Introduction
The endocannabinoid system is composed of endogenous
ligands, cannabinoid receptors, and synthesizing and degrading
enzymes of endogenous ligands. The most extensively investigated
cannabinoid receptors are cannabinoid CB1 and
cannabinoid CB2 receptors (1). Increasing evidence has demonstrated
that cannabinoid CB2 receptor activation decreases
fibrosis in mice exhibiting hepatic fibrosis (2), abates skin fibrosis in a mouse model
of scleroderma (3), and reduces
fibroblast proliferation, and prevents the development of skin and
lung fibrosis in a systemic sclerosis mouse model (4). In addition, our previous study
demonstrated that cannabinoid CB2 receptors are
expressed in a time-dependent manner in neutrophils, macrophages
and myofibroblasts during skin wound healing in mice (5). These findings suggest a potential
role of cannabinoids in alleviating, or even reversing, skin
fibrosis following traumatic damage to the skin.
Wound healing is a dynamically complex but ordered
process, which is tightly regulated and comprises an inflammatory
stage, a fibrotic stage and a remodeling stage (6). The progress of wound healing requires
close interaction between cells and extracellular matrix
components, which is controlled through various cytokines,
including transforming growth factor (TGF)-α, TGF-β, interleukin-1
and insulin-like growth factor I, (7). Among the multitude of growth factors
involved in wound healing, TGF-β has the broadest spectrum of
effects (8). TGF-β is closely
involved in fibrosis, which appears to markedly enhance the
expression levels of matrix components, including fibronectin and
collagens (9). Previous evidence
has confirmed that cannabinoid CB2 receptor stimulation
decreases the expression levels of TGF-β (10) and the downstream mediators,
phosphorylated (P)-small mothers against decapentaplegic (Smad)2/3
(3), which suggests that TGF-β is
one of the pathways by which cannabinoid CB2 receptors
modulates fibrotic events. The present study aimed to investigate
the roles of cannabinoid CB2 receptors in the regulation
of fibrogenesis and the TGF-β/Smad signaling pathway during skin
wound healing in mice.
Materials and methods
Animals and experimental protocol
A total of 155 male, wild-type BALB/c mice (7–9
weeks old; 25±3 g) were housed individually and acclimated to their
environment for at least 1 week prior to surgery, in a
temperature-controlled animal facility with a 12-h light/dark cycle
and ad libitum access to water and chow. The present study
was approved by the Ethics Committee of China Medical University
(Shenyang, China). Experiments conformed to the 'Principles of
Laboratory Animal Care' (11),
which required minimization of the number of animals included and
any suffering that they may experience, and were performed
according to the Guidelines for the Care and Use of Laboratory
Animals of China Medical University (Shenyang, China).
An animal model of excisional skin wounding was
constructed on the basis of previous reports (12–14).
Briefly, following intraperitoneal injection with 2% sodium
pentobarbital (15 mg/kg; Sigma-Aldrich, St. Louis, MO, USA), two
full-thickness circular punch wounds of 6 mm diameter were created
symmetrically over the midline of the mouse dorsum.
Postoperatively, the mice were housed individually to minimize
wound disruption, with access to food and water ad
libitum.
To evaluate the effects of GP1a and AM630, one group
of the injured mice was treated with GP1a, a highly selective
cannabinoid CB2 receptor agonist (Ki: 0.037 and 353 nM
for cannabinoid CB2 and CB1 receptors,
respectively; Tocris Bioscience, Ellisville, MO, USA). A second
group was treated with AM630, a cannabinoid CB2 receptor
antagonist/inverse agonist (Ki=31.2 nM; Tocris Bioscience), which
has 165-fold higher selectivity than the cannabinoid CB1
receptor. GP1a or AM630 was dissolved in dimethylsulfoxide
(DMSOU)/Tween-80/physiological saline (5:2:100; all Sigma-Aldrich)
at a concentration of 3 mg/kg/day, and was administered by
intraperitoneal injection (15–17).
Vehicle control mice were injected with 100 µl solvent to
determine potential effects of DMSO and Tween-80. Treatment with
GP1a or AM630 was started in parallel to excisional challenge and
maintained on each subsequent day until the day prior to sacrifice.
All mice were sacrificed by intraperitoneal injection with an
overdose of sodium pentobarbital. Following sacrifice (12 h and 1,
3, 5, 7, 9, 11, 13, 17 and 21 days post-injury), 1×1 cm specimens
were removed from the epicenter of the wound from five mice for
each post-traumatic interval. One wound specimen (left or right)
was randomly allocated for morphological analyses and the other was
allocated for western blot and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analyses. Five mice without
excision were used as a control group.
Tissue preparation and histological
analysis
Skin specimens were immediately fixed in 4%
paraformaldehyde (Sigma-Aldrich) with phosphate-buffered saline (pH
7.4) and embedded in paraffin. From the specimens, 5
µm-thick sections were obtained and stained using
hematoxylin and eosin (HE; Sigma-Aldrich and Perfemiker, Shanghai,
China, respectively) and Masson's trichrome staining (Perfemiker),
composed of 1 g acid fuchsin, 2 g ponceau and 2 g orange G in 300
ml acetic acid (0.25%). Skin thickness was evaluated under
microscopic magnification (×50; cat. no. DM400 B; Leica
Microsystems, GmbH, Wetzlar, Germany), by measuring the distance
between the epidermis and the dermal-subcuneous fat junction, in
five randomly selected fields for each skin section.
Immunohistochemistry and
immunofluorescence staining
Skin sections (5 µm) were processed in order
to evaluate the expression levels of TGF-β1 (rabbit polyclonal
antibody; cat. no. ab92486; Abcam, Cambridge, UK; 1:300 dilution);
TGF-β receptor I (TβRI; rabbit polyclonal antibody; cat. no.
ab31013; Abcam; 1:3000 dilution); Smad3 ser 423/425 (rabbit
polyclonal antibody; cat. no. PAB11304; Abnova; Taipei, China;
1:500 dilution) and Smad7 (rabbit polyclonal antibody; cat. no.
ab90085; Abcam; 1:500 dilution). A Histostain-Plus kit (Zymed
Laboratories, South San Francisco, CA, USA) was used, according to
the manufacturer's protocol. The positive cells were visualized
using diaminobenzidine (OriGene Technologies, Beijing, China).
Collagen I-positive cells were detected using an immunofluorescence
technique by incubating 3 µm skin sections with
anti-Collagen I (goat polyclonal antibody; sc-25974; Santa Cruz
Biotechnology, Inc., Texas, UT, USA; 1:100 dilution). Hoechst 33258
(cat. no. sc-394039; Santa Cruz Biotechnology, Inc.) was used for
nuclei staining. As immunohistochemical controls for the
immunostaining procedures, additional sections were incubated with
non-immune goat serum or phosphate-buffered saline (pH 7.4) in
place of the primary antibodies. Collagen I-positive fibroblast
cells (FBCs) were counted independently by two pathologists
(magnification, ×400) in three sections (five non-contiguous
microscope fields for each section) from each lesional skin sample
using a Leica DM400 B microscope.
