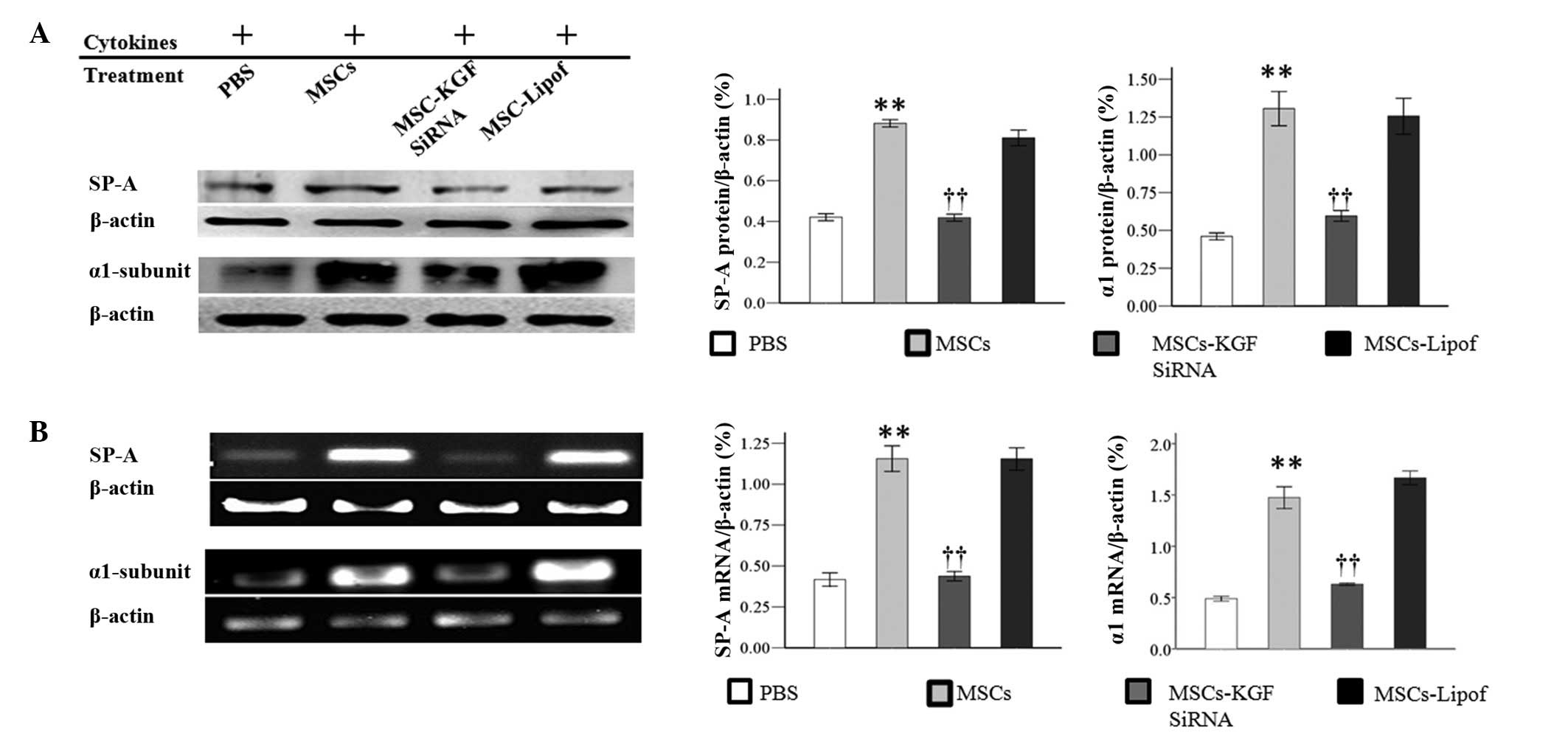
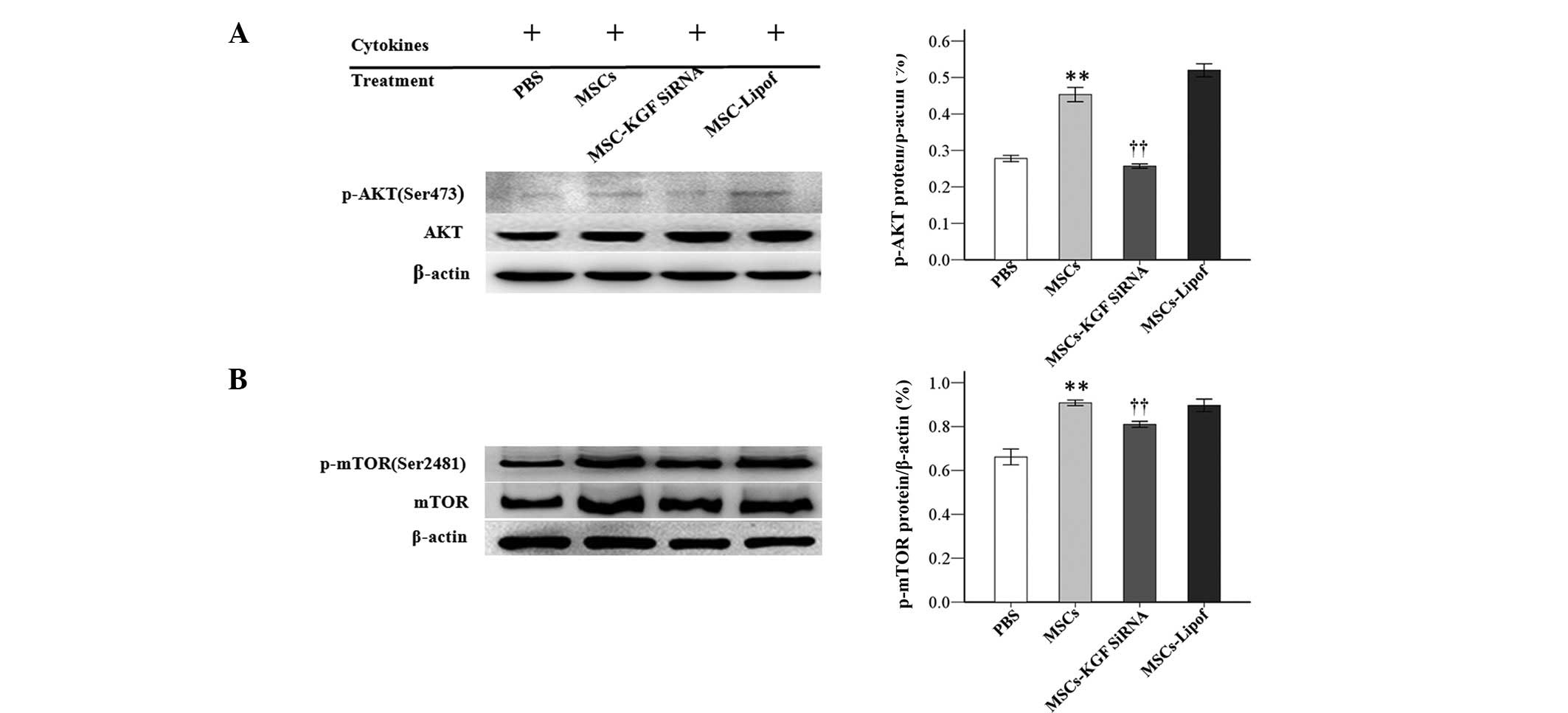
|
1
|
Matthay MA, Goolaerts A, Howard JP and Lee
JW: Mesenchymal stem cells for acute lung injury: Preclinical
evidence. Crit Care Med. 38(Suppl 10): S569–S573. 2010. View Article : Google Scholar
|
|
2
|
Lee JW, Gupta N, Serikov V and Matthay MA:
Potential application of mesenchymal stem cells in acute lung
injury. Expert Opin Biol Ther. 9:1259–1270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JW, Fang X, Dolganov G, Fremont RD,
Bastarache JA, Ware LB and Matthay MA: Acute lung injury edema
fluid decreases net fluid transport across human alveolar
epithelial type II cells. J Biol Chem. 282:24109–24119. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berthiaume Y and Matthay MA: Alveolar
edema fluid clearance and acute lung injury. Respir Physiol
Neurobiol. 159:350–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mutlu GM and Sznajder JI: Mechanisms of
pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol.
