Introduction
Osteoarthritis (OA) is the most common chronic
degenerative joint disease, affecting millions of individuals
worldwide and approximately 2.7 millions in the USA alone (1). Pro-inflammatory cytokines, including
interleukin (IL)-1β and tumor necrosis factor (TNF)-α, are critical
in the development of OA by stimulating chondrocyte responses that
promote catabolism of type II collagen and proteoglycans, thus,
compromising cartilage extracellular matrix integrity and tissue
homeostasis in OA (2). OA is
characterized by progressive loss of articular cartilage and
degradation of the cartilage matrix (3), which is primarily attributed to
proteolysis of proteoglycans and collagens, the major structural
components of cartilage matrix (4). Aggrecanases are a class of
proteinases belonging to the A disintegrin and metalloproteinase
with thrombospondin motifs (ADAMTS) family. Previous studies have
demonstrated that ADAMTS-1, -4, -5, -8, -9, and -15 possess
aggrecanase activity (3,4). The degradation of aggrecan by
aggrecanase-1 (also known as ADAMTS-4) is an early event in
osteoarthritic cartilage damage (5). Song et al (6) reported that knockdown of ADAMTS-4
attenuates the degradation of aggrecan in human cartilage
stimulated by TNF-α and oncostatin M. Other studies have
demonstrated that ADAMTS-4 is selectively overexpressed in human OA
cartilage and is positively correlated with the degree of cartilage
destruction (3,4), suggesting that ADAMTS-4 is an
important aggrecanase in human OA cartilage and its activity is
closely associated with the pathogenesis of OA.
Syndecans are a family of cell-surface heparan
sulfate proteoglycans comprising four members: Syndecan-1, -2, -3
and -4 (7). Syndecans interact
with a variety of extracellular matrix molecules, growth factors
and cytokines via their glycosaminoglycan chains (5,8).
Expression of all four syndecans has been observed in chondrocytes
(5,9). A previous study also demonstrated
that ADAMTS-4 activation in human chondrosarcoma cells requires
syndecan-1, suggesting that syndecan-1 is critical for the
activation of ADAMTS-4 in human chondrocytes (10).
Cannabinoids, which have anti-inflammatory effects
and reduce joint damage (11),
predominantly function through G protein-coupled membrane receptors
(12). Two major cannabinoid
receptors, cannabinoid receptor type 1 (CB1) and 2 (CB2), are
primarily expressed in the nervous and immune system, respectively
(12). A recent report
demonstrated that CB1 and CB2 are both expressed in human OA
articular chondrocytes (11).
Accumulating evidence suggests that cannabinoids have
chondroprotective effects and may be useful for the treatment of OA
(11,13). Furthermore, it has been
demonstrated that biologically stable synthetic cannabinoids have
direct protective effects against cartilage matrix breakdown by
reducing IL-1-induced proteoglycan and collagen degradation in
bovine cartilage, potentially via CB receptors (11,12).
The present study explored the effects of synthetic
cannabinoid WIN-55,212-2 mesylate (WIN-55) on the expression of
syndecan-1 and ADAMTS-4, as well as ADAMTS-4 activity, in
unstimulated and IL-1β-stimulated primary human OA articular
chondrocytes.
Materials and methods
Chondrocyte culture and treatments
Primary human OA articular chondrocytes (cat no.
402OAK-05a) and human chondrocyte growth medium (cat no. 411–500)
were purchased from Cell Applications, Inc. (San Diego, CA, USA).
The cells were cultured in the growth medium supplemented with 5%
fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 100 U/ml penicillin-streptomycin (Sigma-Aldrich, Beijing,
China) in an incubator with a humidified atmosphere of 95% air and
5% CO2 at 37°C. The cells were cultured in the presence
or absence of 10 ng/ml IL-1β (R&D Systems, Inc., MN, USA) for
24 h. Subsequently, the cells were treated with or without
selective CB1 antagonist MJ15 (1, 10 or 50 μM; cat no. 4063;
Tocris Bioscience, Bristol, UK) or selective CB2 antagonist JTE907
(0.1, 0.3, or 0.6 μM; cat no. 2479; Tocris Bioscience) for
30 min prior to the addition of WIN-55 (0.5, 1.0, 2.0, 4.0 or 8.0
μM; cat no. 1038; Tocris Bioscience) for a further 24 h.
ADAMTS-4 activity assay
ADAMTS-4 activity was determined using a
fluorimetric SensoLyte 520 Aggrecanase-1 Assay kit (cat no.
AS-72114; AnaSpec, Inc., Fremont, CA, USA), according to the
manufacturer's protocol (14,15).
Briefly, a fluorogenic substrate (5-FAM/TAMRA) was incubated with
whole cell lysates (25 μg in 50 μl). Measurements
were performed at 37°C over 90 min using a multiplate reader
(SpectraMax Plus 384; Molecular Devices, LLC, Beijing, China) at
excitation/emission wavelengths of 490/520 nm. At the time of the
assay, viable cells were counted using trypan blue and a a Levy
Counting Chambers hemocytometer (Thomas Scientific Inc, Swedesboro,
NJ, USA), as previously described (16). The measured fluorescence in the
assay was normalized against the number of viable cells (per
104 viable cells) to exclude the effects of IL-1β and
WIN-55 on chondrocyte proliferation and survival.
Western blot analysis
Whole cell lysates were extracted by incubating the
cells with lysis buffer [50 mM Tris/HCl, (pH7.2), 150 mM NaCl, l%
(v/v) Triton X-100, 1 mM sodium orthovanadate, 50 mM sodium
pyrophosphate, 100 mM sodium fluoride, 0.01% (v/v) aprotinin, 4
μg/ml pepstatin A, 10 μg/ml leupeptin and 1 mM
phenylmethanesulfonyl fluoride; all purchased from Sigma-Aldrich]
on ice for 30 min and removal of cell debris by centrifugation at
2,000 × g for 15 min at 4°C. The protein concentration was
determination by the Coomassie blue (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) method. Briefly, 50 ml 0.1 M NaOH were added to
each well and the plate was incubated at 37°C for 2 h. Following
incubation, 200 ml of a 1:6 dilution of Coomassie blue were added
to each well and left at room temperature for 30 min. The
absorbance was determined with a SmartSpec Plus spectrophotometer
(Bio-Rad Laboratories, Inc.) at 405 nm. Equal concentrations of
protein for each sample were separated by 10% SDS-polyacrylamide
gel electrophoresis (at 100 V for 90 min) and blotted onto a
polyvinylidene difluoride microporous membrane (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% skim milk
powder in Tris-buffered saline-Tween 20 (Sigma-Aldrich) for 2 h and
subsequently incubated for 1 h with a 1:500 dilution of goat
anti-human ADAMTS-4 polyclonal antibody (cat no. sc-16533), rabbit
anti-human syndecan-1 polyclonal antibody (cat no. sc-5632) or
mouse anti-human β-actin monoclonal antibody (cat no. sc-81178).
The membranes were washed and proteins detected using bovine
anti-goat (cat no. sc-2350), anti-rabbit (cat no. sc-2370) or
anti-mouse (cat no. sc-2371) horseradish peroxidase-conjugated
secondary antibody (1:5,000) for 1 h. All antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Peroxidase
was detected using an enhanced chemiluminescence kit (GE Healthcare
Life Sciences, Shanghai, China). Three independent experiments were
performed.
