Introduction
Researchers in the field of anesthesia are actively
investigating inhalation anesthetics due to their rapid effects,
protective effect against ischemic injury of vital organs,
including the heart, brain and kidneys, and possible neurotoxicity
(1). Previous in vitro and
in vivo studies at a molecular and cellular level suggested
that numerous ion channel receptors and classical neurotransmitters
may be potential molecular targets of general anesthetics (2–4).
Evidence regarding the neurotoxicity of inhalation anesthetics are
primarily from animal studies, and the mechanism of activation has
been investigated in certain experimental studies focussing upon
apoptosis (4–6).
Currently used inhalation anesthetics include
enflurane, halothane, isoflurane, sevoflurane, desflurane and
nitrous oxide (2,7). Isoflurane is a structural isomer of
enflurane, and a colorless transparent liquid with a pungent aroma
of ether (1). Isoflurane is widely
used as an inhalation anesthetic in the clinic due to chemical
stability, high efficiency, ease of regulating the depth of
anesthesia, low metabolic rate and excretion of the liver, low
toxicity to the kidneys and rapid, significant clinical effects
(7). Numerous anesthesiologists
consider it as the first choice of inhalational anesthetics
(2). Previous clinical
applications suggested that the effect of isoflurane on the nervous
system was beneficial (1).
Compared with other anesthetics, isoflurane suppresses the increase
of blood flow to the brain (7,8),
thus it may be used for brain surgery. Furthermore, the superficial
anesthesia of isoflurane does not affect the brain waves of
patients. Whether the anesthesia is repeated or extended, it has
been reported that it will not lead to lasting dysfunction of the
central nervous system (5).
Natural astaxanthin is a type of lutein, obtained by
oxidation of carotenoids, and it is abundant in certain species,
such as salmon and shellfish (9).
Previous studies investigated the chemical structure and excellent
performance of astaxanthin in anti-oxidation, anti-inflammatory,
antitumor, anti-Helicobacter pylori infection and anti-UV
processes (10,11). Whether astaxanthin reduces the
isoflurane-induced neuroapoptosis through the phosphatidylinositol
3-kinase (PI3K)/protein kinase B (Akt) pathway is undetermined. The
aim of the present study was to elucidate whether astaxanthin
reduces isoflurane-induced neuroapoptosis, utilizing validated
in vivo and in vitro models, and to explore its
possible mechanisms.
Materials and methods
In vivo model experiments and
grouping
A total of 30 male Sprague-Dawley rats (weight,
250–300 g; age, 7 days) were purchased from the Experimental Center
of Dalian Medical University (Dalian, China), and were maintained
at 22–25°C and 40–50% humidity, under a 12-h light/dark cycle with
ad libitum access to food and water. The rats were randomly
assigned into three groups as follows: i) Control group, rats were
administered saline by intraperitoneal injection (i.p.); ii) model
group, rats were exposed to 0.75% isoflurane (Sigma-Aldrich, St.
Louis, MO, USA) for 6 h in 25% oxygen or air in a
temperature-controlled chamber, and administered saline (i.p.); and
iii) treated group, randomly assigned rats from the model group
were further administered astaxanthin (100 mg/kg/day, i.p.; 97%
purity; Sigma-Aldrich). All groups were treated for 7 days.
Arterial blood gases and glucose levels in each group were detected
using an automated biochemistry analyzer (SMT100V; Robonik India
Pvt. Ltd., Maharashtra, India) at the Clinical Laboratory of The
First Affiliated Hospital of Dalian Medical University. The present
study was approved by the ethics committee of The First Affiliated
Hospital of Dalian Medical University.
Quantitative histology of in vivo
neurodegeneration in isofluorane-induced rats
An optical dissector and fractionator method (Stereo
Investigator System; MBF Bioscience, Williston, VT, USA) was
utilized to measure neurodegeneration in the rat hippocampi.
Briefly, following anesthetization with 1.5% sodium pentobarbital
(Sigma-Aldrich), the rats were sacrificed by decollation and the
rat hippocampi were harvested. A counting frame (0.05×0.05×0.05 mm)
and a high numerical aperture objective lens were used to visualize
the hippocampi neurons. Sampling of the hippocampus was performed
by randomly selecting 10–15 viewing fields for which the counting
frame was positioned to count at different focal levels (Stereo
Investigator System; Microbright Field, Williston, VT, USA).
Measurement of caspase-3 activity
Tissue and cell samples were dissociated with 10
volumes of tissue lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) and centrifuged at 12,300 × g for 10
min at 4°C. Protein concentrations were measured using the
bicinchoninic acid (BCA) protein assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) in accordance with the manufacturer's
instructions. Cleavage of chromogenic caspase substrate Ac-DEVD-pNA
and equal protein were mixed in accordance to manufacturer's
instructions (Promega Corporation, Madison, WI, USA), and used to
measure caspase-3 activity at 405 nm optical density with a
spectrophotometer (BioTek Synergy™ Microplate Reader; BioTek
Instruments, Inc., Winooski, VT, USA).
Western blot analysis of phosphorylated
(p)-Akt and Akt protein expression
Proteins were extracted and quantified using the BCA
protein assay kit as described above. Equal amounts of protein (50
µg) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinyl difluoride membranes (Amersham; GE Healthcare Life
Sciences, Chalfont, UK). Membranes were blocked with 5% non-fat
milk diluted in phosphate-buffered saline (PBS), and then were
incubated with rabbit anti-p-Akt (sc-33437; 1:2,000), anti-Akt
(sc-8312; 1:1,000; both Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) and anti-β-actin (D110007; 1:500; Sangon Biotech Co., Ltd.,
Shanghai, China) polyclonal antibodies overnight at 4°C. Membranes
were then washed with Tris-buffered saline and Tween-20 (Sangon
Biotech Co., Ltd.) for 2 h at room temperature, and incubated with
horseradish peroxidase-conjugated secondary antibody (SN134;
1:5,000; Sunshine Biotechnology Co., Ltd., Nanjing, China) for 2 h
at room temperature. The membranes were incubated with enhanced
chemiluminescence reagent (Amersham; GE Healthcare Life Sciences),
and protein expression was analyzed using ImageQuant TL software,
version 2003.03 (GE Healthcare Life Sciences).
In vitro model experiments and
grouping
A total of 24 male C57Bl/6 mice (age, 7–8 weeks;
weight, 250–300 g) were obtained from the SPF Animal Experiment
Center of Dalian Medical University. The mice were maintained at
22±1°C and 50–60% humidity under a 12-h light/dark cycle. The mice
were sacrificed by cervical dislocation following anesthetization
with 40 mg/kg sodium pentobarbital, after which organotypic
hippocampal tissue was immediately harvested and seeded into 25
cm2 plastic bottles to separate the cells. Cells were
cultivated in Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), for 24 h
in a humidified atmosphere enriched with 5% CO2, at
37°C. Following a 24-h cultivation, non-adherent cells were
discarded and 0.25% trypsin (Sunshine Biotechnology Co., Ltd.) was
utilized to transfer adherent cells into 25 cm2 plastic
bottles. Cells were cultivated with DMEM/F-12 (Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin/streptomycin (Sunshine Biotechnology Co., Ltd.) at
37°Control, untreated; ii) model group, the cells were treated with
0.75% isoflurane + 50 µM gabazine (Sigma-Aldrich) for 24 h;
iii) treated, the cells were treated with 0.75% isoflurane + 50
µM gabazine + 8 µM astaxanthin for 24 h; iv) treated
+ LY294002 (LP), the cells were treated with 20 µM LP
(Sigma-Aldrich) +0.75% isoflurane + 50 µM gabazine + 8
µM astaxanthin for 24 h.
