Introduction
Stroke is the predominant cause of mortality and a
long-term disability worldwide (1). Ischemic stroke is more common than
hemorrhagic stroke and accounts for >80% of all stroke cases
(2). Angiogenesis is associated
with neurological functional recovery and it has been demonstrated
to be critical in improving brain recovery following ischemic
insults (3,4).
Increasing evidence demonstrated that microRNAs
(miRNAs or miRs) are involved in stroke (5). Peng et al (6) demonstrated that in the pathogenesis
of hypoxic-ischemic brain damage, numerous miRNAs were
downregulated, in particular miR-140 was downregulated >2 fold.
miR-140 has been investigated extensively in cancer research
(7–11), however, its role in angiogenesis
following ischemic stroke remains unclear.
In the present study, the expression of miR-140-5p
and vascular endothelial growth factor A (VEGFA) was examined in a
rat model of middle cerebral artery occlusion (MCAO). Furthermore,
the present study demonstrated the role of miR-140-5p in
angiogenesis and the molecular mechanism mediated by VEGFA in an
in vitro model for brain ischemia.
Materials and methods
Permanent rat model of MCAO
The present study was approved by the Ethics
Committee of Liaocheng People's Hospital (Shandong, China). All
animal experiments were performed in compliance with the guidelines
for the Care and Use of Laboratory Animals published by the
National Institutes of Health (12). Sprague-Dawley male rats (n=32),
weighing 250–300 g, aged 6–8 weeks old were purchased from SLAC
Laboratory Animal, Inc. (Shanghai, China). Rats were housed in
individual cages with of a 12-h light/dark cycle and a controlled
temperature (22–24°C), with free access to food and fresh water.
The rats were sacrificed at 12, 24 and 48 h following treatments
via an intraperitoneal injection of pentobarbital (200 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA). Each rat was anesthetized with
10% chloral hydrate (4 ml/kg; Shuanghe, Ltd., Beijing, China)
intraperitoneally. Following the exposure of the right common
carotid artery, a 4-0 nylon monofilament coated with a silicone tip
was inserted into the internal carotid artery until mild resistance
was felt, to occlude the middle cerebral artery. Rats in the MCAO
group (n=24) were divided into three subgroups based on the
duration of ischemia for 12, 24 and 48 h. Rats in the sham group
(n=8) underwent anesthesia and surgery without the occlusion of the
middle cerebral artery. Following MCAO, neurological function was
tested, and the score was evaluated as previously described
(13). Rats with scores of 1–3
were included.
Cell culture
Human umbilical vein endothelial cells (HUVECs) and
human embryonic kidney (HEK)293 cells were purchased from American
Type Culture Collection (Manassas, VA, USA). HEK293 cells and
HUVECs in normoxic conditions were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) at 37°C, in a humidified atmosphere of 95% air
and 5% CO2. HUVECs in hypoxic conditions were cultured
in DMEM with FBS at 37°C, in a humidified atmosphere of 94%
N2, 5% CO2 and 1% O2.
Cell transfection
The miRNA control, miR-140-5p mimic and miR-140-5p
inhibitor were synthesized by Bioneer (Shanghai, China). The
pEGFP-C1 plasmid was purchased from Clontech Laboratories, Inc.
(Mountain View, CA, USA). Transfection of miRNA control, miR-140-5p
mimic, miR-140-5p inhibitor, pEGFP-C1 plasmid and VEGFA-pEGFP-C1
was performed in HUVECs and/or HEK293 cells using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The oligonucleotides and plasmids were
transfected at a concentration of 20 and 50 nM, respectively.
Briefly, a day prior to transfection, the cells were seeded into
6-well plates. Lipofectamine (10 µl) and
oligonucleotides/plasmids were mixed in 250 µl Opti-MEM
(Invitrogen; Thermo Fisher Scientific, Inc.) and incubated at room
temperature for 20 min to form a complex. The cells were incubated
with the complex at 37°C for 6 h, and the medium was subsequently
replaced with fresh medium.
Luciferase reporter assay
The wild type VEGFA-3′ untranslated region (UTR) and
mutant VEGFA-3′UTR were cloned into the psiCHECK-2 vector (Promega
Corporation, Madison, WI, USA). A luciferase reporter assay was
performed in HEK293 cells. The miRNA control, miR-140-5p mimic and
psiCHECK-2-UTR vectors were transfected into the cells using
Lipofectamine, as described above. The cells were lysed 24 h
following the transfection and the luciferase activity was
determined by the Dual-Luciferase Reporter Assay system (Promega
Corporation), according to the manufacturer's protocol.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The cells were plated into 96-well plates and
allowed to grow for 24, 48 and 72 h prior to incubation with 10
µl MTT solution (0.5 mg/ml; Beyotime Institute of
Biotechnology, Shanghai, China) for 4 h at 37°C. The formazan
crystals were solubilized in dimethyl sulfoxide (Sigma-Aldrich) and
the optical density was measured at 570 nm using a microplate
reader (SpectraMax M2e; Molecular Devices, LLC, Sunnyvale, CA,
USA).
Transwell migration assay
A Transwell migration assay was performed using a
6-well Transwell plate with 8.0 µm pore polycarbonate
membrane inserts (Corning, Inc., Corning, NY, USA). The HUVECs were
suspended and seeded at a density of 5×104 cells/ml into
the upper chambers with serum-free medium. Culture medium with 10%
FBS was added to the lower chambers. Following 24 h incubation, the
cells that migrated were fixed in 95% ethanol and stained with
hematoxylin solution (Sigma-Aldrich) for 15 min at room
temperature. The cells were then counted under a TS100 inverted
microscope (Nikon Corporation, Tokyo, Japan).
