Introduction
In 2002, nucleostemin (NS) was detected in the
nucleoli of early pluripotent cells, and was found to be associated
with cell proliferation; it is also crucial for supporting the
undifferentiated properties of self-renewal certain types of stem
cell (1,2) as well as germ cell tumors (3). Furthermore, NS is abundantly
expressed in numerous tumor types, including prostate cancer
(4), esophageal cancer (5), breast carcinoma (6) and gastric adenocarcinoma (7). Elevated NS expression is associated
with poor prognosis of patients with various types of cancer
(8,9). Furthermore, knockdown of NS was shown
to inhibit cell proliferation as well as induce cell cycle arrest
and apoptosis (6,10–13).
NS is therefore a potential biomarker for tumor diagnosis and
prognosis (7,14).
Originally, NS was reported to combine with p53 to
inhibit its function as a tumor suppressor (1). However, subsequent studies have
revealed an additional p53-independent role for NS (15–17).
It has been reported that NS regulates the cell cycle by modulating
the stability of ARF tumor suppressor (18). However, to date, the detailed
mechanism of the p53-independent NS pathway has remained
elusive.
Patients with wild-type p53 tumors generally have a
better prognosis than those p53-null or p53-mutant tumors; however,
the latter represent the majority of tumors, which is a reason for
drug-resistance and poor therapeutic effects (19). Thus, it is urgent to explore
effective treatment targets for tumors which are p53-null or
p53-mutant. A previous study by our group has performed gene
expression profiling of the p53-null HL-60 leukemia cell line
following NS knockdown (20). In
order to further explore the p53-independent NS pathway, the
present study subjected the p53-mutant NB4 leukemia cell line to
DNA microarray analysis and gene expression profiling following NS
knockdown. The present study shed light on the mechanisms of the
p53-independent NS pathway in NB4 cells and provided a foundation
for the discovery of promising targets for the treatment of
p53-mutant leukemia.
Materials and methods
Cell culture
NB4 cells (GeneChem Co., Ltd., Shanghai, China) were
maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin at 37°C in a humidified
atmosphere containing 5% CO2. The medium was replaced
every two days.
Lentiviral NS-small interfering (si)RNA
vector construction, packaging and transfection
The siRNA target sequence
(5′-CAAGTATTGAAGTAGTAAA-3′) for the NS gene (Genbank ID, NM_004196)
was designed by GeneChem Co., Ltd. The two different
single-stranded DNA oligonucleotides (Table I, designed by GeneChem Co., Ltd.)
were matched to generate the NS-siRNA constructs by being dissolved
in buffer, placed in 90°C water bath for 15 min and then naturally
cooled to room temperature. Then the NS-siRNA constructs were
inserted into the green fluorescent protein-labelled lentiviral
expression vector GV248 (Genechem Co., Ltd.) to form the
recombinant vector named NS-RNAi-GV248 vector. Next, the
recombinant NS-RNAi-GV248 vectors were transformed into competent
Escherichia coli cells (GeneChem Co., Ltd.) and then
verified by DNA sequencing using 3730XL Genetic analyzer (Applied
Biosystems, Thermo Fisher Scientific, Inc.). Subsequently, the
recombinant vectors NS-RNAi-GV248, the packaging vectors pHelper
1.0 and pHelper 2.0 (GeneChem Co., Ltd.) were co-transfected into
293T cells (GeneChem Co., Ltd.). The packaged vectors were
collected from the supernatants of the cell culture medium at 48 h
after transfection. Then the Lentiviral Purification kit (GeneChem
Co., Ltd.) was used to concentrate and purify the packaged
recombinant lentiviral vectors according to the manufacturer's
protocol.
 | Table ISequences of the two single-stranded
DNA oligonucleotides. |
Table I
Sequences of the two single-stranded
DNA oligonucleotides.
| ID | Sequence
(5′–3′) |
|---|
| Single-stranded DNA
oligo 1 |
CCGGAGCAAGTATTGAAGTAGTAAACTCGAGTTTACTACTTCAATACTTGCTTTTTTG |
| Single-stranded DNA
oligo 2 |
AATTCAAAAACAAGTATTGAAGTAGTAAACTCGAGTTTACTACTTCAATACTTGCT |
For lentiviral transfection, 1×106 NB4
cells in the logarithmic growth phase were seeded into six-well
plates with 2 ml fresh medium well. According to the titer of NB4
cells (4×108 ml) and multiplicity of infection (MOI,
30), 80 µl of lentivirus were added to each well. A negative
control group treated with lentiviral vectors containing negative
control sequence: Sense 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense
5′-ACGUGACACGUUCGGAGAATT-3′ (GeneChem Co., Ltd.) and a blank
control group without any lentivirus treatment was also
established. At 16 h after infection, the culture medium was
replaced with pure medium and at 72 h after infection, the cells
were observed under a fluorescence microscope (Eclipse TS100; Nikon
Corporation, Tokyo, Japan) to evaluate the transfection
efficiency.
