Introduction
Asthma is one of the most common lifelong chronic
diseases, it is a reversible airway obstruction that is
characterized by constriction of smooth muscle of the airway,
hypersecretion of mucus, edema and airway hyperresponsiveness
(AHR), and thickening of the basement membrane of the airway
epithelium. During airway inflammation, complex interactions of
innate and adaptive immune cells, structural cells and cytokines,
exert numerous key effects. Following allergen exposure, airway
inflammation is predominantly the result of allergen specific T
helper (Th) 2 and other T cells, which recruit and accumulate in
the lungs and produce a range of different effector cytokines
(1). Th2 cells are important as
they produce a number of key cytokines, including interleukin
(IL)-4, IL-5 and IL-13, which have been demonstrated to contribute
to numerous pathophysiological features of asthma (2). However, the identification of further
subtypes of helper T cells and their cytokines has resulted in
reconsideration of the immunopathology of asthma (3).
Th9 cells were first identified as a Th2
subpopulation that produced large quantities of IL-9. However,
experimental analyses demonstrated that Th9 cells have divergent
regulatory capabilities and are involved in different immune
processes (4,5). As the predominant product of Th9
cells, IL-9 is a pleiotropic cytokine that has multiple effects on
numerous hematopoietic cells, which are central to the pathogenesis
of asthma. IL-9 stimulates the proliferation of activated T cells,
promotes the proliferation and differentiation of mast cells and
increases production of immunoglobulin (Ig)E by B cells (6). It also promotes expression of mast
cell proteases, upregulates the high-affinity IgE receptor
(FcεR) and induces IL-6 production (7,8).
Evidence from human and murine studies has suggested that IL-9 is
associated with susceptibility to develop AHR (9,10).
Innate lymphoid cells (ILCs) are important in innate
immunity and tissue remodeling via production of various cytokines
and growth factors. The ILCs include ILC1 (interferon-γ-expressing
natural killer cells), ILC2 (IL-5 and IL-13-expressing nuocytes)
and RAR-related orphan receptor (ROR)γ ILCs (IL-17 and
IL-22-expressing 'ILC3'). Group 2 ILCs (ILC2s) have recently been
demonstrated to mediate the immune pathology of asthma even without
adaptive immunity, and are found in human respiratory and
gastrointestinal tissues, as well as in skin (11–14).
The present study described the response state of lung tissue to
Th9 cells and ILC2s in mouse models of asthma by detecting
associated receptors, and analyzing the association between the
receptors and IgE levels in sera samples from the mice.
Materials and methods
Animals and asthma induction
Female BALB/c mice (age, 6 weeks; weight, 18±2 g)
were purchased from the Laboratory Animal Center of Yangzhou
University (Yangzhou, China). Mice were housed in plastic cages
with sterilized wood-chip bedding, and bred in animal rooms
maintained at a temperature of 23±2°C and a relative humidity of 55
± 10% with a 12 h light-dark cycle. They had access to tap water
and normal diet ad libitum. The mouse model of allergic
airway inflammation was established, as described by Wang et
al (15) with slight
modification. Briefly, the mice were sensitized by intraperitoneal
(i.p.) injection of 50 μg ovalbumin (OVA; Sigma-Aldrich, St.
Louis, MO, USA) protein and 2 mg aluminum hydroxide gel
(Sigma-Aldrich) in saline (Sinopharm Group Co., Ltd., Shanghai,
China). A second sensitization was given 10 days after the initial
sensitization. On day 22 after initial sensitization, mice were
placed in a Plexiglas chamber linked to an ultrasonic nebulizer
(NE-C29; Dalian Medical Ultrasonic Instrument Co., Ltd., Dalian,
China) and challenged with aerosolized OVA solution (10 mg/ml in
saline) for 60 min. The provocation was performed once a day for 5
consecutive days and then the mice were assessed for allergic
inflammation of the lungs 24 h after the final aerosol exposure.
Subsequent to this, the mice were anesthetized with ketamine (100
mg/kg i.p.) and the left lung was obtained by aseptic surgery, from
which the tissue samples were randomly selected. The present study
was approved by the Ethical Committee of Jiangsu University
(Zhenjiang, China).
Hematoxylin and eosin (H&E) and
immunofluorescence staining
Lung tissue samples from mouse models of asthma were
obtained and fixed in 4% paraformaldehyde (Sinopharm Group Co.,
Ltd.) and embedded in paraffin (Sinopharm Group Co., Ltd.). The
blocks were cut into 4-μm sections, heated for 3 h in a 37°C
incubator, and then dewaxed and stained with H&E (BaSo
Diagnostics Inc., Zhuhai, China). One section was selected from
each mouse and observed under a microscope (CX21BIM-SET5; Olympus
Corporation, Tokyo, Japan).
Immunofluorescence staining
Immunofluorescence staining was performed on
paraffin-embedded mouse lung tissue as described previously
(16). Briefly, following dewaxing
and antigen retrieval (in 1% sodium citrate solution for 10 min at
90–95°C.), the slides were blocked in 1% (weight/volume) bovine
serum albumin (Sigma-Aldrich) for 60 min at 37°C, and then the
slides were incubated with primary antibodies for 2 h at room
temperature, as follows: Polyclonal goat anti-mouse IL-9 (cat. no.
sc-1272; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; dilution,
1:100), rabbit anti-mouse cluster of differentiation (CD)3 (cat.
no. ab16669; Abcam, Cambridge, UK; dilution, 1:100), polyclonal rat
anti-mouse CD117 (c-kit)-PE (cat. no. 135105; BioLegend, Inc., San
Diego, CA, USA; dilution, 1:100), polyclonal Armenian hamster
anti-mice FcεRIα-FITC (cat. no. 134305; BioLegend, Inc.;
dilution, 1:100), polyclonal rabbit anti-mouse RORα (cat. no.
ab60134; Abcam, Cambridge, UK; dilution, 1:100) and polyclonal goat
anti-mouse ST2 (cat. no. sc-18687; Biotechnology, Inc.; dilution,
1:100). Subsequent to washing, the labeled secondary antibodies
were incubated with the slides for 90 min at 37°C, as follows:
Polyclonal donkey anti-goat PE (cat. no. sc-3743; Santa Cruz
Biotechnology, Inc.; dilution, 1:200) and polyclonal donkey
anti-rabbit FITC (cat. no. sc-2090; Santa Cruz Biotechnology, Inc.;
dilution, 1:200). Finally, the slides were dyed in Hoechst 33342
(Beyotime Institute of Biotechnology, Shanghai, China) for 10 min.
All the sections were coverslipped with Vectashield mounting medium
(Vector Laboratories, Inc., Burlingame, CA, USA), viewed with a
fluorescence microscope (Olympus Corporation, Tokyo, Japan), and
analyzed using ImageJ software (imagej.nih.gov/ij/).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using Invitrogen TRIzol
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 500 ng RNA
was reverse transcribed using PrimeScript®RT reagent kit
(Takara Bio, Inc., Otsu, Japan), according to the manufacturer's
protocols at 37°C for 15 min and 85°C for 5 sec. On the basis of
Genebank sequences (www.ncbi.nlm.nih.gov/genbank), the primers used in the
present study were designed by Primer Premier 5.0 software
(http://www.premierbiosoft.com/primerdesign/index.html)
and synthesized by Invitrogen (Thermo Fisher Scientific, Inc.). The
sequences of the primers are presented in Table I. qPCR was conducted using a CFX96
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and SYBR® Premix Ex Taq™ (Takara Bio,
Inc.) according to the manufacturer's protocols. Fold changes in
the expression of each objective gene relative to β-actin were
calculated based on the quantification cycle (Cq) (17). All samples were performed in
triplicate.
 | Table IPrimer sequences for reverse
transcription-polymerase chain reaction. |
Table I
Primer sequences for reverse
transcription-polymerase chain reaction.