In vitro tube formation assay
Pre-cooled Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA) was added to the 24-well plates and allowed to solidify at
37°C for 30 min. The cells were subsequently suspended and seeded
at a density of 4×105 cells/ml into the 24-well plates.
Following incubation at 37°C, in 5% CO2 for 18 h, the
formation of tubes was observed under the microscope and images
were captured (Olympus Corporation, Tokyo, Japan). The tube lengths
were analyzed using Image-Pro Plus software, version 5.0 (BD
Biosciences) in five randomly selected fields per well.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA from the ipsilateral ischemic cerebral
cortex and HUVECs was extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). For miRNA isolation, the mirVana
miRNA Isolation kit (Ambion; Thermo Fisher Scientific, Inc.) was
used, according to the manufacturer's protocol. The total RNA (1
µg) was reverse-transcribed into cDNA using the Transcriptor
First Strand cDNA Synthesis kit (Roche Diagnostics, Basel,
Switzerland). The primer sequences used were as follows:
miR-140-5p, forward: 5′-ACACTCCAGCTGGGCAGTGGTTTTACCCTA-3′ and
reverse: 5′-TGGTGTCGTGGAGTCG-3′; U6, forward:
5′-CTCGCTTCGGCAGCACA-3′ and reverse: 5′-AACGCTTCACGAATTTGCGT-3′.
PCR was performed using a SYBR Green PCR kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) in a 7300 Sequence Detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.), with an
initial denaturation step at 95°C for 5 min, followed by 40 cycles
of amplification (95°C for 30 sec, 60°C for 30 sec and 72°C for 40
sec) and a final extension step at 72°C for 10 min. The relative
mRNA expression of miR-140-5p was calculated using the
2−ΔΔCt method and normalized to the U6 expression
(14).
Western blot analysis
The total protein was extracted from the ipsilateral
ischemic cerebral cortex and cultured cells using ice-cold lysis
buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 2
mM EDTA and protease inhibitor cocktail). Following centrifugation
at 10,000 × g for 15 min at 4°C, the supernatant was collected and
the protein concentration was determined using the Pierce BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.). A total of 20
µg protein was separated by 12% SDS-PAGE and transferred
onto polyvinylidene fluoride membranes (EMD Millipore, Billerica,
MA, USA). The membranes were blocked with 5% non-fat milk in