Plasmid construction and stable
transfection
Human syndecan-1 cDNA clone (cat no. SC118272) was
purchased from OriGene Technologies, Inc. (Beijing, China) and the
full-length syndecan-1 cDNA sequence was subcloned into a pcDNA 3.1
(+) expression vector (Thermo Fisher Scientific, Inc.) at the
KpnI and XhoI restriction sites, using the empty
pcDNA 3.1 (+) vector as the vector control. The syndecan-1
expression vector was transfected into cells using Lipofectamine
2000 transfection reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Pools of stable
transfectants were generated via selection with G418 (600
μg/ml; Sigma-Aldrich), according to the manufacturer's
protocol (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was prepared from cells using TRIzol reagent
(Thermo Fisher Scientific, Inc.) followed by purification with the
TURBO DNA-free System (Ambion; Thermo Fisher Scientific, Inc.).
cDNA was synthesized using SuperScript II reverse transcriptase
(Thermo Fisher Scientific, Inc.). RT-qPCR was performed using an
ABI-PRISM 7700 Sequence Detection System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and the fluorescent dye SYBR Green
Master Mix (Thermo Fisher Scientific, Inc.) as described by the
manufacturer. The primers used were as follows: Forward,
5′-GCCGCAAATTGTGGCTACT-3′ and reverse, 5′-GCTGCGTGTCCTTCCAAGT-3′
for syndecan-1; forward, 5′-GACTCATGACCACAGTCCATGC-3′ and reverse,
5′-AGAGGCAGGGATGATGTTCTG-3′ for GAPDH. The PCR amplification
conditions were as follows: 20 sec at 95°C followed by 40 cycles of
3 sec at 95°C and 30 sec at 60°C. Relative quantification of the
level of the syndecan-1 mRNA was determined using the
2−ΔΔCq method (17) and
normalized against that of GAPDH in the same sample. Each
experiment was repeated three times in duplicate.
Luciferase reporter assay
Cells were transfected with a commercially available
human syndecan-1 promoter/luciferase reporter (cat no. S710408;
SwitchGear Genomics, Shanghai, China) using Lipofectamine 2000
transfection reagent (Thermo Fisher Scientific, Inc.) and then
treated with or without WIN-55 (8 μM; Tocris Bioscience) for
24 h. Luciferase assays were performed using the LightSwitch
Luciferase Assay kit (cat no. LS010; SwitchGear Genomics) and the
GloMax 96 Micorplate Luminometer (Promega Corporation, Madison, WI,
USA), according to the manufacturer's protocol. Plasmid PRL-CMV
(cat no. E2261; Promega Corporation) encoding Renilla
reniformis luciferase was co-transfected with the reporter
plasmid (at 1:5 molar ratio) in each transfection as an internal
control for data normalization. Each experiment was repeated for
three times in duplicate.
Measurement of mRNA stability
Cells were cultured in the presence or absence of 10
ng/ml IL-1β (R&D Systems, Inc.) for 24 h. Subsequently, the
cells were treated with or without JTE907 (0.6 μM; Tocris
Bioscience) for 30 min prior to the addition of WIN-55 (1.0 or 8.0
μM; Tocris Bioscience) for a further 24-h incubation.
Transcription inhibitor actinomycin D (1 mg/ml; Sigma-Aldrich) was
added. The mRNA level of syndecan-1 was determined with RT-qPCR
after 1, 2 and 4 h of actinomycin D treatment, and expressed as
fold changes to mRNA level in control cells immediately prior to
actinomycin D treatment (designated as 1).
Statistical analysis
Statistical analyses were performed using SPSS
software for Windows (version 19.0; IBM SPSS, Armonk, NY, USA). All
continuous variable values are expressed as the mean ± standard
deviation. Comparisons of means among multiple groups were
performed with one-way analysis of variance followed by post-hoc
pair-wise comparisons using Tukey tests. P<0.05 was considered
to indicate a statistically significant difference in this
study.
Results
WIN-55 inhibits ADAMTS-4 activity via CB2
in unstimulated and IL-1β-stimulated human OA articular
chondrocytes
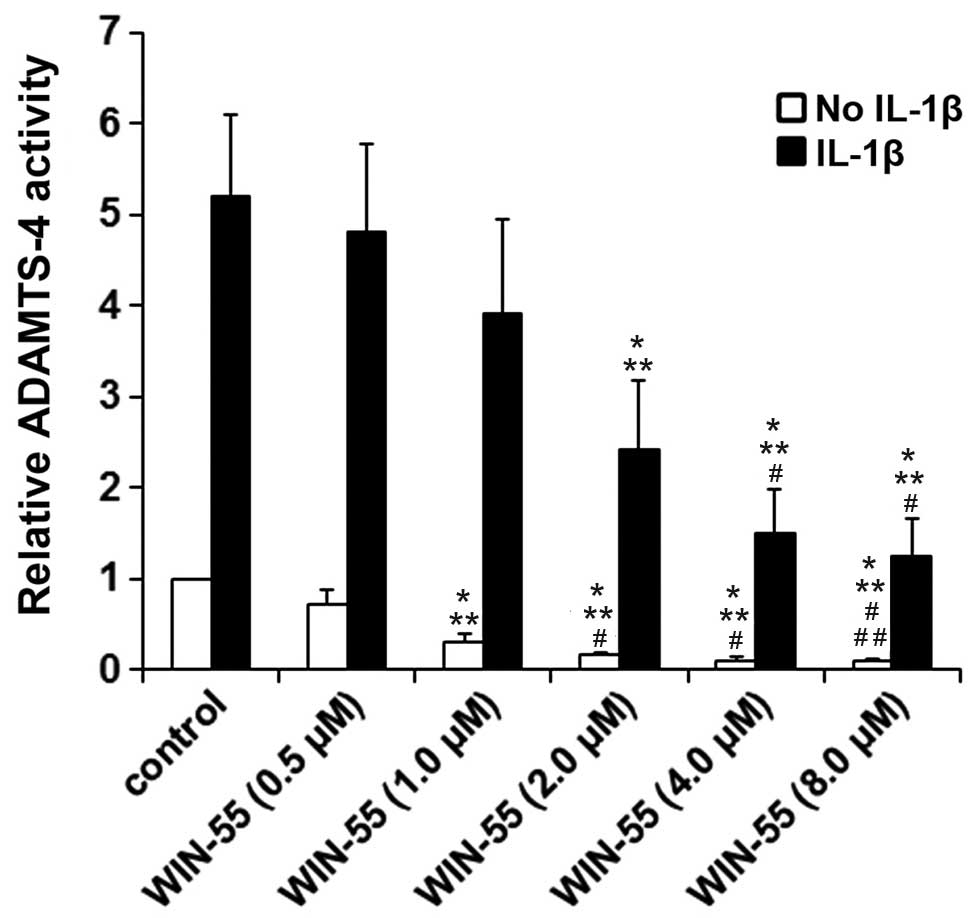
As demonstrated in Fig.