Measurement of cell growth using MTT
Organotypic hippocampal cells were seeded into
96-well plates at a density of 1.5×103cells/well. MTT
(10 µl; Sangon Biotech Co., Ltd.) was added to the cells and
incubated for 4 h in a humidified atmosphere enriched with 5%
CO2 at 37°C. Dimethyl sulfoxide (150 µl;
Invitrogen; Thermo Fisher Scientific, Inc.) was added to each well
and plates were shaken for 20 min at room temperature. Absorbance
was measured at 450 nm with a microplate reader (R&D Systems,
Inc., Minneapolis, MN, USA).
Measurement of cell apoptosis using flow
cytometry
Organotypic hippocampal cells were seeded into
6-well plates at a density of 1–2×106cells/well. Cells
were then washed twice with ice-cold PBS, and 50 µl lysis
buffer was added to each well. Annexin V-fluorescein isothiocyanate
(5 µl) and propidium iodide (5 µl; BD Biosciences,
San Jose, CA, USA) staining was performed, according to the
manufacturer's instructions. Flow cytometry (FACScan; BD
Biosciences) and CellQuest Pro software, version 5.1 (BD
Biosciences) were used to analyze cell apoptosis.
Statistical analysis
Results were analyzed with SPSS software, version 17
(SPSS, Inc., Chicago, IL, USA) using one-way analysis of variance,
followed by Dunnett's post hoc test. Data are expressed as the mean
± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
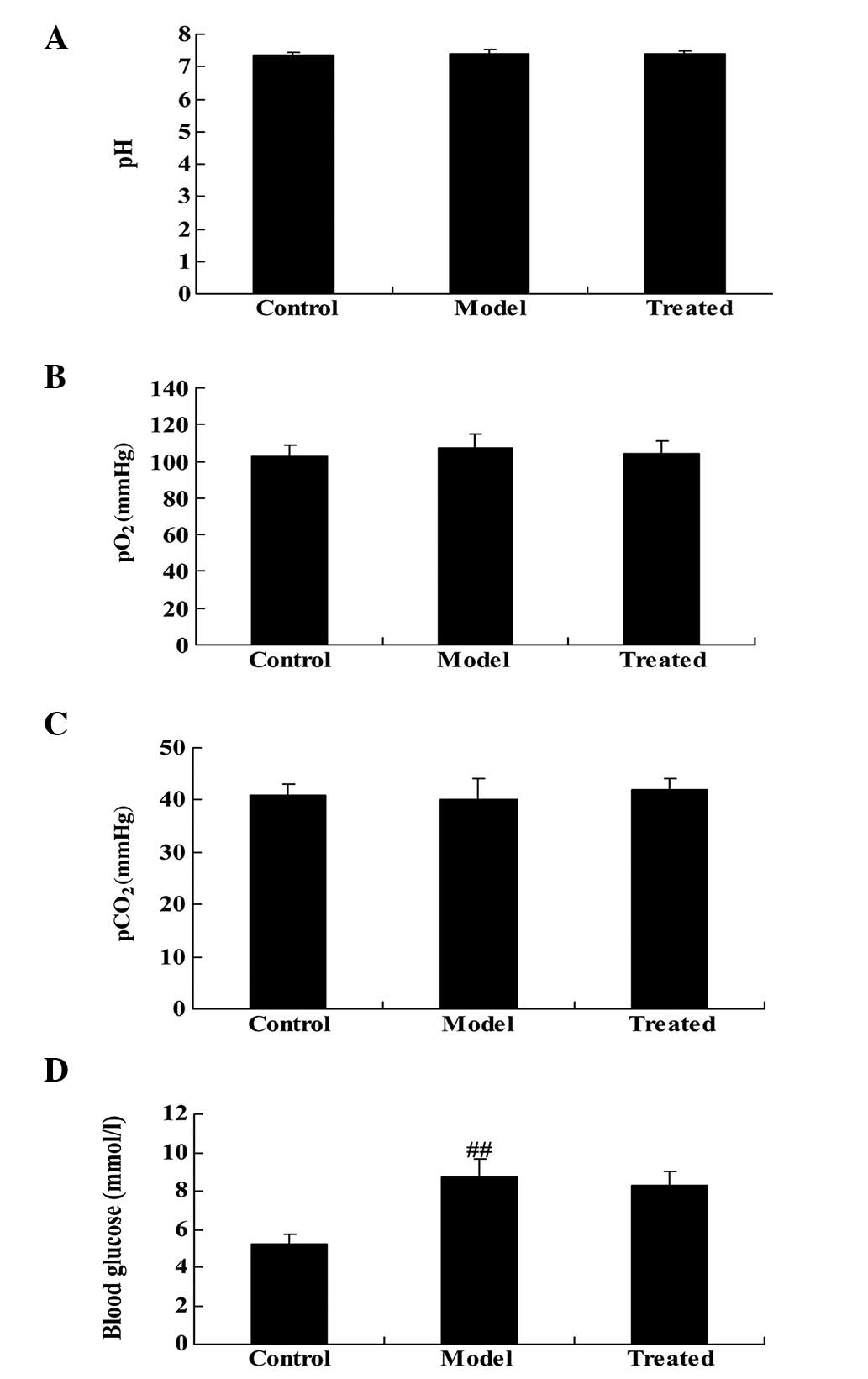
Effect of astaxanthin on arterial blood
gases and glucose levels
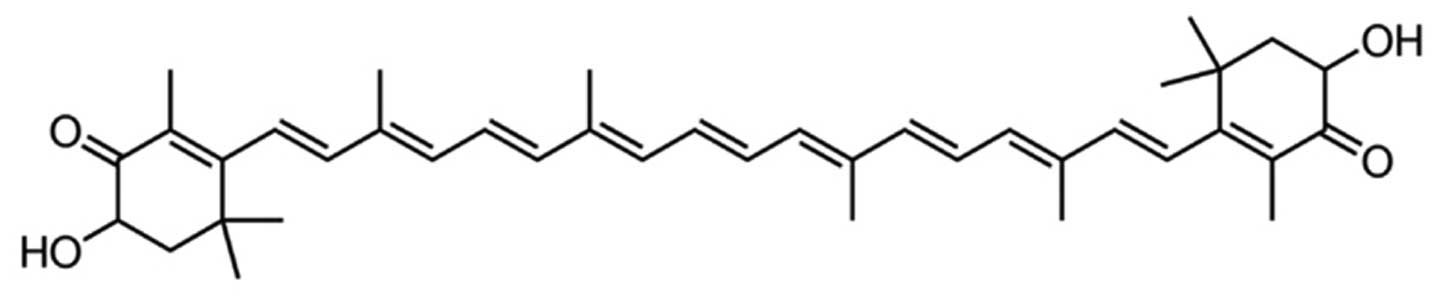
The chemical structure of astaxanthin is presented
in Fig. 1. As demonstrated in
Fig. 2A–C, in vivo
experiments detected no significant differences among groups for
arterial blood gases (pH, pO2 and pCO2).
Blood glucose levels were significantly increased in the model
group compared with the control group (P<0.01), and markedly
increased in the astaxanthin-treated group (Fig. 2D).