Tris-buffered saline, containing 0.01% Tween-20 at 4°C overnight.
Following blocking, the membranes were incubated at 37°C for 2 h
with VEGFA (dilution: 1:800; cat. no: sc-53462, mouse monoclonal;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and β-actin
(dilution: 1:1,000; cat. no: sc-47778, mouse monoclonal; Santa Cruz
Biotechnology, Inc.) primary antibodies. Following washing with
phosphate-buffered saline, the membranes were incubated with the
corresponding horseradish peroxidase-conjugated secondary antibody
(dilution: 1:2,000; cat. no: BA1051, goat polyclonal; Wuhan Boster
Biological Technology, Ltd., Wuhan, China). The reaction was
detected using the Pierce ECL Western Blotting Substrate (Thermo
Fisher Scientific, Inc.). The band intensity was quantified using
Image J 1.48u software (National Institutes of Health, Bethesda,
MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 19.0 (IBM SPSS, Chicago, IL, USA). The data are
presented as the mean ± standard deviation. Student's t-test was
used to identify differences between the two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
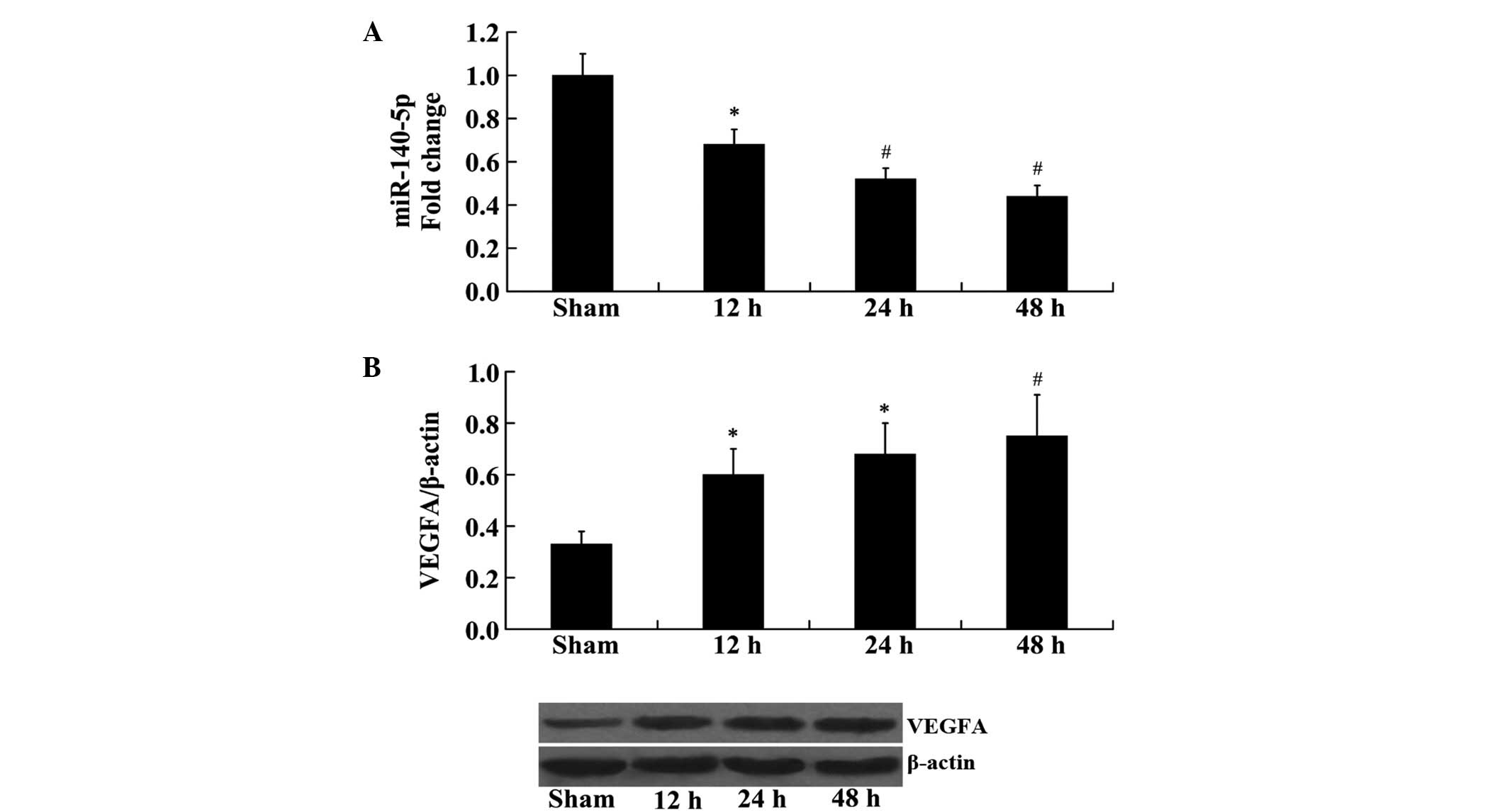
Expression of miR-140-5p and VEGFA in rat
cerebral tissue following MCAO
RT-qPCR and western blot analyses were used to
determine changes in the expression of miR-140-5p and VEGFA in the
rat brain following MCAO at 12, 24 and 48 h. The results from
RT-qPCR analysis demonstrated that the expression of miR-140-5p was
significantly reduced at 12, 24 and 48 h following MCAO, compared
with the sham group (P<0.05, P<0.01 and P<0.01,
respectively; Fig. 1A). However,
western blot analysis demonstrated that the protein expression
levels of VEGFA were significantly increased at 12, 24 and 48 h
following MCAO, compared with the sham group (P<0.05, P<0.05
and P<0.01, respectively; Fig.
1B).
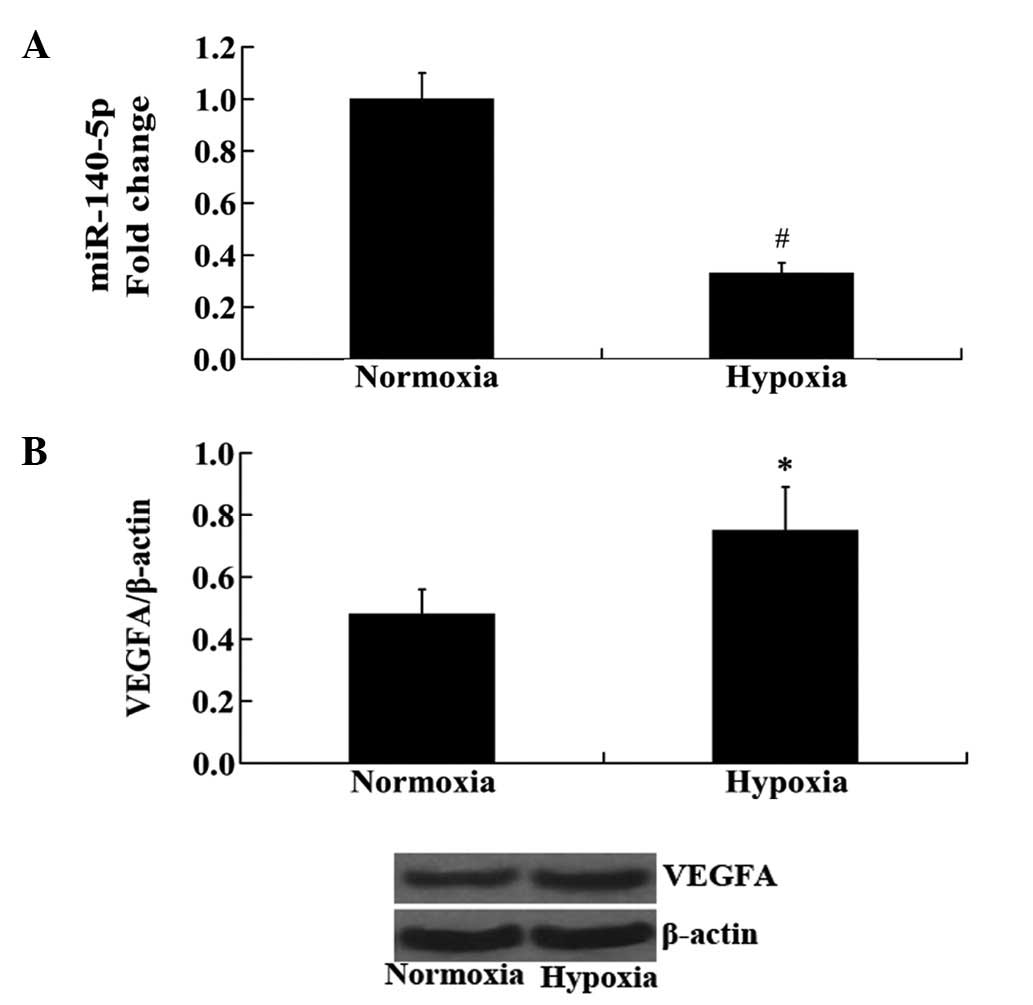
Expression of miR-140-5p and VEGFA in
HUVECs following hypoxia
HUVECs were exposed to hypoxia and the changes in
the expression levels of miR-140-5p and VEGFA were measured using
RT-qPCR and western blot analyses. Consistent with the results from
the in vivo experiment in rat cerebral tissue, the
expression of miR-140-5p was significantly decreased, while the
protein expression levels of VEGFA were significantly increased in
hypoxic HUVECs compared with those in normoxic HUVECs (P<0.01
and P<0.05, respectively; Fig.