1, IL-1β stimulation increased the ADAMTS-4 activity by ~5 fold
in human OA articular chondrocytes. Synthetic cannabinoid WIN-55
concentration-dependently inhibited the ADAMTS-4 activity in the
presence or absence of IL-1β (P<0.05). The inhibitory effect of
WIN-55 reached a plateau at 2 μM in unstimulated cells (~85%
inhibition) and at 4 μM in IL-1β-stimulated cells (~70%
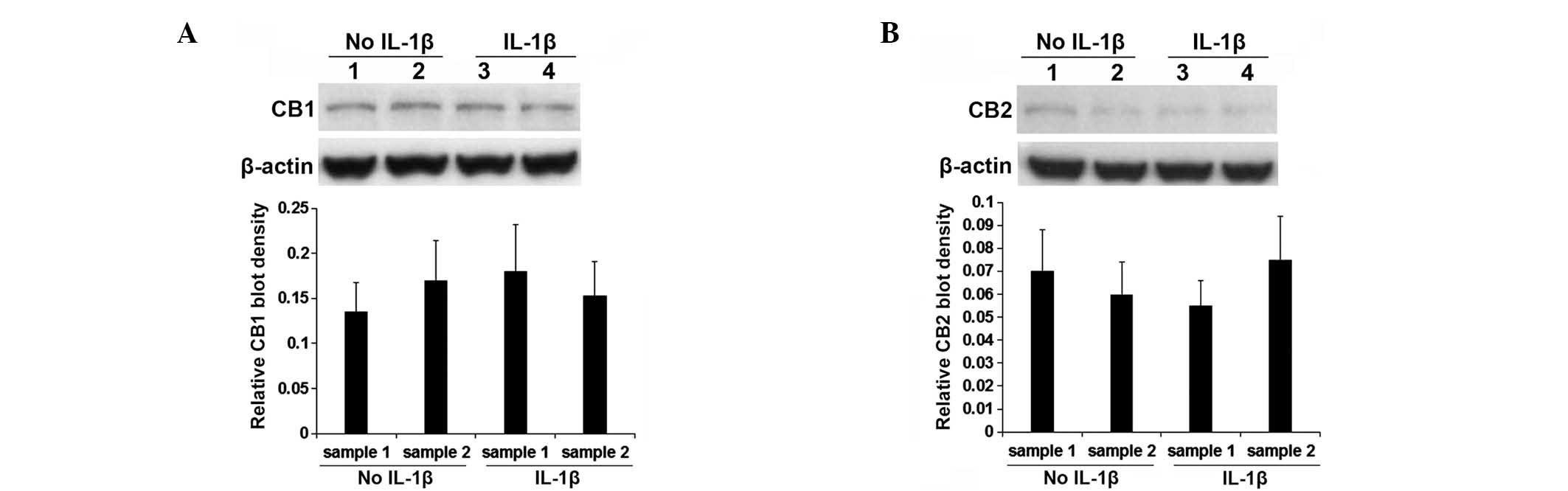
inhibition). As presented in Fig.
2, classical cannabinoid receptors CB1 and CB2 were
constitutively expressed in human OA articular chondrocytes. IL-1β
stimulation demonstrated no significant effect on the expression of
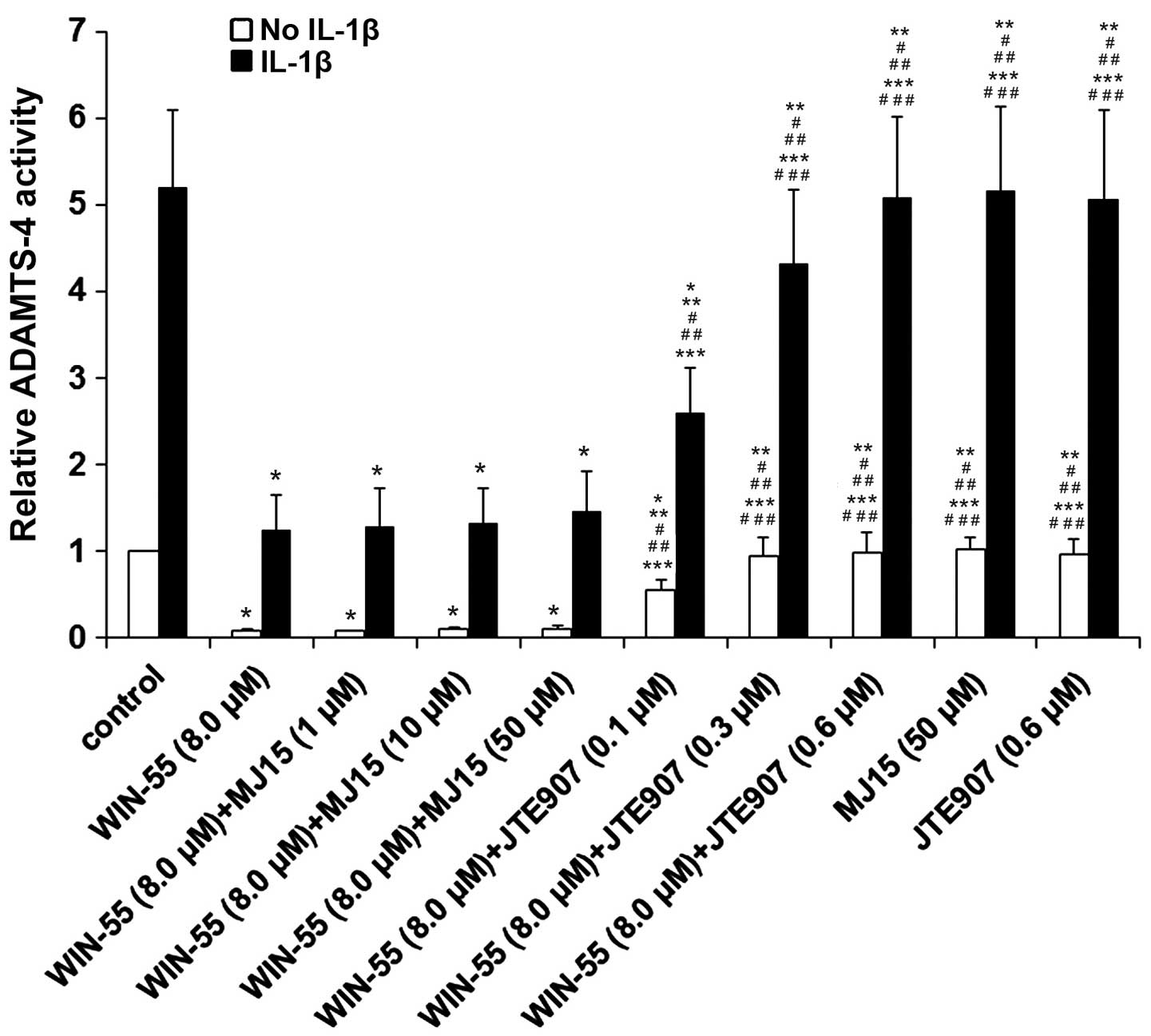
CB1 and CB2 (P>0.05). As presented in Fig. 3, selective CB2 antagonist JTE907
concentration-dependently abolished the inhibitory effect of WIN-55
on the ADAMTS-4 activity in unstimulated and IL-1β-stimulated human
OA articular chondrocytes compared with WIN-55 treatment alone
(P<0.05). Selective CB1 antagonist MJ15 did not cause any
significant change to the effect of WIN-55 (P>0.05).
Furthermore, MJ15 and JTE907 alone demonstrated no significant
effect on ADAMTS-4 activity in the presence or absence of IL-1β
compared with the control (P>0.05). The findings indicate that
WIN-55 inhibits ADAMTS-4 activity via CB2 in unstimulated and
IL-1β-stimulated human OA articular chondrocytes.