RNA isolation and RT-qPCR
Total RNA was isolated from the skin specimens (100
mg) using RNAiso Plus (cat. no. 9109; Takara Bio, Inc., Shiga,
Japan), according to the manufacturer's protocol. Briefly, each
skin specimen was cut to 1 mm3 then treated with 1 ml
TRIzol solution (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for 30 min at room temperature. Following
centrifugation at 12,000 × g for 15 min at 4°C, the supernatant was
obtained, mixed with chloroform, centrifuged again as before and
supplemented with isopropanol (both Perfemiker). Following further
centrifugation as before, the precipitate was collected and washed
with 75% ethanol (Perfemiker), centrifugation as before, then
repeated. The RNA pellet was air-dried and resolved in 60 µl
diethylpyrocarbonate-treated dH2O (Takara Bio, Inc.).
The optical density value for each RNA sample was measured using a
Nanodrop 2000 ultraviolet spectrophotometer (Thermo Fisher
Scientific, Inc.). RNA was reverse transcribed into cDNA using a
PrimeScript™ RT reagent kit (cat. no. RR037A; Takara Bio, Inc.).
RT-qPCR amplification was performed on an ABI 7500 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using a
SYBR® PrimeScript™ RT-qPCR kit (cat. no. RR081A; Takara
Bio, Inc.). The 20 µl reaction system contained the
following: 10 µl SYBR Premix Ex Taq (2X), 0.4
µl ROX Dye II, 6 µl dH2O, 0.8 µl PCR forward
primer, 0.8 µl PCR reverse primer and 2 µl cDNA. qPCR
thermal cycling was performed as follows: One cycle at 95°C for 30
sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec,
and one cycle of 95°C for 15 sec, 60°C for 30 sec and 95°C for 15
sec for fluorescence signal acquisition. Sequence-specific primer
pairs were synthesized by Takara Bio, Inc. (Table I). β-actin (Actb) was used as a
loading control. Relative quantification was performed using the
comparative quantification cycle (ΔΔCQ) method. To exclude any
potential contamination, negative controls were also included, with
dH2O, in place of cDNA, during each run. No
amplification product was detected. The RT-qPCR procedure was
repeated at least three times for each sample.
 | Table ISequences of the primers used for
reverse transcription-quantitative polymerase chain reaction
analysis. |
Table I
Sequences of the primers used for
reverse transcription-quantitative polymerase chain reaction
analysis.
| Gene | GenBank ID | Primer | Sequence (5′-3′) | Product size
(bp) |
|---|
| Actb | NM_007393 | Forward |
ACCTTCTACAATGAGCTGCG | 147 |
| | Reverse |
CTGGATGGCTACGTACATGG | |
| Tgfb1 | NM_011577 | Forward |
CCTGAGTGGCTGTCTTTTGA | 124 |
| | Reverse |
CGTGGAGTTTGTTATCTTTGCTG | |
| Tgfbr1 | NC_000070.6 | Forward |
ATTGCTCGACGCTGTTCTATTGGT | 269 |
| | Reverse |
CCTTCCTGTTGGCTGAGTTGTGA | |
| Smad3 | NM_016769 | Forward |
CCGAGAACACTAACTTCCCTG | 84 |
| | Reverse |
CATCTTCACTCAGGTAGCCAG | |
| Smad7 | NM_001042660 | Forward |
GTGTTGCTGTGAATCTTACGG | 118 |
| | Reverse |
CATTGGGTATCTGGAGTAAGGAG | |
| Col1a1 | NM_007742 | Forward |
CATAAAGGGTCATCGTGGCT | 150 |
| | Reverse |
TTGAGTCCGTCTTTGCCAG | |
| Col3a1 | NM_009930 | Forward |
GAAGTCTCTGAAGCTGATGGG | 149 |
| | Reverse |
TTGCCTTGCGTGTTTGATATTC | |
| Acta2 | NM_007392 | Forward |
GTGAAGAGGAAGACAGCACAG | 146 |
| | Reverse |
GCCCATTCCAACCATTACTCC | |
Protein preparation and immunoblotting
assay
Skin samples were homogenized in phosphorylated
protein lysis buffer (cat. no. KGP9100; KeyGEN Biotech Co., Ltd.,
Nanjing, China) using a Sonic Ruptor 400 ultrasound (Omni, Inc.,
Kennesaw, GA, USA) at 4°C. The homogenates were centrifuged three
times at 12,000 × g for 30 min at 4°C, and the resulting
supernatants were collected. Protein concentrations were determined
using a Bicinchoninic Acid kit (cat. no. P0010; Beyotime Institute
of Biotechnology; Shanghai, China), according to the manufacturer's
protocol. Subsequently, 30 µg protein was separated on 12%
polyacrylamide gels (Sigma-Aldrich). The protein lysates were then
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA) for 100 V for 1 h at room temperature.
Membranes were subsequently incubated with 8% skimmed milk for 4 h,
washed for a few seconds with Tris-buffered saline containing 0.1%
Tween-20 (TBS-T; Perfemiker) and were then incubated with primary
antibodies overnight at 4°C. The specifications and dilutions for
the primary antibodies were as follows: Rabbit anti-TGF-β1
polyclonal antibody (cat. no. ab92486; Abcam; 1:400 dilution);
rabbit anti-TβRI polyclonal antibody (cat. no. ab31013; Abcam;
1:1,000 dilution); rabbit anti-Smad3 ser 423/425 polyclonal
antibody (cat. no. PAB11304; Abnova; 1:10,000 dilution) and rabbit
anti-Smad7 polyclonal antibody (cat. no. ab90085; Abcam; 1:500
dilution). Mouse anti-β-actin monoclonal antibody (cat. no.
sc-47778; Santa Cruz Biotechnology, Inc; 1:5,000 dilution) was used
as a loading control. Following rinsing with TBS-T, the membranes
were incubated with polyclonal goat anti-rabbit (cat. no. sc-2054)
and anti-mouse (cat. no. sc-2055; both 1:5,000 dilution) secondary
antibodies for 90 min at room temperature. Blots were visualized
using western blotting luminal reagent (cat. no. sc-2048; all Santa
Cruz Biotechnology, Inc.) on an Electrophoresis Gel Imaging
Analysis system (cat. no. 5500; Tanon, Shanghai, China). The bands
of the blot were quantified by densitometry using ImageJ software
(ImageJ 1.48 v; National Institutes of Health, Bethesda, MA,
USA).