2).
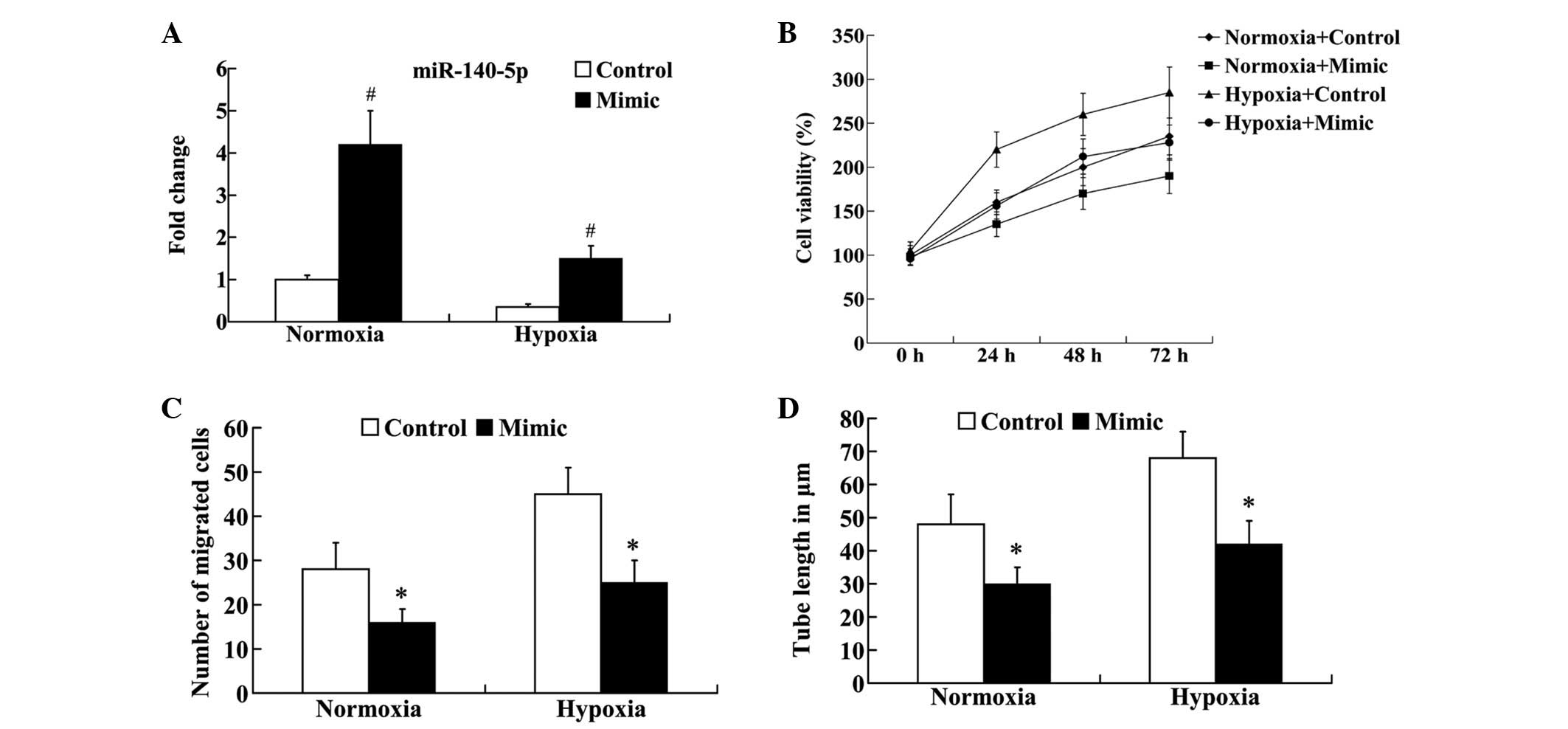
Effect of miR-140-5p on the in vitro
angiogenesis of HUVECs following hypoxia
To investigate the effect of miR-140-5p on
angiogenesis, the miR-140-5p mimic was transfected into normoxic
and hypoxic HUVECs. RT-qPCR analysis demonstrated that, compared
with the control group, the expression of miR-140-5p was
significantly increased in normoxic and hypoxic HUVECs following
transfection (P<0.01; Fig. 3A).
Subsequently, cell viability, migration and tube formation were
examined. An MTT assay demonstrated that cell viability was
markedly decreased in normoxic and hypoxic HUVECs following
transfection (Fig. 3B). As
demonstrated in Fig. 3C, the
migrated cell number was significantly different between the
control and the miR-140-5p mimic groups (P<0.05). The results
suggested that the enhanced expression of miR-140-5p led to
decreased cell migration in the normoxic and hypoxic HUVECs. In
addition, compared with the control group, the tube length was
significantly decreased in the normoxic and hypoxic HUVECs
following transfection (P<0.05; Fig. 3D).
