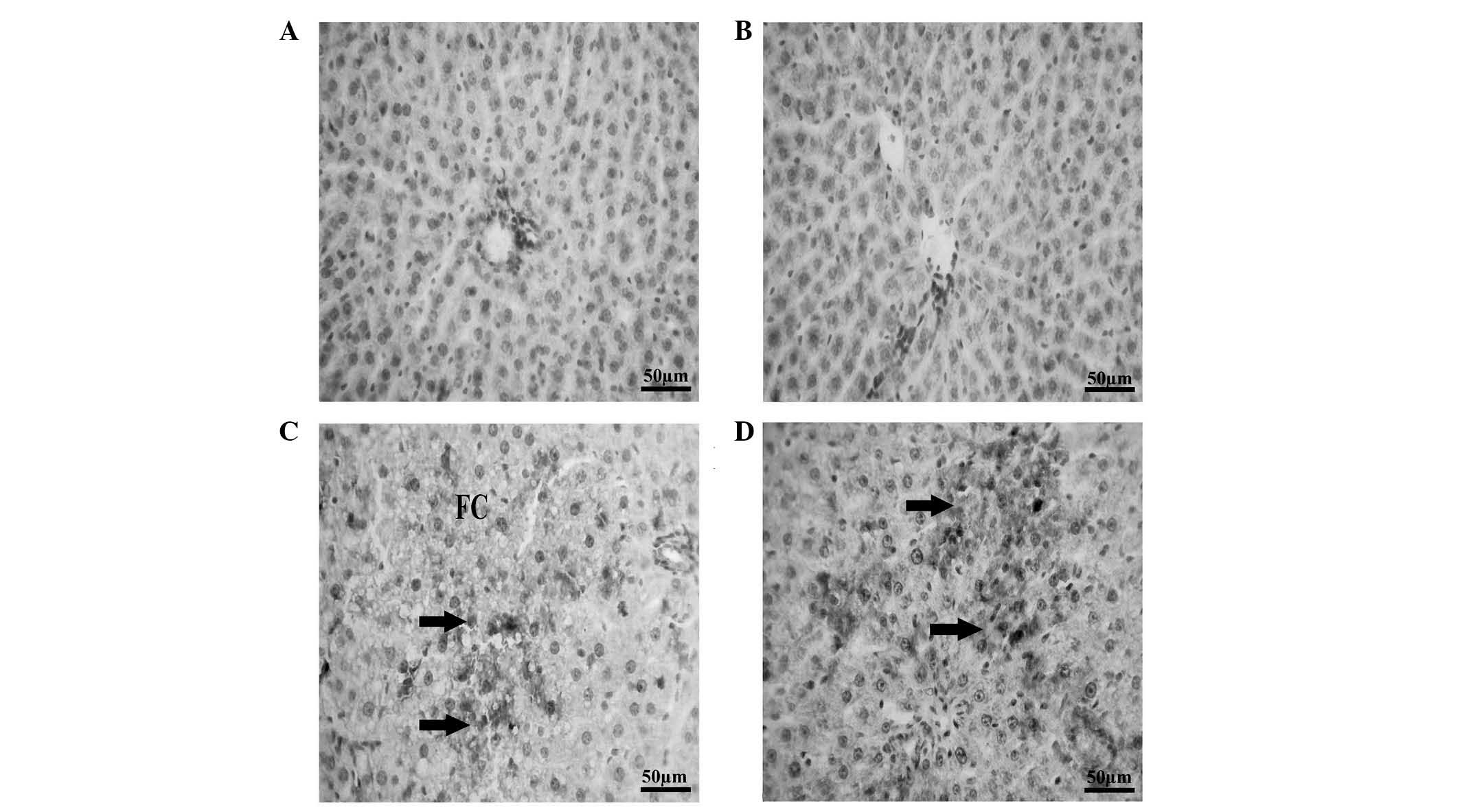
Effects of POM on hepatic HSP70, as
revealed by immunohistochemical staining
Immunostaining of liver samples from control rats
with HSP70 antibodies indicated normal hepatic architecture, with
normal hepatic cords, hepatic sinusoids and normal portal area, and
an absence of HSP70 expression (Fig.
