Introduction
Atherosclerosis is a chronic inflammatory response
to injury in the arterial wall (1). It is harmful to human health and
responsible for the majority of fatalities in the senior population
(2). The initial step of
atherogenesis is intimal injury and subsequent platelet aggregation
and leukocyte invasion beneath the endothelial monolayer.
Furthermore, through macrophage activation and foam cell formation,
vascular smooth muscle cell (VSMC) proliferation and migration lead
to atherosclerosis (3).
Increased proliferation and migration of VSMCs are
critical events in the pathophysiology of atherosclerosis (4). The proliferation of VSMCs can be
induced by cytokines and growth factors, such as tumor necrosis
factor-α, platelet-derived growth factor (PDGF) and transforming
growth factor (TGF)-β (3). It has
been reported that PDGF initiates numerous biological effects via
the activation of intracellular signal transduction pathways that
are critical in the proliferation and migration of VSMCs (5). Therefore, preventing PDGF-mediated
VSMC proliferation and migration may be an important therapeutic
approach for atherosclerosis.
Class A scavenger receptors (SR-As) are cell surface
receptors that bind a range of ligands, including modified
low-density lipoproteins and nucleic acids (6). The SR-A types found in mammals are
macrophage scavenger receptor type A (SR-A, SCARA1), MARCO
(SCARA2), CSR1 (SCARA3), SRCL4 (SCARA4) and SCARA5 (7). Several studies have demonstrated that
SCARA5 is critical in cancer cell migration and invasion (8–10).
More recently, one study showed that catechin supplementation
reduced the atherosclerotic lesion area, and downregulated the
expression levels of SCARA5 (11).
These results suggest that SCARA5 may be involved in
atherosclerosis. However, the role of SCARA5 in VSMCs remains to be
elucidated in the development of atherosclerosis. Therefore, the
role of SCARA5 in PDGF-BB-stimulated HASMC proliferation and
migration was investigated.
Materials and methods
Cell culture
Human aortic smooth muscle cells (HASMCs) were
obtained from the American Type Culture Collection (Manassas, VA,
USA). Cells were cultured in SmGM-2 growth media (Lonza, Basel,
Switzerland) in 5% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). at 37°C in a humidified 5%
CO2 incubator. Cells from passages 3 to 5 were used in
the experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from HASMCs using the RNA
plus kit (Takara Biotechnologies Inc., Dalian, China). cDNA was
synthesized from 0.5 µg of total RNA with superscriptor
reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.).
The levels of gene mRNA transcripts were analyzed using the
specific primers and SYBR Green I reagent (Takara Biotechnologies
Inc.) and the RT-PCR kit (Takara Biotechnologies Inc.), on the
Bio-Rad iQ5 Quantitative PCR system (Bio-Rad, Hercules, CA, USA),
according to the manufacturer's instructions. The following primers
were used: SCARA5, 5′-CAGCTGGTTTCTTACCACGTAT-3′ (sense),
5′-GCACAAGTTCTCCCACACTTAG-3′ (antisense); and β-actin
5′-CCGTGAAAAGATGACCCAGATC-3′ (sense), 5′-CACAGCCTGGATGGCTACGT-3′
(antisense) (all obtained from Takara Biotechnologies Inc.). For
relative quantification, the levels of individual gene mRNA
transcripts were firstly normalized to the control β-actin.
Subsequently, the differential expression of these genes was
analyzed by the DDCt method and expressed as the fold changes. The
PCR procedure was as follows: Initial denaturation at 94°C for 5
min; 40 cycles of denaturation at 94°C for 30 sec, annealing at
55°C for 30 sec and extension at 72°C for 20 sec; and the melt
curve was analyzed from 65 to 95°C. Expression levels of the
relative genes were calculated using the 2−ΔΔCq method
(12) and with β-actin mRNA as an
internal control.
Western blotting
Total protein extracts were prepared using
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology, Nantong, China) according to the
manufacturer's instructions. Protein concentration was determined
by a bicinchoninic acid protein assay (Sangon Biotech, Shanghai,
China) using bovine serum albumin (Gibco; Thermo Fisher Scientific,
Inc.) as the standard. Aliquots (30 µl) were separated on a
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto polyvinylidene difluoride membranes (Whatman
Schleicher & Schuell, Middlesex, UK). Membranes were blocked
with Tris-buffered saline (TBS) containing 1% (w/v) non-fat dry
milk and 0.1% (v/v) Tween-20 (TBST) for >2 h. After blocking,
the target proteins were probed with the following primary
antibodies overnight at 4°C: Rabbit anti-SCARA5 (1:1,500;
sc-98123), mouse anti-platelet-derived growth factor receptor β
(PDGFRβ) (1:1,500; sc-19995), mouse anti-AKT (1:2,000; sc-5298),
rabbit anti-phospho-AKT (1:2,000; sc-135650), rabbit
anti-extracellular signal-regulated kinase (ERK) (1:2,000;
sc-292838), mouse anti-phospho-ERK (1:2,000; sc-81492) and
anti-β-actin (1:1500; sc-47778) (all from Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). Subsequently, the blots were washed
with TBST and incubated with Subsequently, the blots were washed
with TBST and incubated with horseradish peroxidase
(HRP)-conjugated goat anti-mouse immunoglobulin G (sc-395761) and
bovine anti-rabbit horseradish peroxidase conjugated secondary
antibody (sc-2370) (both 1:3,000; Santa Cruz Biotechnology, Inc.)
for 1 h. After washing, the sites of antibody binding were
visualized by chemiluminescence (Boehringer Mannheim, Mannheim,
Germany). β-actin protein levels were used as an endogenous control
to allow the normalization of target proteins. The relative protein
expression levels were quantified using Image-Pro Plus 6.0 software
(Media Cybernetics, Silver Spring, MD, USA) and normalized to
β-actin.