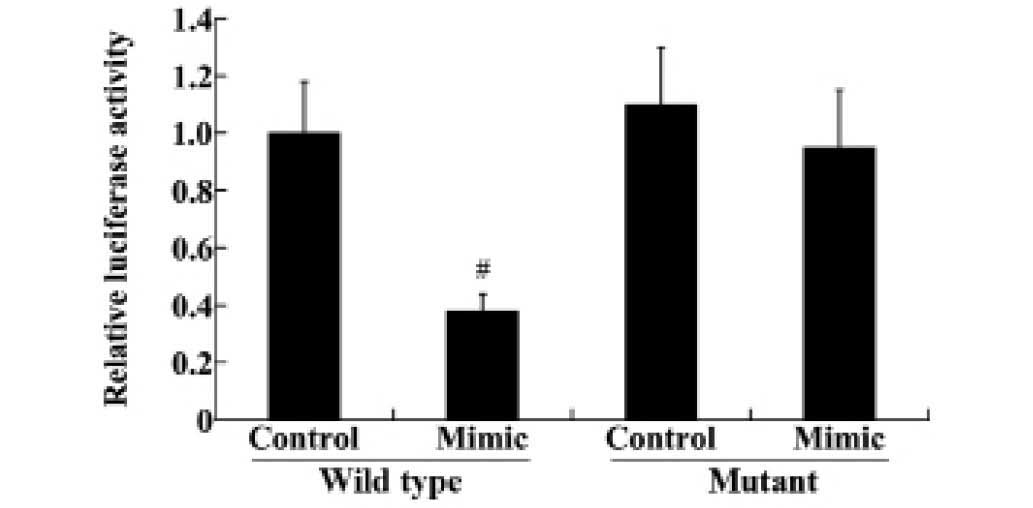
miR-140-5p directly targets the 3′UTR of
VEGFA and regulates the expression of VEGFA
To reveal the association between miR-140-5p and
VEGFA, wild type and mutant 3′UTR of VEGFA were transfected into
HEK293 cells, along with the miR-140-5p mimic and miRNA control.
The luciferase reporter assay demonstrated that transfection of the
miR-140-5p mimic significantly downregulated the luciferase
activity in the wild type VEGFA-3′UTR compared with the control
group (P<0.01; Fig. 4).
However, the mutant VEGFA-3′UTR levels were not significantly
altered following transfection (Fig.
4).
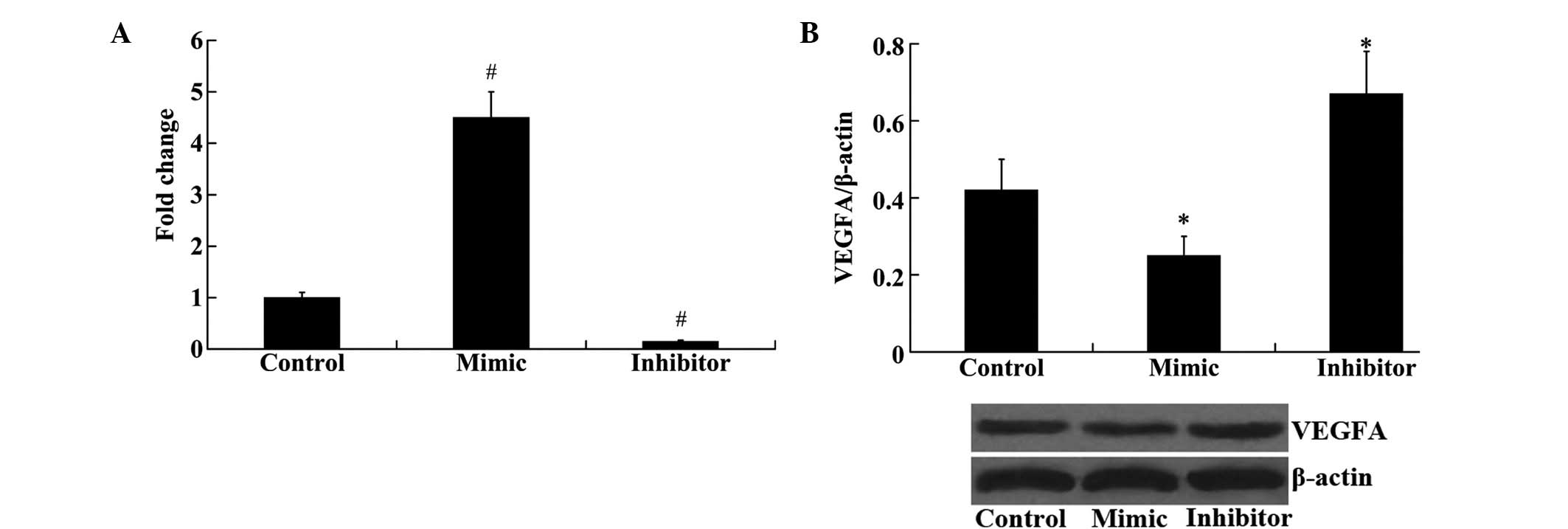
Furthermore, the effect of miR-140-5p on the
expression of VEGFA in HEK293 cells was examined. As demonstrated
in Fig. 5A, the expression of
miR-140-5p was significantly upregulated following transfection
with the miR-140-5p mimic and downregulated following transfection
with the miR-140-5p inhibitor, compared with the control group
(P<0.01). Western blot analysis demonstrated that the protein
expression levels of VEGFA were significantly decreased following
transfection with the miR-140-5p mimic and increased following
miR-140-5p inhibitor transfection, compared with the control group
(P<0.01; Fig. 5B).