Statistical analysis
Results are presented as the mean ± standard
deviation. One-way analysis of variance was then used to determine
the significant differences using SPSS for Windows 13.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of GP1a and AM630 on
fibrosis
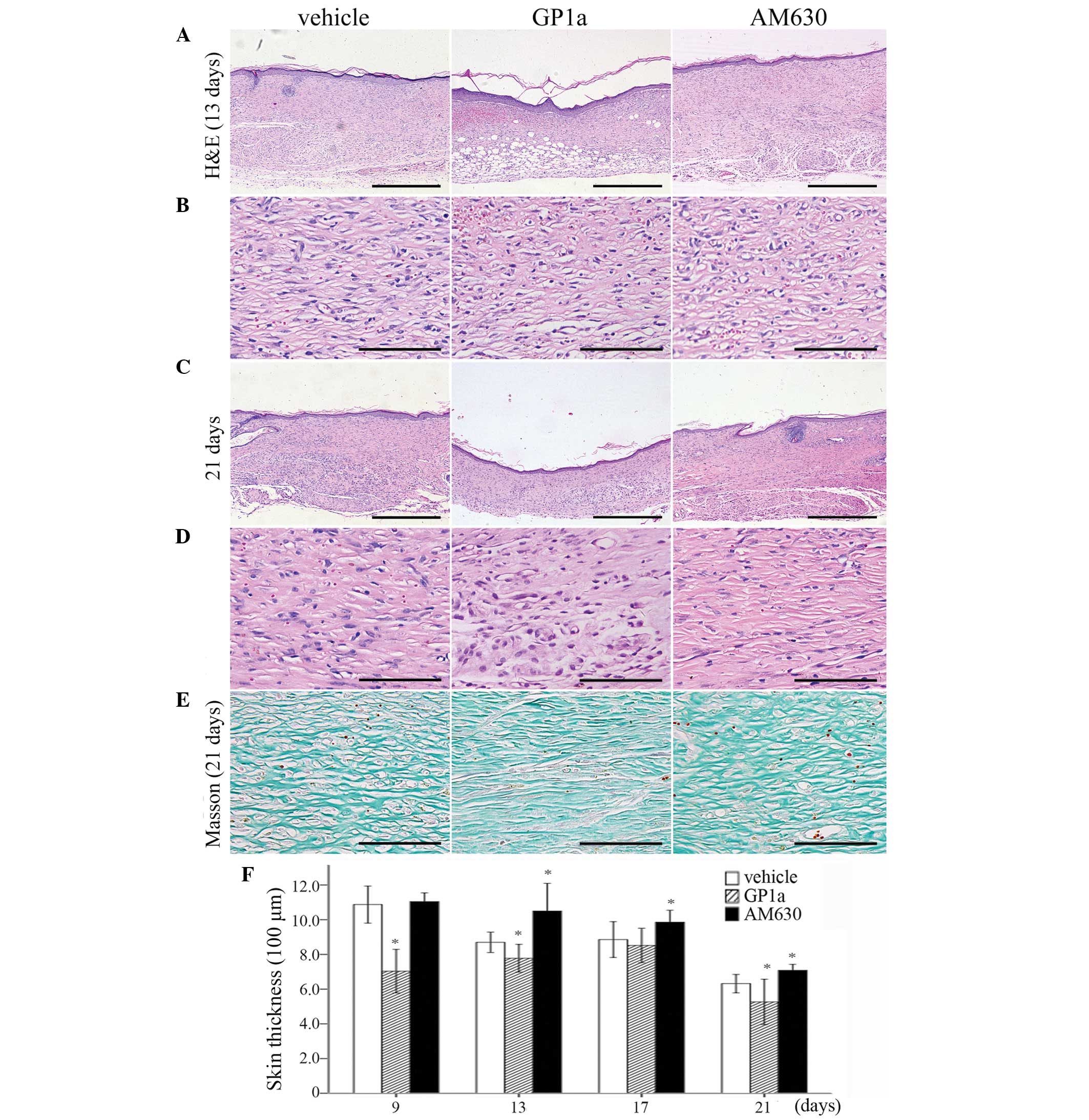
HE staining revealed that, in the vehicle-treated
group, numerous polymorphonuclear cells were detected
microscopically at 12 h and 1 day post-injury. Mononuclear cells
(MNCs), spindle-shaped FBCs and endothelial cells appeared in the
wounded zone at 3 days post-injury. Fibrotic tissue formation was
observed at 5 days, and became more evident between 9 and 21 days
post-injury. The results of the HE and Masson's trichrome staining
revealed that the GP1a-treated mice exhibited less collagen
deposition and slender fibers, compared with the vehicle-treated
mice. By contrast, the mice treated with AM630 exhibited increased
collagen deposition and thicker fibers between days 9 and 21
post-injury (Fig. 1A–E).
Skin fibrosis was evaluated by measuring the skin
thickness of the wounded area. Between days 9 and 21 post-injury,
GP1a-treated group exhibited a considerable decrease in skin
thickness, whereas the AM630-treated group exhibited increased skin
thickness in the HE-stained sections, compared with the vehicle
group (Fig. 1F, P<0.05).
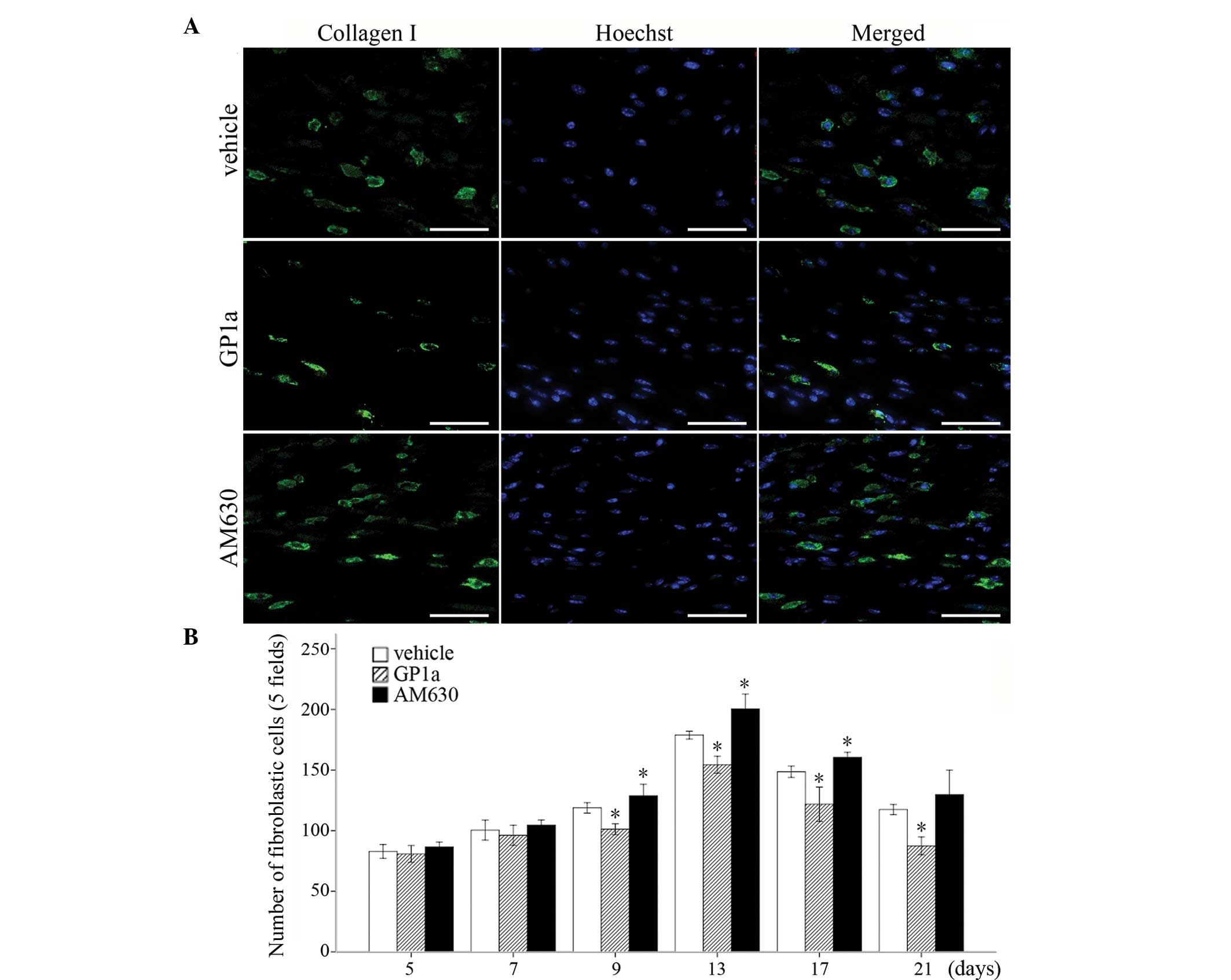
Collagen I, for the detection of FBCs, was detected using
immunofluorescence staining. FBCs began to emerge 5 days
post-injury. No significant differences were detected in the number
of FBCs among the vehicle, GP1a and AM630 groups at days 5 and 7
post-injury. Following GP1a treatment, there was a decrease in the