4A). Liver samples from the CCl4 group exhibited
moderate HSP70 expression in the hepatocytes with increased fat
infiltration (Fig. 4B). Liver
samples from the POM-supplemented group exhibited a lack of HSP70
expression in hepatocytes, alongside normal hepatic architecture
(Fig. 4C). Liver samples from the
rats co-treated with CCl4 and POM exhibited
significantly increased HSP70 expression in the hepatocytes
(P<0.05), alongside a decrease in the aberrant alterations
detected in the CCl4 group (Fig. 4D).
Discussion
The present study demonstrated that POM exerted a
protective effect on the liver against CCl4-induced
oxidative stress. CCl4 is one of the most commonly used
hepatotoxins, and its metabolites trichloromethyl radical
(CCl3) and trichloromethyl peroxy radical
(Cl3COO•) are involved in the pathogenesis of liver and
kidney damage (7,21). Both radicals are able to covalently
bind macromolecules and induce peroxidative degradation of the cell
membrane of liver cells.
IL-6 is produced in the liver by several types of
cells, including biliary epithelial cells and cholangiocytes, in
response to inflammatory mediators (22,23).
IL-6 is a commonly used marker of inflammation, the expression of
which has been demonstrated to be markedly increased in the liver
of patients with non-alcoholic fatty liver disease and
non-alcoholic steatohepatitis (24). In the present study,
CCl4 induced inflammation, as demonstrated by an
upregulation in hepatic IL-6 expression. However, downregulation of
basal IL-6 mRNA expression levels, and attenuation of the
CCl4-induced upregulation of IL-6 by POM, indicates its
anti-inflammatory effects. Parallel with these results, increased
levels of the proinflammatory cytokine IL-6 in the ileum of a rat
model of necrotizing enterocolitis were normalized following
treatment with POM seed oil (25).
SREBP-1c is an important transcription factor, which
regulates the hepatic expression of enzymes associated with the
de novo synthesis of fatty acids (26). Upregulation of the gene expression
levels of SREBP-1c has previously been shown to accompany
ethanol-induced fatty liver (27).
In the present study, CCl4 induced the upregulation of
SREBP-1c mRNA expression, thus indicating the disturbance of lipid
metabolism through which CCl4 may induce liver
degeneration. In addition, treatment with POM normalized SREBP-1c
hepatic mRNA expression, thus suggesting that the hepatoprotective
effects of POM operate via normalization of lipid metabolism.
Consistently, a previous study demonstrated that strawberry seed
oil was able to decrease SREBP-1c hepatic content, which was
associated with reduced plasma triglycerides and a decreased
proportion of liver fat (28).
α-2M is the largest major non-immunoglobulin plasma
protein, which is synthesized by numerous cell lineages, including
lung fibroblasts, monocytes, macrophages, hepatocytes, astrocytes
and adrenocortical cells (29).
α-2M is expressed in hepatocytes where it has an important role in
the regulation of proteolytic activity in the tissue and
pericellular space, and contributes to the clearance of
α-2M-proteinase complexes from the circulation (30). In addition, α-2M exerts an
inhibitory effect on various types of nonspecific proteases and
possesses pronounced immunosuppressive activity (31). The results of the present study
demonstrated that treatment with CCl4 resulted in
downregulation of hepatic α-2M mRNA expression compared with the
control group. The ability of POM to upregulate basal and
CCl4-suppressed α-2M mRNA expression indicated that the
hepatoprotective effects of POM may operate via upregulation of
α-2M expression. Notably, increased α-2M levels have been reported
to have an important role in radioprotection via anti-fibrotic,
anti-inflammatory, antioxidant, homeostatic, and repair and
remodeling mechanisms (32).
TGF-β1 is a powerful pleiotropic cytokine that
possesses immune-suppressing and anti-inflammatory properties
(33). In the present study,
treatment with POM alone elevated TGF-β1 mRNA expression levels,
thus indicating that the modulatory effects of POM on immune status
may be caused by TGF-β1 upregulation. Treatment with
CCl4 induced the upregulation of TGF-β1 mRNA expression,
the expression of which was further upregulated in the rats
co-treated with POM and CCl4. These results suggested
that the anti-inflammatory effects of POM may be due to TGF-β1
upregulation. In patient-tolerated kidney or liver allografts, type
1 regulatory T cells were demonstrated to exist, and were capable
of suppressing naïve T-cell activation and producing high levels of
both IL-10 and TGF-β1 (34).