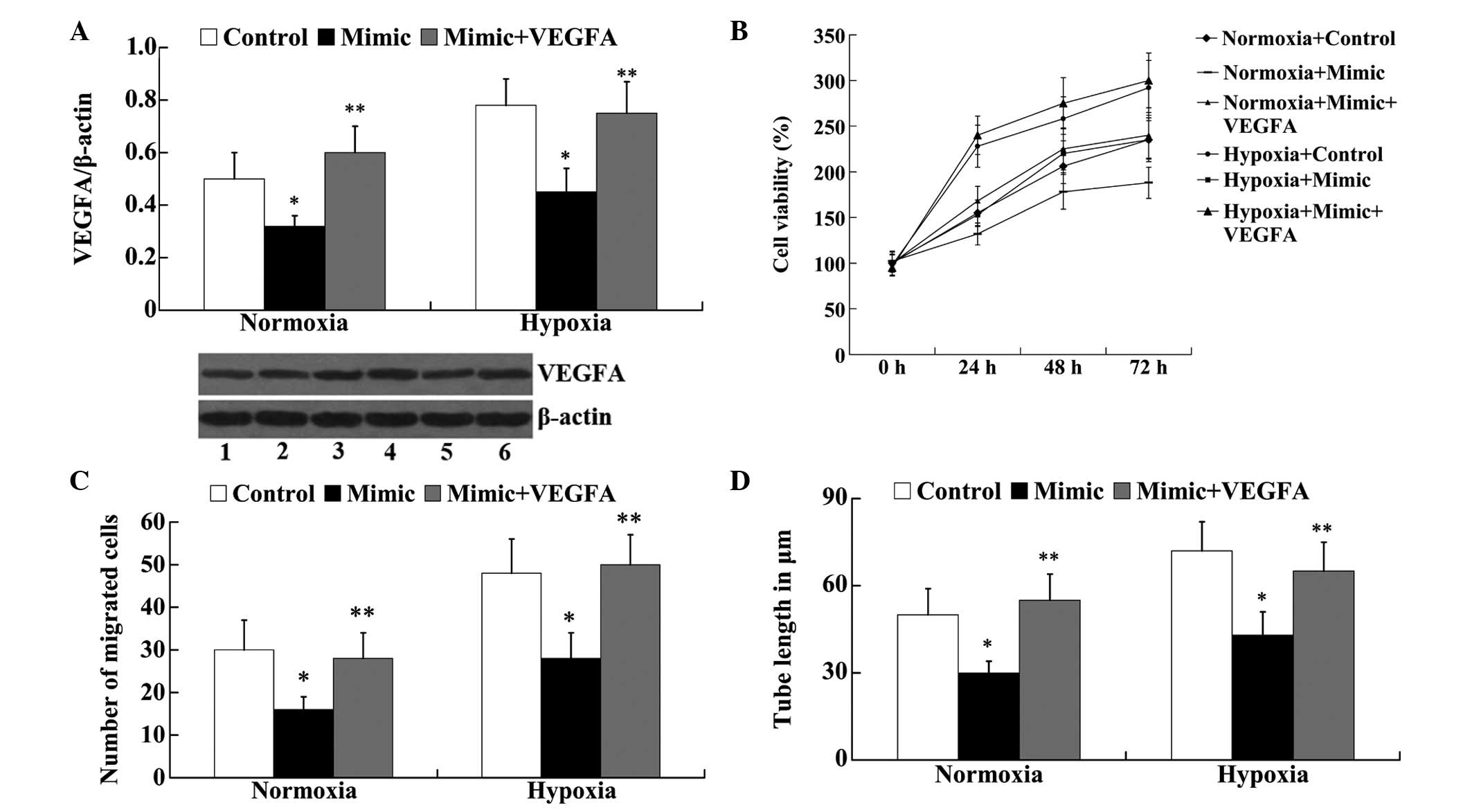
VEGFA attenuates the effect of miR-140-5p
on in vitro angiogenesis of HUVECs following hypoxia
To investigate whether the effect of miR-140-5p on
angiogenesis was mediated by VEGFA, VEGFA-pEGFP-C1 was transfected
into the normoxic and hypoxic HUVECs, together with the miR-140-5p
mimic. Western blot analysis demonstrated that in the normoxic and
hypoxic HUVECs, miR-140-5p mimic transfection significantly
downregulated the protein expression levels of VEGFA compared with
the control group (P<0.05; Fig.
6A). However, following mimic + VEGFA transfection, the protein
expression levels of VEGFA were significantly increased compared
with the mimic group (P<0.05; Fig.
6A). An MTT assay demonstrated that the decreased cell
viability observed due to the transfection of the miR-140-5p mimic
in normoxic and hypoxic HUVECs was reversed by the overexpression
of VEGFA (Fig. 6B). The results
from the Transwell migration assay revealed that overexpression of
VEGFA attenuated the effect of miR-140-5p on cell migration in
normoxic and hypoxic HUVECs (Fig.
6C). In addition, compared with the mimic group, the tube
length was significantly increased in the mimic + VEGFA group in
normoxic and hypoxic HUVECs (P<0.05; Fig. 6D).
Discussion
miRNAs are a family of small non-coding RNAs that
are 19–24 nucleotides in length. They serve important roles in the
regulation of various biological processes, including cell
proliferation, differentiation, apoptosis and motility (15–17).
Aberrant miRNA expression profiles have been linked to the
pathophysiology of numerous human diseases. Previous studies
demonstrated that miRNA profiles were altered in tissues, including
myocardial, retina, liver and brain tissues, under hypoxic-ischemic
conditions (18–21). The miR-140-5p expression profile of
rat hippocampus may be detected by microarray (22), and it has been demonstrated that
miR-140-5p modulates myelination in the dorsal root
ganglion/Schwann cell co-cultures (23). In addition, these previous studies
suggested that miR-140-5p may be a functional miRNA of the central
nervous system. In the present study, it was demonstrated that the
expression of miR-140-5p was significantly decreased in rat
cerebral ischemia and in the in vitro hypoxic condition.