| Gene | Sequence
(5′-3′) | Length (bp) |
|---|
| RORα | F:
CTCGTGGCTTCAGGAAAAGGTA | 151 |
| R:
TCGTCCACATAGGGCTCTTAAC | |
| IL-9 | F:
CACAAATGCACCTTCTGGGACA | 119 |
| R:
TCACTCCAACGATACGGTCCTT | |
| IL-5 | F:
AGGATGCTTCTGCACTTGAG | 144 |
| R:
CCTCATCGTCTCATTGCTTG | |
| IL-13 | F:
TGAGCAACATCACACAAGACC | 157 |
| R:
AGGCCATGCAATATCCTCTG | |
| PU.1 | F:
CCCTCCATCGGATGACTTGGTT | 142 |
| R:
GTTGTTGTGGACATGGTGTGCG | |
| IL-13Rα2 | F:
GGACTCATCAGACTATAAAGATT | 176 |
| R:
GTGTGCTCCATTTCATTCTA | |
| IL-5Rα | F:
CCTATACTACAGGTTTGGTGT | 152 |
| R:
GCTTGCTTGAGCCATTAATGT | |
| IRF4 | F:
GGTGTGGGAGAACGAGGAGAAG | 221 |
| R:
TCCTCTCGACCAATTCCTCAAA | |
| MBP-1 | F:
TCTGACTCCAAAAGCCCATT | 195 |
| R:
AAGGTCCACGTCTGTCACAT | |
|
FcεR1α | F:
GAACTCTTCACATTTGGTCA | 110 |
| R:
GCGTTACATTCAAGTACACA | |
| β-actin | F:
TGGAATCCTGTGGCATTCATGAAAC | 349 |
| R:
TAAAACGCAGCTCAGTAACAGTCCG | |
Flow cytometric quantification of ILC2s and Th9. The
ILC2s or Th9 population was defined as
CD278+ST2+ lineage (LIN)− or
IL-9+IL-4−. Lung tissue samples or PBMCs were
freshly obtained from mouse models of asthma. A lung tissue single
cell suspension was isolated by sieving, and peripheral blood
mononuclear cells (PBMCs) were obtained by standard Ficoll-Hypaque
density centrifugation (Tian Jin Hao Yang Biological Manufacture
Co., Ltd., Tianjin, China). Subsequently, the cells were cultured
at 37°C in an atmosphere of 5% CO2 for 5 h, and
incubated with antibodies, as follows: Polyclonal rat anti-mouse
ST2-PE (cat. no. 145303; BioLegend, Inc.; dilution, 1:200),
polyclonal Syrian hamster anti-mouse-CD278 (inducible T-cell
costimulator)-PerCP/Cy5.5 (cat. no. 107705; BioLegend, Inc.;
dilution, 1:200), polyclonal mouse anti-mouse hematopoietic
LIN-FITC (cat. no. 22-7770-72; eBioscience, Inc., San Diego, CA,
USA; dilution, 1:200) for the ILC2 cells; polyclonal rat anti-mouse
IL-9-PE (cat. no. 514103; BioLegend, Inc.; dilution, 1:200),
polyclonal rat anti-mouse IL-4-PerCP/Cy5.5 (cat. no. 504123;
BioLegend, Inc.; dilution, 1:200) for the Th9 cells. The following
isotype control antibodies were used in all the investigations:
Polyclonal rat immunoglobulin (Ig) G-PE (cat. no. B157311;
Biolegend, Inc.; dilution, 1:200) and polyclonal rat
IgG-PerCP/Cy5.5 (cat. no. 400425; Biolegend, Inc.; dilution,
1:200). For intracellular cytokine measurement, the single cell
suspension was treated with cell lysis buffer (Hanks' Balanced Salt
Solution; eBioscience, Inc., San Diego, CA, USA) for 10 min prior
to flow cytometric analysis using a BD FACSCalibur™ (BD
Biosciences, Franklin Lakes, NJ, USA). Cells were then analyzed
using FlowJo software version 10.0.7 (http://www.flowjo.com/download-flowjo/).
Enzyme-linked immunosorbent assay (ELISA)
for serum IgE
The serum IgE was measured by ELISA kit according to
the manufacturer's protocols (eBioscience, Inc.). All samples were
measured in triplicate, and the mean concentration was calculated
from a standard curve.
Statistical analysis
All statistical analysis was performed using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA) and data were
expressed as the mean ± standard deviation. Comparisons between
groups were performed using the unpaired Student's t-test and
Pearson's correlation was used to test correlation between two
continuous variables. P<0.05 was considered to indicate a
statistically significant difference.
Results
Increased ILC2s frequency and their
associated factors in lung tissue samples from mouse models of
asthma
The transcription factor RORα is essential for the
development and function of ILC2s. ST2 is relatively specific
receptor or surface marker expressed by ILC2s and IL-5 and IL-13
are characteristic cytokines. To analyze the level of ILC2s in lung
tissue samples from mouse models of asthma, the RORα, ST2, IL-5 and
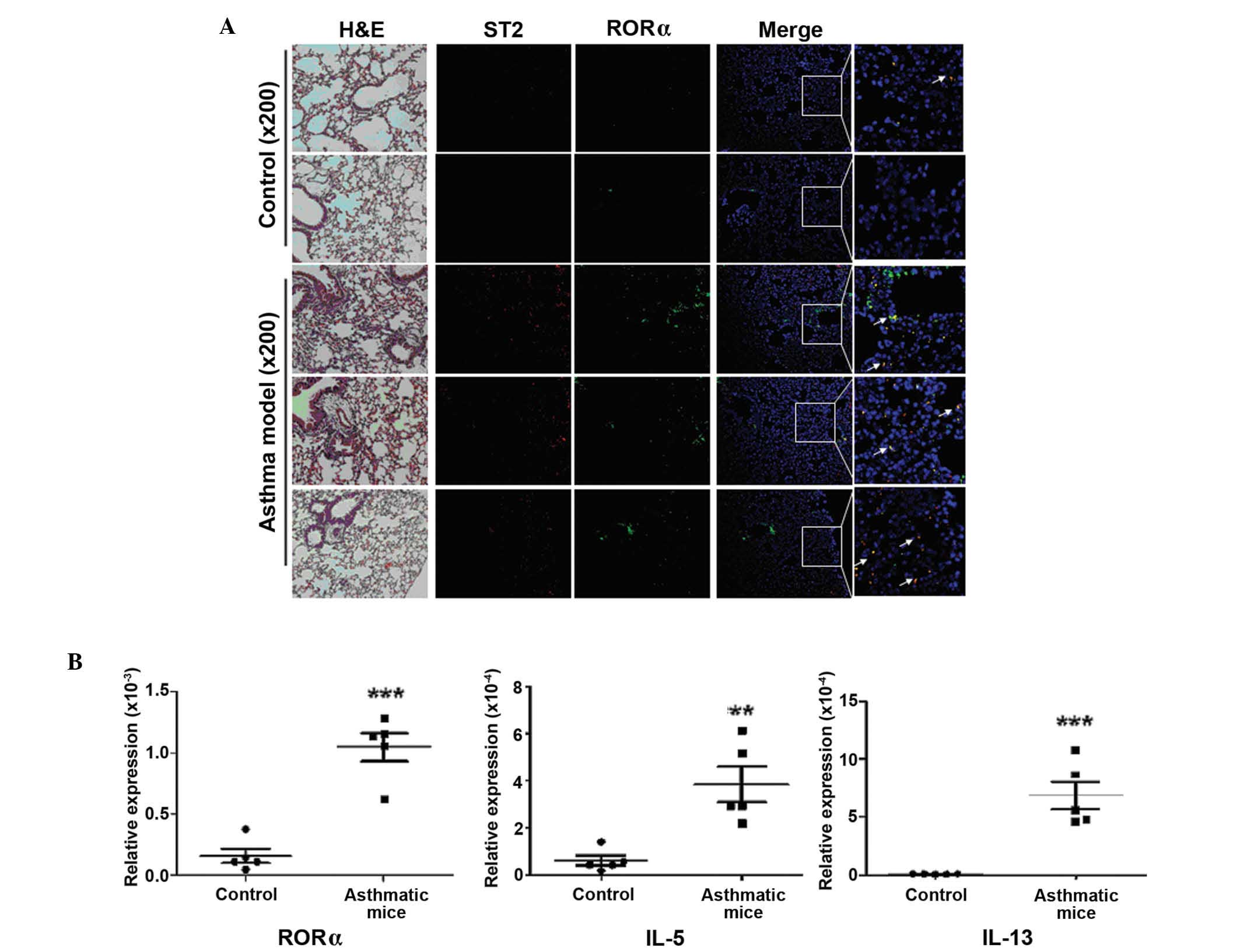
IL-13 were detected by immunofluorescence staining or RT-qPCR. As
presented in Fig. 1, the mRNA
expression levels of RORα, IL-5 and IL-13 were significantly
increased in asthmatic mice (P=0.0001, P=0.0003 and P=0.0005,
respectively), and the results of immunofluorescence staining
indicated ST2 and RORα positive cells in lung tissue samples of
asthmatic mice. In addition, the results of flow cytometry analysis
demonstrated that the percentage of ILC2s was increased in the lung
tissue samples of asthma mouse models, but no marked changes were
observed in PBMCs (Fig. 2).