RNA extraction and reverse-transcription
quantitative polymerase chain reaction analysis (RT-qPCR)
At 96 h after transfection, 5–10×106
cells per group were collected and total RNA was isolated using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The total extracted RNA
was used to synthesize cDNA by reverse transcription reaction using
the PrimeScript RT Reagent kit with gDNA Eraser (Takara Bio, Inc.,
Otsu, Japan). PCR amplification of NS, GAPDH, CCND2, CHOP, MTIE,
MTIF and MAPK9 was performed in an ABI7500 quantitative real-time
PCR instrument (Thermo Fisher Scientific, Inc.) using the SYBR
Premix Ex Taq II (Tli RNaseH Plus) kit (Takara Bio, Inc.) and the
corresponding primers as listed in Table II were obtained from Sangon
Biotech Co., Ltd. (Shanghai, China). The following thermocycling
conditions were used: 95°C for 30 sec; 95°C for 5 sec, 60°C for 34
sec, 40 cycles; 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec.
The PCR products were quantified using the 2−ΔΔCq method
(21)
 | Table IIPrimer pairs used for quantitative
polymerase chain reaction analysis. |
Table II
Primer pairs used for quantitative
polymerase chain reaction analysis.
| Gene | Primer pair |
|---|
| CCND2 | F:
5′-ATTTCAGGCACAACGATA-3′ |
| R:
5′-ATTTGCTGATGGCTTCTC-3′ |
| CHOP | F:
5′-CTGACCAGGGAAGTAGAGG-3′ |
| R:
5′-TGCGTATGTGGGATTGAG-3′ |
| MT1E | F:
5′-GTGGGCTGTGCCAAGTGT-3′ |
| R:
5′-CAGCAAATGGCTCAGTGTT-3′ |
| MT1F | F:
5′-CGACTGATGCCAGGACAA-3′ |
| R:
5′-CAAATGGGTCAAGGTGGT-3′ |
| MAPK9 | F:
5′-CTGCGTCACCCATACATCAC-3′ |
| R:
5′-CTTTCTTCCAACTGGGCATC-3′ |
| GAPDH | F:
5′-TGACTTCAACAGCGACACCCA-3′ |
| R:
5′-CACCCTGTTGCTGTAGCCAAA-3′ |
| NS | F:
5′-TAGAGGTGTTGGATGCCAGAG-3′ |
| R:
5′-CACGCTTGGTTATCTTCCCTTTA-3′ |
Microarray hybridization and data
processing
For each treatment group of cells, the total
extracted RNA was purified using an RNase Mini kit (cat. no. 74104;
Qiagen, Hilden, Germany) and reversely transcribed into cDNA.
Cy3-labelled cRNA was synthesized from cDNA using the Quick Amp
Labeling kit, One-Color (cat. no. 5190-0442; Agilent Technologies,
Inc., Santa Clara, CA, USA). The purified labelled cRNA was then
subjected to hybirdization using Agilent 4×44K Human Whole-Genome
60-mer oligonucleotide microarrays with utilization of the Agilent
Gene Expression Hybridization kit (cat. no. 5188-5242; Agilent
Technologies, Inc.) following the manufacturer's instructions.
An Agilent DNA microarray scanner (cat. no. G2565BA;
Agilent Technologies, Inc.) was used to scan the microarrays, with
the parameters set as follows: Green photomultiplier tube, external
data representation (XDR) Hi 100% and XDR Lo 10%; scan resolution,
5 µm. Next, the acquired microarray images were analyzed
using Feature Extraction v 11.01.1 software (Agilent Technologies,
Inc.), and the resulting text files extracted from it were further
analyzed by GeneSpring GX v 12.0 software (Agilent Technologies,
Inc.). The data were normalized through logarithmic transformation.