SCARA5 small interfering (si)RNA
transfection
SCARA5 siRNA was designed by Genepharma (Shanghai,
China). Sequences corresponding to the siRNA of SCARA5 were: Sense,
5′-GCUCCAUCUGUGAGGAUUCdTdT-3′ and antisense,
5′-GAAUCCUCAGAUGGAGdTdT-3′. For in vitro transfection,
HASMCs were seeded at a density of 1×104 cells/well in
each well of a 96-well microplate and grown for 24 h to reach 60%
confluence. The cells were transfected with either siRNA-scramble
or siRNA-SCARA5 using HiPerFect Transfection reagent (Qiagen)
according to the manufacturer's instructions. A scramble Stealth
RNAi duplex served as a negative control (sense,
5′-UUCUCGAACGUGUCACGUdTdT-3′; and antisense,
5′-ACGUACACGUUCGGAGAAdTdT-3′).
HASMC proliferation assay
HASMC proliferation was measured by an MTT assay. In
brief, HASMCs (4×104 cells/ml) were growth-arrested in a
96-well microplate. After being grown to 60% confluence, HASMCs
were incubated in serum-free medium for 24 h prior to stimulation
with 20 ng/ml PDGF-BB and/or siRNA-SCARA5 in serum free medium.
After 24 h, 0.5 µg/ml MTT Sigma-Aldrich (St. Louis, MO, USA)
was added and incubated for 4 h. Subsequently, 400 µl
dimethyl sulfoxide was added to dissolve the formazan crystals
formed. The absorbance value was measured at 450 nm using a
microplate reader (model 680, Bio-Rad).
HASMC migration assay
HASMCs migration was measured with the Transwell
migration assay. In brief, HASMCs were resuspended in serum-free
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.), and placed in the upper compartment of Transwell
chambers, while the lower chamber was supplemented with serum-free
medium (Sigma-Aldrich) containing PDGF-BB (Sigma-Aldrich) as a
positive control. After incubation for 16 h at 37°C, the Transwells
(Sigma-Aldrich) were washed twice with phosphate-buffered saline.