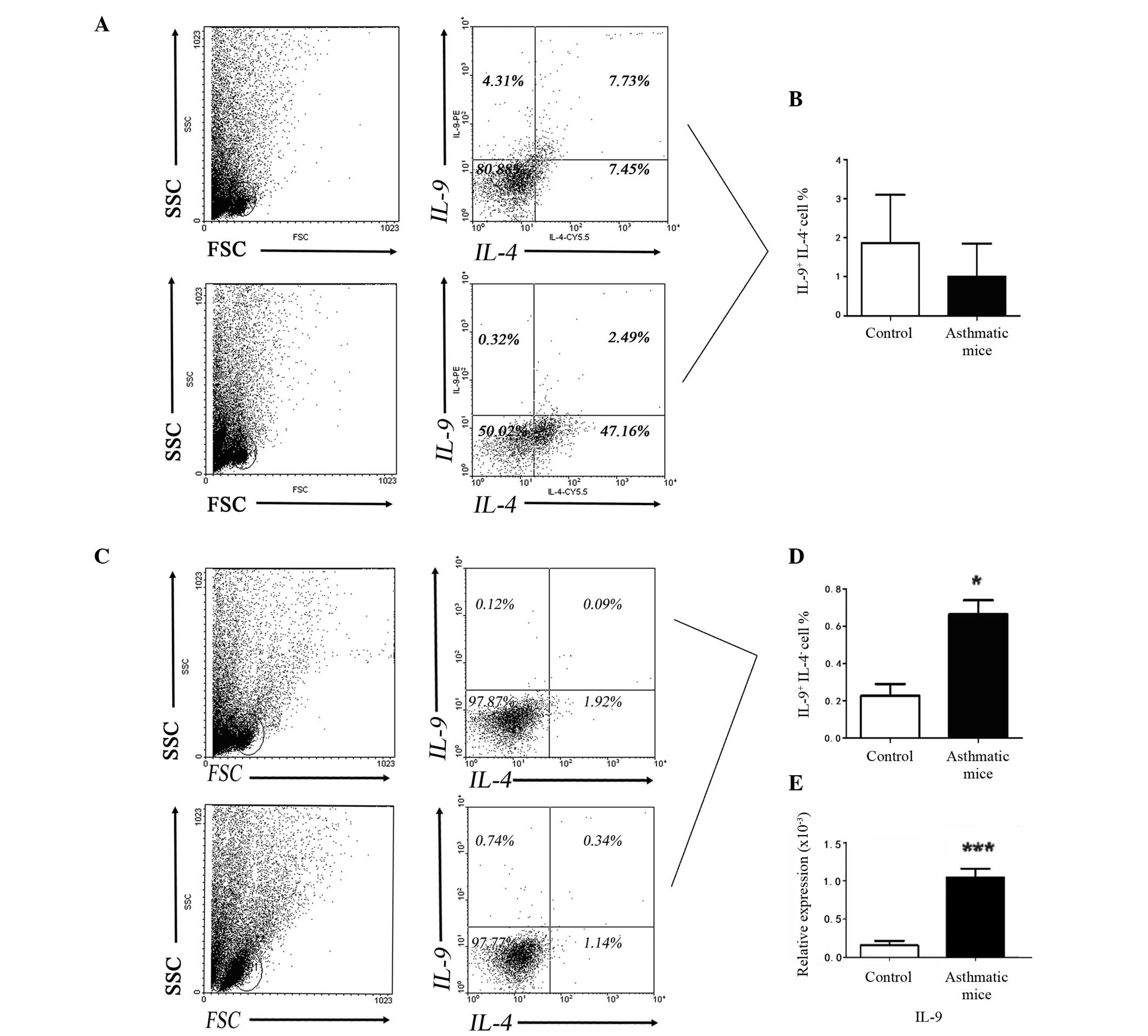
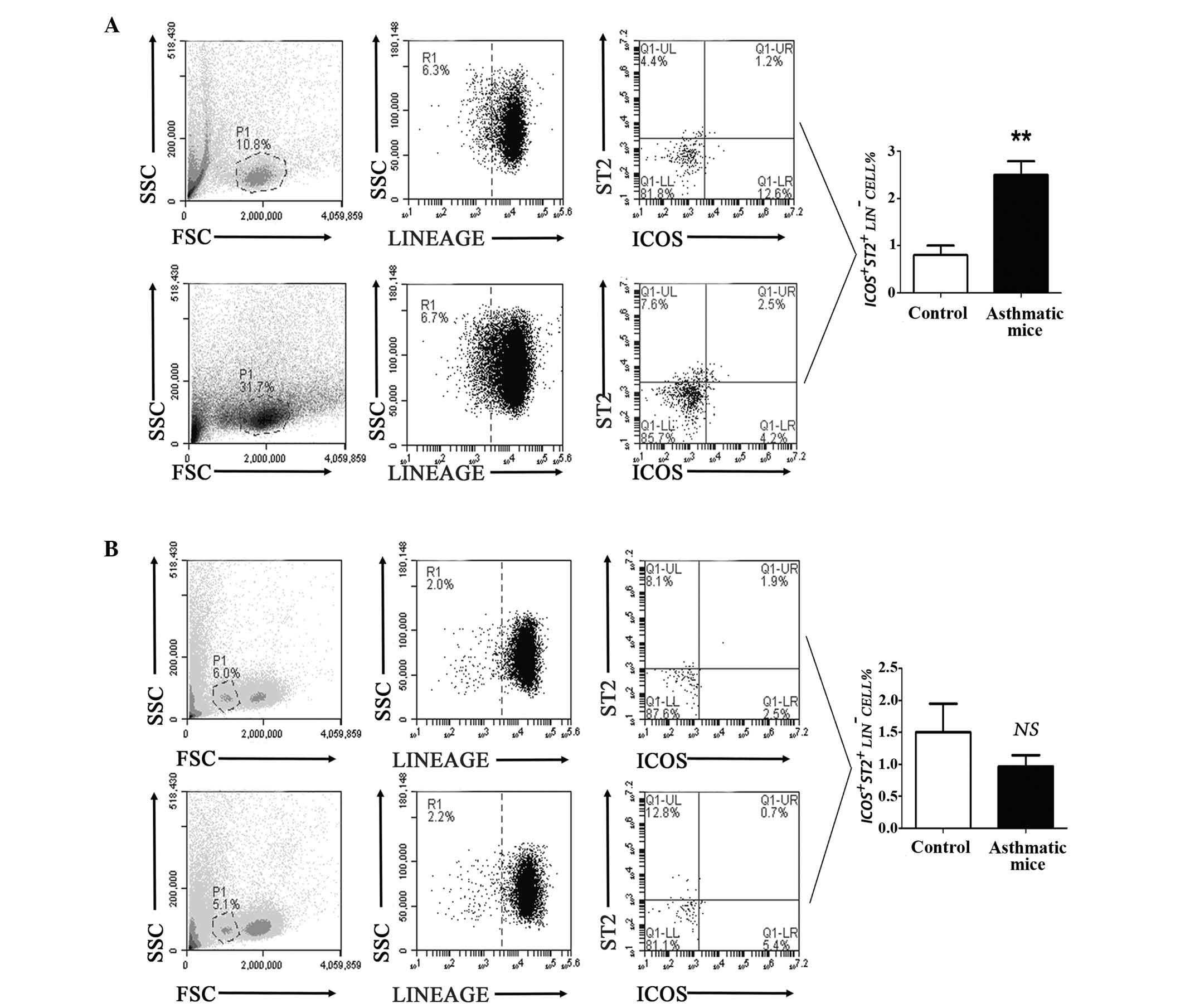 | Figure 2Frequency of ILC2s was significantly
increased in the lung tissue samples from asthmatic mice, but not
in peripheral blood samples. (A) Flow cytometric analysis showed
that the number of ILC2s in lung tissue samples from asthmatic mice
was higher than that of the control group (**P<0.01
vs. the control group). (B) The result of flow cytometric analysis
indicated that the number of ILC2s in peripheral blood samples from
asthmatic mice was not significantly different, compared with the
control mice (P=0.3325 vs. the control group). FSC corresponds to
the relative size of the cell, the greater the value of FSC, the
larger the cell; SSC corresponds to the degree of internal
complexity of the cell, the greater the value of SSC, the greater
the number of particles inside the cell. LIN, cell lineage; ILC2s,
innate lymphoid cells 2; ICOS, inducible T-cell costimulator; NS,
not significant; ST2, interleukin 1 receptor-like 1. |
These results suggested that ILC2s and their
characteristic transcription factors or cytokines were notably
upregulated in lung tissue samples in asthmatic mice.
