Introduction
Late-onset hypogonadism (LOH) has been defined as a
syndrome in middle-aged and elderly men, who report symptoms in the
presence of low levels of testosterone (1). Testosterone deficiency is associated
with alterations in reproductive function, muscle strength, bone
density and other physiological parameters (2).
Testosterone is predominantly produced by Leydig
cells. One of the major reasons for the reduced production of
testosterone is oxidative stress, which usually results from an
imbalance between the production of reactive oxygen species (ROS)
and the scavenging ability of cellular antioxidant defense systems.
ROS, including H2O2 and superoxides, are
produced by cells as by-products of normal cellular metabolism.
Elevated levels of ROS have been shown to be associated with
several diseases, including neurodegenerative disease and cancer
(3,4). Multiple studies have indicated that
ROS inhibits testosterone production in Leydig cells by dissipating
mitochondrial membrane potential, and reducing the expression and
activity of testicular steroidogenic enzymes (5–7).
Thus, the accumulation of ROS during the ageing process results in
reduced levels of testosterone (8).
Peroxiredoxins (Prdxs) are a family of antioxidant
enzymes, which are capable of metabolizing
H2O2. Prdxs are thioredoxin-specific
antioxidants, which were first identified in yeast, and are found
in archea, prokaryotes and eukaryotes (9). Prdx2, a member of the peroxiredoxin
family, is considered to regulate multiple cellular functions,
including cell proliferation, differentiation and intracellular
signaling. Of note, through the clearance of excessive
H2O2, Prdx2 is critical in the modulation of
cell survival. For example, previous studies have indicated that
Prdx2 is upregulated in colorectal cancer and protects cells from
oxidative stress (10), whereas
Prdx2 knockdown by RNA interference inhibits the growth of
colorectal cancer cells (11), and
attenuation of Prdx2 inhibits proliferation and induces apoptosis
in granulosa cells (12).
Despite the protective effect of Prdx2 in several
cell types, its biological function in Leydig cell remains to be
elucidated. In the present study, primary Leydig cells were treated
with H2O2 to induce oxidative stress,
following which cell apoptosis, testosterone production and changes
in the expression of Prdx2 were investigated. These investigations
were performed to determine whether Prdx2 is involved in the
modulation of cell survival and testosterone production in Leydig
cells.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM)/Ham's
nutrient mixture F12 (DMEM/F12) was purchased from Invitrogen;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Percoll, HEPES
and collagenase type I were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Hanks' balanced salt solution (HBSS) without
Ca2+ or Mg2+, and penicillin-streptomycin
were purchased from Life Technologies, Inc. (Paisley, UK).
H2O2 was purchased from Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, China). The Annexin V Apoptosis
Detection kit APC was purchased from eBioScience, Inc. (San Diego,
CA, USA). Mouse monoclonal antibody against 3β-hydroxysteroid
dehydrogenase (HSD) was purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA; cat. no. 515120; dilution, 1:200).
Rabbit monoclonal antibody against Prdx2 was purchased from Abcam
(Cambridge, MA, USA; cat. no. ab133481; dilution, 1:50,000).
Monoclonal rabbit antibodies against β-actin (cat. no. AC-40;
dilution, 1:10,000), secondary horseradish peroxidase-conjugated
goat anti-mouse (cat. no. BA1050; dilution, 1:10,000) and goat
anti-rabbit antibodies (cat. no. BA1054; dilution, 1:10,000) were
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China).
Animals
Male Sprague-Dawley rats (n=20; age, 9-10 weeks;
weight, 200±20 g) were obtained from the Center of Comparitive
Medicine, Nanjing Jinling Hospital (Nanjing, China) and bred in the
laboratory of the Center of Reproductive Medicine, Nanjing Jinling
Hospital, Nanjing University School of Medicine (Nanjing, China).
The animal room was maintained at 22–24°C under a constant 12 h
light: 12 h dark cycle. The animals were fed with standard pellet
diet and water ad libitum. The procedures involving the
animals were performed following the guidelines for animal
treatment of Nanjing Jinling Hospital and approved by the ethics
committee of Nanjing Jinling Hospital, in accordance with the
principles and procedure of the National Institute of Health
guidelines for the care and use of laboratory animals (13).
Leydig cell isolation and culture
The Leydig cells were prepared from the immature rat
testes by collagenase treatment, as described previously (14). Briefly, the rats were sacrificed by
cervical dislocation and immersed in 75% ethanol for 5 min. The
sterile testes were dissected and washed three times in
phosphate-buffered saline (PBS) chilled to 4°C. The epididymis,
visible vessels, adipose and connective tissues were removed from
the testes using microscissors. The tunica albuginea was dissected
and the decapsulated testes were incubated with collagenase (0.25
mg/ml) for 20 min at 37°C. The crude interstitial cells were
collected by centrifugation at 1,000 g for 10 min at 4°C, and then
washed twice in HBSS containing 0.1% (w/v) bovine serum albumin
(BSA; Sigma-Aldrich). To purify the Leydig cells, the crude cell
suspension was loaded onto a discontinuous Percoll gradient (20,
40, 60 and 90% Percoll in HBSS) and subsequently centrifuged at 800
g for 20 min at 4°C. The fractions enriched in the Leydig cells
were obtained and further centrifuged in a continuous,
self-generating density gradient (starting at 60% Percoll), at
20,000 g for 30 min at 4°C.
The cells were then resuspended at a density of
105 cells/cm2 in 24-well plates (Costar;
Corning, NY, USA) at 0.5 ml/well. A total of 1.0×106 The
total numbers of purified cells were analyzed for the expression of
3β-HSD to determine the purity of the Leydig cells (15). The purity was found to be ~85–90%,
and >90% of these cells were viable, as determined using Trypan
blue exclusion dye (Sigma-Aldrich). The purified Leydig cells were
then washed twice with DMEM-F/12 and resuspended in DMEM-F/12
supplemented with 15 mmol/l HEPES (pH 7.4), 1 mg/ml BSA, 365 mg/l
glutamine (Invitrogen; Thermo Fisher Scientific, Inc.), 100 IU/ml
penicillin and 100 μg/ml streptomycin.