number of FBCs in the lesional skin, compared with the vehicle
group, whereas treatment with AM630 resulted in an increase in the
number of FBCs, compared with the vehicle group between days 9 and
21 post-injury (Fig. 2;
P<0.05). These findings were consistent with the results of the
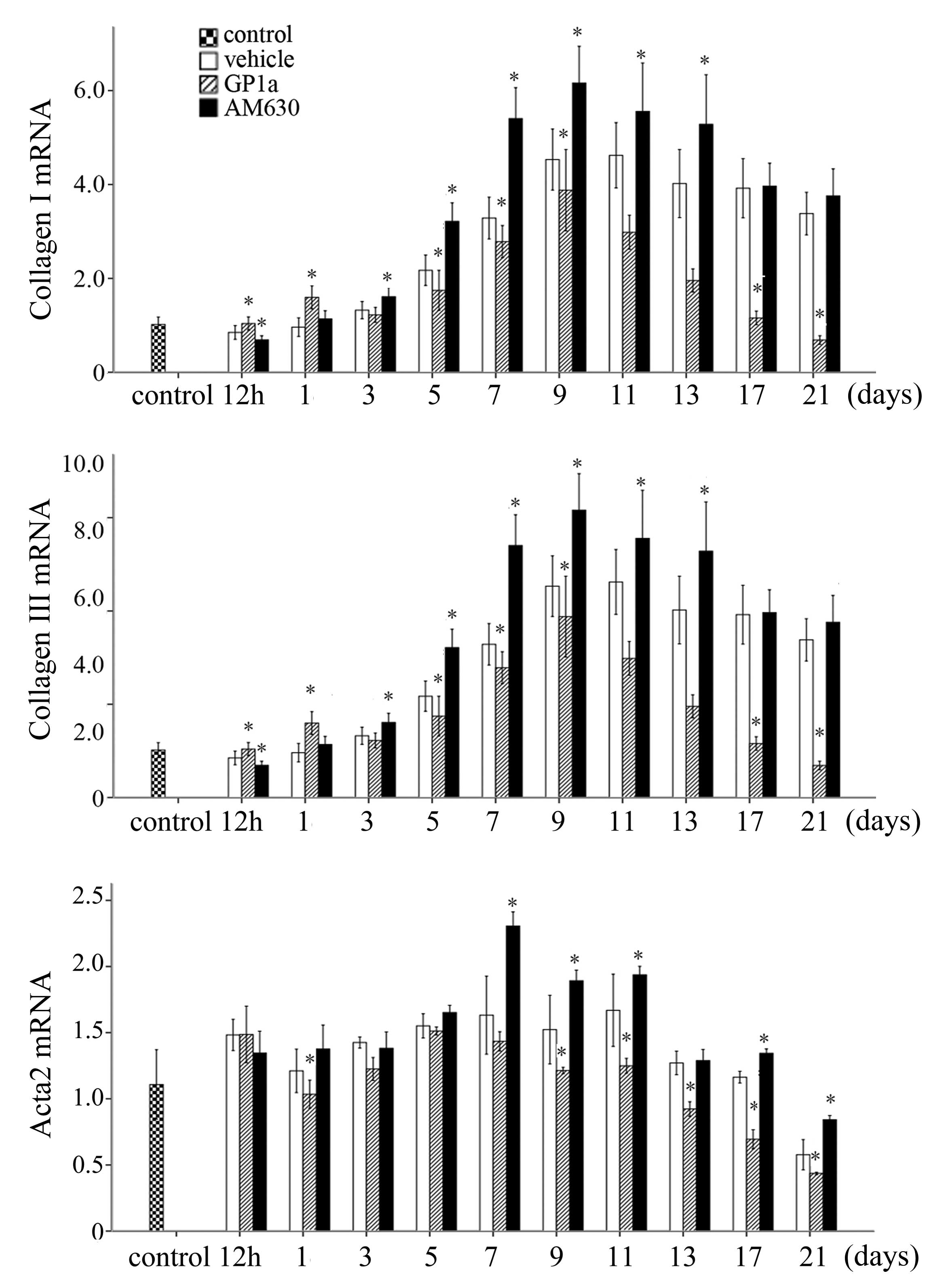
RT-qPCR analysis. The mRNA expression levels of Col1a1, Col3a1 and
actin α (Acta)2 were marginally elevated at 12 h, peaked for Col3a1
and Acta2 at 7 days, and for Col1a1 at 9 days, and then reduced
gradually in the vehicle group. Treatment with GP1a significantly
downregulated the mRNA expression levels of Col1a1, Col3a1 and
Acta2 in the GP1a-treated group, compared with the vehicle group,
whereas AM630 upregulated mRNA expression levels of Col1a1, Col3a1
and Acta2 (Fig. 3; P<0.05).
Effects of GP1a and AM630 on the
TGF-β/Smad signaling pathway
Immunostaining indicated that, in the control skin
specimens, positive cytoplasmic immunoreactivities of TGF-β1, TβRI
and Smad7 were detected in the epidermis, hair follicles, cutaneous
muscle layer and vascular walls, whereas P-Smad3 immunostaining was
detected in the nuclei. In the excisional skin samples, no
immunostaining for TGF-β1, TβRI, P-Smad3 or Smad7 were observed in
poly-morphonuclear cells at 12 h or on day 1. Immunoreactivities
for TGF-β1, TβRI, P-Smad3 and Smad7 were predominantly observed in
the MNCs and FBCs between 3 and 7 days post-injury. Between days 9
and 21 post-wounding, the immunoreactivities were predominantly
detected in the FBCs. At 21 days post-injury, the majority of the
FBCs exhibited weak immunostaining for TGF-β1, TβRI, P-Smad3 and
Smad7 (Fig. 4).