These findings suggested that miR-140-5p may be involved in the
pathogenesis of hypoxic-ischemic brain damage.
Ischemic stroke results from the blockage of a brain
arterial blood vessel, therefore, the improvement of blood supply
in the ischemic brain tissue is the fundamental approach for the
treatment of ischemic stroke. Previous clinical studies indicated
that good collateral circulation may influence the infarct volume
and aid in the prognosis of patients with ischemic stroke (24–27).
Cerebral collateral circulation is a secondary compensatory
mechanism following cerebral ischemia, and it refers to the
collateral or the newly formed vascular anastomosis that stabilize
cerebral blood flow when the arteries are blocked.
Angiogenesis is the formation of new vessels
sprouting from pre-existing capillaries (28), and it was suggested to be a potent
therapy for ischemic stroke through increasing the cerebral blood
flow (29). A previous study
indicated that angiogenesis is associated with the neurological
functional recovery and has a beneficial effect on the long-term
survival of patients following cerebral ischemia (30).
Cell proliferation, migration and tube formation in
endothelial cells are important processes in angiogenesis. Previous
studies in cancer research indicated that miR-140-5p suppresses
cancer cell proliferation and metastasis (9,11).
In the present study, an in vitro study was performed for
the first time to the best of our knowledge, to investigate the
effect of miR-140-5p on angiogenesis in normoxic and hypoxic
HUVECs. The results demonstrated that miR-140-5p exerts inhibitory
effects against angiogenesis in normoxic and hypoxic HUVECs, as
indicated by decreased cell proliferation, migration and tube
formation in HUVECs following transfection with the miR-140-5p
mimic. These results suggested that miR-140-5p may regulate
angiogenesis following hypoxic-ischemic brain damage.
VEGFA is a notable pro-angiogenic factor, which
promotes angiogenesis through numerous mechanisms (31). It has been demonstrated that VEGFA
may induce the proliferation and migration of endothelial cells,
and increase vascular permeability (32). VEGFA has important roles in the
angiogenesis following cerebral ischemia (33–35).
Several animal and clinical studies have demonstrated that VEGFA is
significantly upregulated following stroke (36,37).
Administration of VEGFA appears to increase microvessel density in
the ischemic penumbra following MCAO in rats, thus leading to an
enhanced post-ischemic angiogenesis (38). To investigate the association
between miR-140-5p and VEGFA, potential targets of miR-140-5p were
identified using the miRanda algorithm (www.microrna.org) and VEGFA was predicted to be a
direct target. The luciferase reporter assay demonstrated that
miR-140-5p may directly target the 3′UTR of VEGFA and downregulated
the protein expression of VEGFA. Furthermore, overexpression of
VEGFA may attenuate the effect of miR-140-5p on angiogenesis.
In conclusion, the results of the present study
demonstrated that miR-140-5p suppresses angiogenesis following
cerebral ischemia, and this effect is achieved by directly
targeting VEGFA. The present study provided evidence that
miR-140-5p may be a potential candidate for the treatment of
ischemic brain injury.
References
|
1
|
Wang X: Investigational anti-inflammatory
agents for the treatment of ischaemic brain injury. Expert Opin
Investig Drugs. 14:393–409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Durukan A and Tatlisumak T: Acute ischemic
stroke: Overview of major experimental rodent models,
pathophysiology, and therapy of focal cerebral ischemia. Pharmacol
Biochem Behav. 87:179–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arenillas JF, Sobrino T, Castillo J and
Dávalos A: The role of angiogenesis in damage and recovery from
ischemic stroke. Curr Treat Options Cardiovasc Med. 9:205–212.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Navarro-Sobrino M, Rosell A,
Hernández-Guillamon M, Penalba A, Boada C, Domingues-Montanari S,
Ribó M, Alvarez-Sabín J and Montaner J: A large screening of
angiogenesis biomarkers and their association with neurological
outcome after ischemic stroke. Atherosclerosis. 216:205–211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koutsis G, Siasos G and Spengos K: The
emerging role of microRNA in stroke. Curr Top Med Chem.
13:1573–1588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng T, Jia YJ, Wen QQ, Guan WJ, Zhao EY
and Zhang BA: Expression of microRNA in neonatal rats with
hypoxic-ischemic brain damage. Chin J Contemp Pediatr. 12:373–376.
2012.In Chinese.
|
|
7
|
Hatse S, Brouwers B, Dalmasso B, Laenen A,
Kenis C, Schöffski P and Wildiers H: Circulating MicroRNAs as
easy-to-measure aging biomarkers in older breast cancer patients:
Correlation with chronological age but not with fitness/frailty
status. PLoS One. 9:e1106442014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mosakhani N, Lahti L, Borze I,
Karjalainen-Lindsberg ML, Sundström J, Ristamäki R, Osterlund P,
Knuutila S and Sarhadi VK: MicroRNA profiling predicts survival in
anti-EGFR treated chemorefractory metastatic colorectal cancer
patients with wild-type KRAS and BRAF. Cancer Genet. 205:545–551.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang H, Fang F, Chang R and Yang L:
MicroRNA-140-5p suppresses tumor growth and metastasis by targeting
transforming growth factor β receptor 1 and fibroblast growth
factor 9 in hepatocellular carcinoma. Hepatology. 58:205–217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W and He F: Monocyte to macrophage
differentiation-associated (MMD) targeted by miR-140-5p regulates
tumor growth in non-small cell lung cancer. Biochem Biophys Res
Commun. 450:844–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kai Y, Peng W, Ling W, Jiebing h and Zhuan
B: Reciprocal effects between microRNA-140-5p and ADAM10 suppress
migration and invasion of human tongue cancer cells. Biochem
Biophys Res Commun. 448:308–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. National Academies
Press; 2011
|
|
13
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wen KC, Sung PL, Yen MS, Chuang CM, Liou
WS and Wang PH: MicroRNAs regulate several functions of normal
tissues and malignancies. Taiwan J Obstet Gynecol. 52:465–469.