Increased Th9 and ILC2s frequency in lung
tissue samples from mouse models of asthma
A number of groups have reported that IL-9
production is increased in the lungs of human patients with chronic
asthma (18,19). However, although Th9 cells have
recently been identified in vitro (20,21),
it remains to be elucidated whether these are the predominant
cellular sources of IL-9 during chronic allergic inflammation.
Mouse models of asthma were used to investigate the development of
IL-9 and other key cytokines by effector T cells. The results of
flow cytometric analysis and RT-qPCR (Fig. 3) demonstrated a decreased
percentage of IL-9+ IL-4− cells (P>0.05)
in the peripheral blood from the asthmatic mouse samples compared
with the control mice (Fig. 3B).
However, Fig. 3C and D demonstrate
that the percentage of IL-9+ IL-4− cells and
expression levels of IL-9 mRNA were significantly increased
(P<0.05 and P<0.0001, respectively) in lung tissue samples
from the asthmatic mice when compared with the control mice.
Results from the present study indicate that Th9 cells may be
important in the local inflammation of lung tissue.
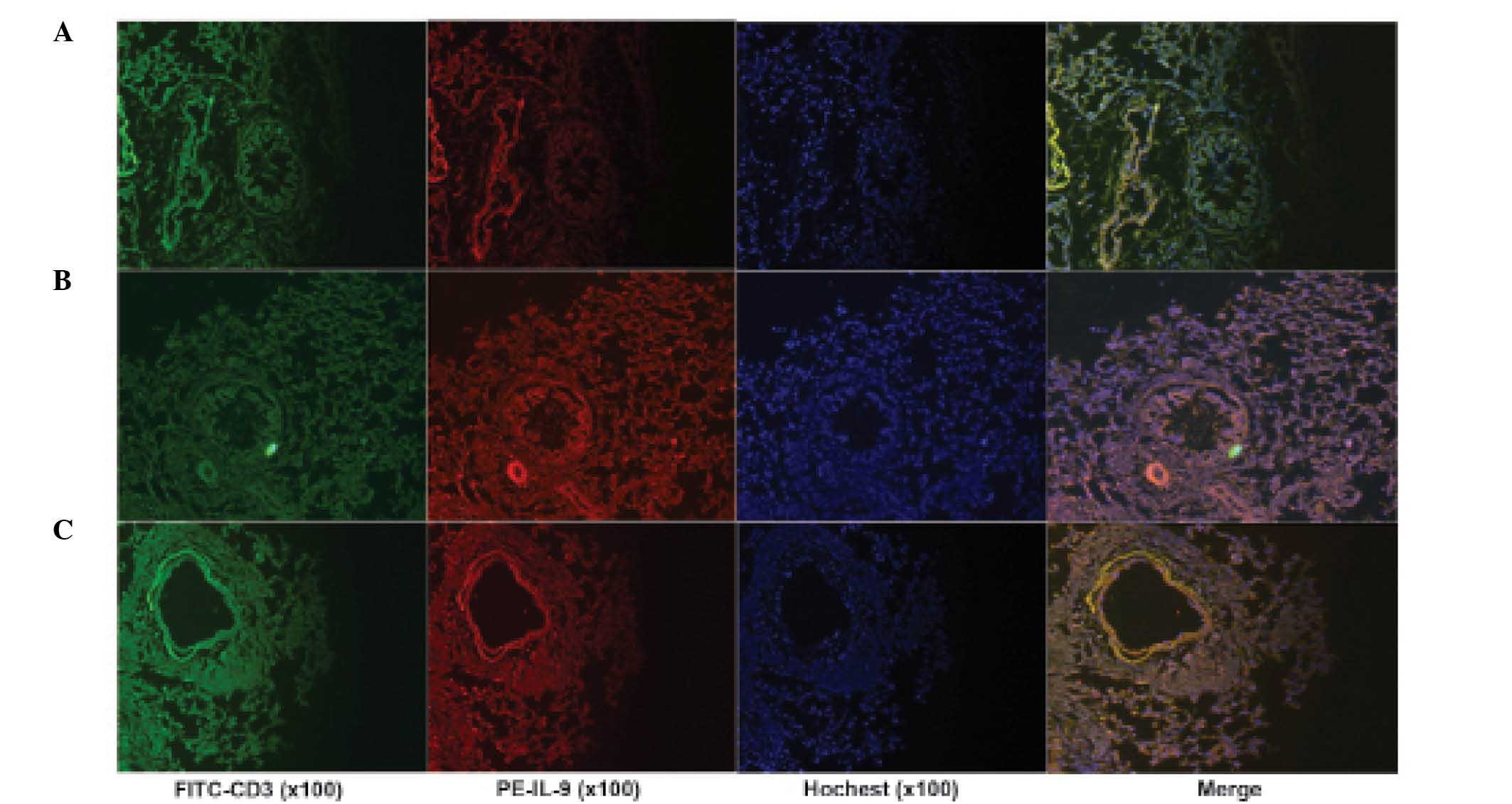
Immunofluorescence staining also indicated that IL-9+
cells were observed in inflammatory lung tissue (Fig. 4). Results from the present study
suggested that Th9 cells may be important in the local inflammation
of lung tissue.
PU.1 is reported to be an important transcriptional
regulator of Th9 cell differentiation. In addition, a number of
studies have indicated that other factors, including IFN regulatory
factor 4 (IRF-4), NF-κB and c-Myc binding protein 1 (MBP-1)
regulate IL-9 production in Th9 cells (22,23).
In the present study, increased mRNA expression levels were also
observed for PU.1, IRF-4 and MBP-1 were increased (Fig. 5).