IL-10 has been reported to exert both pro- and
antitumoral effects through the inhibition of nuclear factor-κB
signaling; therefore, it is able to downregulate proinflammatory
cytokine expression (35). IL-10
configures development of the immune response and suppresses
proinflammatory cytokine expression (36). In addition, IL-10 induces the
downregulation of T helper 1 cytokine mRNA expression, and inhibits
IL-1 and IL-6 production (37,38).
In the present study, CCl4-induced downregulation of
IL-10 is consistent with the findings of a previous study, which
reported that CCl4 reduced hepatic IL-10 expression, as
compared with in the control group (39). In the present study, POM
upregulated IL-10 expression, thus suggesting that POM exerts an
anti-inflammatory effect, which protects the liver from
CCl4-induced inflammation. Notably, previous studies
have reported that IL-10 may exert anti-fibrotic effects during
CCl4-induced hepatic fibrosis (40,41).
Therefore, POM may protect the liver from CCl4-induced
inflammation and fibrosis by upregulating the expression of IL-10
(42).
Delaying or inhibiting the oxidation of easily
oxidizable macromolecules, such as lipids, is accomplished by
antioxidants, including SOD, CAT and GST, which have a major role
in protecting these molecules from the actions of free radicals or
ROS (43). Conversion of
superoxide to less toxic H2O2 is catalyzed by
SOD, whereas conversion of H2O2 into nontoxic
H2O is catalyzed by CAT (44). The present study demonstrated that
administration of POM in CCl4-administered rats
significantly restored mRNA expression of the antioxidants
examined, thus suggesting that POM possesses anti-lipid
peroxidation and antioxidative properties. Parallel with these
results, Ocimum sanctum extract has been shown to cause a
significant decrease in lipid peroxidation, coupled with a
significant increase in SOD and CAT expression in the liver
homogenates of rats exposed to oxidative stress (45). The present study suggested that
CCl4-induced suppression of SOD mRNA expression may be
caused by enhancement of lipid peroxidation or inactivation of
antioxidant enzymes, which leads to increased accumulation of
superoxide radicals and lipid peroxidation acceleration. However,
when administered alongside CCl4, POM prevented
CCl4-induced antioxidant suppression. These results
indicated that the ROS scavenging activities of POM juice may
protect the liver from CCl4-induced oxidative stress. A
similar protective effect of flavonoids has been reported in a
previous study, which detected the ability of flavonoids to
scavenge oxidative radicals in the liver of mice and rats following
exposure to CCl4 (46).
In accordance with the present results, POM has been reported to
exhibit a promising antioxidant capacity, and is an effective
scavenger for several ROS, primarily due to its high levels of
phenolic acids, flavonoids and other polyphenolic compounds
(47), through which POM may
protect against free radical-mediated oxidative stress and
attenuate CCL4-induced antioxidant depletion (48).
HSPs are induced in response to various stressors,
in order to protect cells from such effects (49). HSP70 has been reported to protect
cells from tumor necrosis factor-α, prostaglandin, hydrogen
peroxide, ethanol and UV (50). In
the present study, HSP70 expression was slightly increased
following treatment with CCl4, which is parallel to the
previously reported induction of HSP70 in acute liver damage by
CCl4 (51). POM
administration, together with CCl4, induced strong HSP70
immunoreactivity, thus preventing protein denaturation, which may
act as a mechanism to overcome CCl4-induced hepatic
oxidative stress. Parallel with this assumption,
CCl4-induced liver damage has been shown to be
attenuated by pre-induction of chaperones by heat treatment
(52).
In conclusion, the present study confirmed that POM
exerts hepatoprotective effects against CCl4-induced
oxidative stress. POM was demonstrated to possess anti-inflammatory
effects by suppressing CCl4-induced IL-6 expression, and
was able to normalize lipid peroxidation by decreasing
CCl4-induced SREBP-1c expression and increasing
CCl4-suppressed α-2M mRNA expression. In addition, POM
may exert hepatoprotective activity by its immunosuppressive,
anti-inflammatory and regenerative effects via upregulation of
TGF-β1, HSP70 and IL-10, and may increase ROS scavenging activities
by augmenting the antioxidant defense mechanism against
CCl4-induced hepatotoxicity and preventing
CCl4-induced SOD, CAT and GST downregulation.