2013. View Article : Google Scholar
|
|
17
|
Chan SH and Wang LH: Regulation of cancer
metastasis by microRNAs. J Biomed Sci. 22:92015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roy S, Khanna S, Hussain SR, Biswas S,
Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ and Sen CK: MicroRNA
expression in response to murine myocardial infarction: miR-21
regulates fibroblast metalloprotease-2 via phosphatase and tensin
homologue. Cardiovasc Res. 82:21–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen J, Yang X, Xie B, Chen Y, Swaim M,
Hackett SF and Campochiaro PA: MicroRNAs regulate ocular
neovascularization. Mol Ther. 16:1208–1216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dharap A, Bowen K, Place R, Li LC and
Vemuganti R: Transient focal ischemia induces extensive temporal
changes in rat cerebral microRNAome. J Cereb Blood Flow Metab.
29:675–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu CF, Yu CH and Li YM: Regulation of
hepatic microRNA expression in response to ischemic preconditioning
following ischemia/reperfusion injury in mice. OMICS. 13:513–520.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park CS and Tang SJ: Regulation of
microRNA expression by induction of bidirectional synaptic
plasticity. J Mol Neurosci. 38:50–56. 2009. View Article : Google Scholar
|
|
23
|
Viader A, Chang LW, Fahrner T, Nagarajan R
and Milbrandt J: MicroRNAs modulate Schwann cell response to nerve
injury by reinforcing transcriptional silencing of
dedifferentiation-related genes. J Neurosci. 31:17358–17369. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miteff F, Levi CR, Bateman GA, Spratt N,
McElduff P and Parsons MW: The independent predictive utility of
computed tomography angiographic collateral status in acute
ischaemic stroke. Brain. 132:2231–2238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liebeskind DS, Cotsonis GA, Saver JL, Lynn
MJ, Turan TN, Cloft HJ and Chimowitz MI; Warfarin-Aspirin
Symptomatic Intracranial Disease (WASID) Investigators: Collaterals
dramatically alter stroke risk in intracranial atherosclerosis. Ann
Neurol. 69:963–974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rusanen H, Saarinen JT and Sillanpää N:
Collateral Circulation Predicts the Size of the Infarct Core and
the Proportion of Salvageable Penumbra in Hyperacute Ischemic
Stroke Patients Treated with Intravenous Thrombolysis. Cerebrovasc
Dis. 40:182–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ernst M, Forkert ND, Brehmer L, Thomalla
G, Siemonsen S, Fiehler J and Kemmling A: Prediction of infarction
and reperfusion in stroke by flow- and volume-weighted collateral
signal in MR angiography. AJNR Am J Neuroradiol. 36:275–282. 2015.
View Article : Google Scholar
|
|
28
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.PubMed/NCBI
|
|
29
|
Henry TD: Therapeutic angiogenesis. BMJ.
318:1536–1539. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beck H and Plate KH: Angiogenesis after
cerebral ischemia. Acta Neuropathol. 117:481–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsui M and Tabata Y: Enhanced
angiogenesis by multiple release of platelet-rich plasma contents
and basic fibroblast growth factor from gelatin hydrogels. Acta
Biomater. 8:1792–1801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spyridopoulos I, Luedemann C, Chen D,
Kearney M, Chen D, Murohara T, Principe N, Isner JM and Losordo DW:
Divergence of angiogenic and vascular permeability signaling by
VEGF: Inhibition of protein kinase C suppresses VEGF-induced
angiogenesis, but promotes VEGF-induced, NO-dependent vascular
permeability. Arterioscler Thromb Vasc Biol. 22:901–906. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Plate KH, Beck H, Danner S, Allegrini PR
and Wiessner C: Cell type specific upregulation of vascular
endothelial growth factor in an MCA-occlusion model of cerebral
infarct. J Neuropathol Exp Neurol. 58:654–666. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Banai S, Jaklitsch MT, Shou M, Lazarous
DF, Scheinowitz M, Biro S, Epstein SE and Unger EF:
Angiogenic-induced enhancement of collateral blood flow to ischemic
myocardium by vascular endothelial growth factor in dogs.
Circulation. 89:2183–2189. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lennmyr F, Ata KA, Funa K, Olsson Y and
Terént A: Expression of vascular endothelial growth factor (VEGF)
and its receptors (Flt-1 and Flk-1) following permanent and
transient occlusion of the middle cerebral artery in the rat. J
Neuropathol Exp Neurol. 57:874–882. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marti HJ, Bernaudin M, Bellail A, Schoch
H, Euler M, Petit E and Risau W: Hypoxia-induced vascular
endothelial growth factor expression precedes neovascularization
after cerebral ischemia. Am J Pathol. 156:965–976. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hayashi T, Abe K, Suzuki H and Itoyama Y:
Rapid induction of vascular endothelial growth factor gene
expression after transient middle cerebral artery occlusion in
rats. Stroke. 28:2039–2044. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang ZG, Zhang L, Jiang Q, Zhang R,
Davies K, Powers C, Bruggen N and Chopp M: VEGF enhances
angiogenesis and promotes blood-brain barrier leakage in the
ischemic brain. J Clin Invest. 106:829–838. 2000. View Article : Google Scholar : PubMed/NCBI
|