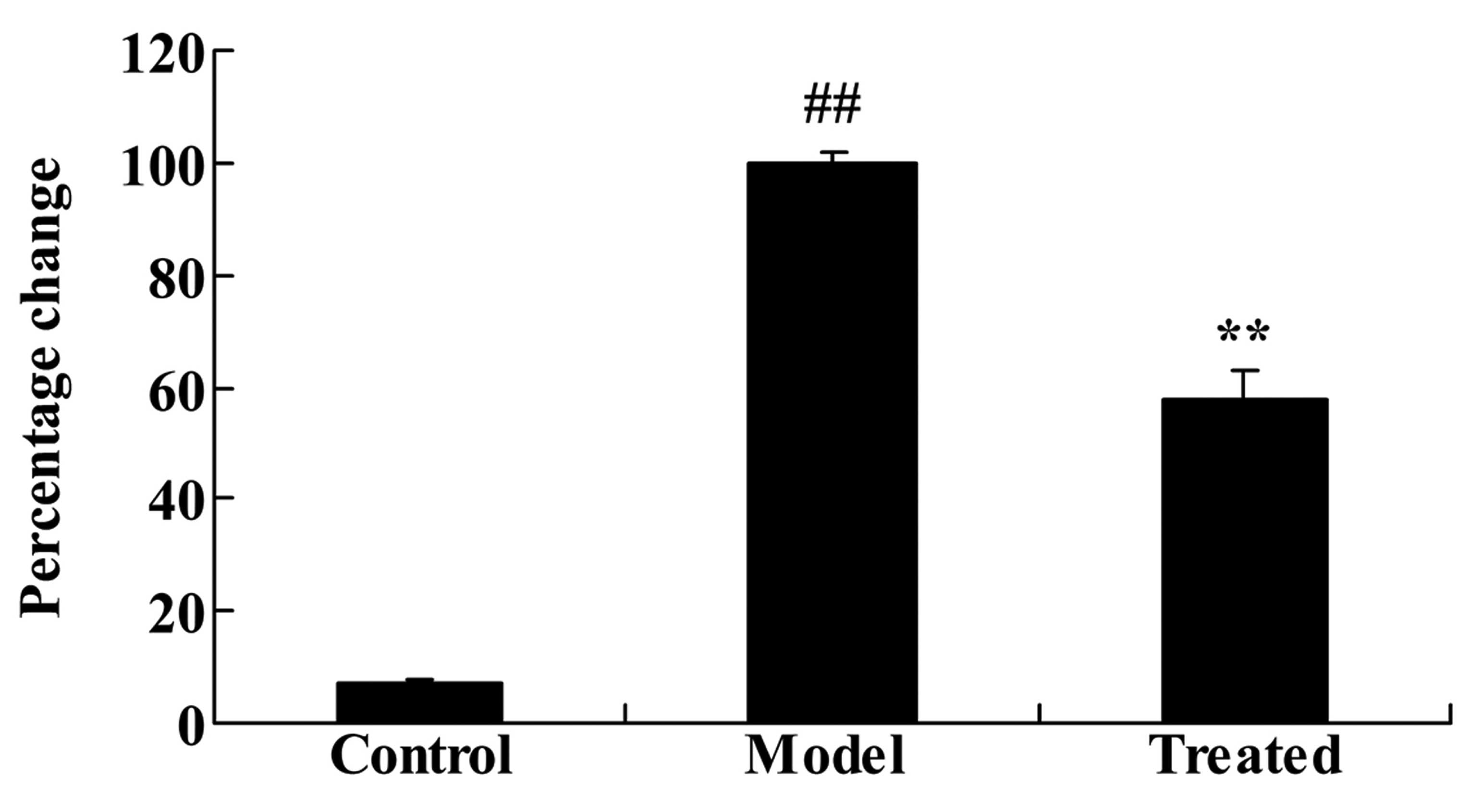
Astaxanthin protects against
isoflurane-induced brain damage in Sprague-Dawley rats
As demonstrated in Fig.
3, administration of isoflurane significantly increased the
rate of neuronal cell apoptosis compared with the control group
(P<0.01). Additional treatment with astaxanthin significantly
reduced the isoflurane-induced brain damage, compared with the
model group (P<0.01; Fig.
3).
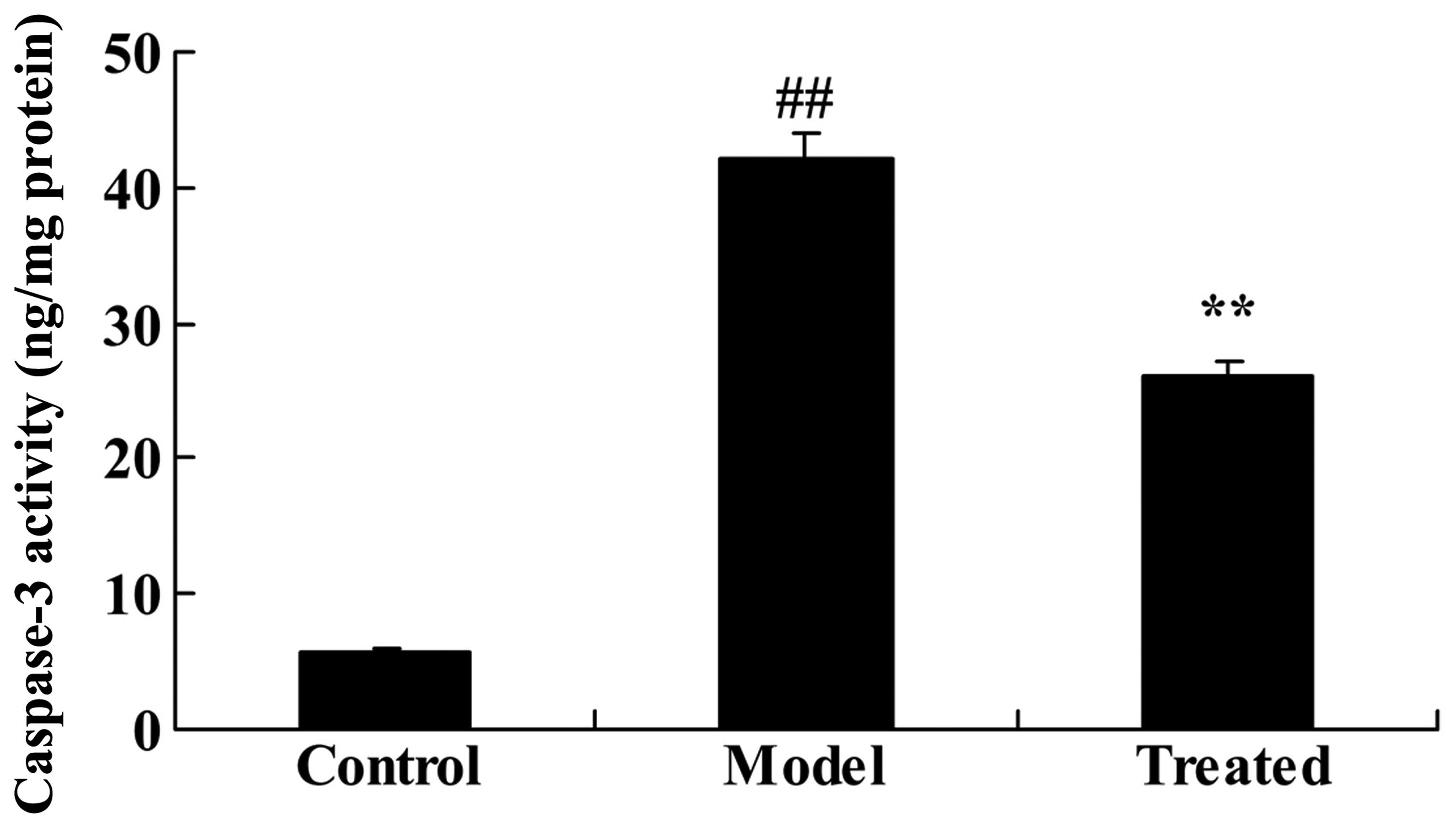
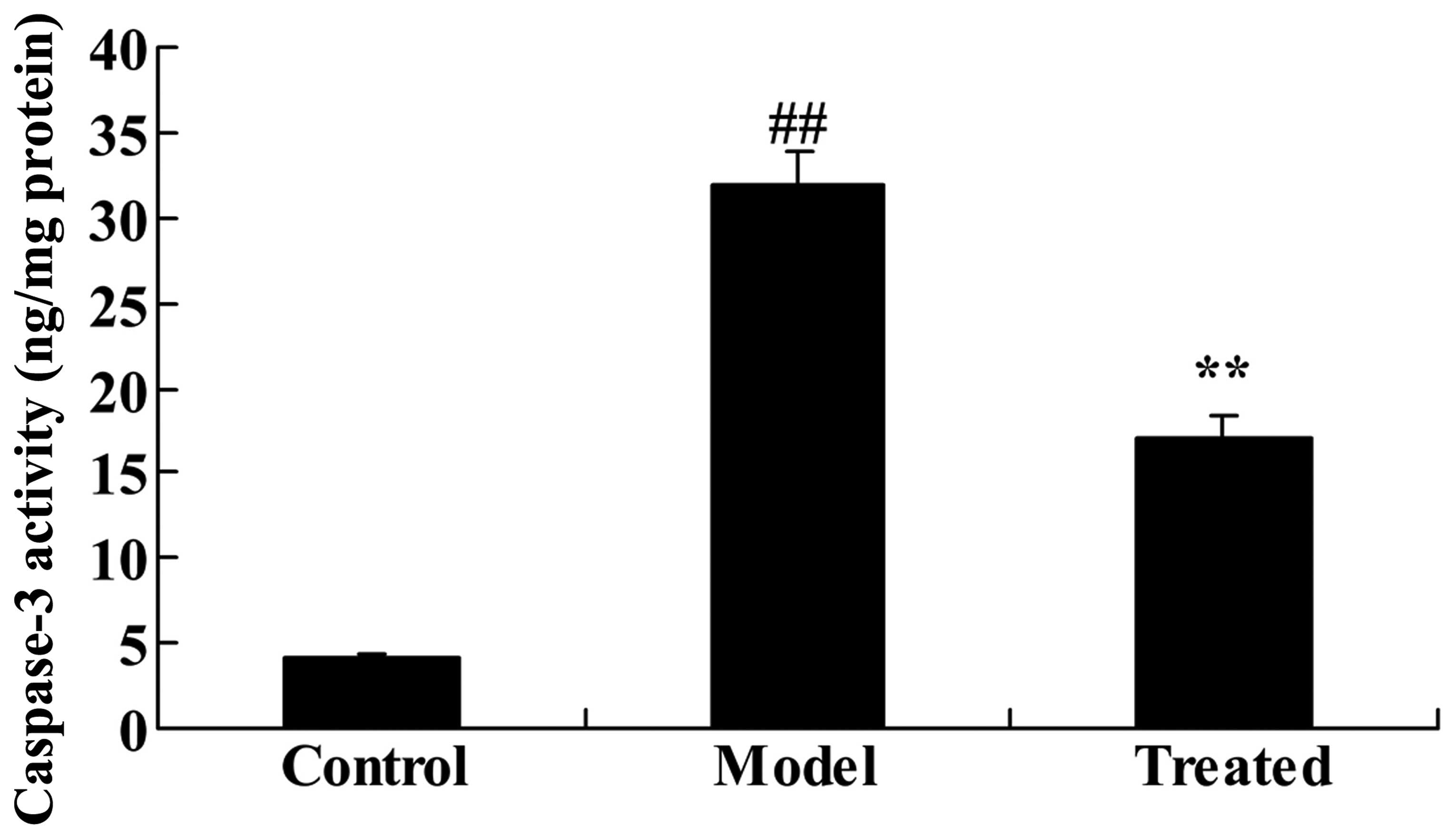
The effect of astaxanthin on caspase-3