The cells from the top side of the filter were removed with a
cotton swab, and cells on the bottom side of the filter were
stained for 30 min with Gill hematoxylin (Sigma-Aldrich). The
number of cells on the lower surface in five random fields was
counted under a microscope (SZX7-1063, Olympus, Tokyo, Japan). in
order to determine the average number of cells that had
migrated.
Statistical analysis
Values are presented as the mean ± standard
deviation. Data were analyzed using one-way analysis of variance
followed by Fisher's Least Significant Differences post hoc test.
All analyses were conducted using 13.0 SPSS software (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
SCARA5 is enhanced by growth factor
PDGF-BB
It has been reported that PDGF-BB promotes the
dedifferentiation and proliferation of SMCs, and is upregulated at
the site of vascular injury and in atherosclerotic plaque specimens
(13). Thus, the present study
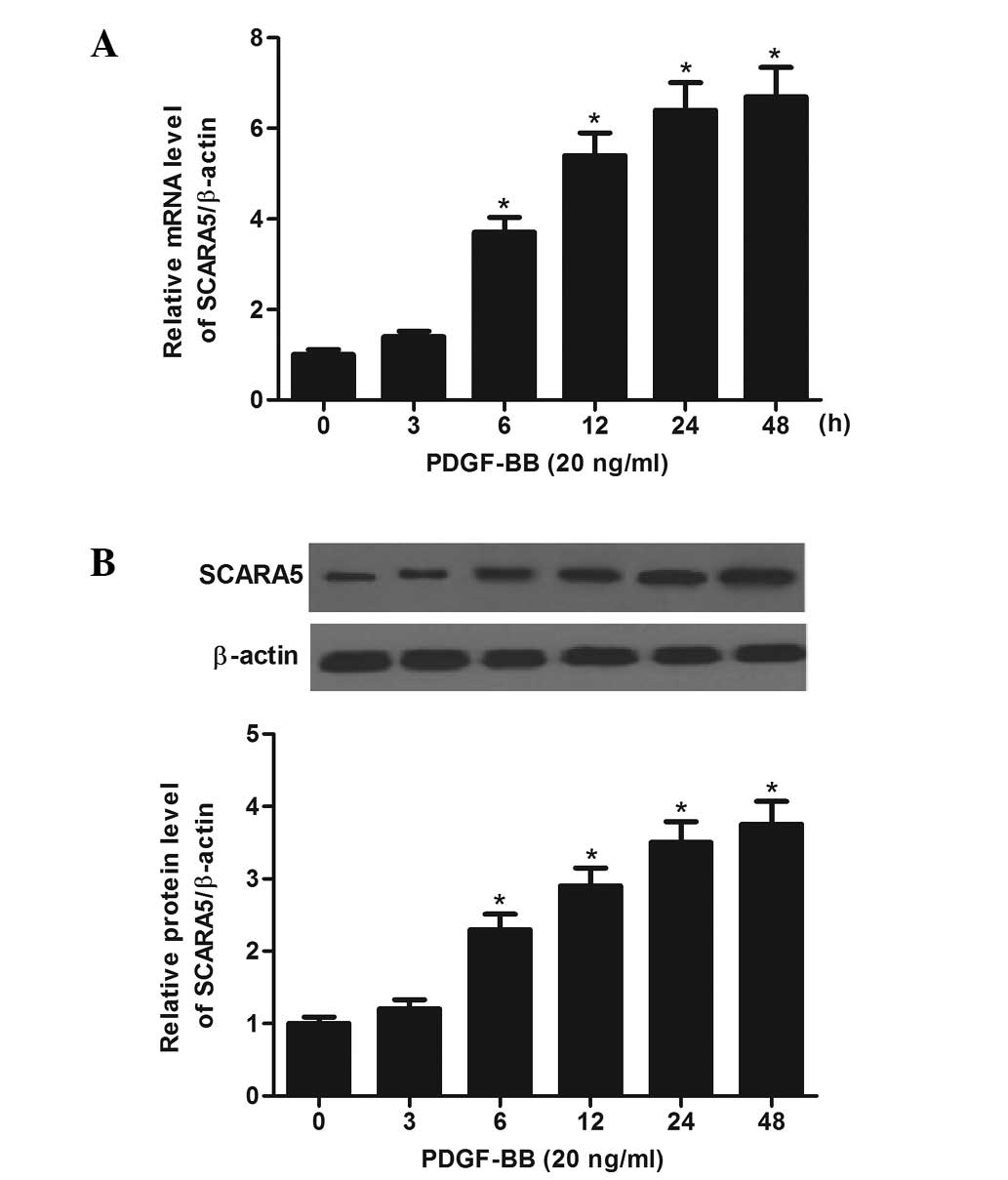
examined the response of SCARA5 to PDGF-BB in cultured HASMCs. As
shown in Fig. 1A, the mRNA level
of SCARA5 was increased in a time-dependent manner in response to
20 ng/ml PDGF-BB. Similarly, western blotting demonstrated that the
level of SCARA5 protein was also increased by PDGF-BB (Fig. 1B).
Knockdown of SCARA5 markedly inhibits
HASMC proliferation in vitro
Evidence shows that HASMCs are critical in the
development of atherosclerosis and that SCARA5 is notably reduced
in PDGF-stimulated HASMCs. Thus, siRNA-SCARA5 was designed and
transfected into the HASMCs in order to investigate the role of
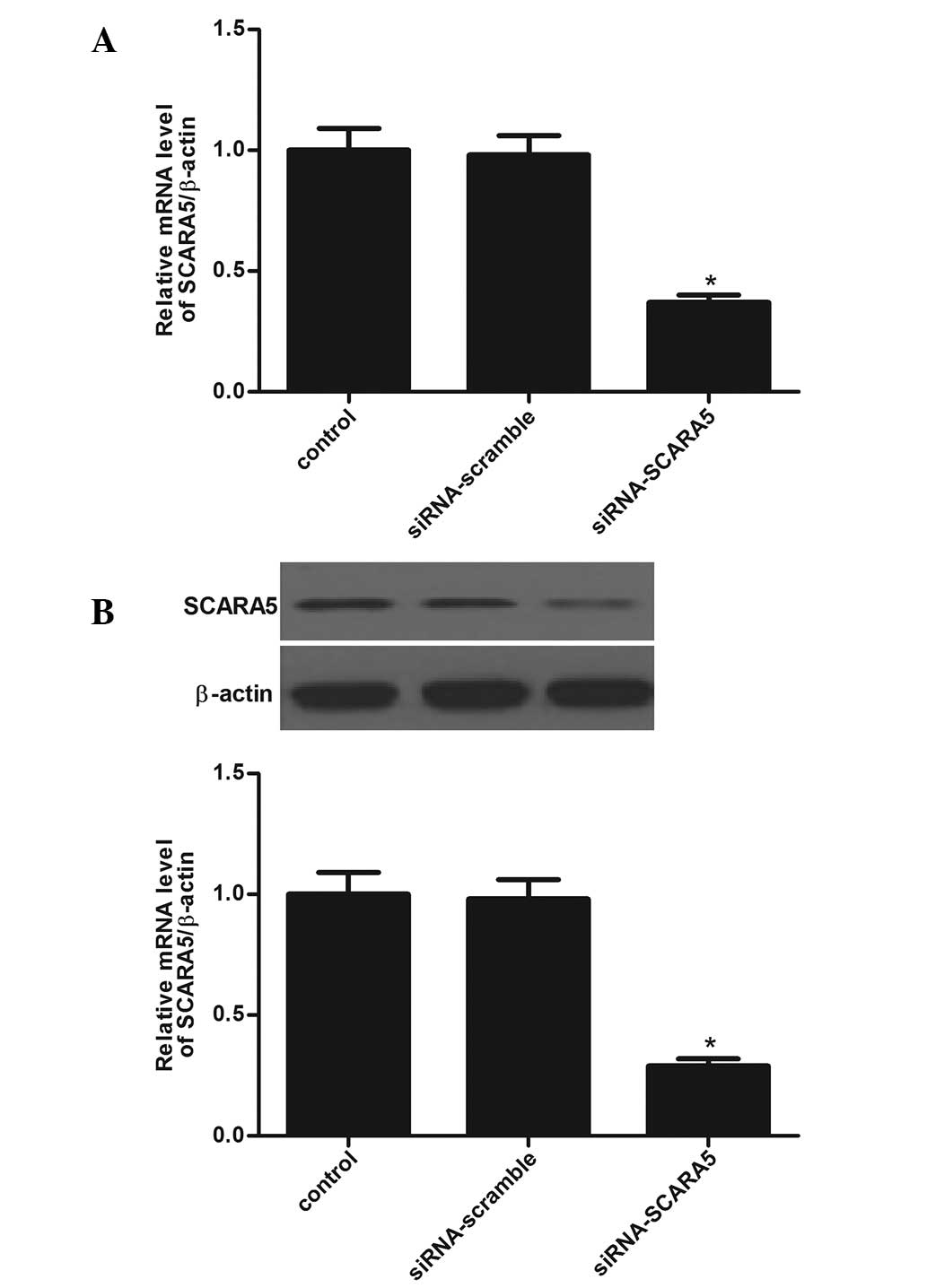
SCARA5 in HASMC. The transfection efficiency was determined by
RT-qPCR and western blot analysis in HASMCs. As shown in Fig. 2A and B, the mRNA and protein
expression levels of SCARA5 were significantly decreased in HASMCs.