For subsequent culturing, 2×106 cells
were plated into each well of a 6-well plate (Costar; Corning, NY,
USA) and incubated at 34°C in a humidified atmosphere of 5%
CO2.
R2C cell line and granulosa cell
culture
The R2C cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and cultured in DMEM/F12
with 15% horse serum (Gibco; Thermo Fisher Scientific, Inc.), 2.5%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
100 U/l penicillin-100 μg/l streptomycin, at 34°C in a
humidified atmosphere of 5% CO2. The granulosa cells
were obtained from the Center of Comparative Medicine, Nanjing
Jinling Hospital and cultured in 6-well plates in DMEM/F12 medium,
10% FBS, at 34°C in a humidified atmosphere of 5% CO2.
The cells were used for reverse transcription-polymerase chain
reaction (RT-PCR) and western blotting, as described below.
Measurement of malondialdehyde (MDA)
levels
Following 24 h of Leydig cell separation and
culturing, the cells were treated with different concentrations of
H2O2 (50, 100 and 200 μM) for 4 and 6
h. At the end of the treatment, the cells were harvested and
sonicated with phosphate buffer (pH 6.8) containing 1.0 mM
phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich), to obtain cell
homogenates. The homogenates were centrifuged at 3,000 × g at 4°C
for 10 min and the supernatants were used for measuring cellular
levels of MDA using an MDA assay kit (Jiancheng Biochemical, Inc.,
Nanjing, China). The MDA levels were calculated by evaluating the
thiobarbituric acid reacting substance at a wavelength of 532 nm
using an Infinite M200 microplate reader (Tecan Group, Ltd.,
Mannedorf, Switzerland). All values were normalized against the
total protein concentration of the corresponding samples. The units
of MDA measurements were μmol/g.
Analyses of Leydig cell viability and
apoptosis
The Leydig cells were cultured and subsequently
treated with different doses of H2O2 (50, 100
and 200 μM) for 4 and 6 h. This was followed by assessment
of their viability and apoptosis using an Annexin V-Propidium
iodide (PI) Apoptosis Detection kit (cat. no. 88-8007) according to
the manufacturer's protocol (eBioScience, Inc.). Briefly, the cells
pellets were resuspended in 100 μl of 1X binding buffer
(eBioscience, Inc.) and incubated with 5 μl of
fluorochrome-conjugated Annexin V for 10–15 min at room
temperature. The cells were then washed with 1X binding buffer and
resuspended in 400 μl of the same binding buffer. Following
this, 5 μl of PI staining solution was added to the cells,
and each sample was analyzed immediately using a Cell Lab BD
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Data analysis was performed using FlowJo version 7.6.1 software
(Tree Star, Inc., Ashland, OR, USA).
Radioimmunoassay of testosterone
To determine the levels of testosterone, the Leydig
cells were incubated with fresh medium containing increasing
concentrations of H2O2 (50, 100 and 200
μmol/l) for 4 and 6 h in the presence of human chorionic
gonadotropin (hCG; 2 ng/ml; Sigma-Aldrich). The levels of
testosterone were determined using a chemiluminescence assay with
an Access Testosterone assay kit, according to the manufacturer's
protocol (Beckman Coulter, Brea, CA, USA).
RT-PCR analysis
Total RNA was extracted from the rat Leydig cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The quality and
concentration of the RNA was determined using an Eppendorf
BioPhotometer® D30 (Eppendorf, Germany). Total RNA was
reverse-transcribed using the PrimeScript™RT-PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China) in a total volume of 25
μl, comprising 5xAMV buffer, 2.5 mmol/l dNTPs, OligdT and 10
U/μl AMV, together with RNA as the template. The reaction
was performed at 42°C for 60 min, following which the samples were
incubated at 95°C for 5 min to terminate the reaction.
The RT-PCR was performed using an AffinityScript
One-Step RT-PCR kit (Stratagene, Mississauga, ON, Canada). The
primer sequences (synthesized by Shanghai Sangong Pharmaceutical
Co., Ltd., Shanghai, China) were as follows: Sense
3′-ATGATGAGGGCATCG CTTAC-5′ and antisense
3′-CATTGGGTTTGATGGTGTCA-5′ for Prdx2; and sense,
5′-GACATGCCGCCTGGAGAAAC-3′ and anti-sense,
5′-AGCCCAGGATGCCCTTTAGT-3′. The DNA was first denatured at 95°C for
30 sec, followed by annealing at 60°C for 30 sec and extension at
72°C for 30 sec. This was repeated for 30 cycles, prior to a final
extension step at 72°C for 10 min. The PCR product was finally run
on a 1.5% agarose gel (Invitrogen; Thermo Fisher Scientific, Inc.)
and visualized using ethidium bromide (Sigma-Aldrich).
Similarly, for quantitative (q)PCR, the RNA samples
from the Leydig cells treated with the various concentrations of
H2O2 for different durations were prepared
and processed, as described above. To determine the expression
levels of Prdx2 RT-qPCR was performed using an ABI Prism 7000
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The GAPDH gene was used as an internal control
for Prdx2 template normalization. Fluorescent signals were
normalized to that of the internal reference, and the
quantification cycle (Cq) was set within the exponential phase of
the PCR. The relative mRNA expression levels were calculated using
the 2-(ΔCt sample-ΔCt control) method (16).