289:L685–L695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fehrenbach H: Alveolar epithelial type II
cell: Defender of the alveolus revisited. Respir Res. 2:33–46.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kotton DN, Ma BY, Cardoso WV, Sanderson
EA, Summer RS, Williams MC and Fine A: Bone marrow-derived cells as
progenitors of lung alveolar epithelium. Development.
128:5181–5188. 2001.PubMed/NCBI
|
|
8
|
Krause DS, Theise ND, Collector MI,
Henegariu O, Hwang S, Gardner R, Neutzel S and Sharkis SJ:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Bunnell BA, Painter RG, Quiniones
BC, Tom S, Lanson NA Jr, Spees JL, Bertucci D, Peister A, Weiss DJ,
et al: Adult stem cells from bone marrow stroma differentiate into
airway epithelial cells: Potential therapy for cystic fibrosis.
Proc Natl Acad Sci USA. 102:186–191. 2005. View Article : Google Scholar :
|
|
10
|
Mei SH, McCarter SD, Deng Y, Parker CH,
Liles WC and Stewart DJ: Prevention of LPS-induced acute lung
injury in mice by mesenchymal stem cells overexpressing
angiopoietin-1. PLoS Med. 4:e2692007. View Article : Google Scholar
|
|
11
|
Ortiz LA, Dutreil M, Fattman C, Pandey AC,
Torres G, Go K and Phinney DG: Interleukin 1 receptor antagonist
mediates the antiinflammatory and antifibrotic effect of
mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA.
104:11002–11007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotton DN, Fabian AJ and Mulligan RC:
Failure of bone marrow to reconstitute lung epithelium. Am J Respir
Cell Mol Biol. 33:328–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JW, Fang X, Krasnodembskaya A, Howard
JP and Matthay MA: Concise review: Mesenchymal stm cells for acute
lung injury: Role of paracrine soluble factors. Stem Cells.
29:913–919. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayes M, Curley G and Laffey JG:
Mesenchymal stem cells - a promising therapy for acute respiratory
distress syndrome. F1000. Med Rep. 4:22012.
|
|
15
|
Yang Y, Cheng Y, Lian QQ, Yang L, Qi W, Wu
DR, Zheng X, Liu YJ, Li WJ, Jin SW and Smith FG: Contribution of
CFTR to alveolar fluid clearance by lipoxin A4 via PI3K/Akt pathway
in LPS-induced acute lung injury. Mediators Inflamm.
2013:8626282013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semaeva E, Tenstad O, Bletsa A, Gjerde EA
and Wiig H: Isolation of rat trachea interstitial fluid and
demonstration of local cytokine production in
lipopolysaccharide-induced systemic inflammation. J Appl Physiol
(1985). 104:809–820. 2008. View Article : Google Scholar
|
|
17
|
Crosby LM and Waters CM: Epithelial repair
mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol.
298:L715–L731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panos RJ, Bak PM, Simonet WS, Rubin JS and
Smith LJ: Intratracheal instillation of keratinocyte growth factor
decreases hyperoxia-induced mortality in rats. J Clin Invest.
96:2026–2033. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hubbard PA, Moody CL and Murali R:
Allosteric modulation of Ras and the PI3K/AKT/mTOR pathway:
Emerging therapeutic opportunities. Front Physiol. 5:4782014.
View Article : Google Scholar
|
|
20
|
LoPiccolo J, Blumenthal GM, Bernstein WB
and Dennis PA: Targeting the PI3K/Akt/Mtor pathway: Effective
combinations and clinical considerations. Drug Resist Updat.
11:32–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: Acquisition of
epithelial-mesenchymal transition and cancer stem cell phenotypes
is associated with activation of the PI3K/Akt/mTOR pathway in
prostate cancer radioresistance. Cell Death Dis. 4:e8752013.
View Article : Google Scholar : PubMed/NCBI
|