Genes with low expression were removed genes detected in all
samples were selected for further data analysis. Only genes with a
fold change ≥2 or ≤0.5 were considered as differentially expressed
between the experimental and the negative control groups. Finally,
the differentially expressed genes were subjected to functional
analysis using the Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway database (http://www.genome.jp/kegg).
Statistical analysis
Each assay was performed in triplicate, and values
are expressed as the mean ± standard deviation. SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA) was used for
analysis. The means of two groups were compared using Student's
t-test. Fisher's exact test was applied to assess the significance
in the pathway analysis. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Transfection of NB4 cells with NS-siRNA
lentiviral vectors
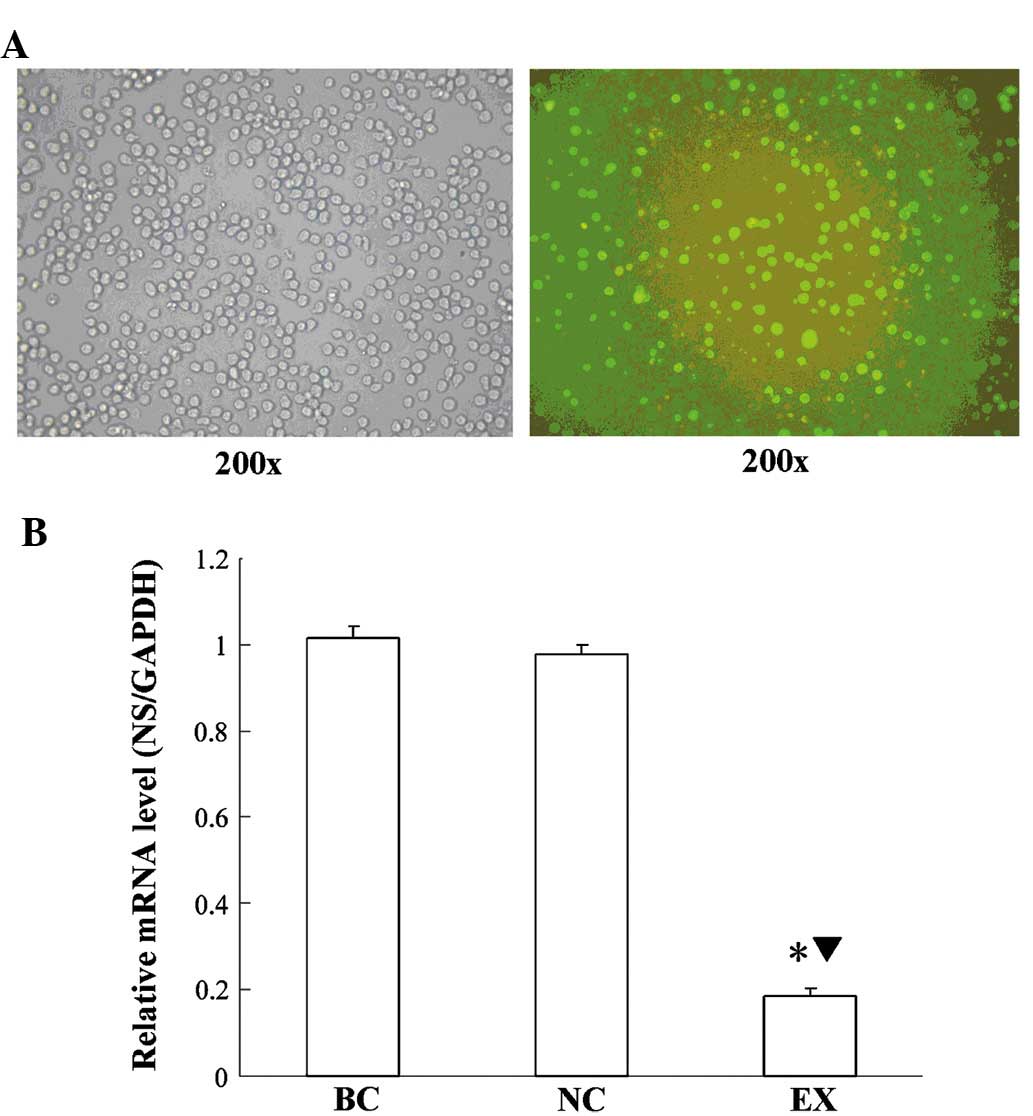
Observation under the inverted fluorescence
microscope revealed that the transfection efficiency of the
lentiviral vectors was >80% (Fig.