Western blot analysis
The Leydig cells were serum-starved for 4 h
following washing once with fresh medium. Subsequently, the cells
were stimulated by increasing concentrations of
H2O2 (50, 100 and 200 μM) for 2, 4 and
6 h). Following treatment, the cells were washed twice with
ice-cold PBS and lysed in 120 μl of ice-cold
radioimmunoprecipitation assay buffer (Sangon Biotech Co., Ltd.,
Shanghai, China), containing 150 mmol/l NaCl, 1% Nonidet P-40,
0.25% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 50 mmol/l
Tris (pH 7.4), 1 mmol/l PMSF, 1 mmol/l Na3VO4
and 1 mmol/l NaF. The cell lysates were harvested and centrifuged
at 10,000 g for 20 min at 4°C. The supernatants were transferred to
new tubes, and the protein concentrations were determined using the
Bradford method (17). The total
protein (30 μg) was then mixed with loading buffer (Sangon
Biotech Co., Ltd.) and boiled for 5 min. These protein samples were
subsequently separated by running them on 15% SDS-PAGE gels (Sangon
Biotech Co., Ltd.) in 1X running buffer (Sangon Biotech Co., Ltd.)
at 25 mA for 2 h. The proteins were transferred onto a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA) at 100 V for 1 h in transfer buffer at 4°C (Sangon Biotech
Co., Ltd.) at 4°C. Subsequently, the membrane was blocked with 5%
non-fat milk powder in Tris-buffered saline with 0.5% Tween 20
(TBST) for 1.5 h at 37°C, and washed three times with TBST for 30
min. The membrane was then incubated with Prdx2 primary antibody
for 16–18 h at 4°C. Following washing in TBST, the membrane was
incubated with horseradish peroxidase-conjugated secondary
antibody, and the bands were visualized with enhanced
chemiluminescence (Promega), according to the manufacturer's
protocol. The intensities of the bands were quantitated using
Quantity-One version 4.6.2 software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean, with standard deviation shown as bars in the figures.
The differences between means were analyzed using one-way analysis
of variance and the least significance difference method using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
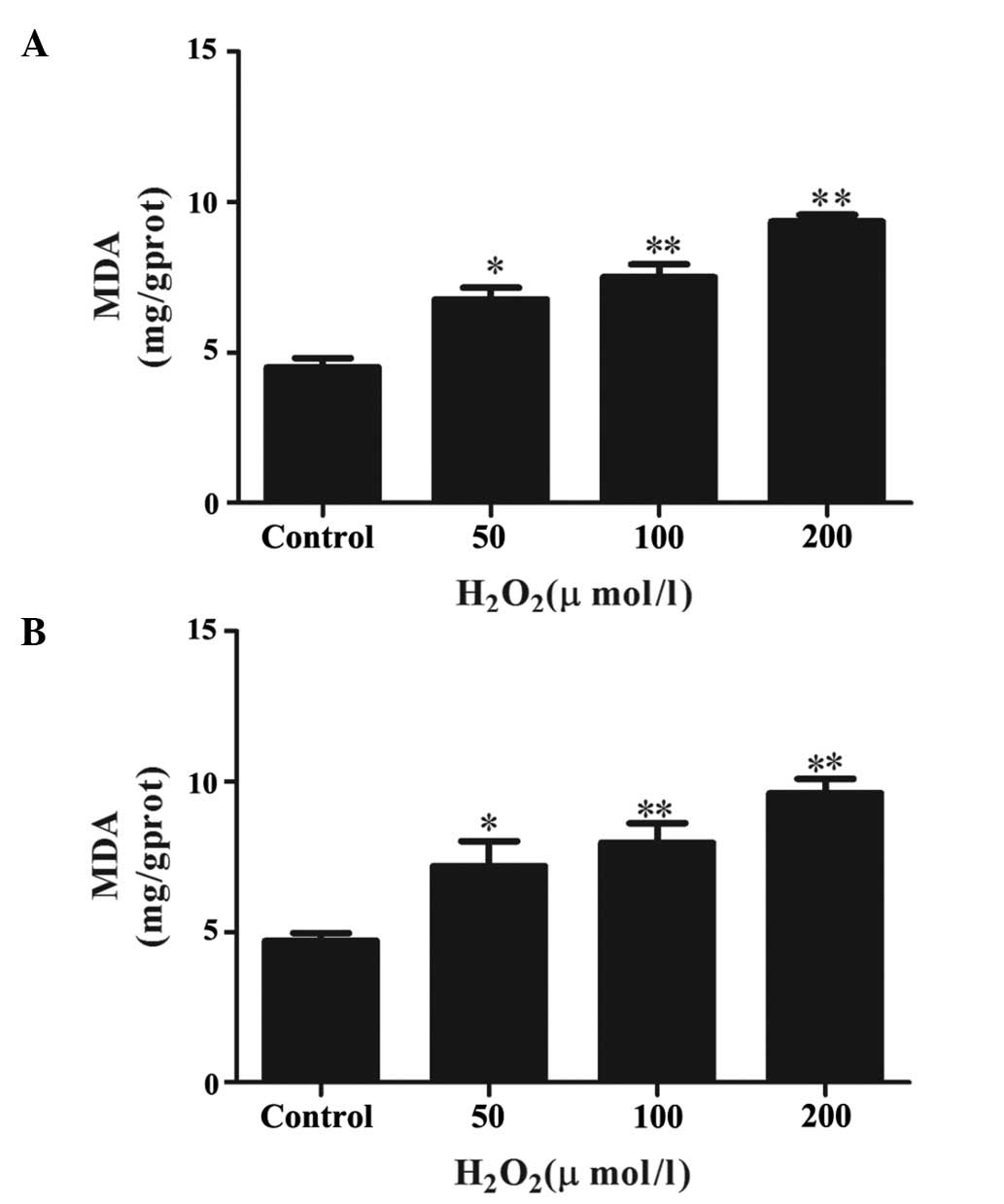
H2O2 induces lipid
peroxidation in primary Leydig cells
The levels of lipid peroxidation in the Leydig cells
following H2O2 treatment were assessed by
measuring the levels of MDA, which is the most frequently used
biomarker to detect oxidative changes. As shown in Fig. 1A, treatment of these cells with
different concentrations of H2O2 for 4 h
caused significant, concentration-dependent increases in MDA
levels, compared with the control group. However, increasing the
duration of H2O2 treatment to 6 h (Fig. 1B) had no additional significant
effect on the levels of MDA, compared with the cells treated for 4
h.