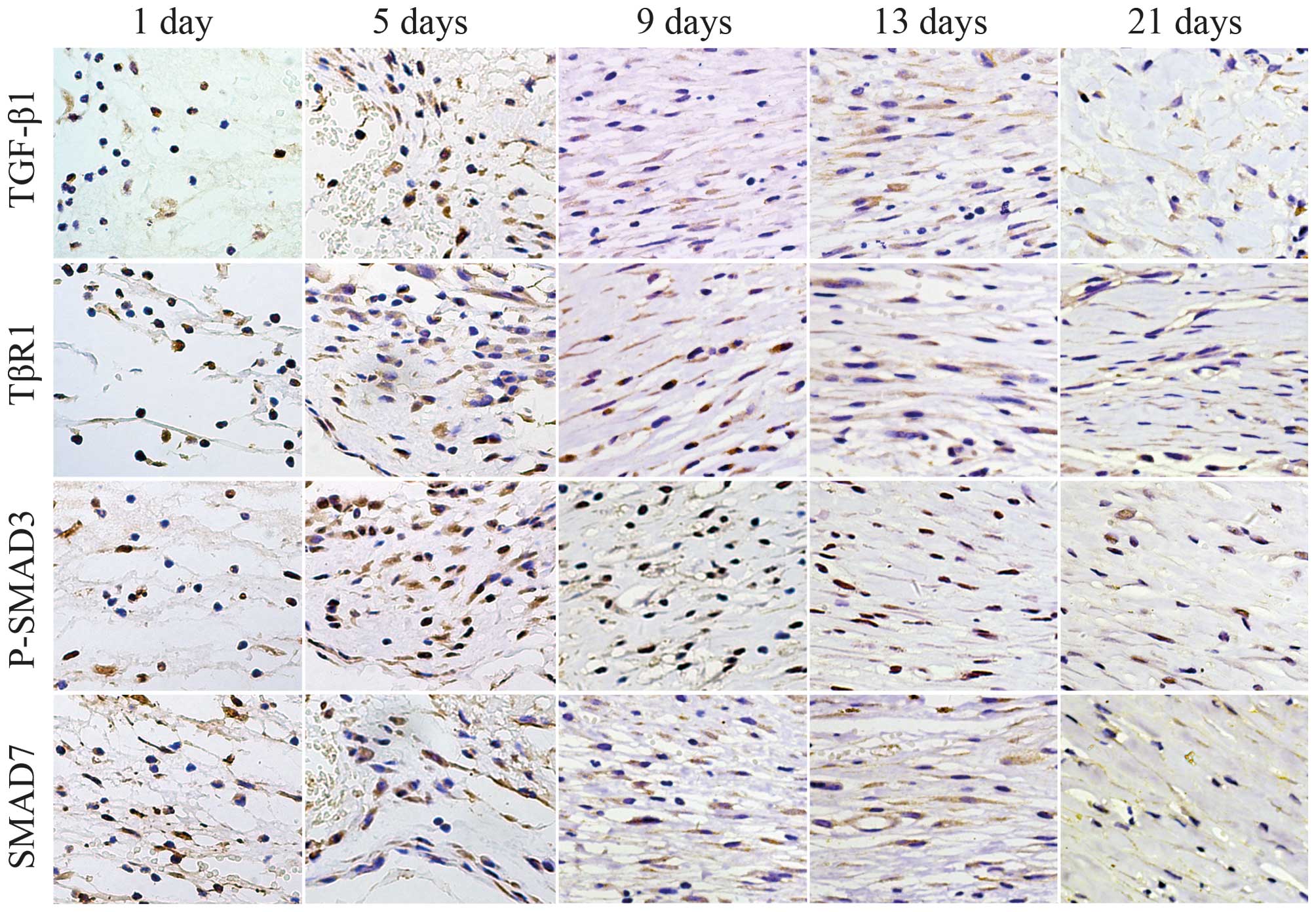 | Figure 4Immunostaining of TGF-β1, TβRI,
P-Smad3 and Smad7 at 1, 5, 9, 13 and 21 days post-trauma. In the
excisional skin samples, no immunostaining for TGF-β1, TβRI,
P-Smad3 or Smad7 were identified in the polymorphonuclear cells at
day 1. Immunoreactivities for TGF-β1, TβRI, P-Smad3 and Smad7 were
predominantly observed in the mononuclear cells and fibroblast
cells at days 5 post-injury. At days 9 and 13 post-injury,
immunoreactivities were predominantly detected in the fibroblast
cells. Scale bar=50 µm. TGF-β1, transforming growth
factor-β1; TβRI, TGF-β receptor type 1; Smad, small mothers against
decapentaplegic; P-Smad, phosphorylated Smad. |
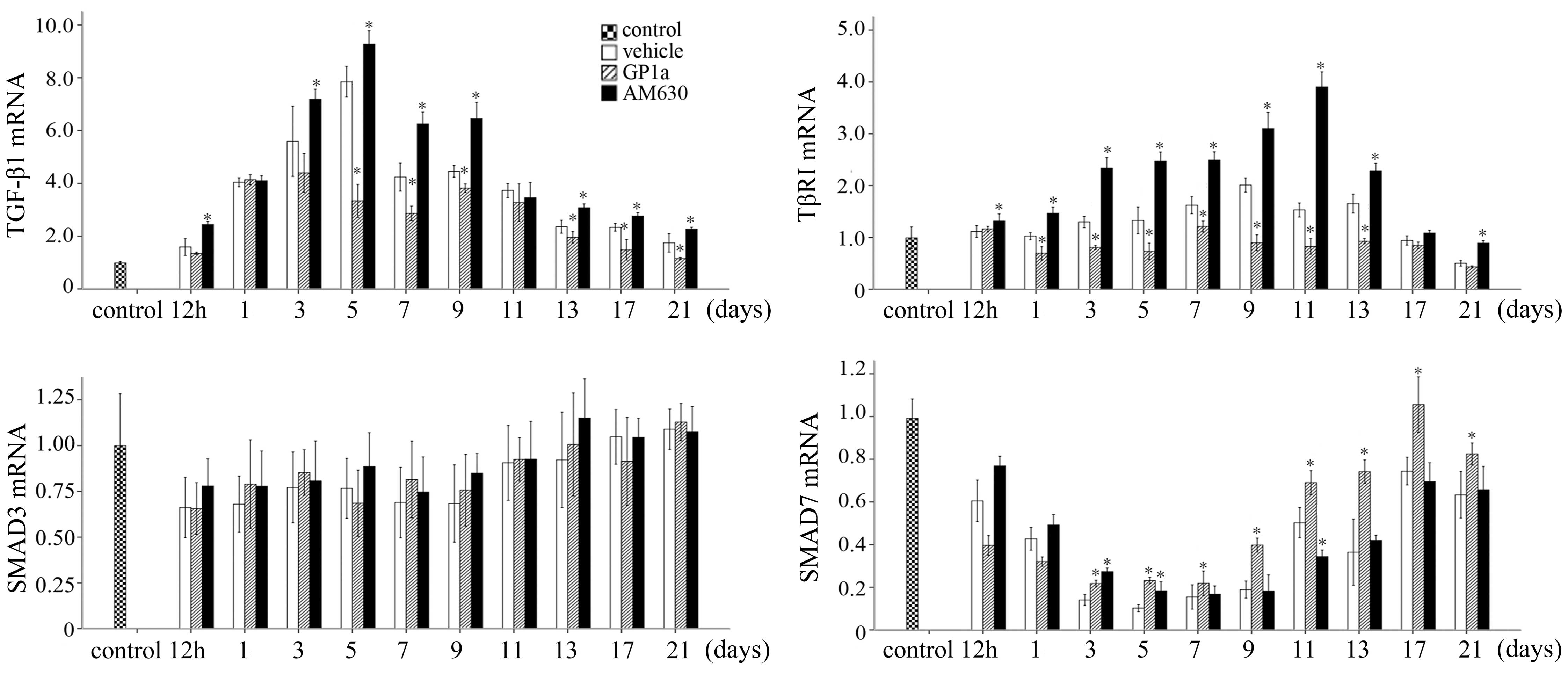
To evaluate the effects of GP1a and AM630 on the
TGF-β/Smad signaling, the relative mRNA expression levels of Tgfb1,
Tgfbr1, Smad3 and Smad7 in the skin specimens were assayed using
RT-qPCR at each of the post-traumatic intervals following excision
(Fig. 5). The mRNA expression of
Tgfb1 was upregulated markedly within 5 days, and reduced gradually
between 7 and 21 days post-injury. The expression of Tgfbr1
increased slowly and reached a peak at 9 days. The mRNA expression
of Smad3 was marginally and persistently raised at post-traumatic
intervals between 12 h and 21 days, whereas the mRNA expression of
Smad7 decreased between 12 h and 5 days, but elevated between 7 and
21 days. The mRNA expression levels of Tgfb1 and Tgfbr1 were
downregulated in the GP1a-treated mice, compared with the
vehicle-treated mice, whereas the opposite was noted in the mice
treated with AM630. In the GP1a group of mice, the mRNA expression
of Smad7 was upregulated between 3 and 21 days post-trauma.