activity in isoflurane-treated rats
As demonstrated in Fig.
4, caspase-3 activity was significantly increased following
isoflurane treatment, compared with the control group (P<0.01).
Further treatment with astaxanthin significantly suppressed the
isoflurane-induced caspase-3 activity, compared with the model
group (P<0.01; Fig. 4).
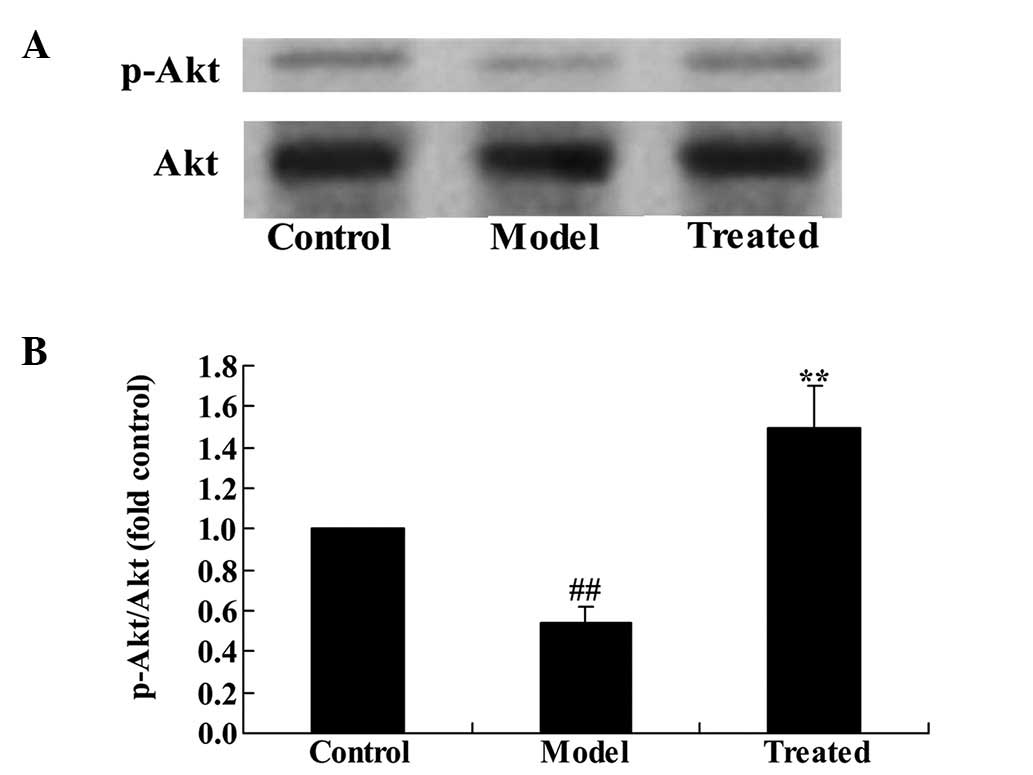
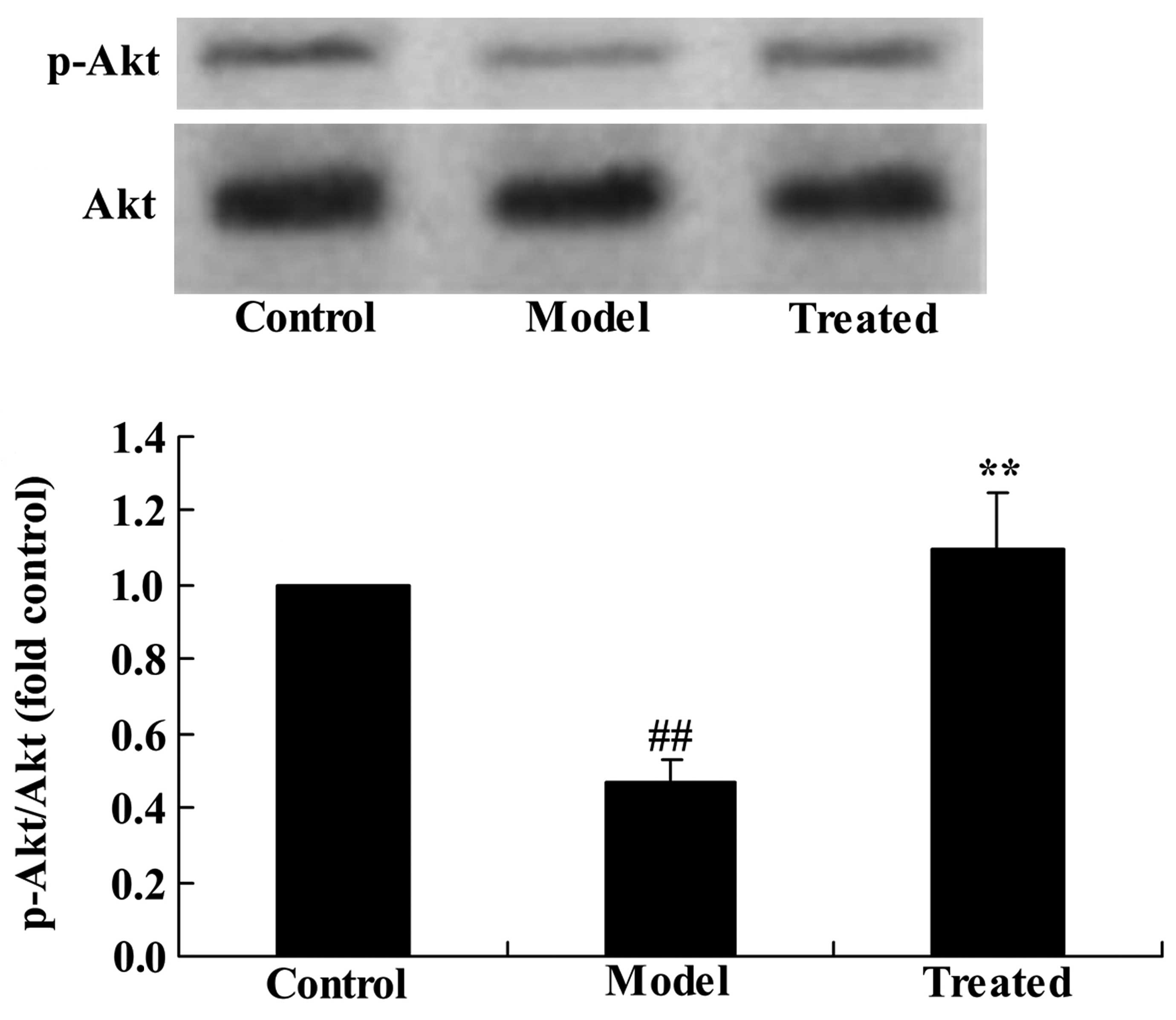
The effect of astaxanthin on the PI3K/Akt
signaling pathway in isoflurane-treated rats
To determine whether astaxanthin protects against