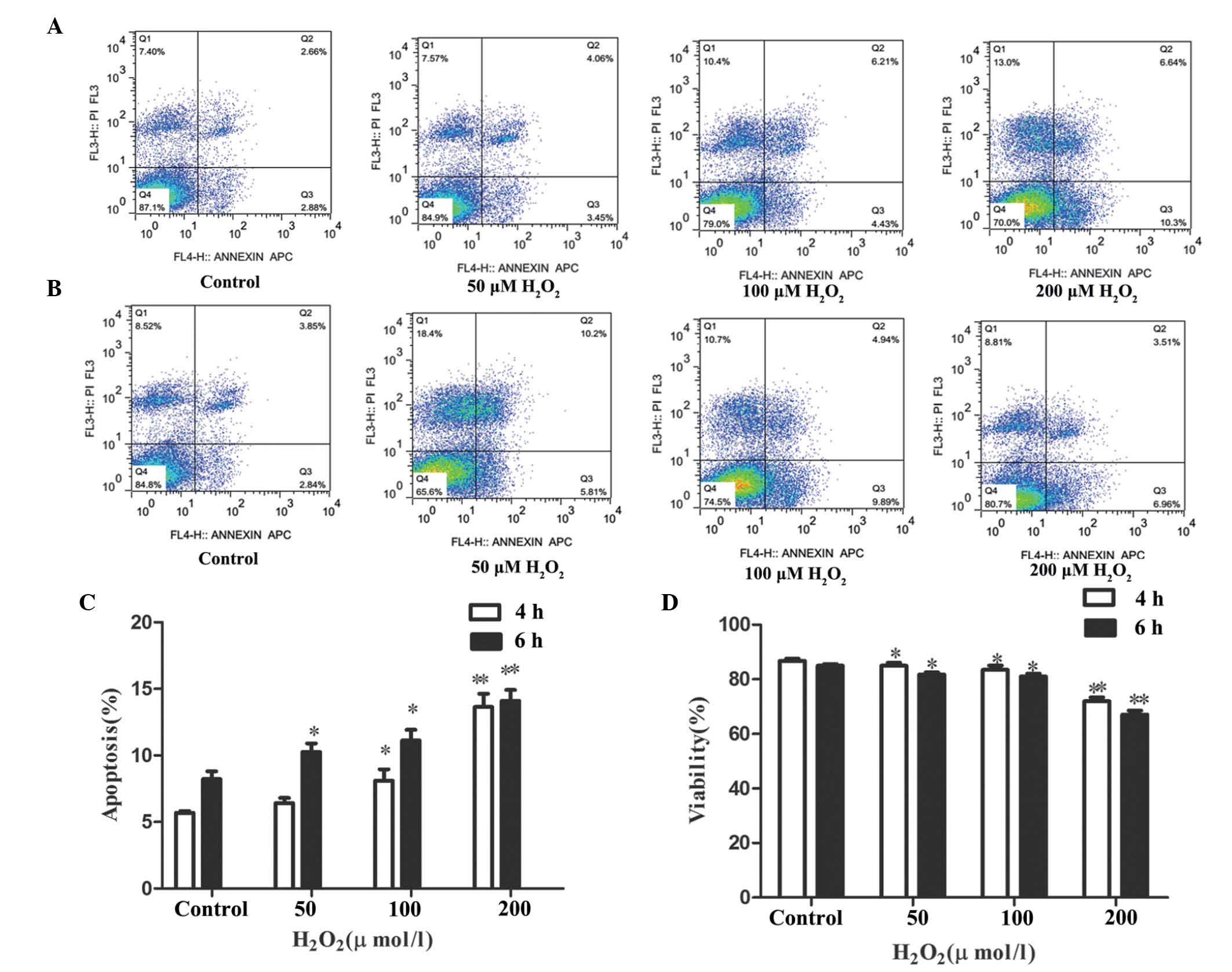
Effects of H2O2
treatment on primary Leydig cell viability and apoptosis
Following exposure of the Leydig cells with varying
concentrations of H2O2 (50, 100 and 200
μM) for 4 and 6 h, the cell viability and rates of apoptosis
were determined using PI and Annexin-V staining, respectively
(Fig. 2). It was observed that
H2O2 treatment for 4 h led to a decrease in
cell viability, whereas the apoptotic rate increased in a
dose-dependent manner, as seen in Fig.
2A. Following 4 h treatment, the apoptotic rate was highest
(13.65±2.44%) in the group treated with 200 μM
H2O2, compared with the control group
(Fig. 2C). This was simultaneously
associated with decreased cell viability (77%; Fig. 2D). Prolonged treatment for 6 h with
the same concentrations of H2O2 led to no
significant enhancement of apoptosis or cell viability, compared
with the 4 h treatment group (Fig.
2B), and the two time points had relatively similar effects.
The apoptotic rate of the cells treated with 200 μM
H2O2 was 14.1±0.02% (Fig. 2C) following 6 h treatment, and cell
viability decreased to a similar extent as that observed following
4 h treatment (Fig. 2D).