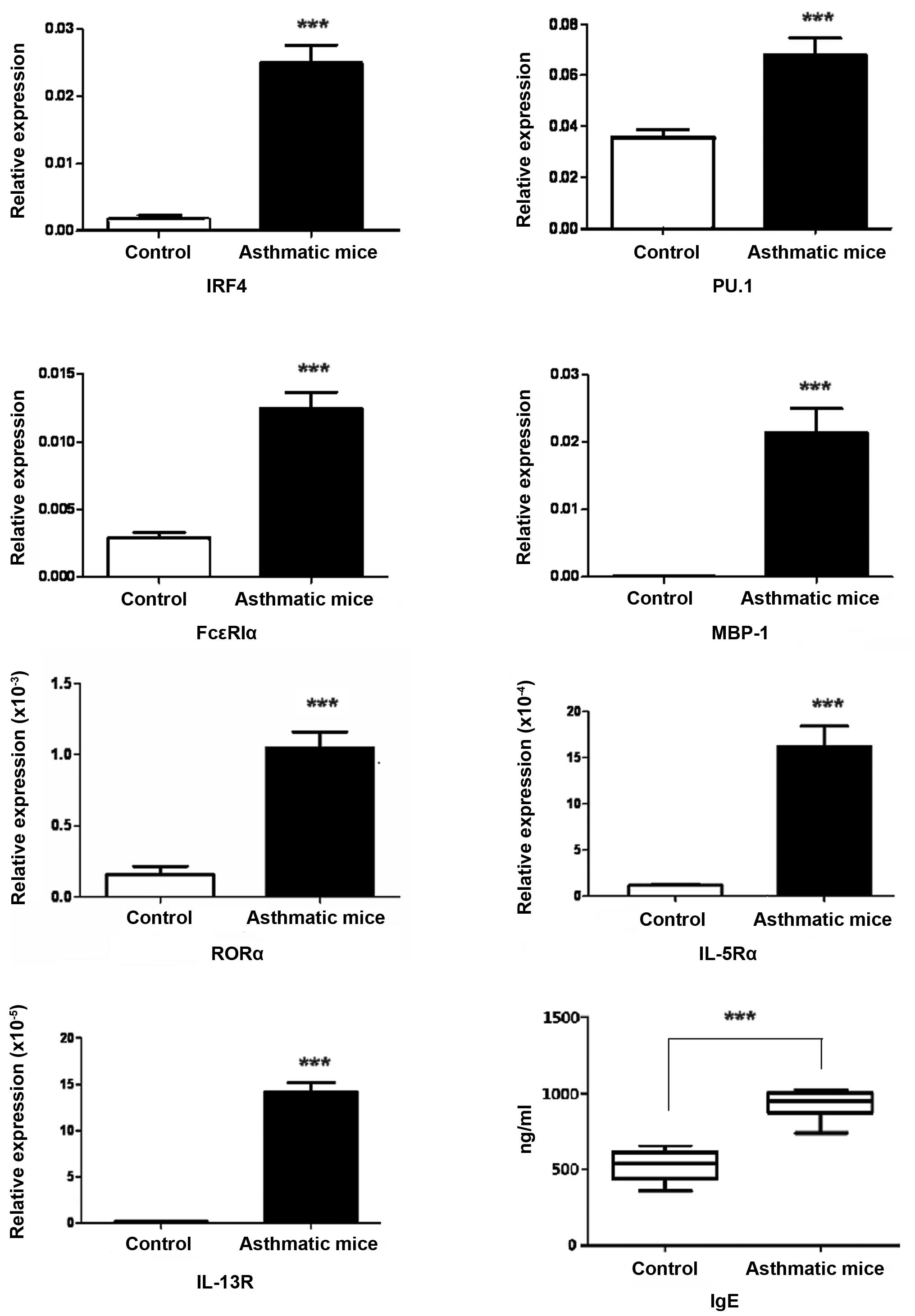 | Figure 5Increased levels of ILC2- and Th9
cell-associated molecules or receptors in inflammatory lung tissue.
The expression levels of ILC2- and Th9 cell-associated molecules or
receptors in the inflammatory lung tissue were detected by RT-qPCR,
all the molecules and receptors were significantly increased. The
values indicating the relative expression (fold changes in the
expression of each objective gene relative to β-actin), which were
calculated based on the quantification cycle. All analysis of
samples was performed in triplicate. RT-qPCR was also conducted to
detect FcεRIα in the lung tissue of asthma and
enzyme-linked immunosorbent assay was performed to detect serum
IgE, the two parameters were observed to be increased.
***P<0.0001 vs. the control. ILC2, innate lymphoid
cells 2; Th9, T helper 9; RT-qPCR, reverse transcription-polymerase
chain reaction; IRF4, interferon regulatory factor 4; PU.1,
transcription factor PU.1; IgE, immunoglobulin E;
FcεRIα, high-affinity IgE receptor 1α; MBP-1, c-myc
binding protein 1; RORα, RAR-related orphan receptor α; IL-5Rα,
interleukin 5 receptor α; IL-13, interleukin 13 receptor. |
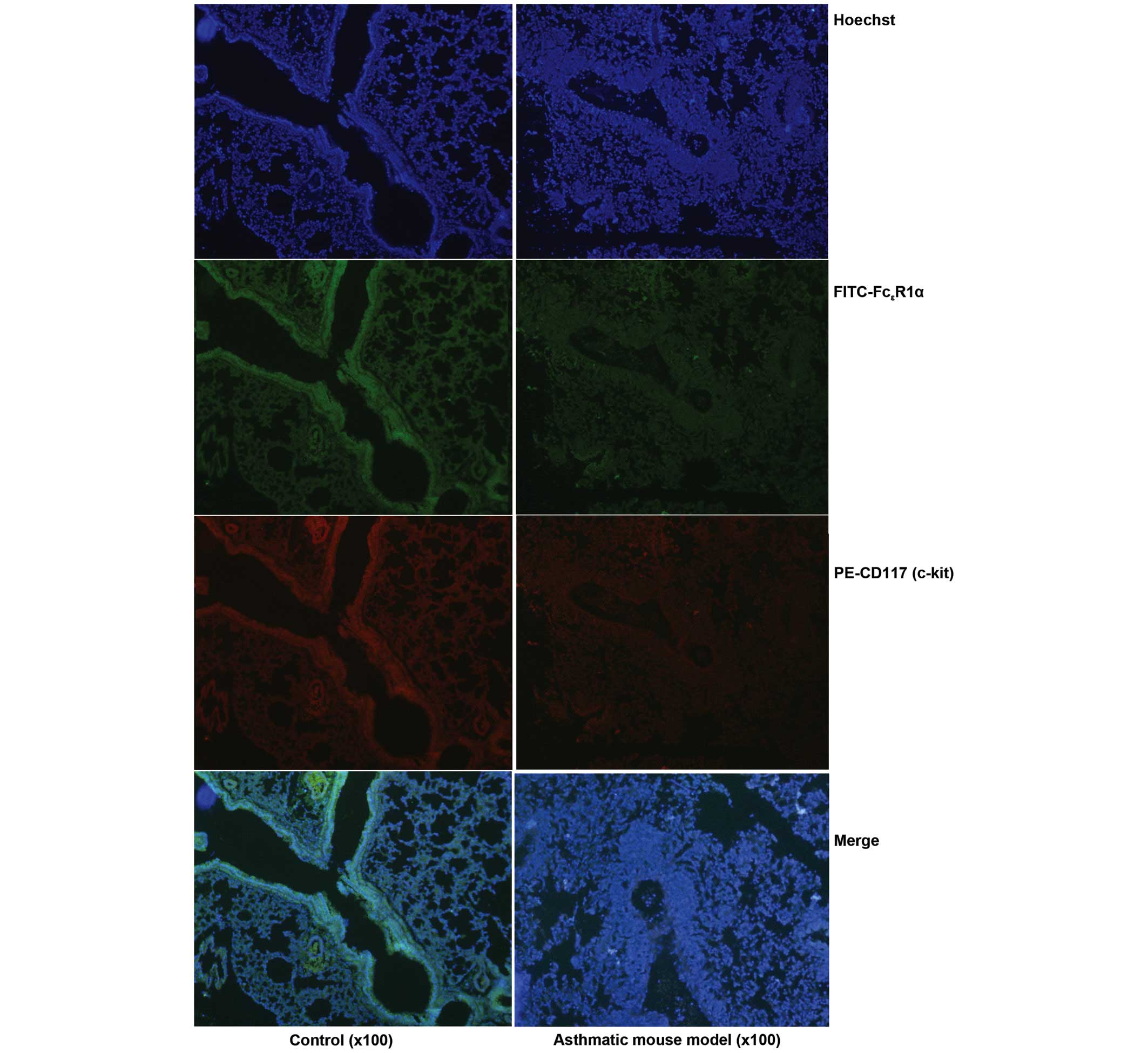
IgE in serum and FcεR in lung
tissue were increased in mouse models of asthma
Elevated serum IgE levels is the predominant
pathological manifestation of asthma inflammation. Thus, ELISA was
conducted in the present study to investigate the total IgE content
in serum samples from asthmatic mice. The result demonstrated that
the level of total IgE was significantly increased in peripheral
blood (Fig. 5). In addition, the
immunofluorescence staining indicated that the number of
FcεR positive cells in inflammatory lung tissue samples
was also increased (Fig. 6).