Abbreviations:
|
α-2M
|
alpha 2-macroglobulin
|
|
CAT
|
catalase
|
|
GST
|
glutathione S-transferase
|
|
HSP70
|
heat shock protein 70
|
|
i.p.
|
intraperitoneal injection
|
|
IL-6
|
interleukin-6
|
|
IL-10
|
interleukin-10
|
|
SOD
|
superoxide dismutase
|
|
ROS
|
reactive oxygen species
|
|
POM
|
pomegranate
|
|
SREBP-1c
|
sterol regulatory element-binding
protein 1c
|
|
TGF-β1
|
transforming growth factor-β1
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
Acknowledgments
The present study was supported by a research
project grant (no. 1/436/3989) from Taif University (Taif, Saudi
Arabia).
References
|
1
|
Hensley K, Robinson KA, Gabbita SP,
Salsman S and Floyd RA: Reactive oxygen species, cell signaling and
cell injury. Free Radic Biol Med. 28:1456–1462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ateşşahin A, Karahan I, Yilmaz S, Ceribaşi
AO and Princci I: The effect of manganese chloride on
gentamicin-induced nephrotoxicity in rats. Pharmacol Res.
48:637–642. 2003. View Article : Google Scholar
|
|
3
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan RA, Khan MR and Sahreen S:
CCl4-induced hepatotoxicity: Protective effect of rutin
on p53, CYP2E1 and the antioxidative status in rat. BMC Complement
Altern Med. 12:1782012. View Article : Google Scholar
|
|
5
|
Es Haghi M, Dehghan G, Banihabib N, Zare
S, Mikaili P and Panahi F: Protective effects of Cornus mas fruit
extract on carbon tetrachloride induced nephrotoxicity in rats.
Indian J Nephrol. 24:291–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cemek M, Aymelek F, Büyükokuroğlu ME,
Karaca T, Büyükben A and Yilmaz F: Protective potential of Royal
Jelly against carbon tetrachloride induced-toxicity and changes in
the serum sialic acid levels. Food Chem Toxicol. 48:2827–2832.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh K, Khanna AK and Chander R:
Hepatoprotective activity of ellagic acid against carbon
tetrachloride induced hepatotoxicity in rats. Indian J Exp Biol.
37:1025–1026. 1999.
|
|
8
|
Ogeturk M, Kus I, Kavakli A, Oner J,
Kukner A and Sarsilmaz M: Reduction of carbon tetrachloride-induced
nephropathy by melatonin administration. Cell Biochem Funct.
23:85–92. 2005. View
Article : Google Scholar
|
|
9
|
Bhadauria M, Nirala SK, Shrivastava S,
Sharma A, Johri S, Chandan BK, Singh B, Saxena AK and Shukla S:
Emodin reverses CCl induced hepatic cytochrome P450 (CYP) enzymatic
and ultrastructural changes: The in vivo evidence. Hepatol Res.
39:290–300. 2009. View Article : Google Scholar
|
|
10
|
Rajesh MG and Latha MS: Preliminary
evaluation of the antihepatotoxic activity of Kamilari, a
polyherbal formulation. J Ethnopharmacol. 91:99–104. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Olayan EM, El-Khadragy MF, Metwally DM
and Abdel Moneim AE: Protective effects of pomegranate (Punica
granatum) juice on testes against carbon tetrachloride intoxication
in rats. BMC Complement Altern Med. 14:1642014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmed MM, Samir el-SA, El-Shehawi AM and
Alkafafy ME: Anti-obesity effects of Taif and Egyptian
pomegranates: Molecular study. Biosci Biotechnol Biochem.
79:598–609. 2015. View Article : Google Scholar
|
|
13
|
Kaur G, Jabbar Z, Athar M and Alam MS:
Punica granatum (pomegranate) flower extract possesses potent
antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in
mice. Food Chem Toxicol. 44:984–993. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malik A, Afaq F, Sarfaraz S, Adhami VM,
Syed DN and Mukhtar H: Pomegranate fruit juice for chemoprevention
and chemotherapy of prostate cancer. Proc Natl Acad Sci USA.
102:14813–14818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seeram NP, Adams LS, Henning SM, Niu Y,
Zhang Y, Nair MG and Heber D: In vitro antiproliferative, apoptotic
and antioxidant activities of punicalagin, ellagic acid and a total
pomegranate tannin extract are enhanced in combination with other
polyphenols as found in pomegranate juice. J Nutr Biochem.
16:360–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Türk G, Sönmez M, Aydin M, Yüce A, Gür S,
Yüksel M, Aksu EH and Aksoy H: Effects of pomegranate juice
consumption on sperm quality, spermatogenic cell density,
antioxidant activity and testosterone level in male rats. Clin
Nutr. 27:289–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rozenberg O, Howell A and Aviram M:
Pomegranate juice sugar fraction reduces macrophage oxidative
state, whereas white grape juice sugar fraction increases it.