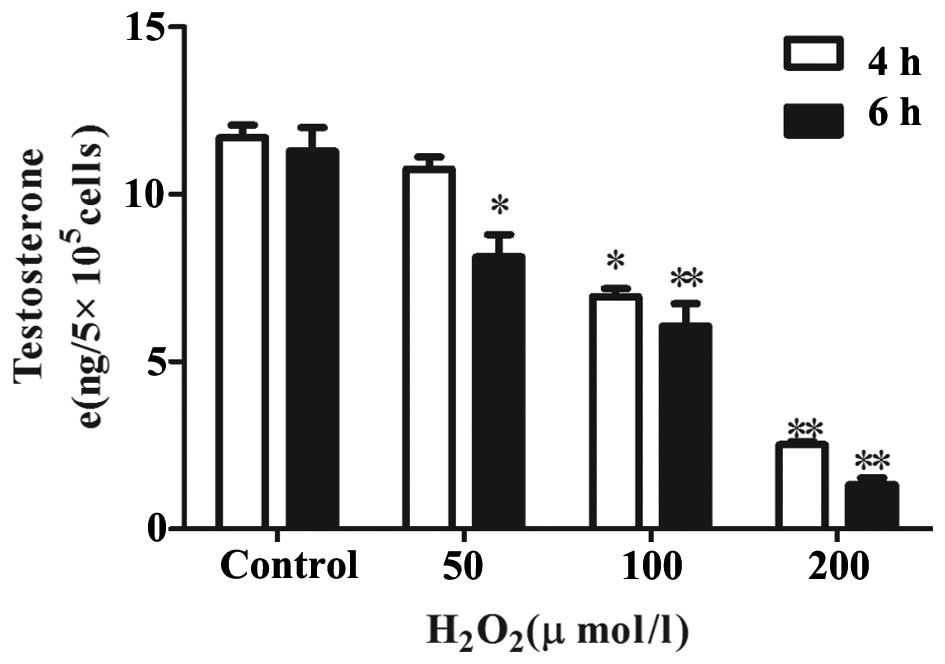
H2O2 inhibits
testosterone production by primary Leydig cells
To determine whether H2O2 has
any effect on the hCG-stimulated production of testosterone in
Leydig cells, the cells were treated with varying concentration of
H2O2 for 4 and 6 h in the presence of hCG. A
dose-dependant reduction in the levels of testosterone produced by
the Leydig cells was observed (Fig.
3). At 4 h, 200 μM of H2O2
inhibited the production of testosterone to the lowest level
(2.36±0.29 ng), compared with the control group. At the two time
points, significant decreases in testosterone production were
observed at all concentrations, with the exception of the cells
treated with 50 μM H2O2 for 4 h.
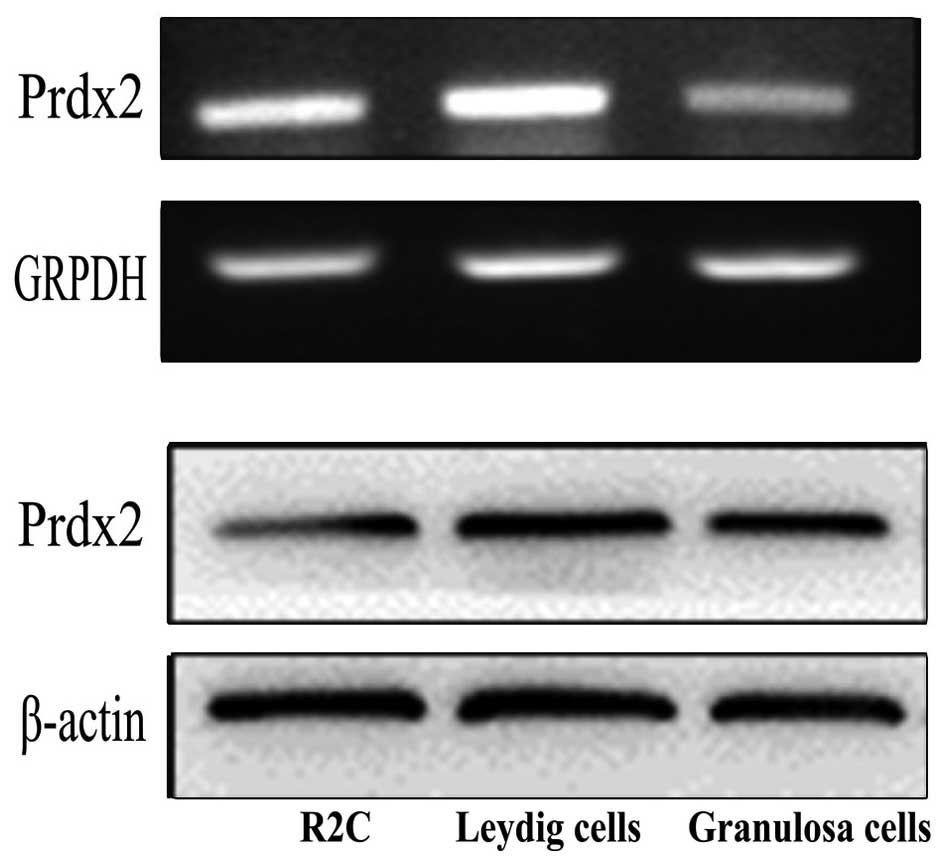
Detection of the mRNA and protein
expression levels of Prdx2 in Leydig cells
It has been previously reported that Prdx2 is
expressed in a variety of cells and tissues (9). To determine whether Prdx2 is
expressed in Leydig cells, the present study performed RT-PCR and
Western blot analyses. The Prdx2 transcripts and proteins were
detected in the R2C Leydig tumor cell line and in the primary
Leydig cells (Fig. 4).