1). The NS mRNA expression levels in the experimental group
were decreased by ~81% compared with the blank control and the
negative control group (P<0.05), as revealed by RT-qPCR. In
order to minimize the off-target effect of NS-siRNA lentiviral
vectors, cells from the negative control-transfected group were
then subjected to DNA micro-array analysis alongside the
experimental group.
DNA microarray data analysis
With the filter cutoff set at a 2.0-fold change in
the microarray data analysis, a total of 1,953 differentially
expressed genes were identified in NB4 cells following knockdown of
NS. Of these genes, 943 were upregulated and 1,010 genes were
downregulated.
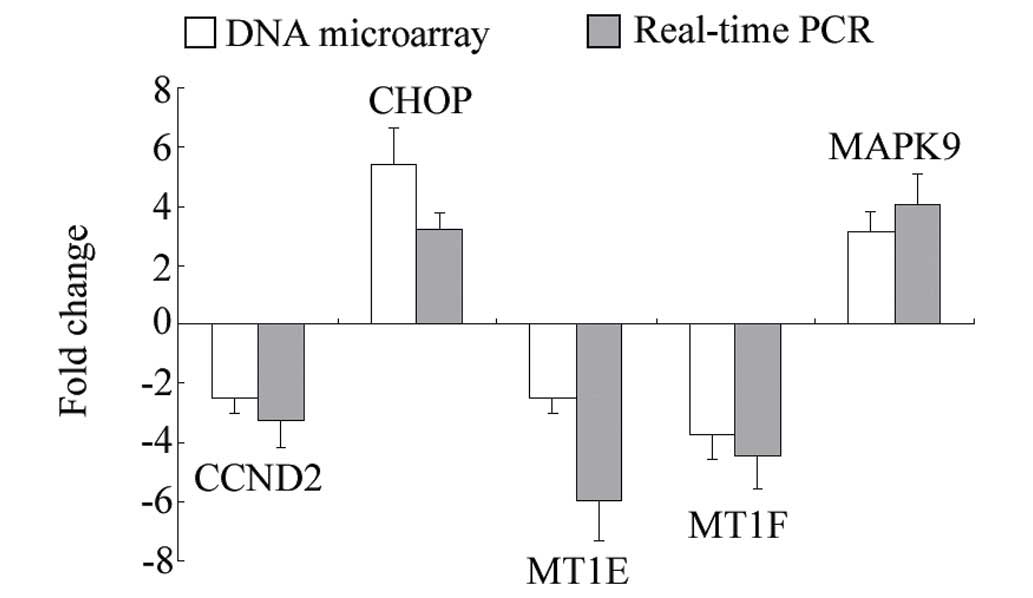
Confirmation of the microarray data by
RT-qPCR analysis
To further confirm the reliability of the microarray
data, four significantly differentially expressed genes, CCND2,
CHOP, MT1E, MT1F and MAPK9, were selected for RT-qPCR analysis. The
results of the RT-qPCR analysis were in general agreement with the
microarray data, as they showed the same trends (Fig. 2).
Pathway analysis
The differentially expressed genes were subjected to
pathway analysis based on the KEGG database. The significant
pathways containing an accumulation of upregulated or downregulated
genes are listed in Tables III
and IV, respectively.
 | Table IIIPathway analysis of upregulated
genes. |
Table III
Pathway analysis of upregulated
genes.