WIN-55 inhibits the protein expression
levels of syndecan-1, but not ADAMTS-4, in unstimulated and
IL-1β-stimulated human OA articular chondrocytes
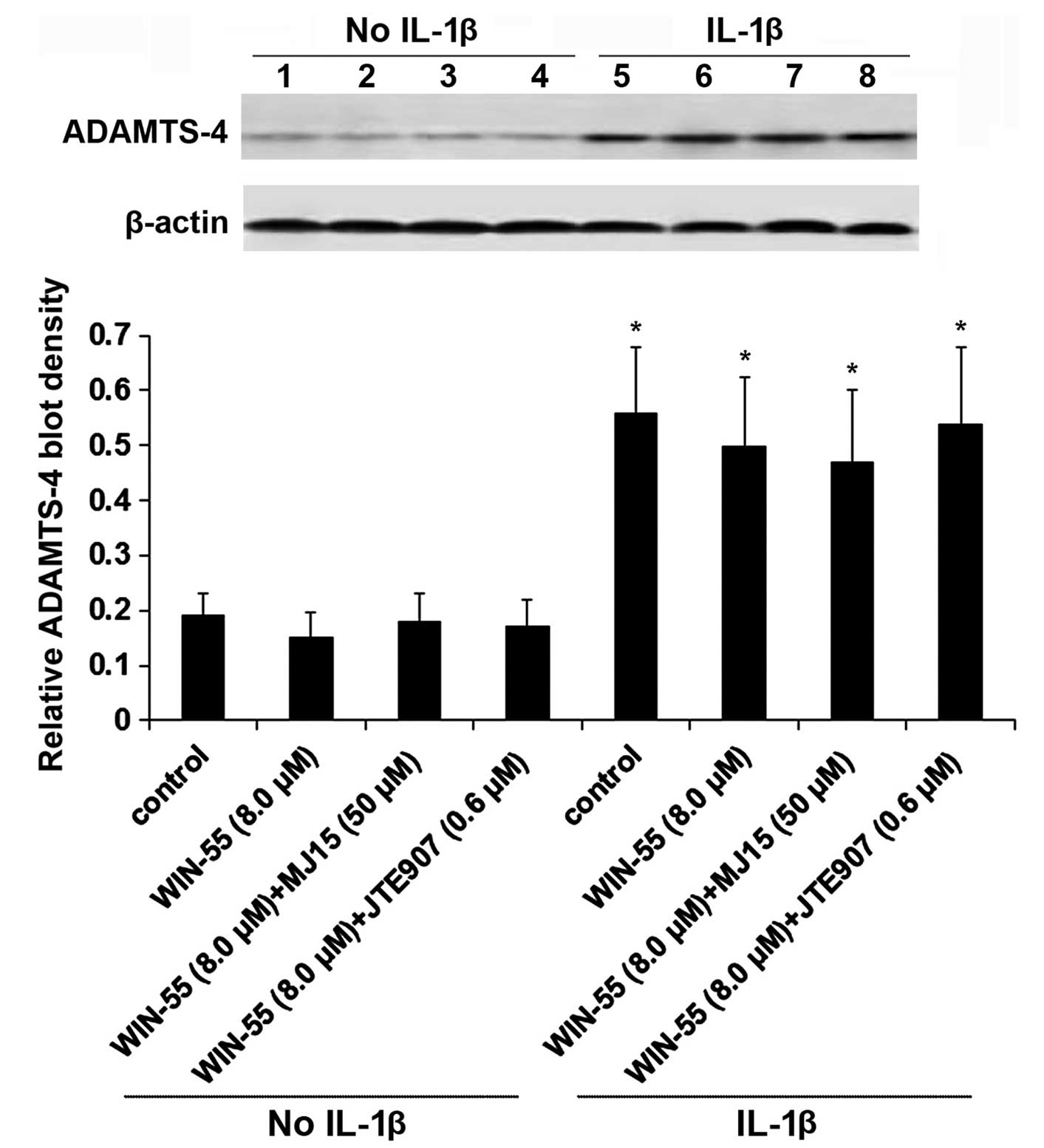
Western blot analyses were performed to examine the
effect of WIN-55 on the expression of ADAMTS-4 in human OA
articular chondrocytes. As presented in Fig. 4, IL-1β stimulation increased the
expression of ADAMTS-4 by ~3 fold in human OA articular
chondrocytes, compared with the unstimulated control (P<0.05).
WIN-55 alone or in the presence of MJ15 or JTE907 demonstrated no
significant effect on the expression of ADAMTS-4 in unstimulated or
IL-1β-stimulated cells (P>0.05). These findings indicate that
WIN-55 inhibits ADAMTS-4 activity in human OA articular
chondrocytes through mechanisms other than altering the protein
expression of ADAMTS-4.
As syndecan-1 is reportedly involved in the
activation of ADAMTS-4 in human chondrosarcoma cells (10), the current study examined the
effect of WIN-55 on the expression of syndecan-1 in human OA
articular chondrocytes. As presented in Fig. 5A, IL-1β stimulation did not
significantly alter the expression of syndecan-1 or the effect of
WIN-55 on syndecan-1 expression in human OA articular chondrocytes
(P>0.05). WIN-55 decreased the constitutive protein level of
syndecan-1 by ~75% in unstimulated and IL-1β-stimulated cells, as
compared with the untreated controls. The effect of WIN-55
treatment was completely abolished by CB2 antagonist JTE907
(Fig. 5A). Stable transfection of
a human syndecan-1 expression vector resulted in a significant
overexpression of syndecan-1 protein, which was inhibited by WIN-55
by ~55%, compared with control and vector control (P<0.05).
JTE907 completely abolished the inhibitory effect of WIN-55
(Fig. 5B). A similar effect on
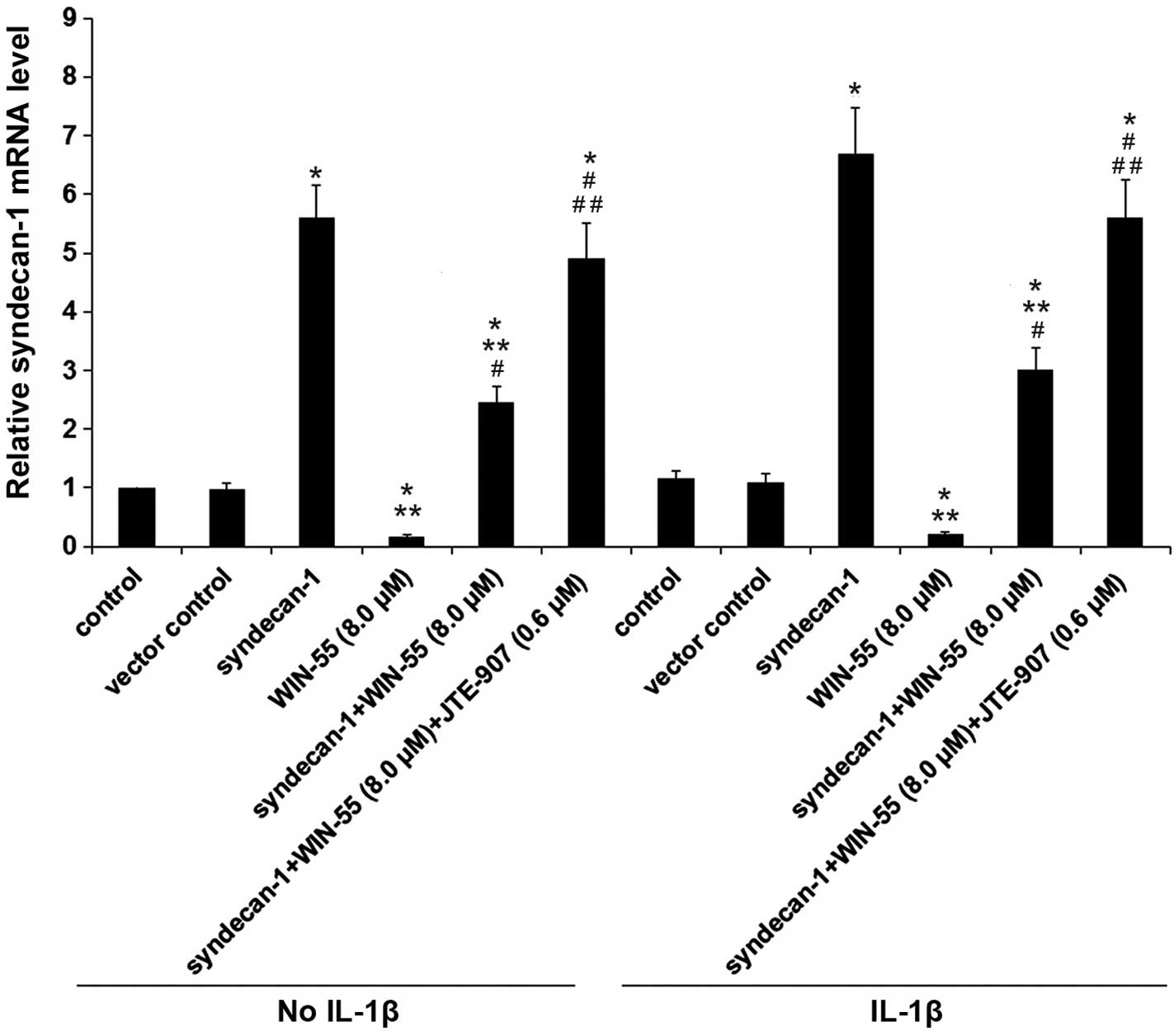
syndecan-1 expression was observed at the mRNA level (Fig. 6). These findings indicate that
WIN-55 exhibits no significant effect on the expression of
ADAMTS-4, but does appear to inhibit the expression of syndecan-1
via CB2 in human OA articular chondrocytes.