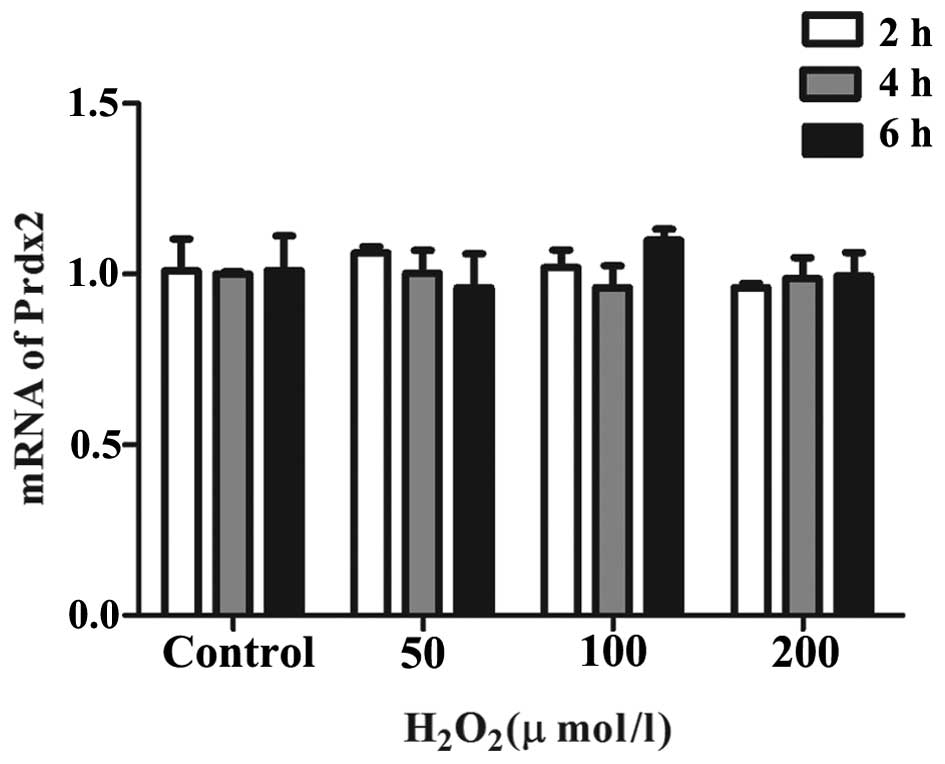
H2O2 treatment has
no effect on the mRNA expression of Prdx2 in primary Leydig
cells
The Leydig cells were treated with different
concentrations of H2O2 (50, 100 and 200
μM) for 2, 4 and 6 h. Subsequently, the mRNA levels of Prdx2
were measured using RT-qPCR. It was observed that, compared with
the control group, none of the concentrations or durations of
H2O2 treatment had any significant effect on
the mRNA levels of Prdx2, as shown in Fig. 5.
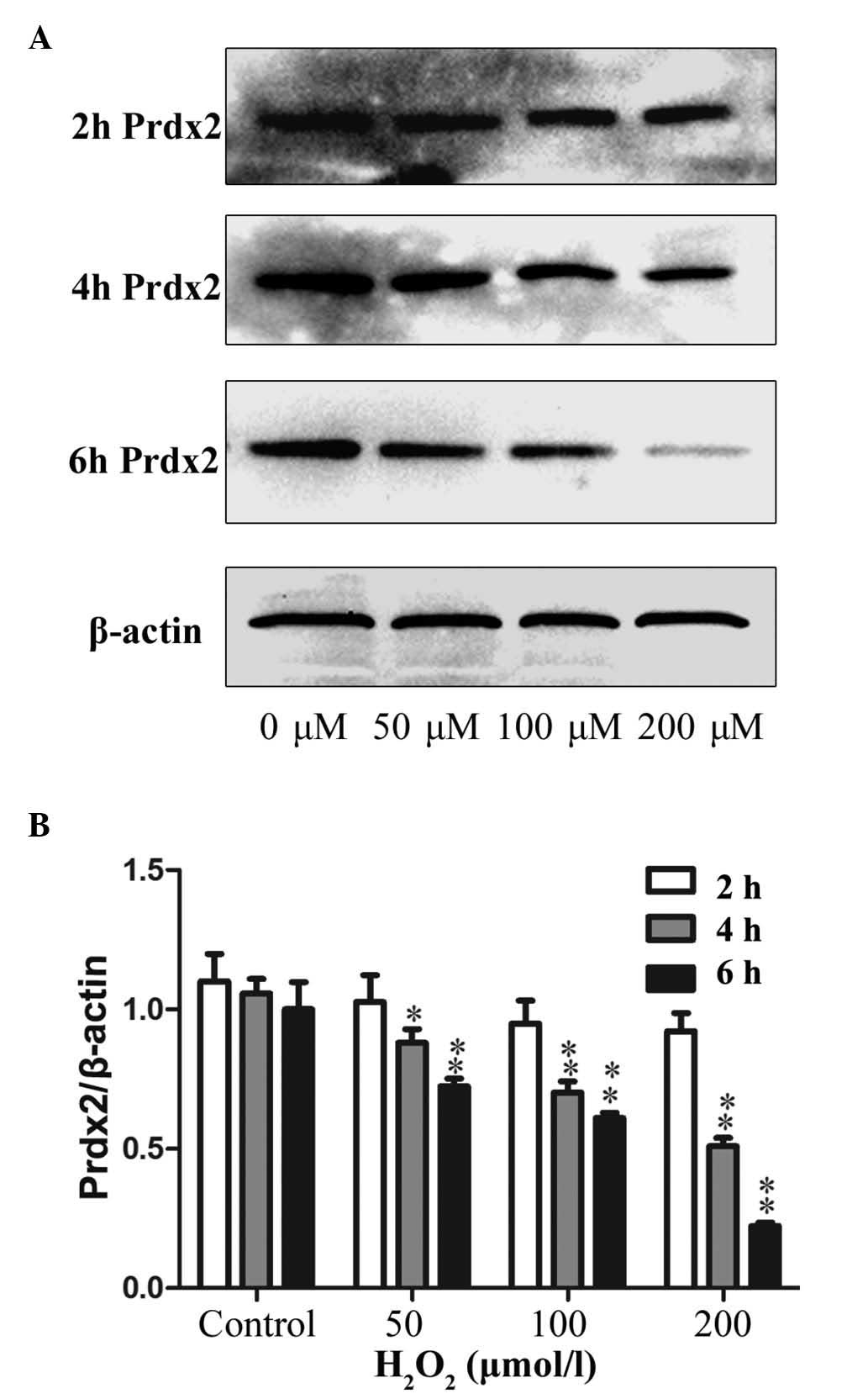
H2O2 treatment
decreases the protein expression of Prdx2 in primary Leydig
cells
The Leydig cells were treated, as above, with
different concentrations of H2O2 for
different durations, and were analyzed for the protein expression
of Prdx2. As shown in Fig. 6A,
compared with the control group, no significant change in the
protein expression of Prdx2 was observed following 2 h treatment
with H2O2. However, the protein expression
levels of Prdx2 decreased following 4 and 6 h of
H2O2 treatment. Furthermore, the expression
of Prdx2 decreased significantly with increased
H2O2 concentration, with the lowest level
observed following 200 μM H2O2
treatment for 6 h, as shown in Fig.