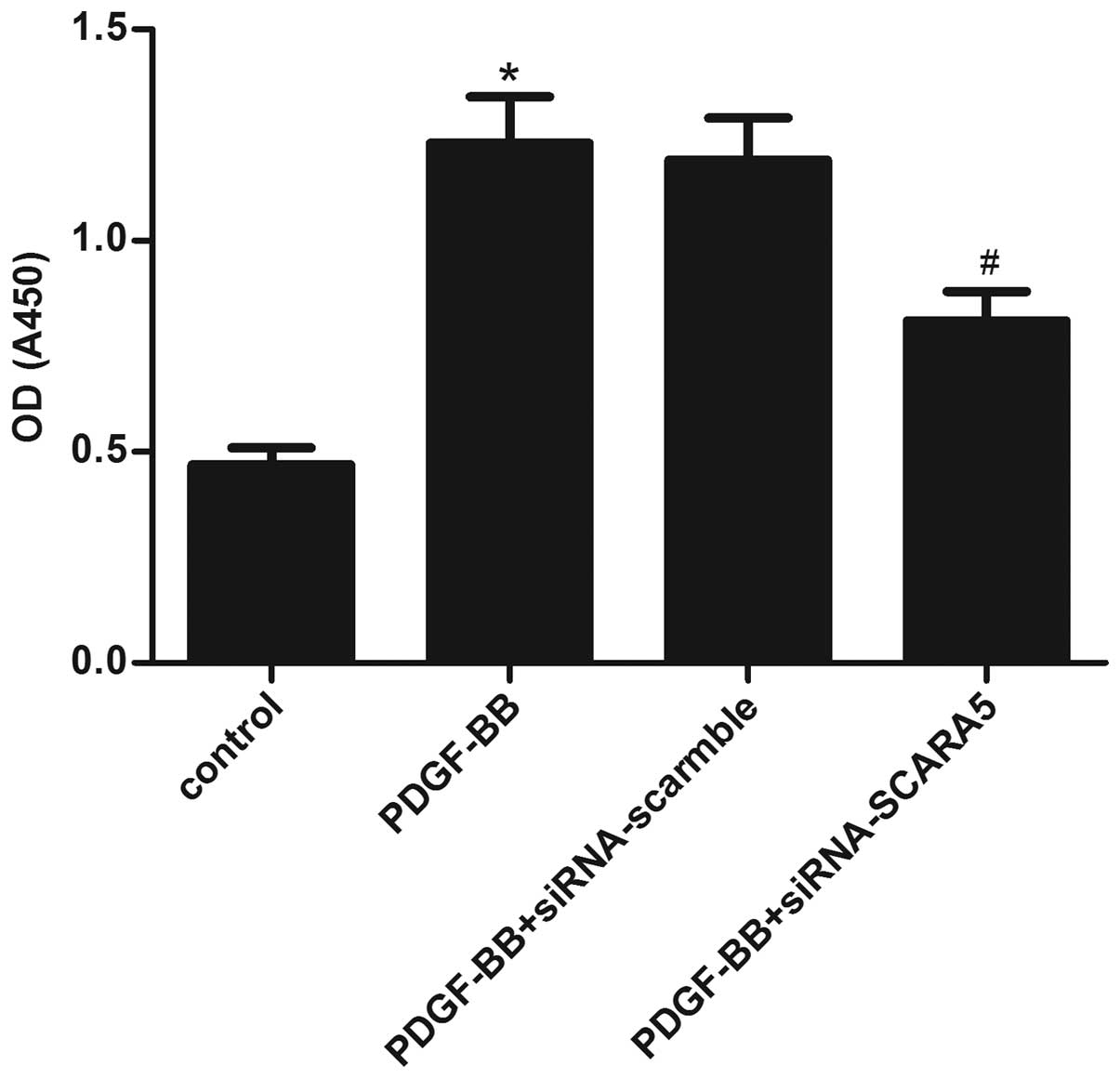
Moreover, an MTT assay was performed to identify the effect of
SCARA5 on HASMC proliferation. HASMCs were pretreated with
siRNA-SCARA5 or siRNA-scramble for 24 h and then stimulated with
PDGF-BB (20 ng/ml) for 24 h. As indicated in Fig. 3, PDGF-BB treatment significantly
increased the proliferation of HASMCs, compared with the untreated
cells; however, siRNA-SCARA5 significantly prevented
PDGF-BB-induced HASMC proliferation. These results suggested that
SCARA5 facilitates HASMC proliferation in vitro.