| Pathway ID | Definition | Fisher P-value | Genes |
|---|
| hsa04141 | Protein processing
in endoplasmic reticulum |
6.058×10−6 | AMFR, BAX, DDIT3,
DERL2, DNAJB1, DNAJC3, EIF2AK3, HERPUD1, HSPA1B, HSPA8, HSPH1,
MAPK9, MARCH6, PDIA3, PDIA4, PPP1R15A, SEC24A, SEC61A2, SSR1,
UBQLN1, YOD1 |
| hsa04621 | NOD-like receptor
signaling pathway |
1.536×10−4 | BIRC3, CCL2, CXCL2,
IL1B, IL8, MAPK9, NFKBIA, TAB2, TAB3, TNFAIP3 |
| hsa05164 | Influenza A |
1.153×10−3 | AKT3, ATF2, CCL2,
DNAJB1, DNAJC3, EIF2AK3, EP300, GSK3B, HLA-DOA, HLA-DRB5, HSPA1B,
HSPA8, ICAM1, IL1B, IL8, MAPK9, NFKBIA |
| hsa05219 | Bladder cancer |
1.683×10−3 | IL8, KRAS, MMP1,
NRAS, RPS6KA5, THBS1, VEGFA |
| hsa05166 | HTLV-I
infection |
1.913×10−3 | AKT3, ATF2, ATF3,
ATM, BAX, BIRC3, EGR1, EGR2, EP300, FZD5, GSK3B, HLA-DOA, HLA-DRB5,
ICAM1, IL15, KRAS, MAPK9, NFKBIA, NRAS, NRP1, TBPL1, ZFP36 |
| hsa04115 | p53 signaling
pathway |
2.297×10−3 | ATM, BAX, BBC3,
CCNE2, MDM4, RRM2, SERPINE1, SESN1, THBS1 |
| hsa05211 | Renal cell
carcinoma |
2.541×10−3 | AKT3, ARNT, EP300,
FLCN, HGF, KRAS, NRAS, RAC1, VEGFA |
| hsa04620 | Toll-like receptor
signaling pathway |
3.649×10−3 | AKT3, CCL4, IL1B,
IL8, LY96, AP3K8, MAPK9, NFKBIA, RAC1, SPP1, TAB2 |
| hsa05162 | Measles |
4.155×10−3 | AKT3, BBC3, CBLB,
CCNE2, CSNK2A1, EIF2AK3, GSK3B, HSPA1B, HSPA8, IL1B, NFKBIA, TAB2,
TNFAIP3 |
| hsa04010 | MAPK signaling
pathway |
4.391×10−3 | AKT3, ATF2,
CACNA1E, DDIT3, DUSP1, DUSP5, HSPA1B, HSPA8, IL1B, KRAS, MAP3K2,
MAP3K8, MAP4K3, MAP4K4, MAPK9, NRAS, PPP3R1, RAC1, RPS6KA5, TAB2,
TAOK1 |
| hsa05323 | Rheumatoid
arthritis |
5.186×10−3 | CCL2, CCL3L3,
HLA-DOA, HLA-DRB5, ICAM1, IL15, IL1B, IL8, MMP1, VEGFA |
| hsa04660 | T-cell receptor
signaling pathway |
5.655×10−3 | AKT3, CBLB, GSK3B,
KRAS, MAP3K8, MAPK9, NCK1, NFKBIA, NRAS, PPP3R1, PTPRC |
| hsa05200 | Pathways in
cancer |
1.067×10−2 | AKT3, ARNT, BAX,
BIRC3, CBLB, CCDC6, CCNE2, CSF1R, EP300, FZD5, GSK3B, HGF, IL8,
ITGA6, KRAS, MAPK9, MITF, MMP1, NFKBIA, NRAS, RAC1, TPR, VEGFA |
| hsa04662 | B-cell receptor
signaling pathway |
1.330×10−2 | AKT3, GSK3B, KRAS,
LILRB3, NFKBIA, NRAS, PPP3R1, RAC1 |
| hsa05144 | Malaria |
1.966×10−2 | CCL2, HGF, ICAM1,
IL1B, IL8, THBS1 |
| hsa05014 | Amyotrophic lateral
sclerosis (ALS) |
2.338×10−2 | ALS2, BAX, PPP3R1,
RAB5A, RAC1, TNFRSF1B |
| hsa05145 | Toxoplasmosis |
2.458×10−2 | AKT3, BIRC3,
HLA-DOA, HLA-DRB5, HSPA1B, HSPA8, ITGA6, LY96, MAPK9, NFKBIA,
TAB2 |
| hsa04210 | Apoptosis |
2.818×10−2 | AKT3, ATM, BAX,
BIRC3, IL1B, IL1RAP, NFKBIA, PPP3R1 |
| hsa04012 | ErbB signaling
pathway |
2.994×10−2 | ABL2, AKT3, CBLB,
GSK3B, KRAS, MAPK9, NCK1, NRAS |
| hsa05132 | Salmonella
infection |
2.995×10−2 | CCL3L3, CCL4,
CXCL2, IL1B, IL8, MAPK9, PKN2, RAC1 |
| hsa05142 | Chagas disease
(American trypanosomiasis) |
3.111×10−2 | AKT3, CCL2, CCL3L3,
GNAQ, IL1B, IL8, MAPK9, NFKBIA, SERPINE1 |
| hsa05216 | Thyroid cancer |
3.224×10−2 | CCDC6, KRAS, NRAS,
TPR |
| hsa04722 | Neurotrophin
signaling pathway |
4.179×10−2 | AKT3, ARHGDIB, BAX,
GSK3B, KRAS, MAPK9, NFKBIA, NRAS, RAC1, RPS6KA5 |
| hsa04380 | Osteoclast
differentiation |
4.372×10−2 | AKT3, CSF1R, GAB2,
IL1B, LILRB3, MAPK9, MITF, NFKBIA, RAC1, TAB2 |
| hsa05210 | Colorectal
cancer |
4.585×10−2 | AKT3, BAX, GSK3B,
KRAS, MAPK9, RAC1 |
 | Table IVPathway analysis of downregulated
genes. |
Table IV
Pathway analysis of downregulated
genes.