6B.
Discussion
LOH has been considered the most common form of male
hypogonadism, with a prevalence rate of ~1 in 100 men (18). Leydig cell dysfunction in older men
leads to lower serum testosterone levels, which may be the
predominant cause of LOH (19-21).
ROS are produced during steroidogenesis,
particularly during steroid hydroxylation by cytochrome P450
enzymes, which are localized in the mitochondria and endoplasmic
reticulum (22,23). In aged animals, the generation of
mitochondrial superoxide is increased in Leydig cells (24). It has been reported that ROS
inhibits the synthesis of testosterone, and that this may be due to
the accumulation of ROS in Leydig cells, resulting in lower levels
of testosterone and enhanced cell death (6,8).
In the present study, the effect of
H2O2 on testosterone production in Leydig
cells at low concentrations was investigated. The levels of MDA,
the most frequently used biomarker to detect oxidative changes,
increased following H2O2 treatment. This
result confirmed that H2O2 induced oxidative
stress. In addition, the present study demonstrated that
H2O2 induced dose-dependant inhibition of
testosterone production in Leydig cells.
High concentrations of H2O2
are likely to affect the survival of cells. Despite the fact that
comparatively low concentrations of H2O2 were
used, Leydig cells apoptosis was enhanced. Following exposure of
the cells to H2O2 at a concentration of 200
μM, an increase in cell apoptosis (>2-fold) was observed.
The present study hypothesized that apoptotic induction may also
have a negative effect on steroidogenic capacity. These
observations suggested that H2O2 at low
levels modulates Leydig cell apoptosis and testosterone production.
These results are consistent with the previous findings of Gautam
et al (25).
Prdx2, a member of the Prdx family, has a crucial
function in eliminating the H2O2, produced
during cell metabolism. The protein eliminates
H2O2 with reducing equivalents provided by
the thioredoxin system. Considering the fact that Prdx2 protects
cells from attack by ROS, and the fact that ROS induced apoptosis
and reduced testosterone levels in the present study, it was
suggested that Prdx2 may be significant in modulating Leydig cell
function.
The present study examined the mRNA and protein
levels of Prdx2 in Leydig cells. No significant changes were
observed in the mRNA levels of Prdx2 following
H2O2 treatment. However, the protein
expression of Prdx2 decreased as the concentration of
H2O2 increased, and this effect may have been
due to the induction of molecular structural transformation. The
thiol (Cys-SH) group of Prdx2 is oxidized to disulfide in the
presence of H2O2. As the level of
H2O2 increases, Prdx2 undergoes further
oxidation to the sulfinic (Cys-SO2H) or sulfonic
(Cys-SO3H) acid forms (26). This hyperoxidation results in the
transition from monomeric Prdx2 to its dimeric form (27). In addition, when Prdx2 is oxidized
to sulfinic or sulfonic acid, it cannot eliminate
H2O2, and this may explain why apoptosis was
evident following treatment with 200 μM
H2O2.
The results of the present study are consistent with
those of Zhao et al (27),
which showed that H2O2 stimulation resulted
in a significant decrease in the expression of Prdx2 in
cardio-myocytes, along with reduced cell viability. Furthermore,
the overexpression of Prdx2 protected cardiomyocytes from oxidative
stress-induced cell death and apoptosis, whereas its ablation
impaired these protective effects. Thus, the present study
hypothesized that Prdx2 may have a protective effect against
H2O2 oxidative damage in Leydig cells.
In conclusion, the results of the present study
demonstrated that low concentrations of H2O2
induced oxidative stress, and modulated cell apoptosis and the
production of testosterone in Leydig cells. In addition,
stimulation with H2O2 resulted in dose- and
time-dependent decreases in the expression levels of Prdx2, which
may have been caused by the induction of molecular structure
transformation due to the elimination of
H2O2. Therefore, it was hypothesized that
Prdx2 may be pivotal in protecting Leydig cells from ROS damage and
preventing the reduction of testosterone. Further investigations
are required to confirm this hypothesis, and further examine the
protective mechanism of Prdx2, which may provide novel insights to
assist in the diagnosis and treatment of LOH.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 31371520) to B. Yao, and the
National Natural Science Foundation of China (grant no. 81300540)
to K. Fan.