Knockdown of SCARA5 markedly retards
HASMC migration in vitro
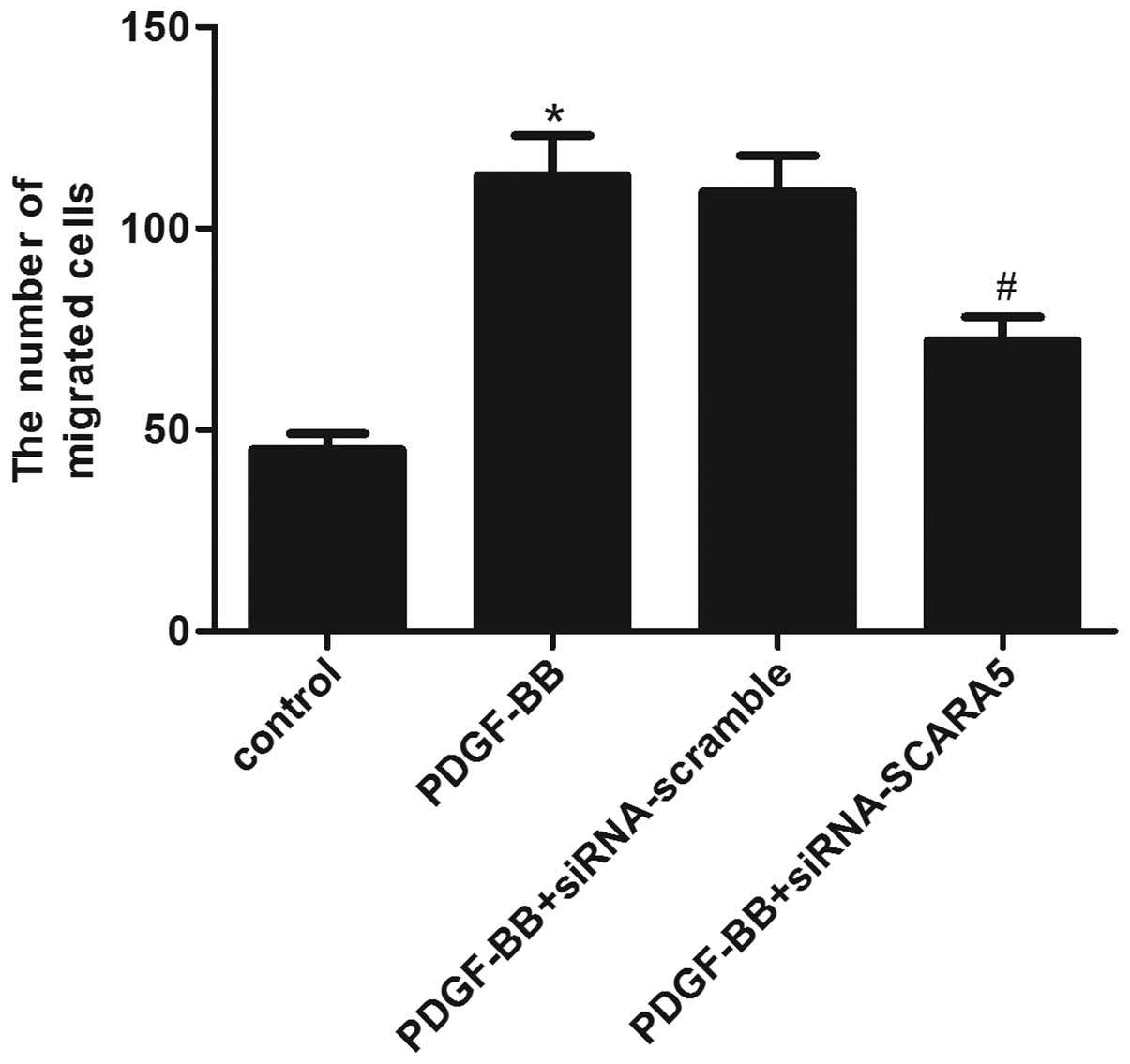
A Transwell migration assay was conducted to
investigate the effect of SCARA5 on HASMC migration. As shown in
Fig. 4, the Transwell assay
revealed that treatment with PDGF-BB markedly increased the number
of HASMCs that migrated through the Transwell chamber, while,
siRNA-SCARA5 significantly reduced the number of cells that
migrated following PDGF-BB stimulation. These results suggested
that SCARA5 facilitates HASMC migration in vitro.