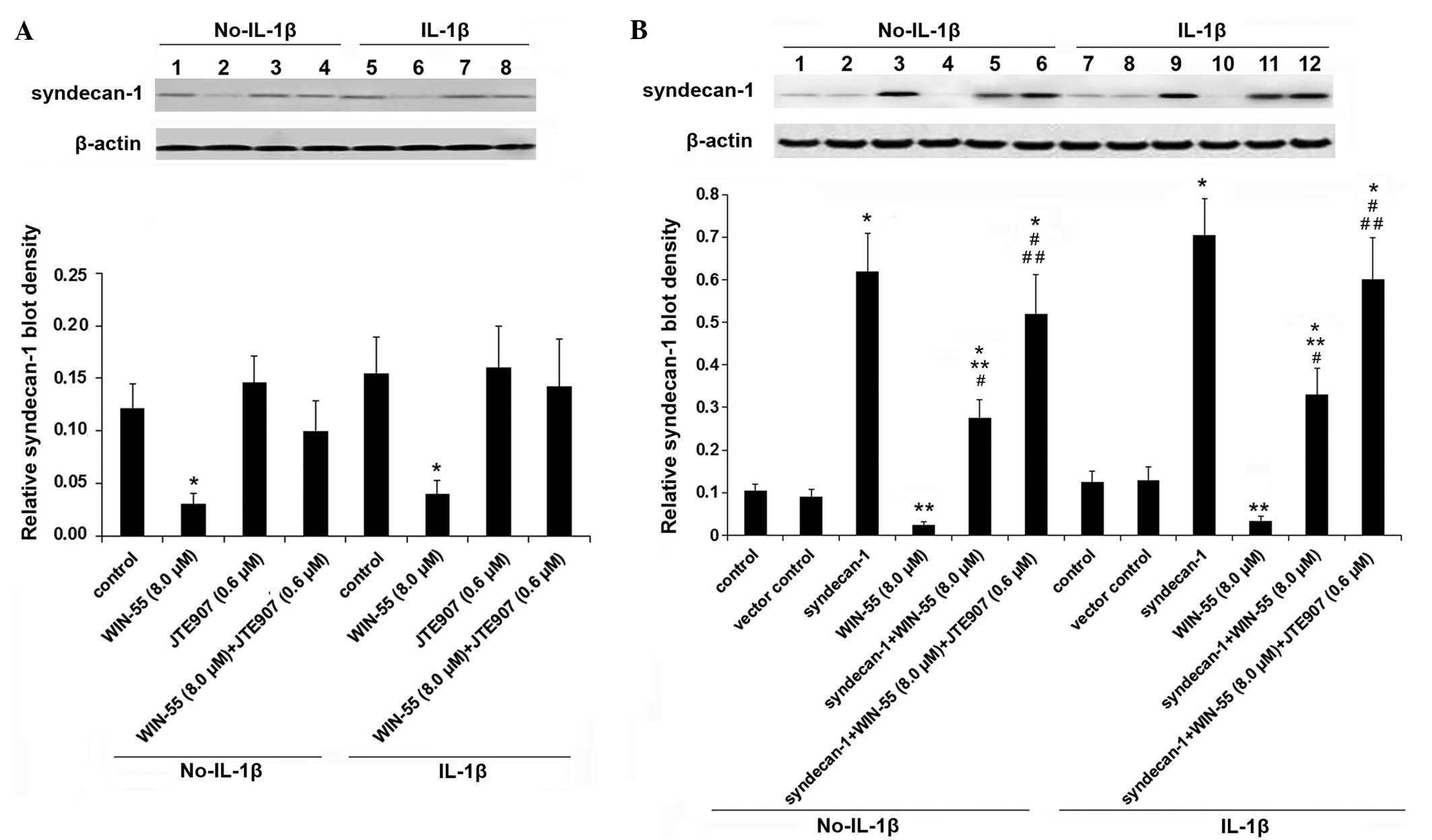 | Figure 5Protein expression levels of
syndecan-1 in human osteoarthritis (OA) articular chondrocytes in
the presence or absence of IL-1β, WIN-55 and cannabinoid receptor
type 2 (CB2) antagonist. Primary human OA articular chondrocytes
were cultured in the presence or absence of 10 ng/ml IL-1β for 24
h. Then the cells were treated with or without selective CB2
antagonist JTE907 (0.6 μM) for 30 min prior to the addition
of WIN-55 (8.0 μM) for a further 24-h incubation. For
syndecan-1 overexpression experiments, human OA articular
chondrocytes were stably transfected with the pcDNA3.1-syndecan-1
plasmid. Cells stably transfected with the pcDNA3.1 plasmid were
used as the vector control. The protein levels of syndecan-1 were
determined by western blot analyses. (A) Lanes 1 and 5, control;
lanes 2 and 6, WIN-55 (8.0 μM); lanes 3 and 7, JTE907 (0.6
μM); lanes 4 and 8, WIN-55 (8.0 μM) + JTE907 (0.6
μM). (B) Lanes 1 and 7, control; lanes 2 and 8, vector
control; lanes 3 and 9, syndecan-1 (overexpression); lanes 4 and
10, WIN-55 (8.0 μM); lanes 5 and 11, syndecan-1 + WIN-55
(8.0 μM); lanes 6 and 12, syndecan-1 + WIN-55 (8.0
μM) + JTE907 (0.6 μM). β-actin was used as a loading
control. Density of the syndecan-1 blot was normalized to that of
the β-actin blot to obtain a relative blot density. Three
independent experiments were performed for each western blot
analysis. Data are presented as the mean ± standard deviation. For
panel (A): *P<0.05 vs. control. For panel (B):
*P<0.05 vs. control and vector control;