Atherosclerosis. 188:68–76. 2006. View Article : Google Scholar
|
|
18
|
Mertens-Talcott SU, Jilma-Stohlawetz P,
Rios J, Hingorani L and Derendorf H: Absorption, metabolism and
antioxidant effects of pomegranate (Punica granatum L.) polyphenols
after ingestion of a standardized extract in healthy human
volunteers. J Agric Food Chem. 54:8956–8961. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faria A, Monteiro R, Mateus N, Azevedo I
and Calhau C: Effect of pomegranate (Punica granatum) juice intake
on hepatic oxidative stress. Eur J Nutr. 46:271–278. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adhami VM, Siddiqui IA, Syed DN, Lall RK
and Mukhtar H: Oral infusion of pomegranate fruit extract inhibits
prostate carcinogenesis in the TRAMP model. Carcinogenesis.
33:644–651. 2012. View Article : Google Scholar :
|
|
21
|
Srivastava SP, Chen NQ and Holtzman JL:
The in vitro NADPH-dependent inhibition by CCl4 of the
ATP-dependent calcium uptake of hepatic microsomes from male rats.
Studies on the mechanism of inactivation of the hepatic microsomal
calcium pump by the CCl3radical. J Biol Chem.
265:8392–8399. 1990.PubMed/NCBI
|
|
22
|
Hirano T: Interleukin 6 and its receptor:
Ten years later. Int Rev Immunol. 16:249–284. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park J, Gores GJ and Patel T:
Lipopolysaccharide induces cholangiocyte proliferation via an
interleukin-6-mediated activation of p44/p42 mitogen-activated
protein kinase. Hepatology. 29:1037–1043. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wieckowska A, Papouchado BG, Li Z, Lopez
R, Zein NN and Feldstein AE: Increased hepatic and circulating
interleukin-6 levels in human nonalcoholic steatohepatitis. Am J
Gastroenterol. 103:1372–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coursodon-Boyiddle CF, Snarrenberg CL,
Adkins-Rieck CK, Bassaganya-Riera J, Hontecillas R, Lawrence P,
Brenna JT, Jouni ZE and Dvorak B: Pomegranate seed oil reduces
intestinal damage in a rat model of necrotizing enterocolitis. Am J
Physiol Gastrointest Liver Physiol. 303:G744–G751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Strable MS and Ntambi JM: Genetic control
of de novo lipogenesis: Role in diet-induced obesity. Crit Rev
Biochem Mol Biol. 45:199–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Takase I, Hakucho A, Okamura N and
Fujimiya T: Carvedilol attenuates the progression of alcohol fatty
liver disease in rats. Alcohol Clin Exp Res. 36:1587–1599. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jurgoński A, Fotschki B and Juśkiewicz J:
Dietary strawberry seed oil affects metabolite formation in the
distal intestine and ameliorates lipid metabolism in rats fed an
obesogenic diet. Food Nutr Res. 59:261042015. View Article : Google Scholar
|
|
29
|
Sottrup-Jensen L: Alpha-macroglobulins:
Structure, shape, and mechanism of proteinase complex formation. J
Biol Chem. 264:11539–11542. 1989.PubMed/NCBI
|
|
30
|
Strickland DK, Kounnas MZ and Argraves WS:
LDL receptor-related protein: A multiligand receptor for
lipoprotein and proteinase catabolism. FASEB J. 9:890–898.
1995.PubMed/NCBI
|
|
31
|
Acosta JA, Hoyt DB, Schmid-Schönbein GW,
Hugli TE, Anjaria DJ, Frankel DA and Coimbra R: Intraluminal
pancreatic serine protease activity, mucosal permeability, and
shock: A review. Shock. 26:3–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Kong X, Zhang Z, Chen W, Chen J,
Li H, Cao W, Ge Y and Fang S: Alpha-2-macroglobulin as a
radioprotective agent: A review. Chin J Cancer Res. 26:611–621.
2014.PubMed/NCBI
|
|
33
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
VanBuskirk AM, Burlingham WJ,
Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier RP and
Orosz CG: Human allograft acceptance is associated with immune
regulation. J Clin Invest. 106:145–155. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schottelius AJ, Mayo MW, Sartor RB and
Baldwin AS Jr: Interleukin-10 signaling blocks inhibitor of kappaB
kinase activity and nuclear factor kappaB DNA binding. J Biol Chem.