Knockdown of SCARA5 inhibits HASMC
proliferation and migration through the suppression of the PDGF
signaling pathway
To investigate the mechanisms of SCARA5-promoted
HASMC proliferation and migration, it was examined whether SCARA5
affected the phosphorylation of AKT and ERK1/2 in
PDGF-BB-stimulated HASMCs. As shown in Fig. 5, treatment with PDGF-BB markedly
increased the phosphorylation of PDGFRβ in HASMCs. In addition, the
phosphorylation of AKT and ERK1/2 were also increased by PDGF-BB in
HASMCs. While, siRNA-SCARA5 significantly inhibited AKT and ERK1/2
phosphorylation by PDGF-BB in HASMCs, which was associated with a
reduction in PDGFRβ phosphorylation by PDGF-BB stimulation.
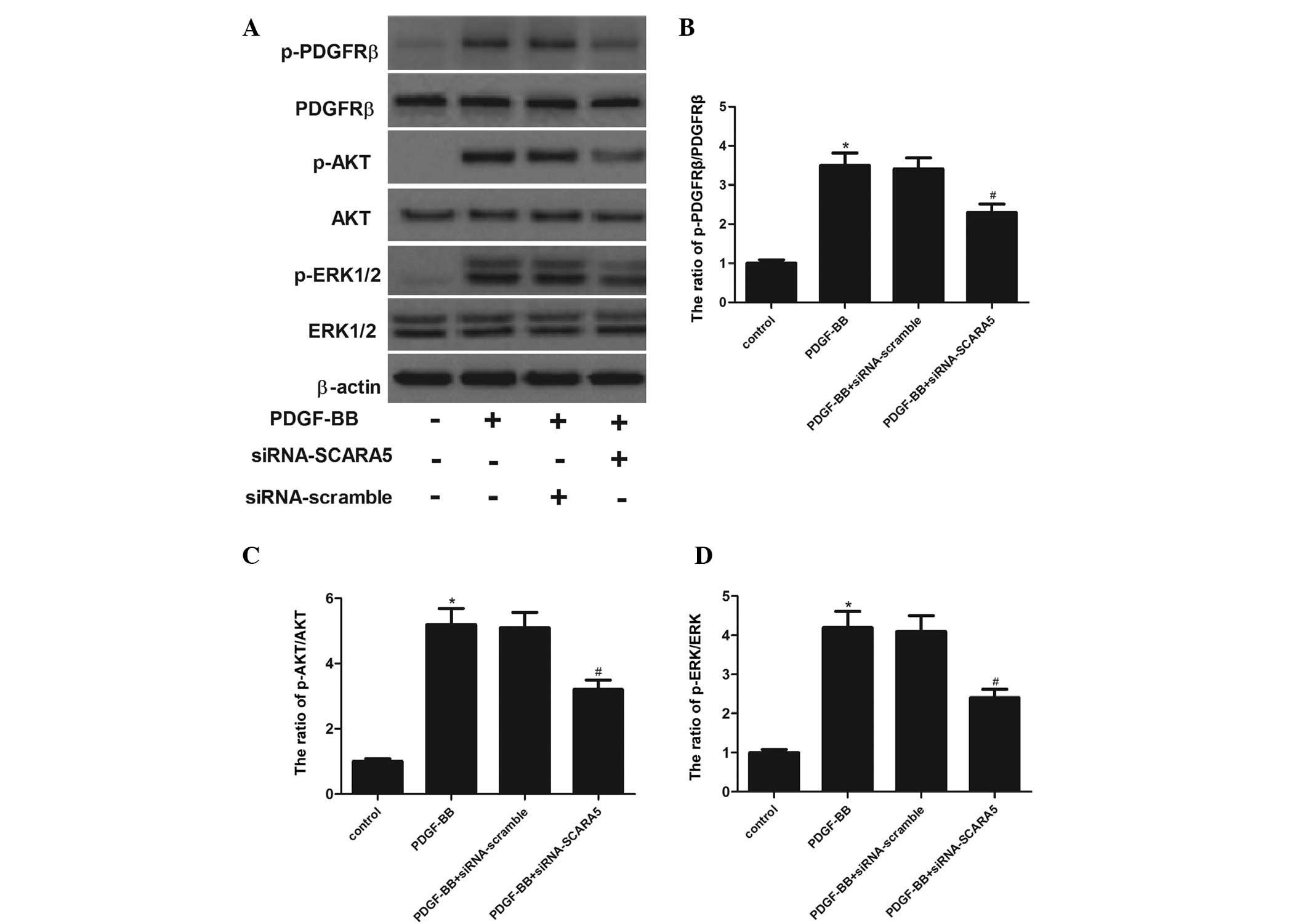 | Figure 5Effects of SCARA5 on PDGF-BB-induced
PDGFRβ, AKT, and ERK1/2 phosphorylation. (A) The cells were
incubated in serum-free medium for 24 h prior to stimulation with
20 ng/ml PDGF-BB and/or siRNA-SCARA5 in serum free medium at 37°C
for 5 min (for PDGFRβ, AKT and ERK1/2 phosphorylation). The cells
were lysed, and proteins were analyzed using 7.5 and 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and
immunoblotting. The relative protein expression levels of (B)
p-PDGFRβ, (C) p-AKT and (D) p-ERK1/2 were quantified using
Image-Pro Plus 6.0 software and normalized to β-actin.
Representative data from three different experiments are presented.