isoflurane-induced brain damage in rats, p-Akt and Akt protein
expression levels were determined using western blotting. The
results demonstrated that the p-Akt/Akt ratio was significantly
downregulated in the isoflurane-treated group compared with the
control group (P<0.01; Fig. 5).
Additional treatment with astaxanthin significantly upregulated the
effect of isoflurane treatment compared with the model group
(P<0.01; Fig. 5).
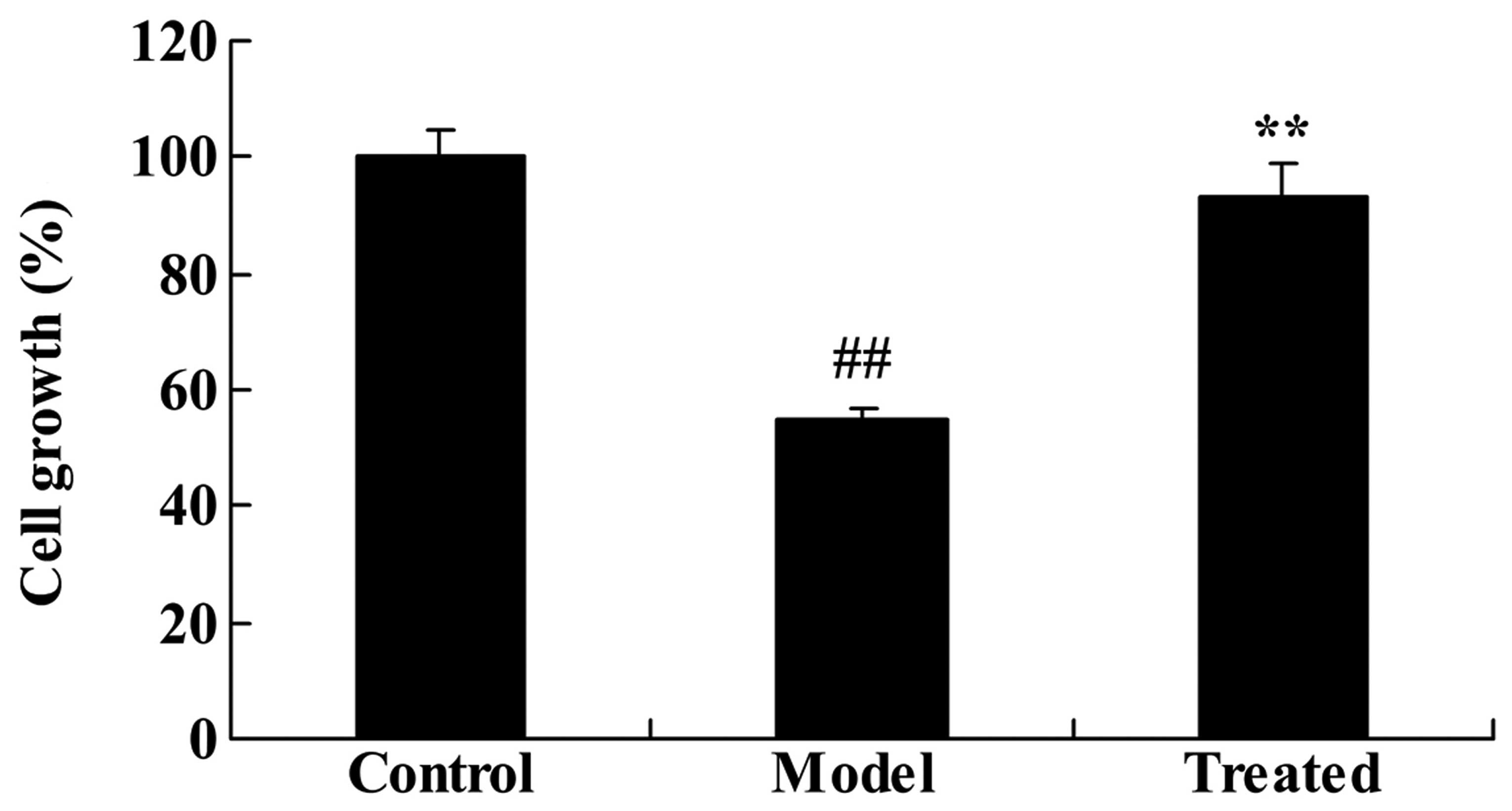
The effect of astaxanthin on the cell
growth of isoflurane-treated rats
As demonstrated in Fig.