Expression levels of cytokines associated
with IgE upregulation were increased
Elevated serum levels of total IgE are associated
with atopic diseases, including allergic rhinitis and allergic
asthma. IRF4 is a key regulator of numerous steps in lymphoid,
myeloid, and dendritic-cell differentiation, including the
differentiation of mature B cells into antibody-secreting plasma
cells that produce IgE and IgG antibodies, among others. The
results from the present study demonstrate that the mRNA expression
levels of FcεR, IRF4 and MBP1 in the inflammatory lung
tissue were increased, similarly to the increased serum IgE level
(Figs. 5 and 6). Furthermore, in the inflammatory lung
tissue samples, the expression levels of Th9- and ILC2-associated
transcription factors, including PU.1 and RORα, or cytokine
receptors, including IL-5 receptor (R)α and
IL-13Rα2, were significantly increased (P<0.0001);
indicating that the lung tissue of asthmatic mice exhibited a
positive response to the two types of cell.
Association between the expression levels
of ILC2 or Th9-associated factors in lung tissue and IgE in serum
from asthmatic mice
IL-5 and IL-13, and IL-9 are characteristic
cytokines produced by ILC2s and Th9 cells, respectively. The
receptors of these cytokines were expressed in inflammatory lung
tissue samples, which was indicative of a positive response to
these two types of cell in the lung tissue. Correlation analysis
indicated that the expression levels of IL-5Rα,
IL-13Rα2 and IL-9Rα within the inflammatory
lung tissue were associated with serum IgE level, and that there
was significantly positive correlation between them in mouse models
of asthma. In addition, the positive correlation was also
demonstrated between the expression levels of ILC2-associated
transcription factors or cytokine receptors and Th9 characteristic
cytokines in the inflammatory lung tissue (Fig. 7). The results from the present
study indicated that ILC2s and Th9 were in a state of polarization
and they may promote each other in the inflammatory lung
tissue.
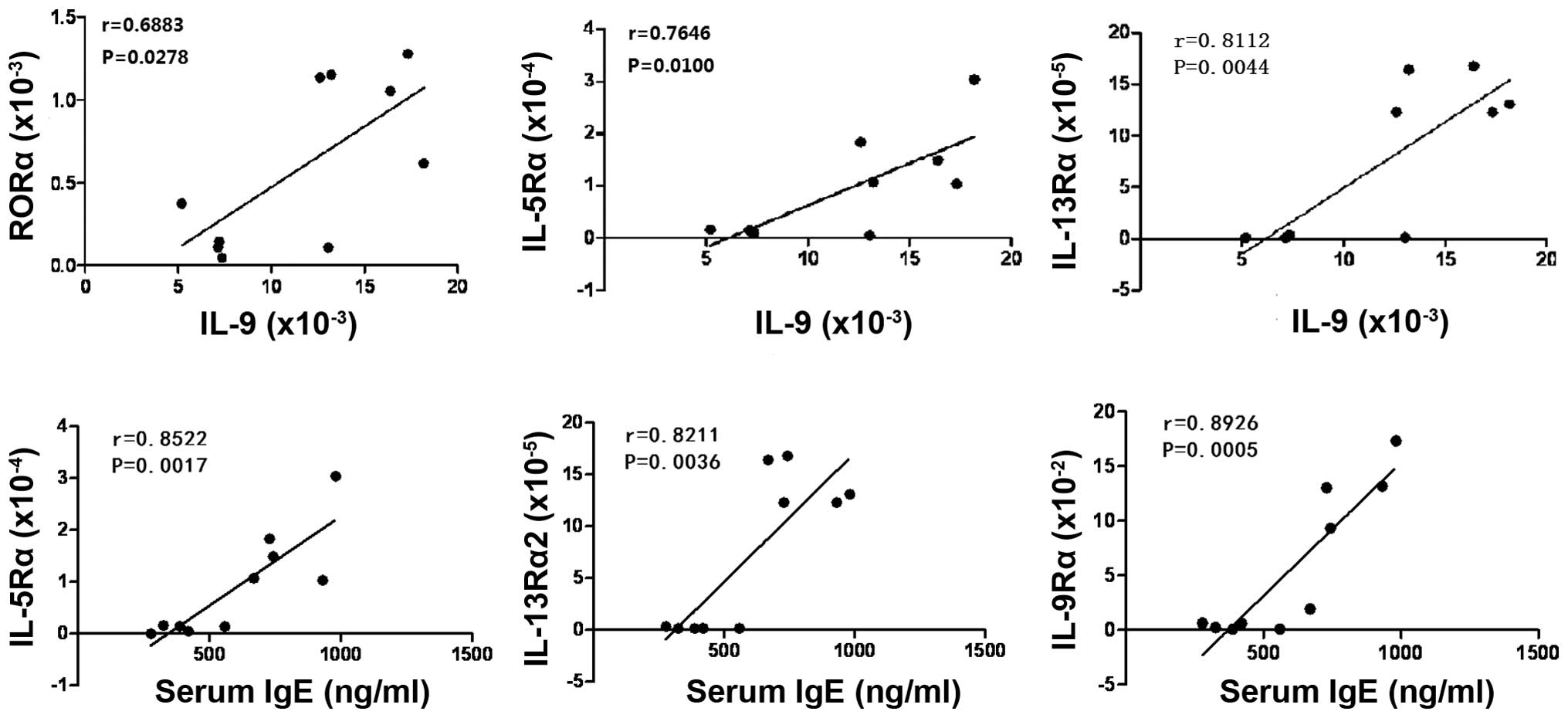 | Figure 7Positive correlation between the
expression levels of ILC2s or Th9-associated factors in lung tissue
samples and IgE in serum samples from asthmatic mice. There were
significantly positive correlations between the expression levels
of IL-5Rα, IL-13Rα2 or IL-9Rα in the inflammatory lung tissue and
serum IgE level in asthma mice. In addition, the positive
correlations were also found between the expression levels of ILC2s
associated transcription factors or cytokine receptors and Th9
characteristic cytokines in the inflammatory lung tissue samples.