**P<0.05 vs. syndecan-1; #P<0.05 vs.
WIN-55; ##P<0.05 vs. syndecan-1 + WIN-55. IL-1β,
interleukin-1β; WIN-55, WIN-55,212-2 mesylate. |
Overexpression of syndecan-1 reverses the
inhibitory effect of WIN-55/CB2 signaling on ADAMTS-4 activity in
unstimulated and IL-1β-stimulated human OA articular
chondrocytes
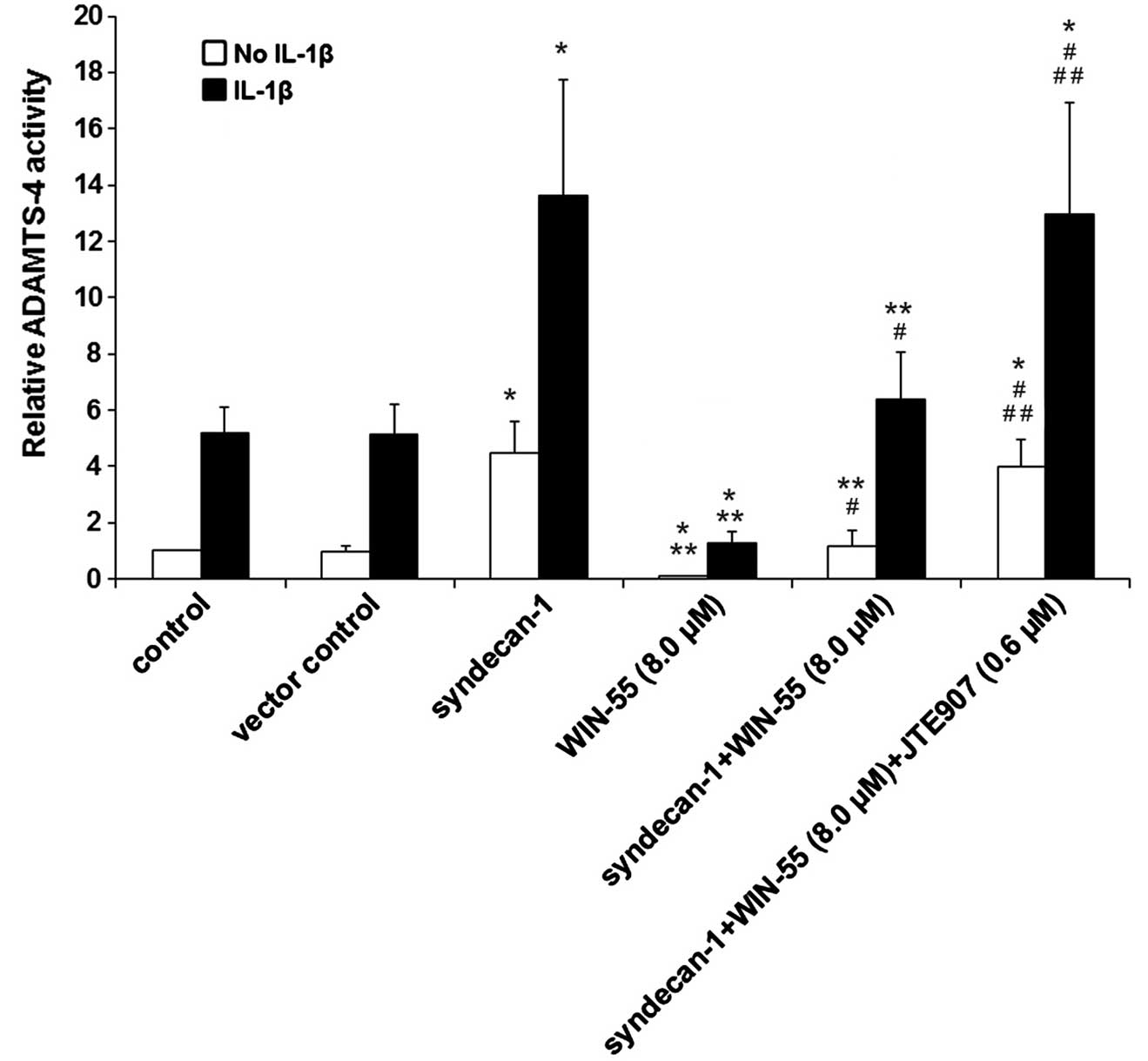
As presented in Fig.
7, overexpression of syndecan-1 significantly increased
ADAMTS-4 activity by 4.5-fold in unstimulated cells and by 2.6-fold
in IL-1β-stimulated human OA articular chondrocytes, compared with
the control and vector control (P<0.05). Furthermore,
overexpression of syndecan-1 completely reversed the inhibitory
effect of WIN-55 on ADAMTS-4 activity, compared with WIN-55
treatment alone. In the presence of WIN-55, JTE907 significantly
increased the inducing effect of syndecan-1 on ADAMTS-4 activity in
unstimulated and IL-1β-stimulated cells, as compared with
syndecan-1 overexpression and WIN-55 treatment (P<0.05; Fig. 7), although JTE907 alone showed no
significant effect on ADAMTS-4 activity (Fig. 1). Taken together, the findings
suggest that inhibiting the expression of syndecan-1 via CB2 may be
a rate-limiting step for WIN55-induced inhibition of ADAMTS-4
activity in unstimulated and IL-1β-stimulated human OA articular
chondrocytes.
WIN-55 decreases the stability of
syndecan-1 mRNA in unstimulated and IL-1β-stimulated human OA
articular chondrocytes
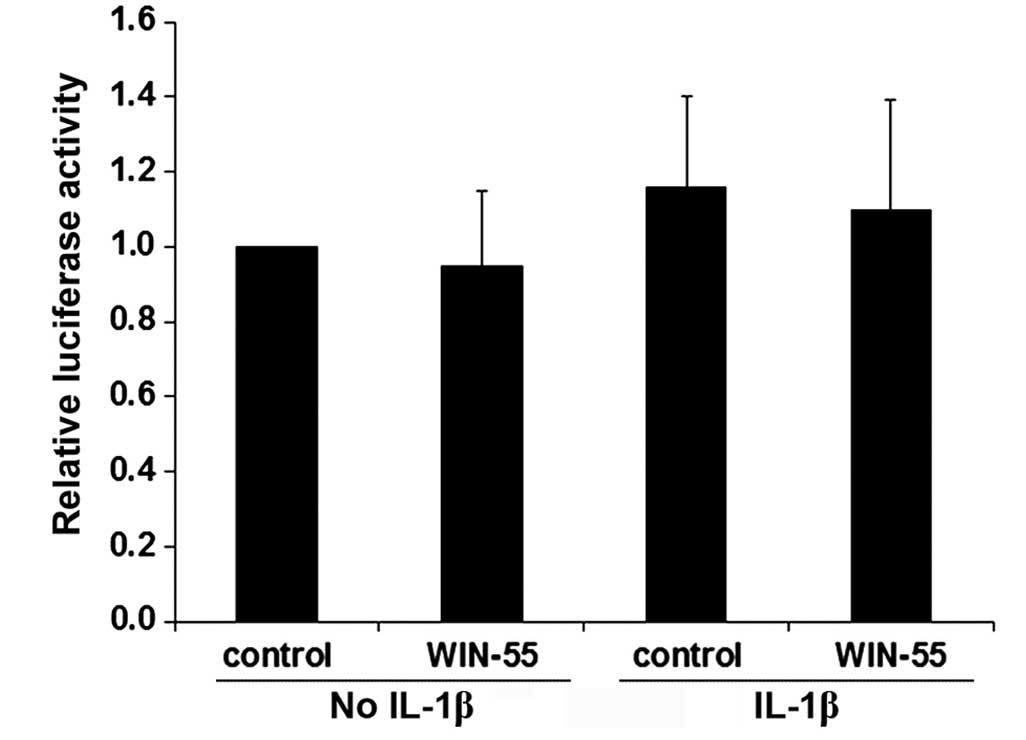
To examine whether WIN-55 decreases syndecan-1 mRNA
levels in OA articular chondrocytes by transcriptionally inhibiting
the syndecan-1 gene promoter, a human syndecan-1 gene
promoter/luciferase reporter was transfected into human OA
articular chondrocytes. As presented in Fig. 8, luciferase reporter assays
demonstrated that IL-1β and WIN-55 did not exhibit any significant
effect on syndecan-1 promoter activity, suggesting that WIN-55
treatment does not decrease syndecan-1 mRNA levels in human OA
articular chondrocytes at the gene promoter/transcriptional level.