Abbreviations:
|
LOH
|
late-onset hypogonadism
|
|
ROS
|
reactive oxygen species
|
|
Prdx2
|
peroxiredoxin 2
|
|
H2O2
|
hydrogen peroxide
|
|
Prdxs
|
peroxiredoxins
|
|
HBSS
|
Hank's balanced salt solution
|
|
MDA
|
malondialdehyde
|
|
SDS
|
sodium dodecyl sulfate
|
|
PMSF
|
phenylmethylsulfonyl fluoride
|
|
PVDF
|
polyvinylidene difluoride
|
|
LSD
|
least significance difference
|
|
Trx
|
thioredoxin
|
References
|
1
|
Pye SR, Huhtaniemi IT, Finn JD, Lee DM,
O'Neill TW, Tajar A, Bartfai G, Boonen S, Casanueva FF, Forti G, et
al: Late-onset hypogonadism and mortality in aging men. J Clin
Endocrinol Metab. 99:1357–1366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee SY, Gong EY, Hong CY, Kim KH, Han JS,
Ryu JC, Chae HZ, Yun CH and Lee K: ROS inhibit the expression of
testicular steroidogenic enzyme genes via the suppression of Nur77
trans-activation. Free Radic Biol Med. 47:1591–1600. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun AY and Chen YM: Oxidative stress and
neurodegenerative disorders. J Biomed Sci. 5:401–414. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gius D and Spitz DR: Redox signaling in
cancer biology. Antioxid Redox Signal. 8:1249–1252. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang JY, Lee YJ, Chou MC, Chang R, Chiu
CH, Liang YJ and Wu LS: Astaxanthin protects steroidogenesis from
hydrogen peroxide-induced oxidative stress in mouse leydig cells.
Mar Drugs. 13:1375–1388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Diemer T, Allen JA, Hales KH and Hales DB:
Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells
and inhibits steroidogenic acute regulatory (StAR) protein and
steroido-genesis. Endocrinology. 144:2882–2891. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsai SC, Lu CC, Lin CS and Wang PS:
Antisteroidogenic actions of hydrogen peroxide on rat Leydig cells.
J Cell Biochem. 90:1276–1286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li WR, Chen L, Chang ZJ, Xin H, Liu T,
Zhang YQ, Li GY, Zhou F, Gong YQ, Gao ZZ and Xin ZC: Autophagic
deficiency is relatedto steroidogenic decline in aged rat Leydig
cells. Asian J Androl. 13:881–888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao F and Wang Q: The protective effect
of peroxiredoxin II on oxidative stress induced apoptosis in
pancreatic β-cells. Cell Biosci. 2:222012. View Article : Google Scholar
|
|
10
|
Lu W, Fu Z, Wang H, Feng J, Wei J and Guo
J: Peroxiredoxin 2 is upregulated in colorectal cancer and
contributes to colorectal cancer cells' survival by protecting
cells from oxidative stress. Mol Cell Biochem. 387:261–270. 2014.
View Article : Google Scholar
|
|
11
|
Lu W, Fu Z, Wang H, Feng J, Wei J and Guo
J: Peroxiredoxin 2 knockdown by RNA interference inhibits the
growth of colorectal cancer cells by downregulating Wnt/β-catenin
signaling. Cancer Lett. 343:190–199. 2014. View Article : Google Scholar
|
|
12
|
Yang S, Luo A, Hao X, Lai Z, Ding T, Ma X,
Mayinuer M, Shen W, Wang X, Lu Y, et al: Peroxiredoxin 2 inhibits
granulosa cell apoptosis during follicle atresia through the NFKB
pathway in mice. Biol Reprod. 84:1182–1189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. National Academies
Press; Washington, DC: 1996
|
|
14
|
Svechnikov KV, Sultana T and Söder O:
Age-dependent stimulation of Leydig cell steroidogenesis by
interleukin-1 isoforms. Mol Cell Endocrinol. 182:193–201. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Payne AH, Downing JR and Wong KL:
Luteinizing hormone receptors and testosterone synthesis in two
distinct populations of Leydig cells. Endocrinology. 106:1424–1429.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Kruger NJ: The Bradford method for protein
quantitation. Methods in Molecular Biology. Walker JM: 32. Humana
Press; Totowa, NJ: pp. 9–15. 1994
|
|
18
|
Corona G, Rastrelli G, Vignozzi L,
Mannucci E and Maggi M: How to recognize late-onset hypogonadism in
men with sexual dysfunction. Asian J Androl. 14:251–259. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arianayagam R, Arianayagam M, McGrath S
and Rashid P: Androgen deficiency in the aging man. Aust Fam
Physician. 39:752–755. 2010.PubMed/NCBI
|
|
20
|
Wylie K and Froggatt N: Late onset
hypogonadism, sexuality and fertility. Hum Fertil (Camb).
13:126–133. 2010. View Article : Google Scholar
|
|
21
|
Bassil N and Morley JE: Late-life onset
hypogonadism: A review. Clin Geriatr Med. 26:197–222. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hall PF: Testicular steroidogenesis:
Organization and regulation. The Physiology of Reproduction. Knobil
E and Neill JD: Raven Press; New York: pp. 1335–1362. 1994
|
|
23
|
Hornsby PJ and Crivello JF: The role of
lipid peroxidation and biological antioxidants in the function of
the adrenal cortex. Part 2. Mol Cell Endocrinol. 30:123–147. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen H, Cangello D, Benson S, Folmer J,
Zhu H, Trush MA and Zirkin BR: Age-related increase in
mitochondrial superoxide generation in the testosterone-producing
cells of Brown Norway rat testes: Relationship to reduced
steroidogenic function? Exp Gerontol. 36:1361–1373. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gautam DK, Misro MM, Chaki SP and Sehgal
N: H2O at physiological concentrations modulates Leydig
cell function inducing oxidative stress and apoptosis. Apoptosis.
11:39–46. 2006. View Article : Google Scholar
|
|
26
|
Woo HA, Chae HZ, Hwang SC, Yang KS, Kang
SW, Kim K and Rhee SG: Reversing the inactivation of peroxiredoxins
caused by cysteine sulfinic acid formation. Science. 300:653–656.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao W, Fan GC, Zhang ZG, Bandyopadhyay A,
Zhou X and Kranias EG: Protection of peroxiredoxin II on oxidative
stress-induced cardiomyocyte death and apoptosis. Basic Res
Cardiol. 104:377–389. 2009. View Article : Google Scholar :
|