Significant differences in Smad7 were detected between the AM630
and vehicle group at 3, 5 and 11 days post-traumatic interval. No
significant differences were identified among the vehicle, GP1a and
AM630 groups for the mRNA expression of Smad3 (Fig. 5).
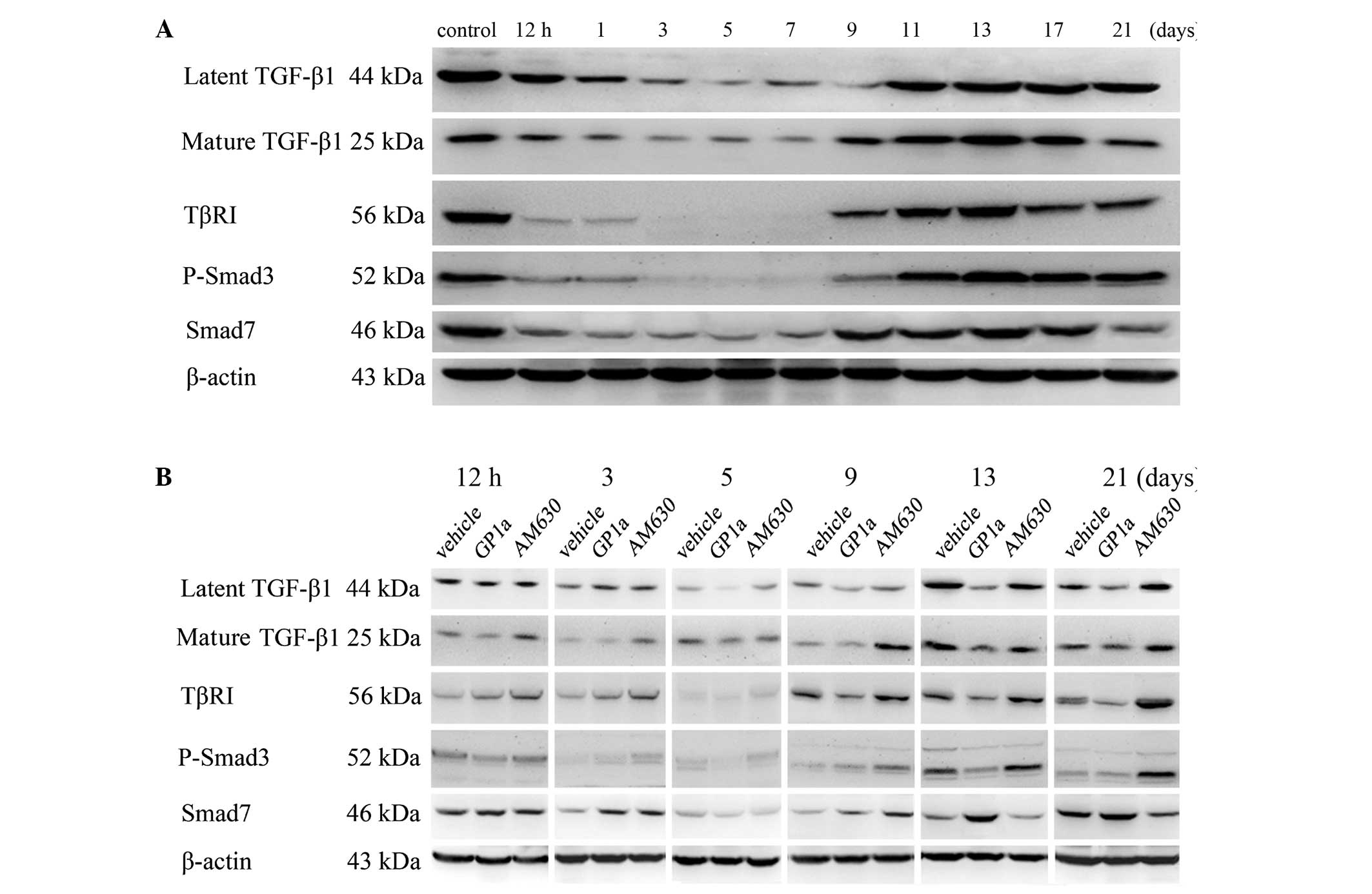
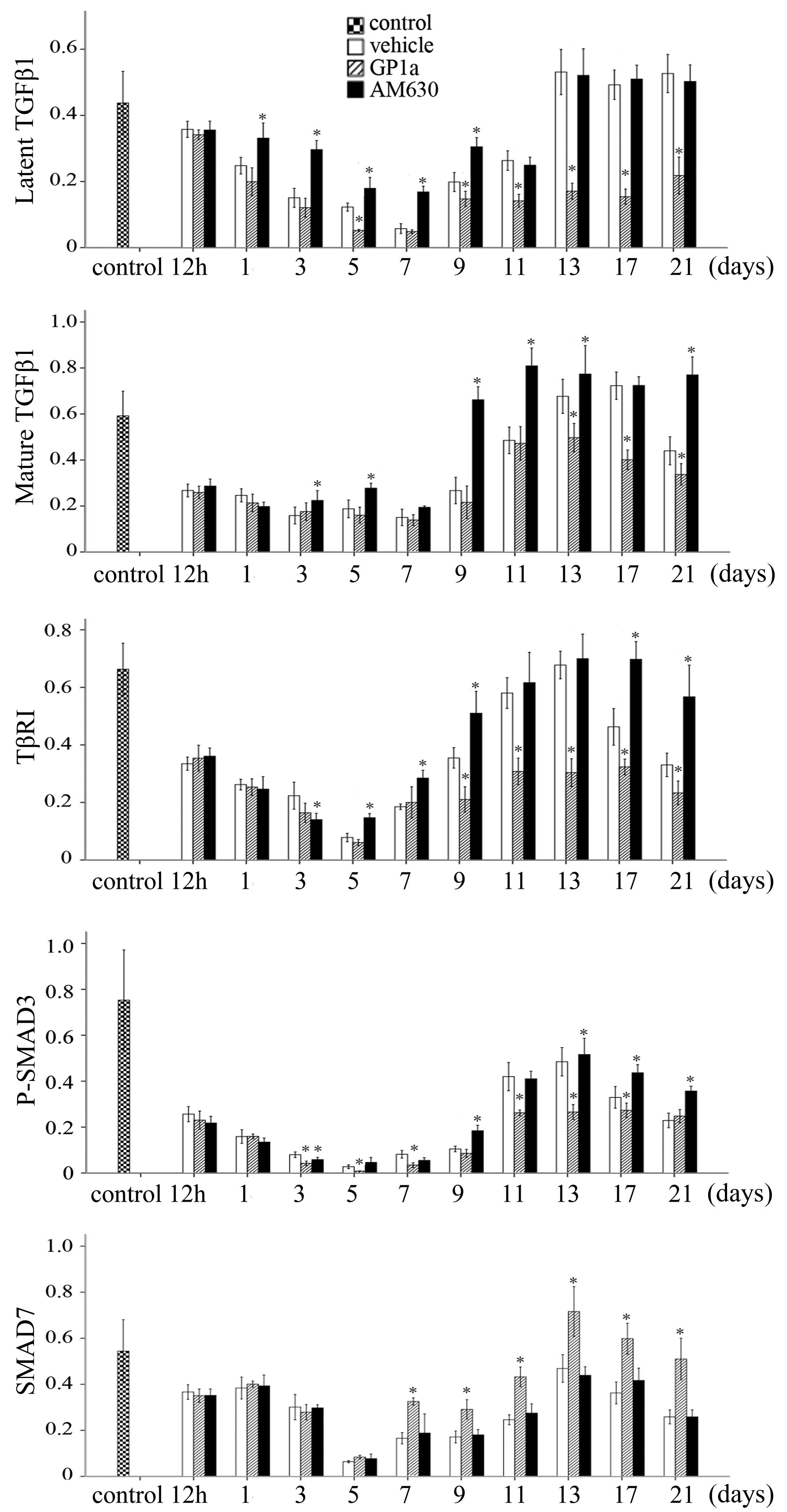
The results of the western blot analysis of TGF-β1,
TβRI, P-Smad3, Smad7 and β-actin for the vehicle-treated samples
are shown in Fig. 6A. The relative
protein expression levels of TGF-β1 (latent and mature), TβRI,
P-Smad3 and Smad7 gradually decreased between 12 h and 3 days, were
decreased at 5 days post-injury, gradually increased from day 7 and
peaked at day 13, prior to decreasing moderately (Fig. 7). The bands of TGF-β1, TβRI,
P-Smad3 and Smad7 proteins in the GP1a- and AM630-treated groups
are shown in Fig. 6B. Between 12 h
and 7 days post-injury, there was a marked upregulation of latent
TGF-β1 in the AM630-treated group, however, minimal change was
observed in the GP1a group, compared with the vehicle group. From 9
days onwards, the expression of latent TGF-β1 in the GP1a-treated
group was substantially lower, compared with that in the vehicle
group. In mature TGF-β1, TβRI and P-Smad3, no significant
differences were observed among the GP1a, AM630 and vehicle groups
between 12 h and 7 days. Between days 9 and 21, the expression
levels of mature TGF-β1, TβRI and P-Smad3 in the GP1a group were
significantly lower, compared with those in the vehicle group,
whereas these levels were increased in the AM630 group. However,
the results of Smad7 differed. Between 12 h and 5 days post-injury,
no significant differences were observed among the three groups.
Between days 7 and 21 days, Smad7 was markedly increased in the
GP1a-treated group, however, minimal change was observed in the
AM630-treated group, compared with the vehicle group (Fig. 7; P<0.05).
Discussion
The present study investigated the effects of GP1a
and AM630 on excisional wound healing in skin. The data
demonstrated that GP1a markedly attenuated fibrogenesis, whereas
AM630 enhanced fibrotic events during skin wound healing. In
addition, the expression levels of fibrosis-associated genes,
Col1a1, Col3a1 and Acta2 were downregulated by GP1a and upregulated
by AM630, indicating that cannabinoid CB2 receptors
modulated fibrogenesis in mouse skin wound repair. Our previous
study showed that cannabinoid CB2 receptors are detected
in the epidermis, hair follicles, sebaceous glands, cutaneous
muscle layer and vascular smooth muscle cells in the skin of mice,
and are dynamically expressed in neutrophils, macrophages and