6, the in vitro experiments indicated that isoflurane
treatment significantly reduced organotypic hippocampal cell
growth, compared with the control group (P<0.01). Further
treatment with astaxanthin significantly reversed the
isoflurane-suppressed cell growth, compared with the model
group.
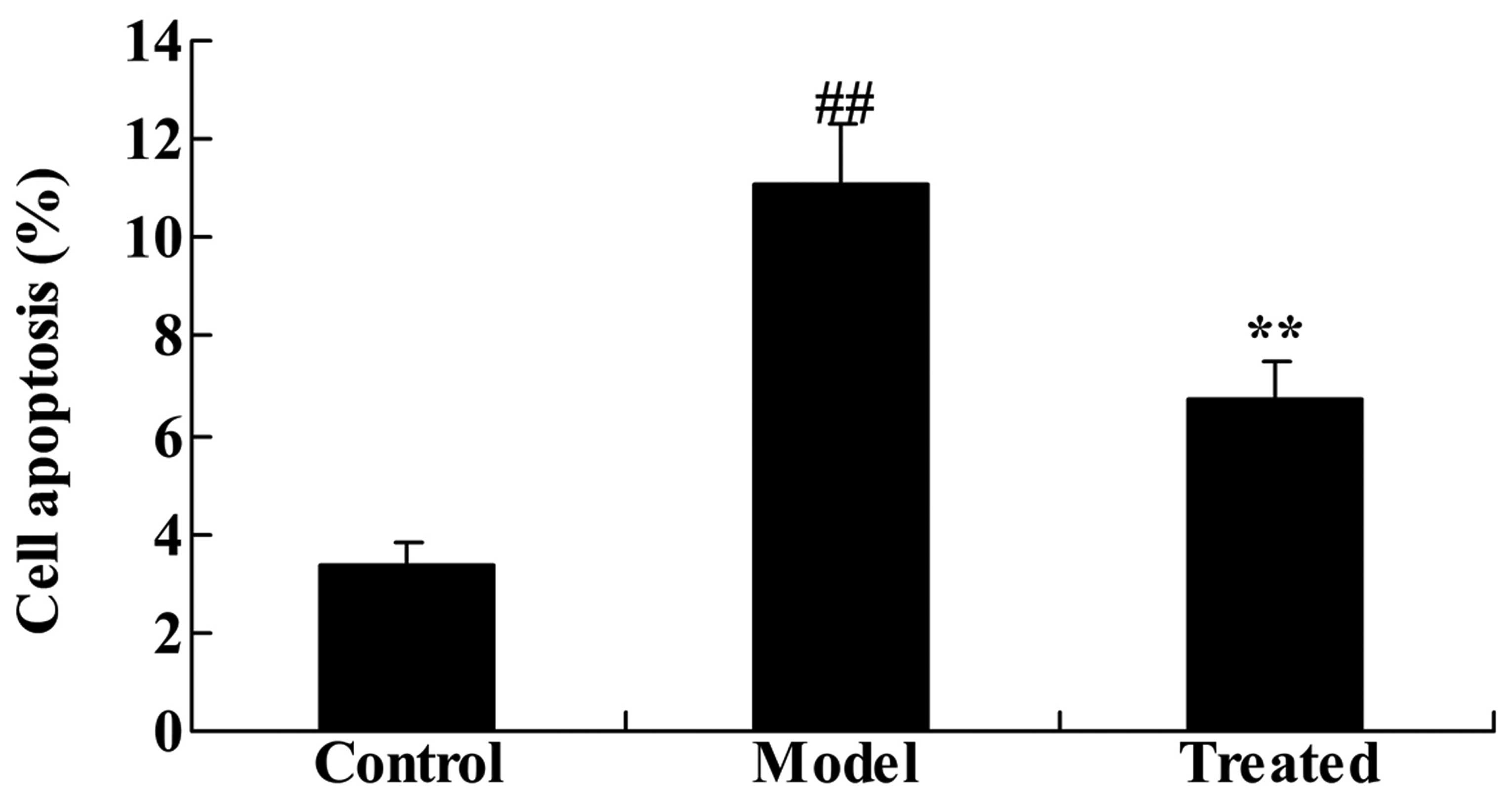
The effect of astaxanthin on the cell
apoptosis of isoflurane-treated rats
As demonstrated in Fig.
7 the in vitro experiments indicated that treatment with
isoflurane significantly increased cell apoptosis, compared with
the control group (P<0.01). Administration of astaxanthin
significantly reduced the effect of isoflurane-treatment, compared
with the model group (P<0.01; Fig.
7).
The effect of astaxanthin reduces the
isoflurane-induced caspase-3 activity in mouse organotypic
hippocampal cells
Isoflurane treatment significantly induced the
caspase-3 activity compared with the control group (P<0.01;
Fig. 8). Furthermore,
supplementary treatment with astaxanthin significantly suppressed
the isoflurane-induced caspase-3 activity compared with the model
group (P<0.01; Fig. 8).