*P<0.05 vs. control. #P<0.05 vs.
PDGF-BB treatment. SCARA5, scavenger receptor class A, member 5;
PDGF-BB, platelet-derived growth factor BB; PDGFRβ,
platelet-derived growth factor receptor β; ERK1/2, extracellular
signal-regulated kinase 1/2; siRNA, small interfering RNA. |
Discussion
VSMC proliferation and migration are critical in the
progression of atherosclerotic lesions. Inhibition of VSMC
proliferation and migration is an important therapeutic strategy
for atherosclerosis (14). In the
present study, it was demonstrated that SCARA5 expression was
enhanced by PDGF-BB in HASMCs. Knockdown of SCARA5 by siRNA
significantly inhibited PDGF-BB-induced HASMC proliferation and
migration. Furthermore, siRNA-SCARA5 significantly inhibited AKT
and ERK1/2 phosphorylation by PDGF-BB in HASMCs.
SR-As are membrane glycoproteins that can form
homotrimers. This receptor was originally defined by its ability to
mediate the accumulation of lipids in macrophages. Recently,
Syväranta et al (15)
showed that the mRNA expression level of SR-A1 was upregulated in
stenotic human aortic valves. In this study, it was demonstrated
that SCARA5 expression was enhanced by PDGF-BB in HASMCs. These
results suggest that SCARA5 may be important in the development of
atherosclerosis.
PDGF and its β receptor subtype (PDGFRβ), which is
abundantly expressed in VSMCs, are significantly upregulated and
activated at sites of vascular injury (16,17).
In this study, PDGF was used as a proliferative agent as studies
have demonstrated that PDGF is a critical mediator of VSMC growth
(18,19). In line with these previous studies,
it was demonstrated that PDGF-BB increased HASMC proliferation and
migration. However, siRNA-SCARA5 inhibited the proliferation and
migration of PDGF-BB-stimulated HASMCs.
PDGF-BB, one of the most potent mitogens and
chemoattractants for VSMCs, is important in the development of
atherosclerosis (16). Upon
binding to PDGFRβ on VSMCs, PDGF-BB initiates a multitude of
biological effects through the activation of ERK1/2 MAP kinase.
ERK1/2 transduces mitogenic signals to the nucleus by
phosphorylating and activating specific transcription factors,
thereby leading to the formation of hyperplastic lesions in
response to vascular injury (20,21).
ERK1/2 has been involved in PDGF-induced upregulation of the
neuron-derived orphan receptor-1 in VSMCs that is required for cell
cycle progression (22). Moreover,
PDGF-BB binds to PDGFRβ and activates the phosphatidylinositol
3-kinase (PI3K)/AKT pathway in VSMCs. Activated AKT has been
implicated in the PDGF-BB-induced proliferation, migration and
changes in the cytoskeleton of VSMCs (23,24).
A PDGFRβ antagonist or protein kinase inhibitor suppressed VMSC
proliferation in vitro and prevented cardiovascular
disorders in several animal experiments (25,26).
In this study, it was demonstrated that PDGF-BB markedly increased
the phosphorylation of PDGFRβ, AKT and ERK1/2 in PDGF-BB-stimulated
HASMCs. While, siRNA-SCARA5 inhibited PDGF-BB-induced
phosphorylation of PDGFRβ, AKT and ERK1/2. These results indicate
that inhibiting PDGF-BB-induced activation of the PDGF signaling
pathway may have contributed to the inhibition of HASMC
proliferation and migration exerted by siRNA-SCARA5.
In conclusion, this study demonstrated that
knockdown of SCARA5 significantly inhibited HASMC proliferation and
migration induced by PDGF-BB. In addition, knockdown of SCARA5
notably inhibited the activation of the PDGF signaling pathway.
Therefore, SCARA5 may be a novel therapeutic target for preventing
or treating vascular diseases involving VSMC proliferation and
migration.
References
|
1
|
Ross R and Agius L: The process of
atherogenesis-cellular and molecular interaction: From experimental
animal models to humans. Diabetologia. 35(Suppl 2): S34–S40. 1992.
View Article : Google Scholar
|
|
2
|
Wang JC and Bennett M: Aging and
atherosclerosis: Mechanisms, functional consequences and potential
therapeutics for cellular senescence. Circ Res. 111:245–259. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross R: Atherosclerosis-an inflammatory
disease. New Engl J Med. 340:115–126. 1999. View Article : Google Scholar
|
|
4
|
Owens GK: Regulation of differentiation of
vascular smooth muscle cells. Physiol Rev. 75:487–517.
1995.PubMed/NCBI
|
|
5
|
Heldin CH and Westermark B: Mechanism of
action and in vivo role of platelet-derived growth factor. Physiol
Rev. 79:1283–1316. 1999.PubMed/NCBI
|
|
6
|
Peiser L and Gordon S: The function of
scavenger receptors expressed by macrophages and their role in the
regulation of inflammation. Microbes Infect. 3:149–159. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whelan FJ, Meehan CJ, Golding GB, McConkey
BJ and Bowdish DM: The evolution of the class A scavenger
receptors. BMC Evol Biol. 12:227–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Hu G, Chen D, Gong AY, Soori GS,
Dobleman TJ and Chen XM: Suppression of SCARA5 by Snail1 is
essential for EMT-associated cell migration of A549 cells.