myofibroblasts during skin wound healing in mice (5). The primary physiological function of
the cutaneous endocannabinoid system is to constitutively control
balanced proliferation, differentiation and survival, as well as
immune competence and/or tolerance of the skin cells (18). Previous studies have shown that
long-term cannabinoid CB2 receptor stimulation
ameliorates cirrhosis in rates subjected to bile duct ligation
(19) and the synthetic
cannabinoid, WIN55, 212-2 is capable of preventing skin fibrosis in
a mouse model of scleroderma (3).
TGF-β is a well-studied fibrotic cytokine and
originates from injured epidermis, blood and exudate. Platelets are
considered to be the primary source of activated TGF-β immediately
following injury (20). Previous
studies have demonstrated that cannabinoid CB2 receptor
activation attenuates the expression of TGF-β in hepatic fibrosis
(21), myocardial fibrosis
(22), subarachnoid hemorrhage
(23) and skeletal muscle
contusion (24), and disrupts
dermal fibrogenesis in a model of bleomycin-induced scleroderma by
promoting downregulation of the TGF-β signaling pathway (3). The role of TGF-β in wound healing has
been well characterized. Targeting the TGF-β signaling pathway
using therapeutic agents to improve wound healing and/or reduce
scarring has been successful in pre-clinical investigations
(25).
In the present study, TGF-β1 and TβRI were decreased
in the GP1a-treated group, but increased in the AM630-treated
group, indicating that cannabinoid CB2 receptors are
important in modulating the TGF-β signaling pathway. Smad proteins
are the downstream mediators of canonical TGF-β signaling. The
protein level of P-Smad3 was decreased in the GP1a-treated group
but increased in the AM630-treated group. However, no significant
difference was detected in the mRNA expression of Smad3 between the
post-traumatic intervals. These findings suggested that neither
GP1a or AM630 modulated the expression of Smad3 at the gene level.
As the Smad7 protein is induced by P-Smad3 but inhibits TGF-β
signaling by binding to activated TβRI and preventing Smad3
phosphorylation (26), the
increased level of Smad7 in fibrosis following treatment with GP1a
indicated the inhibition of TGF-β1/Smad3 signaling by negative
feedback. Although significant differences were detected between
the mRNA expression levels of Smad7 on 3, 5 and 11 days
post-traumatic interval, no significant differences in Smad7
protein expression were detected. Taken together, these data
suggested that cannabinoid CB2 receptors exerted a
regulatory effect on the TGF-β/Smad signaling pathway, although the
role of the endocannabinoid system in fibrogenesis remains to be
fully elucidated.