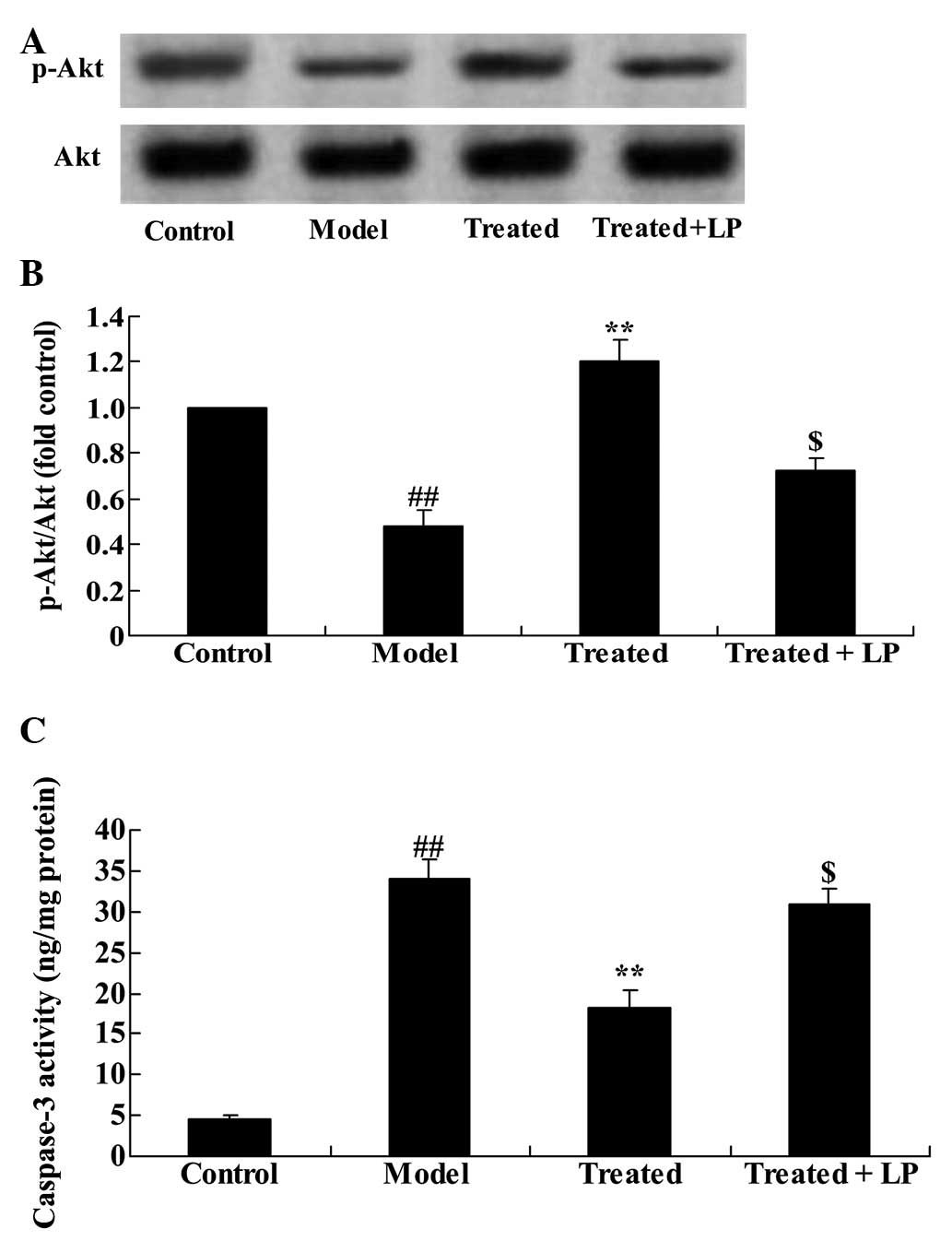
Astaxanthin activates the
isoflurane-suppressed PI3K/Akt pathway in mouse organotypic
hippocampal cells
To explore whether the anti-apoptotic effects of
astaxanthin protect against the isoflurane-induced PI3K/Akt pathway
activation in organotypic hippocampal cells, p-Akt and Akt protein
expression levels were determined with western blotting. The
results indicated that isoflurane treatment significantly
suppressed the p-Akt/Akt ratio compared with the control group
(P<0.01; Fig. 9). In addition,
supplementary treatment with astaxanthin significantly activated
the isoflurane-suppressed PI3K/Akt pathway, compared with the model
group (P<0.01; Fig. 9).
Downregulation of the PI3K/Akt pathway
reduces the effect of astaxanthin and protects against
isoflurane-induced neuroapoptosis
To further assess the effect of the PI3K/Akt
pathway, LY294002 (20 µM) + isoflurane were administered to
the organotypic hippocampal cells and they were incubated for 24 h.
Administration of LY294002 significantly inhibited the effect of
astaxanthin on the p-Akt/Akt ratio (Fig. 10A and B). Furthermore, treatment
with LY294002 significantly reduced the effect of isoflurane
treatment on caspase-3 activity compared with the model group
(Fig. 10C).
Discussion
Neuronal apoptosis resulting from the use of
inhalation anesthetics is detected in the early and late stages of
nervous system development (7).
Inhalation anesthetics do not exert obvious toxic effects in the
late stage (12), due to the
difficulty in inducing neuronal damage (13). In addition, in the early stage,
particularly the stage of rapid formation of synapses, inhalation
anesthetics may activate the cell death pathway (14), and lead to disruption of synaptic
remodeling and differentiation and maturation of axons (15). Neurotoxicity due to calcineurin
(CN) inhibitors has a significant impact on the healing and quality
of life of patients (15). The
neurotoxicity of CN inhibitors has complex mechanisms and clinical
manifestations (16). Elucidation
of the mechanisms of neurotoxicity, and development in clinical
prevention remain emphases (12,13).
The present study demonstrated that astaxanthin significantly
reduced the isoflurane-induced brain damage. Franceschelli et
al (17) demonstrated that
astaxanthin protects against stimulation of U937 cells with
lipopolysaccharide, reducing O2-production through
suppression of oxidative stress. Lu et al (18) demonstrated that astaxanthin
protects against neuron loss through suppression of oxidative
stress in the adult rat hippocampus. The results of these studies
suggest that astaxanthin may reduce isoflurane-induced
neuroapoptosis.
During the developmental process of neurons, the
formation of new neurons is cross-linked with each other between
networks (16). Isoflurane
interferes with the formation of neuronal networks during the
developmental process to hinder the maturation and differentiation
of neurons (19). The effect of
inhalation anesthetics on the developing nervous system is not
limited to neural degeneration and induced neuroapoptosis (20). Other mechanisms are involved in the
toxic isoflurane-induced effect. Numerous studies confirm that
pretreatment with isoflurane for a short period of time results in
a protective effect (16,21,22).
If the time of exposure is extended, there can be toxic effects. A
previous study suggested that pretreatment with isoflurane is
associated with neuroprotection and cardioprotection (23). Therefore, it is suggested that
isoflurane has neuroprotective effects and neurotoxic effects
simultaneously (24). The effect
of isoflurane is dependent on its concentration, exposure time and
the patient tolerance (24). The
results of the present study demonstrated that astaxanthin
significantly inhibited the isoflurane-induced caspase-3 activity
in vivo and in vitro. These results are consistent
with those of Lee et al (25) who demonstrated that astaxanthin
protects against MPP-induced mitochondrial dysfunction through
inhibition of the activation of caspase-3 in vivo and in
vitro. Chan et al (26)
indicated that the neuroprotective effects of astaxanthin
alleviated H2O2- or MPP+-induced
cell death of PC12 cells. Based on these results, the
anti-apoptotic effect of astaxanthin is suggested to serve an
important role in the treatment of isoflurane-induced
neuroapoptosis.
Neuroapoptosis is an important form of programmed
cell death subsequent to anesthesia with isoflurane (27). The activation of signal
transduction processes during the early stage of apoptosis is
required for neuroapoptosis. An early intervention to the signaling
pathways may result in suppression of neuroapoptosis and protection
against brain damage (23). The
PI3K/Akt signaling pathway has been shown to promote cell survival
(28). Neuroprotective agents
targeting Akt have an effect on neuroapoptosis following anesthesia
with isoflurane (7). The PI3K/Akt
signaling pathway is activated by stimulation of endogenous and
exogenous neurotrophic factors (7). The present study demonstrated that
astaxanthin significantly upregulated the PI3K/Akt pathway in the
isoflurane-treated rats and mouse hippocampal cells. Furthermore,
downregulation of the PI3K/Akt pathway reduced the effects of
astaxanthin against isoflurane induced neuroapoptosis in
vitro. Guo et al (29)
suggested that astaxanthin attenuates early acute kidney injury
through the Akt/Bad/Caspases signaling cascade. Li et al
(30) indicated that astaxanthin
protects the ARPE-19 cells through activation of the PI3K/Akt
pathway. In conclusion, the results of the present study indicated
that astaxanthin reversed isoflurane-induced neuroapoptosis through
activation of the PI3K/Akt signaling pathway, in vivo and
in vitro.
References
|
1
|
Johnson SA, Young C and Olney JW:
Isoflurane-induced neuroapoptosis in the developing brain of
nonhypoglycemic mice. J Neurosurg Anesthesiol. 20:21–28. 2008.