ILC2s, innate lymphoid cells 2; Th9, T helper 9; IgE,
immunoglobulin E; IL, interleukin; IL-5Rα, IL-5 receptor α;
IL-13Rα2, IL-13 receptor α2; IL-9Rα, IL-9 receptor α; RORα,
RAR-related orphan receptor α. |
Discussion
Since the identification of polarized T cell
subsets, the type 2 immune response (Th2 mediated response) has
increasingly been identified as a critical component in various
human diseases. Defined by the particular cell types involved,
adaptive and innate immune cells, and the soluble mediators that
they release, type 2 immunity may exert protective and potentially
harmful effects during various disease states, notably cancer,
infection, autoimmunity, and tissue repair. An improved
understanding of immune regulation in the context of various
diseases is important in the development of novel therapeutic
approaches, for example, the epithelium produces cytokines and its
role in the development of inflammatory events requires further
elucidation. Recent progress in the understanding the interaction
between immune and inflammatory cell subsets and ILs aids in the
development of novel ideas for immune intervention, particularly
with regards to reciprocal regulation and counter balance between
Th1, Th2, Th9, Th17 and T regulatory cells, as well as B-cell
subsets. The process of developing allergic diseases is
characterized by effector Th2 cells, which produce characteristic
cytokines, and contribute to tissue inflammation, IgE production,
eosinophilia, mucous production, and activation and cell death in
the epithelium. They are emerging key factors in the pathogenesis
of allergic inflammatory diseases, and they include ILC2s (24,25).
It has been demonstrated that Th9 cells and/or ILC2s are Th2 cell
facilitators, and maintain Th2 cell polarization in certain immune
inflammatory diseases (25–27).
ILC2s are IL-5 and IL-13-expressing nuocytes, the
transcription factor RORα is essential for the development and
function of ILC2s and ST2 and IL-17RB are relatively specific
receptors to ILC2s. Th9 cells are an T-helper cell subset that
produces IL-9 and is also involved in type I hypersensitivity,
including airway inflammation (28). Although its critical roles in
asthma have attracted interest, the physiological regulatory
mechanisms of Th9 cell differentiation and function are largely
unknown. IL-9 influences numerous cell types, including T cells, B
cells, mast cells, eosinophils, lung epithelial cells, and
hematopoietic progenitors (29).
Although type I hypersensitivity had been considered a Th2 cytokine
(IL-4, IL-5, and IL-13)-mediated reaction, recent findings have
shown that blocking IL-4 signaling had little effect on human
asthma, and IL-9 is currently of interest as a potential novel
therapeutic target of type I hypersensitivity (30). During the pathological process of
pulmonary inflammation in asthma, IL-9 promotes the proliferation
and differentiation of mast cells and increases production of IgE
by B cells (6). It also promotes
expression of mast cell proteases and upregulates the
FcεR (7–10).
Asthma is one of the most common lifelong chronic
diseases, it is a reversible airway obstruction that is
characterized by constriction of airway smooth muscle, hyper
secretion of mucus, edema and AHR, mucus secretion and thickening
of the basement membrane underneath the airway epithelium. Th9 has
recently been considered the most important mediators during the
development of immune responses in the lung of asthma, and ILC2s
have been described as a novel type of innate immunocyte with the
ability to enhance IgE production. However, the interaction between
ILC2s and Th9 cells in asthmatic mice pulmonary tissues has not
been unambiguously characterized. The present study described the
response state of lung tissue to Th9 cells and ILC2s in asthmatic
mice by analyzing the associated receptors of these types of cell,
and detected the association between the expression levels of the
characteristic cytokine receptors for the two types of cells in
lung tissue and IgE in sera samples from mouse models of asthma.
Results from the present study demonstrated that the frequency of
ILC2 and Th9 cells was significantly increased in the lung tissue
of asthmatic mice (P=0.007 and P=0.036, respectively), in addition
to the increased mRNA expression levels of RORα, ST2, PU.1 and
IL-9. Immunofluorescence staining of lung tissue samples also
demonstrated similar results. In addition, IL-5Rα, IL-13Rα2 and
FcεR were also enhanced in pulmonary tissue from mouse
models of asthma, and a positive association was observed between
the expression levels of ILC2- or Th9-associated receptors, or
cytokines in tissue samples and IgE in sera. It indicated that
ILC2s and Th9 were in a state of polarization and that they may
promote each other in the lung tissue of mouse models of asthma,
and furthermore, the lung tissue exhibited a positive response to
the two types of cell (indicated by the increased expression of
cytokine receptors).
The present study investigated the changes in the
numbers of ILC2 and Th9 cells in peripheral blood samples, and this
was compared with the frequency of the two classes of cells in the
lung tissue samples from mouse models of asthma. It was observed
that the number of ILC2 and Th9 cells was markedly increased in the
lung tissue samples, but not in peripheral blood samples. Asthma is
a local respiratory allergic inflammatory disease, and pulmonary or
bronchial responses to environmental change are associated with the
pathological process. The results from the present study suggested
that ILC2s and Th9 cells may be important in the local tissue in
asthma. In addition, the predominant expression of ILC2- and
Th9-associated molecules emerged in lung tissue samples from
asthmatic mice, which indicated a positive response to the two
types of cells in inflammatory lung tissue. The present study also
investigated the association between the expression levels of ILC2-
or Th9-associated factors in lung tissue and IgE in serum from
mouse models of asthma and the results indicated that the
expression levels of IL-5Rα, IL-13Rα2 and
IL-9Rα within the inflammatory lung tissue were
associated with serum IgE levels and there were significantly
positive correlations between the two parameters in mouse models of
asthma. The positive correlation was also demonstrated between the
expression levels of ILC2-associated transcription factors or
cytokine receptors and characteristic cytokines of Th9 cells in the
inflammatory lung tissue. It indicated that ILC2s and Th9 cells
were in a state of polarization and they may promote each other in
the lung tissue of asthmatic mice.
MBP-1, IRF4 and PU.1 are key regulators of lymphoid,
myeloid and dendritic cell differentiation, including the
differentiation of mature B cells into antibody-secreting plasma
cells, and Th9 cells to produce IL-9 (31). IL-9 increases the expression of
mast cell surface IgE FcεRIa, and promotes IL-5 to
mediate the maturation of eosinophil precursors via the inhibition
of eosinophil apoptosis (32).
Furthermore, IL-9 generated by Th9 cells may promote the
differentiation of ILC2s and secretion of IL-5, which further
increases IgE production and eosinophil proliferation (33). The results of the present study
demonstrated that these regulatory factors and Th9 or ILC2s
characteristic cytokines, including MBP-1, IRF4, PU.1, IL-5, IL-9
and IL-13, were significantly increased in inflammatory lung
tissue. Thus, the interaction between Th9 and ILC2s was evident,
and the results suggested they may contribute to the IgE production
involved in pathological process of asthma.
In conclusion, ILC2s and Th9 cells were polarized
and may promote each other in the lung tissue of mouse models of
asthma, furthermore, increased expression of associated receptors
in the lung tissue demonstrated the positive response to the two
types of cell. IgE has long been a therapeutic target for allergic
disease, further understanding the interaction of ILC2s and Th9
cells in the development of asthma, and its effect on IgE, will
contribute to the elucidation of the pathogenesis of asthma and aid
in the development of more effective prevention measures and
therapeutic strategies.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31270947,
31170849 and 81370084), the Natural Science Foundation of Jiangsu
Province (grant no. BK2011472) and the Postdoctoral Foundation of
China (grant no. 2013T60508).
References
|
1
|
Guo HW, Yun CX, Hou GH, Du J, Huang X, Lu
Y, Keller ET, Zhang J and Deng JG: Mangiferin attenuates Th1/Th2
cytokine imbalance in an ovalbumin-induced asthmatic mouse model.
PLOS One. 9:e1003942014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Sivaprasad U, Gibson AM,
Cunningham CM, Bass SA, Kinker KG, Finkelman FD, Wills-Karp M and
Khurana Hershey GK: IL-13 receptor α2 contributes to development of
experimental allergic asthma. J Allergy Clin Immunol. 132:951–958.