The results of the present study indicated that
cannabinoid CB2 receptors modulate fibrogenesis and the
TGF-β/Smad profibrotic signaling pathway during skin wound repair
in mice. These findings may improve current understanding of the
molecular mechanisms involved in the action of cannabinoid
CB2 receptors in skin wound healing, and offer a
potential therapeutic strategy.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81273342), a
research fund for the Doctoral Program funded by the Ministry of
Education of China (grant no. 20122104110025), and research funds
from the Shenyang Science and Technology Plan (grant no.
F12-277-1-03) and the Anshan Science and Technology Plan (grant no.
2060499).
References
|
1
|
Mackie K: Cannabinoid receptors: Where
they are and what they do. J Neuroendocrinol. 20(Suppl 1): S10–S14.
2008. View Article : Google Scholar
|
|
2
|
Guillot A, Hamdaoui N, Bizy A, Zoltani K,
Souktani R, Zafrani ES, Mallat A, Lotersztajn S and Lafdil F:
Cannabinoid receptor 2 counteracts interleukin-17-induced immune
and fibrogenic responses in mouse liver. Hepatology. 59:296–306.
2014. View Article : Google Scholar
|
|
3
|
Balistreri E, Garcia-Gonzalez E, Selvi E,
Akhmetshina A, Palumbo K, Lorenzini S, Maggio R, Lucattelli M,
Galeazzi M and Distler JW: The cannabinoid WIN55, 212-2 abrogates
dermal fibrosis in scleroderma bleomycin model. Ann Rheum Dis.
70:695–699. 2011. View Article : Google Scholar
|
|
4
|
Servettaz A, Kavian N, Nicco C, Deveaux V,
Chéreau C, Wang A, Zimmer A, Lotersztajn S, Weill B and Batteux F:
Targeting the cannabinoid pathway limits the development of
fibrosis and autoimmunity in a mouse model of systemic sclerosis.
Am J Pathol. 177:187–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng JL, Yu TS, Li XN, Fan YY, Ma WX, Du
Y, Zhao R and Guan DW: Cannabinoid receptor type 2 is
time-dependently expressed during skin wound healing in mice. Int J
Legal Med. 126:807–814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Artlett CM: Inflammasomes in wound healing
and fibrosis. J Pathol. 229:157–167. 2013. View Article : Google Scholar
|
|
7
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Finnson KW, Arany PR and Philip A:
Transforming growth factor beta signaling in cutaneous wound
healing: Lessons learned from animal studies. Adv Wound Care (New
Rochelle). 2:225–237. 2013. View Article : Google Scholar
|
|
9
|
Branton MH and Kopp JB: TGF-beta and
fibrosis. Microbes Infect. 1:1349–1365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zarruk JG, Fernández-López D,
García-Yébenes I, García-G utiérrez MS, Vivancos J, Nombela F,
Torres M, Burguete MC, Manzanares J, Lizasoain I and Moro MA:
Cannabinoid type 2 receptor activation downregulates stroke-induced
classic and alternative brain macrophage/microglial activation
concomitant to neuroprotection. Stroke. 43:1211–219. 2012.
View Article : Google Scholar
|
|
11
|
US Office of Science and Technology
Policy: Laboratory animal welfare: U.S. government principles for
the utilization and care of vertebrate animals used in testing,
research and training; notice. Fed Regist. 50:20864–20865.
1995.
|
|
12
|
Wang X, Ge J, Tredget EE and Wu Y: The
mouse excisional wound splinting model, including applications for
stem cell transplantation. Nat Protoc. 8:302–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galiano RD, Michaels J V, Dobryansky M,
Levine JP and Gurtner GC: Quantitative and reproducible murine
model of excisional wound healing. Wound Repair Regen. 12:485–492.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ansell DM, Campbell L, Thomason HA, Brass
A and Hardman MJ: A statistical analysis of murine incisional and
excisional acute wound models. Wound Repair Regen. 22:281–287.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guabiraba R1, Russo RC, Coelho AM,
Ferreira MA, Lopes GA, Gomes AK, Andrade SP, Barcelos LS and
Teixeira MM: Blockade of cannabinoid receptors reduces
inflammation, leukocyte accumulation and neovascularization in a
model of sponge-induced inflammatory angiogenesis. Inflamm Res.
62:811–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Del Fabbro L, Borges Filho C, Cattelan
Souza L, Savegnago L, Alves D, Henrique Schneider P, de Salles HD
and Jesse CR: Effects of Se-phenyl thiazolidine-4-carboselenoate on
mechanical and thermal hyperalgesia in brachial plexus avulsion in
mice: Mediation by cannabinoid CB1 and CB2 receptors. Brain Res.
1475:31–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gorantla S, Makarov E, Roy D, Finke-Dwyer
J, Murrin LC, Gendelman HE and Poluektova L: Immunoregulation of a
CB2 receptor agonist in a murine model of neuroAIDS. J Neuroimmune
Pharmacol. 5:456–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bíró T, Tóth BI, Haskó G, Paus R and
Pacher P: The endocannabinoid system of the skin in health and
disease: Novel perspectives and therapeutic opportunities. Trends
Pharmacol Sci. 30:411–420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahmoud MF, Swefy SE, Hasan RA and Ibrahim
A: Role of cannabinoid receptors in hepatic fibrosis and apoptosis
associated with bile duct ligation in rats. Eur J Pharmacol.
742:118–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brunner G and Blakytny R: Extracellular
regulation of TGF-beta activity in wound repair: Growth factor
latency as a sensor mechanism for injury. Thromb Haemost.
92:253–261. 2004.PubMed/NCBI
|
|
21
|
Lee TY, Lee KC and Chang HH: Modulation of
the cannabinoid receptors by andrographolide attenuates hepatic
apoptosis following bile duct ligation in rats with fibrosis.
Apoptosis. 15:904–914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Defer N, Wan J, Souktani R, Escoubet B,
Perier M, Caramelle P, Manin S, Deveaux V, Bourin MC, Zimmer A, et
al: The cannabinoid receptor type 2 promotes cardiac myocyte and
fibroblast survival and protects against
ischemia/reperfusion-induced cardiomyopathy. FASEB J. 23:2120–2130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujii M, Sherchan P, Krafft PR, Rolland
WB, Soejima Y and Zhang JH: Cannabinoid type 2 receptor stimulation
attenuates brain edema by reducing cerebral leukocyte infiltration
following subarachnoid hemorrhage in rats. J Neurol Sci.
342:101–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu T, Wang X, Zhao R, Zheng J, Li L, Ma W,
Zhang S and Guan D: Beneficial effects of cannabinoid receptor type
2 (CB2R) in injured skeletal muscle post-contusion. Histol
Histopathol. 30:737–749. 2015.PubMed/NCBI
|
|
25
|
Finnson KW, McLean S, Di Guglielmo GM and
Philip A: Dynamics of transforming growth factor beta signaling in
wound healing and scarring. Adv Wound Care (New Rochelle).
2:195–214. 2013. View Article : Google Scholar
|
|
26
|
Yan X and Chen YG: Smad7: Not only a
regulator, but also a cross-talk mediator of TGF-β signalling.
Biochem J. 434:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|