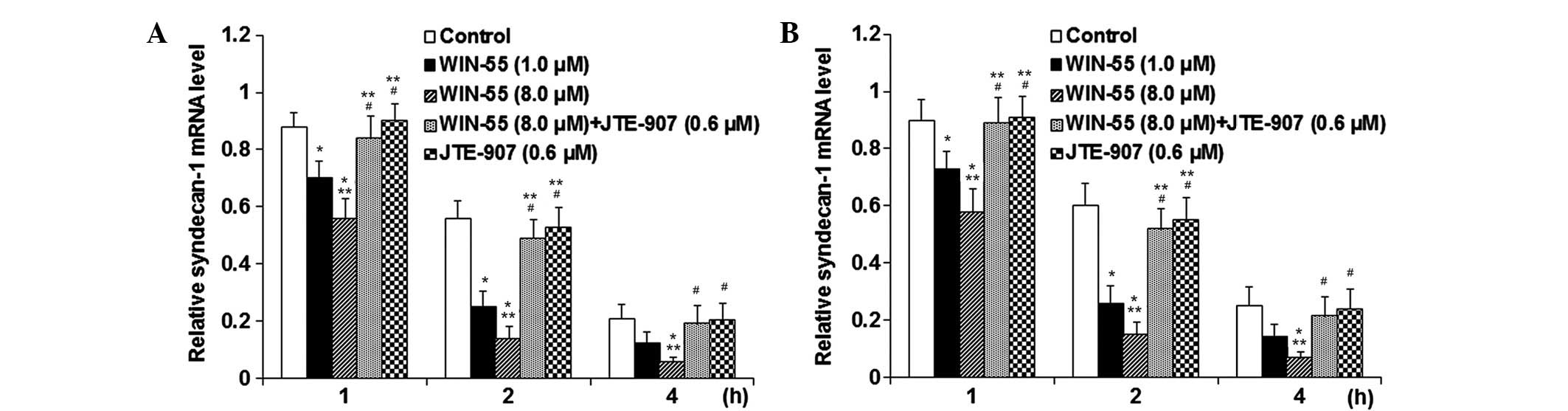
The current study additionally examined the effect of WIN-55 on
syndecan-1 mRNA stability. Following IL-1β stimulation, human OA
articular chondrocytes were treated with or without JTE907 for 30
min prior to the addition of WIN-55 for a further 24-h incubation
period. The transcription inhibitor actinomycin D was then added
and incubated with the cells for 1, 2 or 4 h. The mRNA level of
syndecan-1 was subsequently determined using RT-qPCR and expressed
as the fold change in mRNA levels of control cells at 0 h of
actinomycin D treatment. Under actinomycin D treatment, WIN-55
dose-dependently decreased the syndecan-1 mRNA expression level
compared with the control within 2 h of actinomycin D treatment
(P<0.05; Fig. 9). CB2
antagonism by JTE907 completely abolished the effect of WIN-55,
while JTE907 alone showed no signifi-cant effect on syndecan-1 mRNA
levels compared with the control (Fig.
9). IL-1β stimulation did not significantly alter the effect of
WIN-55 on the syndecan-1 mRNA level (Fig. 9). The findings suggest that WIN-55
decreases the expression of syndecan-1 in human OA articular
chondrocytes by decreasing the stability of syndecan-1 mRNA via
CB2; the effect of WIN-55 treatment was not significantly affected
by IL-1β stimulation.
Discussion
Accumulating evidence suggests that cannabinoids
have chondroprotective effects (11,13).
It has been demonstrated that biologically stable synthetic
cannabinoids, including WIN-55, protect cartilage matrix from
cytokine-induced degradation via CB receptors (12). A central feature of OA is the loss
of articular cartilage. Cartilage breakdown is mediated by the
complex interactions of pro-inflammatory cytokines, such as IL-1β,
inflammatory mediators, such as prostaglandin E2, and proteases,
such as matrix metalloproteinases (13). It has become clear that proteolysis
of the major constituents of the cartilage matrix, such as
collagens and proteoglycans, is achieved not only by the classical
matrix metalloproteinase family, but also by a relatively new group
of metalloproteinases termed the aggrecanase/ADAMTS family. The
degradation of aggrecan by aggrecanase-1 (also known as ADAMTS-4)
is an early event in osteoarthritic cartilage damage (5). Recent studies have suggested that
ADAMTS-4 is a major contributor to the pathogenesis of OA (3,4). To
the best of out knowledge, the present study provides the first
evidence suggesting that cannabinoid WIN-55 inhibits the activity
of ADAMTS-4 in OA chondrocytes.
The current study used IL-1β-stimulated human OA
articular chondrocytes as a cell model, as IL-1β is known to be
important in the development of OA by promoting degradation of the
cartilage matrix (2,3). In the present study, 24-h treatment
with 2 and 4 μM WIN-55 inhibited ADAMTS-4 activity by ~85
and 70% in unstimulated and IL-1β-stimulated human OA articular
chondrocytes, respectively, suggesting that WIN-55 is a potent
inhibitor of ADAMTS-4 activity in OA chondrocytes. Considering that
ADAMTS-4 is a major contributor to the pathogenesis of OA (3,4),
WIN-55 may have therapeutic value in the treatment of OA.
As an agonist of the classical cannabinoid receptors
CB1 and CB2, WIN-55 has also been demonstrated to activate other
receptors, including peroxisome proliferator activated receptor
(PPAR)-α and -γ (11,18-20).
In agreement with a previous investigation (11), the current study observed that CB1
and CB2 were constitutively expressed in human OA articular
chondrocytes. In the present study, a selective CB2 antagonist
completely abolished the inhibitory effect of WN-55 on ADAMTS-4
activity, however, a selective CB1 antagonist exhibited no effect,
indicating that WIN-55 inhibits ADAMTS-4 activity via CB2 in human
OA articular chondrocytes. Although PPAR-α and -γ are reportedly
also expressed in human OA articular chondrocytes (11), these receptors were not
investigated in the present study. In future studies, it may be
important to investigate the role of PPAR receptors in
WIN55-induced inhibition of ADAMTS-4 activity in OA articular
chondrocytes.