274:31868–31874. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moore KW, de Waal Malefyt R, Coffman RL
and O'Garra A: Interleukin-10 and the interleukin-10 receptor. Annu
Rev Immunol. 19:683–765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fiorentino DF, Zlotnik A, Mosmann TR,
Howard M and O'Garra A: IL-10 inhibits cytokine production by
activated macrophages. J Immunol. 147:3815–3822. 1991.PubMed/NCBI
|
|
38
|
Soliman MM, Abdo Nassan M and Ismail TA:
Immunohistochemical and molecular study on the protective effect of
curcumin against hepatic toxicity induced by paracetamol in Wistar
rats. BMC Complement Altern Med. 14:4572014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hou YL, Tsai YH, Lin YH and Chao JC:
Ginseng extract and ginsenoside Rb1 attenuate carbon
tetrachloride-induced liver fibrosis in rats. BMC Complement Altern
Med. 14:4152014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang YH, Shi MN, Zheng WD, Zhang LJ, Chen
ZX and Wang XZ: Therapeutic effect of interleukin-10 on
CCl4-induced hepatic fibrosis in rats. World J
Gastroenterol. 12:1386–1391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang LJ, Zheng WD, Chen YX, Huang YH,
Chen ZX, Zhang SJ, Shi MN and Wang XZ: Antifibrotic effects of
interleukin-10 on experimental hepatic fibrosis.
Hepatogastroenterology. 54:2092–2098. 2007.
|
|
42
|
Pestka S, Krause CD, Sarkar D, Walter MR,
Shi Y and Fisher PB: Interleukin-10 and related cytokines and
receptors. Annu Rev Immunol. 22:929–979. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ramnath V, Rekha PS and Sujatha KS:
Amelioration of heat stress induced disturbances of antioxidant
defense system in chicken by brahma rasayana. Evid Based Complement
Alternat Med. 5:77–84. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Celi P: The role of oxidative stress in
small ruminants health and production. R Bras Zootec. 39:348–363.
2010. View Article : Google Scholar
|
|
45
|
Ramesh B and Satakopan VN: Antioxidant
activities of hydroalcoholic extract of Ocimum sanctum against
cadmium Induced toxicity in rats. Indian J Clin Biochem.
25:307–310. 2010. View Article : Google Scholar
|
|
46
|
Yuan LP, Chen FH, Ling L, Bo H, Chen ZW,
Li F, Zhong MM and Xia LJ: Protective effects of total flavonoids
of Bidens bipinnata L. against carbon tetrachloride-induced liver
fibrosis in rats. J Pharm Pharmacol. 60:1393–1402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Aviram M, Dornfeld L, Rosenbblat M,
Volkova N, Kaplan M, Coleman R, Hayek T, Presser D and Fuhrman B:
Pomegranate juice consumption reduces oxidative stress, atherogenic
modifications to LDL, and platelet aggregation: Studies in humans
and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin
Nutr. 71:1062–1076. 2000.PubMed/NCBI
|
|
48
|
Pirinççioğlu M, Kızıl G, Kızıl M, Kanay Z
and Ketani A: The protective role of pomegranate juice against
carbon tetrachloride-induced oxidative stress in rats. Toxicol Ind
Health. 30:910–918. 2014. View Article : Google Scholar
|
|
49
|
Garrido C, Gurbuxani S, Ravagnan L and
Kroemer G: Heat shock proteins: Endogenous modulators of apoptotic
cell death. Biochem Biophys Res Commun. 286:433–442. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ikeyama S, Kusumoto K, Miyake H, Rokutan K
and Tashiro S: A non-toxic heat shock protein 70 inducer,
geranylgeranylacetone, suppresses apoptosis of cultured rat
hepatocytes caused by hydrogen peroxide and ethanol. J Hepatol.
35:53–61. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schiaffonati L and Tiberio L: Gene
expression in liver after toxic injury: Analysis of heat shock
response and oxidative stress-inducible genes. Liver. 17:183–191.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee KJ, Terada K, Oyadomari S, Inomata Y,
Mori M and Gotoh T: Induction of molecular chaperones in carbon
tetrachloride-treated rat liver: Implications in protection against
liver damage. Cell Stress Chaperones. 9:58–68. 2004. View Article : Google Scholar : PubMed/NCBI
|