View Article : Google Scholar
|
|
2
|
Deng M, Hofacer RD, Jiang C, Joseph B,
Hughes EA, Jia B, Danzer SC and Loepke AW: Brain regional
vulnerability to anaesthesia-induced neuroapoptosis shifts with age
at exposure and extends into adulthood for some regions. Br J
Anaesth. 113:443–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Chen X, Zhang J, Zhao Y, Li S, Tan
L, Gao J, Fang X and Luo A: Glycyrrhizin attenuates
isoflurane-induced cognitive deficits in neonatal rats via its
anti-inflammatory activity. Neuroscience. 316:328–336. 2016.
View Article : Google Scholar
|
|
4
|
Brambrink AM, Back SA, Riddle A, Gong X,
Moravec MD, Dissen GA, Creeley CE, Dikranian KT and Olney JW:
Isoflurane-induced apoptosis of oligodendrocytes in the neonatal
primate brain. Ann Neurol. 72:525–535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cattano D, Williamson P, Fukui K, Avidan
M, Evers AS, Olney JW and Young C: Potential of xenon to induce or
to protect against neuroapoptosis in the developing mouse brain.
Can J Anaesth. 55:429–436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ge HW, Hu WW, Ma LL and Kong FJ:
Endoplasmic reticulum stress pathway mediates isoflurane-induced
neuroapoptosis and cognitive impairments in aged rats. Physiol
Behav. 151:16–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Zeng M, Chen W, Liu C, Wang F, Han
X, Zuo Z and Peng S: Dexmedetomidine reduces isoflurane-induced
neuroapoptosis partly by preserving PI3K/Akt pathway in the
hippocampus of neonatal rats. PLoS One. 9:e936392014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Creeley CE, Dikranian KT, Dissen GA, Back
SA, Olney JW and Brambrink AM: Isoflurane-induced apoptosis of
neurons and oligodendrocytes in the fetal rhesus macaque brain.
Anesthesiology. 120:626–638. 2014. View Article : Google Scholar :
|
|
9
|
Manunta C: Astaxanthin in insects and
other terrestrial arthropods. Nature. 162:2981948. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ambati RR, Phang SM, Ravi S and
Aswathanarayana RG: Astaxanthin: Sources, extraction, stability,
biological activities and its commercial applications - a review.
Mar Drugs. 12:128–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fassett RG and Coombes JS: Astaxanthin in
cardiovascular health and disease. Molecules. 17:2030–2048. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Yuan Z, Liu B, Sailhamer EA, Shults
C, Velmahos GC, Demoya M and Alam HB: Prevention of hypoxia-induced
neuronal apoptosis through histone deacetylase inhibition. J
Trauma. 64:863–870; discussion 870–871. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rivera LR, Thacker M, Pontell L, Cho HJ
and Furness JB: Deleterious effects of intestinal
ischemia/reperfusion injury in the mouse enteric nervous system are
associated with protein nitrosylation. Cell Tissue Res.
344:111–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yel L, Brown LE, Su K, Gollapudi S and
Gupta S: Thimerosal induces neuronal cell apoptosis by causing
cytochrome c and apoptosis-inducing factor release from
mitochondria. Int J Mol Med. 16:971–977. 2005.PubMed/NCBI
|
|
15
|
Payette DJ, Xie J, Shirwany N and Guo Q:
Exacerbation of apoptosis of cortical neurons following traumatic
brain injury in par-4 transgenic mice. Int J Clin Exp Pathol.
1:44–56. 2008.PubMed/NCBI
|
|
16
|
Fujimura M and Usuki F: Methylmercury
causes neuronal cell death through the suppression of the TrkA
pathway: In vitro and in vivo effects of TrkA pathway activators.
Toxicol Appl Pharmacol. 282:259–266. 2015. View Article : Google Scholar
|
|
17
|
Franceschelli S, Pesce M, Ferrone A, De
Lutiis MA, Patruno A, Grilli A, Felaco M and Speranza L:
Astaxanthin treatment confers protection against oxidative stress
in U937 cells stimulated with lipopolysaccharide reducing
O2-production. PLoS One. 9:e883592014. View Article : Google Scholar
|
|
18
|
Lu Y, Xie T, He XX, Mao ZF, Jia LJ, Wang
WP, Zhen JL and Liu LM: Astaxanthin rescues neuron loss and
attenuates oxidative stress induced by amygdala kindling in adult
rat hippocampus. Neurosci Lett. 597:49–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agarwal B, Camara AK, Stowe DF, Bosnjak ZJ
and Dash RK: Enhanced charge-independent mitochondrial free Ca(2+)
and attenuated ADP-induced NADH oxidation by isoflurane:
Implications for cardioprotection. Biochim Biophys Acta.
1817:453–465. 2012. View Article : Google Scholar :
|
|
20
|
Zhong Y, Liang Y, Chen J, Li L, Qin Y,
Guan E, He D, Wei Y, Xie Y and Xiao Q: Propofol inhibits
proliferation and induces neuroapoptosis of hippocampal neurons in
vitro via downregulation of NF-κB p65 and Bcl-2 and upregulation of
caspase-3. Cell Biochem Funct. 32:720–729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng Y and Levy RJ: Subclinical carbon
monoxide limits apoptosis in the developing brain after isoflurane
exposure. Anesth Analg. 118:1284–1292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Makaryus R, Lee H, Yu M, Zhang S, Smith
SD, Rebecchi M, Glass PS and Benveniste H: The metabolomic profile
during isoflurane anesthesia differs from propofol anesthesia in
the live rodent brain. J Cereb Blood Flow Metab. 31:1432–1442.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lang XE, Wang X and Jin JH: Mechanisms of
cardioprotection by isoflurane against I/R injury. Front Biosci
(Landmark Ed). 18:387–393. 2013. View
Article : Google Scholar
|
|
24
|
Bai T, Dong DS and Pei L: Resveratrol
mitigates isoflurane-induced neuroapoptosis by inhibiting the
activation of the Akt-regulated mitochondrial apoptotic signaling
pathway. Int J Mol Med. 32:819–826. 2013.PubMed/NCBI
|
|
25
|
Lee DH, Kim CS and Lee YJ: Astaxanthin
protects against MPTP/MPP+-induced mitochondrial dysfunction and
ROS production in vivo and in vitro. Food Chem Toxicol. 49:271–280.
2011. View Article : Google Scholar
|
|
26
|
Chan KC, Mong MC and Yin MC: Antioxidative
and anti-inflammatory neuroprotective effects of astaxanthin and
canthaxanthin in nerve growth factor differentiated PC12 cells. J
Food Sci. 74:H225–H231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kajimoto M, Atkinson DB, Ledee DR, Kayser
EB, Morgan PG, Sedensky MM, Isern NG, Des Rosiers C and Portman MA:
Propofol compared with isoflurane inhibits mitochondrial metabolism
in immature swine cerebral cortex. J Cereb Blood Flow Metab.
34:514–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo LY, Li YM, Qiao L, Liu T, Du YY, Zhang
JQ, He WT, Zhao YX and He DQ: Notch2 regulates matrix
metallopeptidase 9 via PI3K/AKT signaling in human gastric
carcinoma cell MKN-45. World J Gastroenterol. 18:7262–7270. 2012.
View Article : Google Scholar
|
|
29
|
Guo SX, Zhou HL, Huang CL, You CG, Fang Q,
Wu P, Wang XG and Han CM: Astaxanthin attenuates early acute kidney
injury following severe burns in rats by ameliorating oxidative
stress and mitochondrial-related apoptosis. Mar Drugs.
13:2105–2123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y,
Hou D and Zhang X: Astaxanthin protects ARPE-19 cells from
oxidative stress via upregulation of Nrf2-regulated phase II
enzymes through activation of PI3K/Akt. Mol Vis. 19:1656–1666.
2013.PubMed/NCBI
|