2013. View Article : Google Scholar
|
|
3
|
Farahani R, Sherkat R, Hakemi MG,
Eskandari N and Yazdani R: Cytokines (interleukin-9, IL-17, IL-22,
IL-25 and IL-33) and asthma. Adv Biomed Res. 3:1272014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horka H, Staudt V, Klein M, Taube C,
Reuter S, Dehzad N, Andersen JF, Kopecky J, Schild H, Kotsyfakis M,
et al: The tick salivary protein sialostatin L inhibits the
Th9-derived production of the asthma-promoting cytokine IL-9 and is
effective in the prevention of experimental asthma. J Immunol.
188:2669–2676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Besnard AG, Sabat R, Dumoutier L, Renauld
JC, Willart M, Lambrecht B, Teixeira MM, Charron S, Fick L, Erard
F, et al: Dual Role of IL-22 in allergic airway inflammation and
its cross-talk with IL-17A. Am J Respir Crit Care Med.
183:1153–1163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma L, Xue HB, Guan XH, Shu CM, Zhang JH
and Yu J: Possible pathogenic role of T helper type 9 cells and
interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol.
175:25–31. 2014. View Article : Google Scholar :
|
|
7
|
Pilette C: Pathophysiology of asthma: Data
concerning regulation of IGE and Th2 responses in the lung. Bull
Mem Acad R Med Belg. 166:280–287. 2011.In French.
|
|
8
|
Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM
and Wu R: Stimulation of airway mucin gene expression by
interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol
Chem. 278:17036–17043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gasch M, Goroll T, Bauer M, Hinz D,
Schütze N, Polte T, Kesper D, Simon JC, Hackermüller J, Lehmann I
and Herberth G: Generation of IL-8 and IL-9 producing
CD4+ T cells is affected by Th17 polarizing conditions
and AHR ligands. Mediators Inflamm. 1825492014.
|
|
10
|
Kung TT, Luo B, Crawley Y, Garlisi CG,
Devito K, Minnicozzi M, Egan RW, Kreutner W and Chapman RW: Effect
of anti-mIL-9 antibody on the development of pulmonary inflammation
and airway hyperresponsiveness in allergic mice. Am J Respir Cell
Mol Biol. 25:600–605. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spits H, Artis D, Colonna M, Diefenbach A,
Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius
RE, et al: Innate lymphoid cells-a proposal for uniform
nomenclature. Nat Rev Immunol. 13:145–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lund S, Walford HH and Doherty TA: Type 2
innate lymphoid cells in allergic disease. Curr Immunol Rev.
9:214–221. 2013. View Article : Google Scholar
|
|
13
|
Fukuoka A, Futatsugi-Yumikura S, Takahashi
S, Kazama H, Iyoda T, Yoshimoto T, Inaba K, Nakanishi K and
Yonehara S: Identification of a novel type 2 innate immunocyte with
the ability to enhance IgE production. Int Immunol. 25:373–382.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gold MJ, Antignano F, Halim TY, Hirota JA,
Blanchet MR, Zaph C, Takei F and McNagny KM: Group 2 innate
lymphoid cells facilitate sensitization to local, but not systemic,
TH2-inducing allergen exposures. J Allergy Clin Immunol.
133:1142–1148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang SY, Yang M, Xu XP, Qiu GF, Ma J, Wang
SJ, Huang XX and Xu HX: Intranasal delivery of T-bet modulates the
profile of helper T cell immune responses in experimental asthma. J
Investig Allergol Clin Immunol. 18:357–365. 2008.PubMed/NCBI
|
|
16
|
He Z, Shotorban SS, Jiao Z, Su Z, Tong J,
Liu Y, Shen P, Ma J, Gao J, Wang T, et al: HMGB1 promotes the
differentiation of Th17 via up-regulating TLR2 and IL-23 of CD14+
monocytes from patients with rheumatoid arthritis. Scand J Immunol.
76:483–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Y, Yan Y, Su Z, Bie Q, Wu J, Wang S, Yu
Y, Ding H, Lu P and Xu H: Enhanced circulating ILC2s accompany by
upregulated MDSCs in patients with asthma. Int J Clin Exp Pathol.
8:3568–3579. 2015.PubMed/NCBI
|
|
18
|
Erpenbeck VJ, Hohlfeld JM, Discher M,
Krentel H, Hagenberg A, Braun A and Krug N: Increased expression of
interleukin-9 messenger RNA after segmental allergen challenge in
allergic asthmatics. Chest. 123:370S2003.PubMed/NCBI
|
|
19
|
Shimbara A, Christodoulopoulos P,
Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, Nicolaides
NC, Holroyd KJ, Tsicopoulos A, Lafitte JJ, et al: IL-9 and its
receptor in allergic and nonallergic lung disease: Increased
expression in asthma. J Allergy Clin Immunol. 105:108–115. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dardalhon V, Awasthi A, Kwon H, Galileos
G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et
al: IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together
with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells.
Nat Immunol. 9:1347–1355. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veldhoen M, Uyttenhove C, van Snick J,
Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C and Stockinger
B: Transforming growth factor-beta 'reprograms' the differentiation
of T helper 2 cells and promotes an interleukin 9-producing subset.
Nat Immunol. 9:1341–1346. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang HC, Sehra S, Goswami R, Yao W, Yu Q,
Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, et al: The
transcription factor PU.1 is required for the development of
IL-9-producing T cells and allergic inflammation. Nat Immunol.
11:527–534. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaplan MH: Th9 cells: differentiation and
disease. Immunol Rev. 252:104–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eiwegger T and Akdis CA: IL-33 links
tissue cells, dendritic cells and Th2 cell development in a mouse
model of asthma. Eur J Immunol. 41:1535–1538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bie Q, Zhang P, Su Z, Zheng D, Ying X, Wu
Y, Yang H, Chen D, Wang S and Xu H: Polarization of ILC2s in
peripheral blood might contribute to immunosuppressive
microenvironment in patients with gastric cancer. J Immunol Res.
9231352014.PubMed/NCBI
|
|
26
|
Fahy JV and Locksley RM: The airway
epithelium as a regulator of Th2 responses in asthma. Am J Respir
Crit Care Med. 184:390–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Juncadella IJ, Kadl A, Sharma AK, Shim YM,
Hochreiter-Hufford A, Borish L and Ravichandran KS: Apoptotic cell
clearance by bronchial epithelial cells critically influences
airway inflammation. Nature. 493:547–551. 2013. View Article : Google Scholar :
|
|
28
|
Mikami N, Miyagi Y, Sueda K, Takatsuji M,
Fukada S, Yamamoto H and Tsujikawa K: Calcitonin gene-related
peptide and cyclic adenosine 5′-monophosphate/protein kinase A
pathway promote IL-9 production in Th9 differentiation process. J
Immunol. 190:4046–4055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Temann UA, Ray P and Flavell RA: Pulmonary
overexpression of IL-9 induces Th2 cytokine expression, leading to
immunepathology. J Clin Invest. 109:29–39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Corren J: Cytokine inhibition in severe
asthma: Current knowledge and future directions. Curr Opin Pulm
Med. 17:29–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Flaherty BM, Soni T, Wakeman BS and
Speck SH: The murine gammaherpesvirus immediate-early Rta
synergizes with IRF4, targeting expression of the viral M1
superantigen to plasma cells. PLoS Pathog. 10:e10043022014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Holgate ST: New strategies with anti-IgE
in allergic diseases. World Allergy Organ J. 7:172014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Turner JE, Morrison PJ, Wilhelm C, Wilson
M, Ahlfors H, Renauld JC, Panzer U, Helmby H and Stockinger B:
IL-9-mediated survival of type 2 innate lymphoid cells promotes
damage control in helminth-induced lung inflammation. J Exp Med.
210:2951–2965. 2013. View Article : Google Scholar : PubMed/NCBI
|