Oncogenesis. 2:e732013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan N, Zhang S, Yang Y, Cheng L, Li C, Dai
L, Dai L, Zhang X, Fan P, Tian H, et al: Therapeutic upregulation
of Class A scavenger receptor member 5 inhibits tumor growth and
metastasis. Cancer Sci. 103:1631–1639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang J, Zheng DL, Qin FS, Cheng N, Chen
H, Wan BB, Wang YP, Xiao HS and Han ZG: Genetic and epigenetic
silencing of SCARA5 may contribute to human hepatocellular
carcinoma by activating FAK signaling. J Clin Invest. 120:223–241.
2010. View
Article : Google Scholar :
|
|
11
|
Auclair S, Milenkovic D, Besson C, Chauvet
S, Gueux E, Morand C, Mazur A and Scalbert A: Catechin reduces
atherosclerotic lesion development in apo E-deficient mice: A
transcriptomic study. Atherosclerosis. 204:e21–e27. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chadjichristos CE, Morel S, Derouette JP,
Sutter E, Roth I, Brisset AC, Bochaton-Piallat ML and Kwak BR:
Targeting connexin 43 prevents platelet-derived growth
factor-BB-induced phenotypic change in porcine coronary artery
smooth muscle cells. Circ Res. 102:653–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Andrés V: Control of vascular cell
proliferation and migration by cyclin-dependent kinase signalling:
New perspectives and therapeutic potential. Cardiovasc Res.
63:11–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Syväranta S, Alanne-Kinnunen M, Oörni K,
Oksjoki R, Kupari M, Kovanen PT and Helske-Suihko S: Potential
pathological roles for oxidized low-density lipoprotein and
scavenger receptors SR-AI, CD36 and LOX-1 in aortic valve stenosis.
Atherosclerosis. 235:398–407. 2014. View Article : Google Scholar
|
|
16
|
Raines EW: PDGF and cardiovascular
disease. Cytokine Growth Factor Rev. 15:237–254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abe J, Deguchi J, Takuwa Y, Hara K, Ikari
Y, Tamura T, Ohno M and Kurokawa K: Tyrosine phosphorylation of
platelet derived growth factor beta receptors in coronary artery
lesions: Implications for vascular remodelling after directional
coronary atherectomy and unstable angina pectoris. Heart.
79:400–406. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jawien A, Bowen-Pope DF, Lindner V,
Schwartz SM and Clowes AW: Platelet-derived growth factor promotes
smooth muscle migration and intimal thickening in a rat model of
balloon angioplasty. J Clin Invest. 89:507–511. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bilder G, Wentz T, Leadley R, Amin D, Byan
L, O'Conner B, Needle S, Galczenski H, Bostwick J, Kasiewski C, et
al: Restenosis following angioplasty in the swine coronary artery
is inhibited by an orally active PDGF-receptor tyrosine kinase
inhibitor, RPR101511A. Circulation. 99:3292–3299. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lannoy M, Slove S, Louedec L, Choqueux C,
Journé C, Michel JB and Jacob MP: Inhibition of ERK1/2
phosphorylation: A new strategy to stimulate elastogenesis in the
aorta. Hypertension. 64:423–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mii S, Khalil RA, Morgan K, Ware JA and
Kent KC: Mitogen-activated protein kinase and proliferation of
human vascular smooth muscle cells. Am J Physiol. 270:H142–H150.
1996.PubMed/NCBI
|
|
22
|
Nomiyama T, Nakamachi T, Gizard F, Heywood
EB, Jones KL, Ohkura N, Kawamori R, Conneely OM and Bruemmer D: The
NR4A orphan nuclear receptor NOR1 is induced by platelet-derived
growth factor and mediates vascular smooth muscle cell
proliferation. J Biol Chem. 281:33467–33476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goncharova EA, Ammit AJ, Irani C, Carroll
RG, Eszterhas AJ, Panettieri RA and Krymskaya VP: PI3K is required
for proliferation and migration of human pulmonary vascular smooth
muscle cells. Am J Physiol Lung Cell Mol Physiol. 283:L354–L363.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi KH, Kim JE, Song NR, Son JE, Hwang
MK, Byun S, Kim JH, Lee KW and Lee HJ: Phosphoinositide 3-kinase is
a novel target of piceatannol for inhibiting PDGF-BB-induced
proliferation and migration in human aortic smooth muscle cells.
Cardiovasc Res. 85:836–844. 2010. View Article : Google Scholar
|
|
25
|
Lipson KE, Pang L, Huber LJ, Chen H, Tsai
JM, Hirth P, Gazit A, Levitzki A and McMahon G: Inhibition of
platelet-derived growth factor and epidermal growth factor receptor
signaling events after treatment of cells with specific synthetic
inhibitors of tyrosine kinase phosphorylation. J Pharmacol Exp
Ther. 285:844–852. 1998.PubMed/NCBI
|
|
26
|
Myllärniemi M, Frösen J, Ramirez LG,
Buchdunger E, Lemström K and Häyry P: Selective tyrosine kinase
inhibitor for the platelet-derived growth factor receptor in vitro
inhibits smooth muscle cell proliferation after reinjury of
arterial intima in vivo. Cardiovasc Drug Ther. 13:159–168. 1999.
View Article : Google Scholar
|