In agreement with a previous investigation (4), the current study demonstrated that
IL-1β induces the expression of ADAMTS-4 in human OA articular
chondrocytes. Although WIN-55 demonstrated a potent inhibitory
effect on ADAMTS-4 activity, it produced no direct effect on the
expression of ADAMTS-4 in unstimulated and IL-1β-stimulated human
OA articular chondrocytes. Notably, WIN-55 inhibited the expression
of syndecan-1, which is reportedly associated with the activation
of ADAMTS-4 in human chondrosarcoma cells (10). Furthermore, overexpression of
syndecan-1 reversed the inhibitory effects of WIN-55 on ADAMTS-4
activity, suggesting that inhibiting the expression of syndecan-1
is a rate-limiting step for WIN55-induced inhibition of ADAMTS-4
activity in human OA articular chondrocytes. Although no
significant effect on the syndecan-1 gene promoter activity was
observed, WIN-55 did significantly decrease the stability of
syndecan-1 mRNA via CB2, which provides a mechanistic explanation
for the inhibitory effect of WIN-55 on syndecan-1 expression in
human OA articular chondrocytes. The mechanism by which WIN-55/CB2
signaling decreases syndecan-1 mRNA stability in OA articular
chondrocytes should be explored in future studies. In addition, it
was noted that IL-1β stimulation produced no significant effect on
the WIN55-induced decrease in syndecan-1 mRNA stability. Similarly,
IL-1β stimulation did not significantly alter the inhibitory effect
of WIN-55 on syndecan-1 expression.
Syndecans are transmembrane heparan sulfate
proteoglycans that interact with extracellular matrix molecules,
growth factors and cytokines (5,8). The
syndecan family comprises four members: Syndecan-1, -2, -3 and -4
(7). As modulators of cellular
activities, the syndecans are commonly associated with normal
tissue remodeling or pathological changes in tissue organization
(7). All four syndecans, and two
major aggrecanases (ADAMTS-4 and -5), are expressed in chondrocytes
(5,10). Previous studies have suggested that
the activation of ADAMTS-4 and -5 in chondrocytes requires
syndecan-1 and -4, respectively (5,10).
As the findings of the current study suggest that WIN-55 inhibits
ADAMTS-4 activity in human OA articular chondrocytes by inhibiting
the expression of syndecan-1, several questions arise for future
studies: i) Can synthetic cannabinoids also alter the expression of
syndecan-4 and regulate ADAMTS-5 activity in OA articular
chondrocytes?; and i) as loss of articular cartilage is also a key
pathological feature of rheumatoid arthritis (RA) (11,21),
can synthetic cannabinoids alter the expression of syndecans, in
addition to the activities of ADAMTS, in RA articular chondrocytes
and have potential therapeutic value for the treatment of RA?
In conclusion, the current study provides in
vitro evidence supporting that synthetic cannabinoid WIN-55
inhibits ADAMTS-4 activity in unstimulated and IL-1β-stimulated
human OA articular chondrocytes by decreasing the mRNA
stability/expression of syndecan-1 via CB2. This suggests a novel
mechanism by which cannabinoids may prevent cartilage breakdown in
OA. In addition, it also provides novel insights regarding the
pharmacological effects of synthetic cannabinoids on OA.
References
|
1
|
Lawrence RC, Felson DT, Helmick CG, Arnold
LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG,
et al: National Arthritis Data Workgroup: Estimates of the
prevalence of arthritis and other rheumatic conditions in the
United States: Part II. Arthritis Rheum. 58:26–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xue J, Wang J, Liu Q and Luo A: Tumor
necrosis factor-α induces ADAMTS-4 expression in human
osteoarthritis chondrocytes. Mol Med Rep. 8:1755–1760.
2013.PubMed/NCBI
|
|
4
|
Matsukawa T, Sakai T, Yonezawa T, Hiraiwa
H, Hamada T, Nakashima M, Ono Y, Ishizuka S, Nakahara H, Lotz MK,
et al: MicroRNA-125b regulates the expression of aggrecanase-1
(ADAMTS-4) in human osteoarthritic chondrocytes. Arthritis Res
Ther. 15:R282013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Echtermeyer F, Bertrand J, Dreier R,
Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C,
Theilmeier G and Pap T: Syndecan-4 regulates ADAMTS-5 activation
and cartilage breakdown in osteoarthritis. Nat Med. 15:1072–1076.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song RH, Tortorella MD, Malfait AM, Alston
JT, Yang Z, Arner EC and Griggs DW: Aggrecan degradation in human
articular cartilage explants is mediated by both ADAMTS-4 and
ADAMTS-5. Arthritis Rheum. 56:575–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barre PE, Redini F, Boumediene K, Vielpeau
C and Pujol JP: Semiquantitative reverse transcription-polymerase
chain reaction analysis of syndecan-1 and -4 messages in cartilage
and cultured chondrocytes from osteoarthritic joints.
Osteoarthritis Cartilage. 8:34–43. 2000. View Article : Google Scholar
|
|
8
|
Tkachenko E, Rhodes JM and Simons M:
Syndecans: New kids on the signaling block. Circ Res. 96:488–500.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Molténi A, Modrowski D, Hott M and Marie
PJ: Differential expression of fibroblast growth factor receptor-1,
-2, and -3 and syndecan-1, -2, and -4 in neonatal rat mandibular
condyle and calvaria during osteogenic differentiation in vitro.
Bone. 24:337–347. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao G, Plaas A, Thompson VP, Jin S, Zuo F
and Sandy JD: ADAMTS4 (aggrecanase-1) activation on the cell
surface involves C-terminal cleavage by glycosylphosphatidyl
inositol-anchored membrane type 4-matrix metalloproteinase and
binding of the activated proteinase tochondroitin sulfate and
heparan sulfate on syndecan-1. J Biol Chem. 279:10042–10051. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dunn SL, Wilkinson JM, Crawford A, Le
Maitre CL and Bunning RA: Cannabinoid WIN-55,212-2 mesylate
inhibits interleukin-1β induced matrix metalloproteinase and tissue
inhibitor of matrix metalloproteinase expression in human
chondrocytes. Osteoarthritis Cartilage. 2:133–144. 2014. View Article : Google Scholar
|
|
12
|
Mbvundula EC, Bunning RA and Rainsford KD:
Arthritis and cannabinoids: HU-210 and Win-55,212-2 prevent
IL-1alpha-induced matrix degradation in bovine articular
chondrocytes in-vitro. J Pharm Pharmacol. 58:351–358. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dunn SL, Wilkinson JM, Crawford A and Le
Maitre CL: Novel therapies for arthritis? Future Med Chem.
4:713–725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moncada-Pazos A, Obaya AJ, Viloria CG,
López-Otín C and Cal S: The nutraceutical flavonoid luteolin
inhibits ADAMTS-4 and ADAMTS-5 aggrecanase activities. J Mol Med
Berl. 89:611–619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tauchi R, Imagama S, Natori T, Ohgomori T,
Muramoto A, Shinjo R, Matsuyama Y, Ishiguro N and Kadomatsu K: The
endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes
functional recovery after spinal cord injury. J Neuroinflammation.
9:532012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doyle A and Griffiths JB: Cell and Tissue
Culture: Laboratory Procedures. Cell Quantification. John Wiley
& Sons, Inc; Chichester, England: pp. 58–59. 1998
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Pertwee RG, Howlett AC, Abood ME,
Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos
G, Mackie K, et al: International Union of Basic and Clinical
Pharmacology. LXXIX. Cannabinoid receptors and their ligands:
Beyond CB and CB1 and CB2. Pharmacol Rev.
62:588–631. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Alexander SP, Kendall DA and
Bennett AJ: Cannabinoids and PPARalpha signalling. Biochem Soc
Trans. 34:1095–1097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Sullivan SE and Kendall DA: Cannabinoid
activation of peroxisome proliferator-activated receptors:
Potential for modulation of inflammatory disease. Immunobiology.
215:611–616. 2010. View Article : Google Scholar
|
|
21
|
Goldring MB and Marcu KB: Cartilage
homeostasis in health and rheumatic diseases. Arthritis Res Ther.
11:2242009. View
Article : Google Scholar : PubMed/NCBI
|