| Pathway ID | Definition | Fisher-P-value | Genes |
|---|
| hsa04978 | Mineral
absorption |
8.318×10−4 | ATP1A4, MT1B, MT1E,
MT1F, MT1H, MT1X, MT2A, SLC31A1 |
| hsa00920 | Sulfur
metabolism |
1.338×10−3 | BPNT1, SULT1A2,
SULT1A4, SUOX |
| hsa04146 | Peroxisome |
3.678×10−3 | ACOX1, AMACR, CRAT,
DHRS4, HMGCL, IDH1, IDH2, PEX6, PXMP4 |
| hsa03013 | RNA transport |
7.928×10−3 | C9ORF23, DDX39B,
EIF4B, EIF4E2, EIF4G3, ELAC1, GEMIN4, GEMIN6, NCBP1, PABPC1L,
PRMT5, RPP30, XPO5 |
| hsa00510 | N-Glycan
biosynthesis |
1.269×10−2 | B4GALT2, DOLK,
FUT8, MAN1B1, MAN1C1, MGAT1 |
| hsa00051 | Fructose and
mannose metabolism |
1.339×10−2 | ALDOC, GMPPA, KHK,
PMM2, TSTA3 |
| hsa03008 | Ribosome biogenesis
in eukaryotes |
1.843×10−2 | C9ORF23, GNL3,
GNL3L, IMP4, NOL6, NOP56, RPP30, UTP14A |
| hsa00533 | Glycosaminoglycan
biosynthesis - keratan sulfate |
2.009×10−2 | B4GALT2, FUT8,
ST3GAL3 |
| hsa04622 | RIG-I-like receptor
signaling pathway |
2.255×10−2 | CASP8, DAK, DHX58,
IRF3, MAVS, NLRX1, RIPK1 |
| hsa00020 | Citrate cycle
(tricarboxylic acid cycle) |
3.015×10−2 | IDH1, IDH2, PCK2,
SDHA |
| hsa04623 | Cytosolic
DNA-sensing pathway |
3.642×10−2 | IRF3, MAVS, POLR1C,
POLR3C, POLR3H, RIPK1 |
| hsa00531 | Glycosaminoglycan
degradation |
3.806×10−2 | GALNS, HGSNAT,
NAGLU |
| hsa00010 |
Glycolysis/Gluconeogenesis |
4.438×10−2 | AKR1A1, ALDOC,
ENO3, LDHA, PCK2, PGAM1 |
| hsa03015 | mRNA surveillance
pathway |
4.988×10−2 | CPSF6, DDX39B,
NCBP1, PABPC1L, PAPOLA, PCF11, PPP2R1A |
Discussion
NS is a protein required for the maintenance of stem
cells, the early embryonal development and proliferation of tumor
cells (1,4,5).
While NS was initially indicated to act via combining with p53
(1), increasing evidence suggested
the existence of an additional p53-independent NS pathway (15–17,22–24).
However, the underlying mechanisms of the function of NS in
p53-inactivated tumor cells have largely remained elusive. A
previous study by our group reported that inhibition of the
JAK/STAT, the PI3K/AKT and the RAS/RAF/MEK/ERK1/2 pathways, as well
as the activation of the p38MAPK and JNK pathways may participate
in the induction of apoptosis following NS knockdown in p53-null
HL-60 cells (20).
The present study further explored the mechanisms of
the function of the p53-independent function of NS by using the
p53-mutant NB4 leukemia cell line. Gene expression profiling
indicated that a large number of genes were aberrantly expressed in
NB4 cells following knockdown of NS.
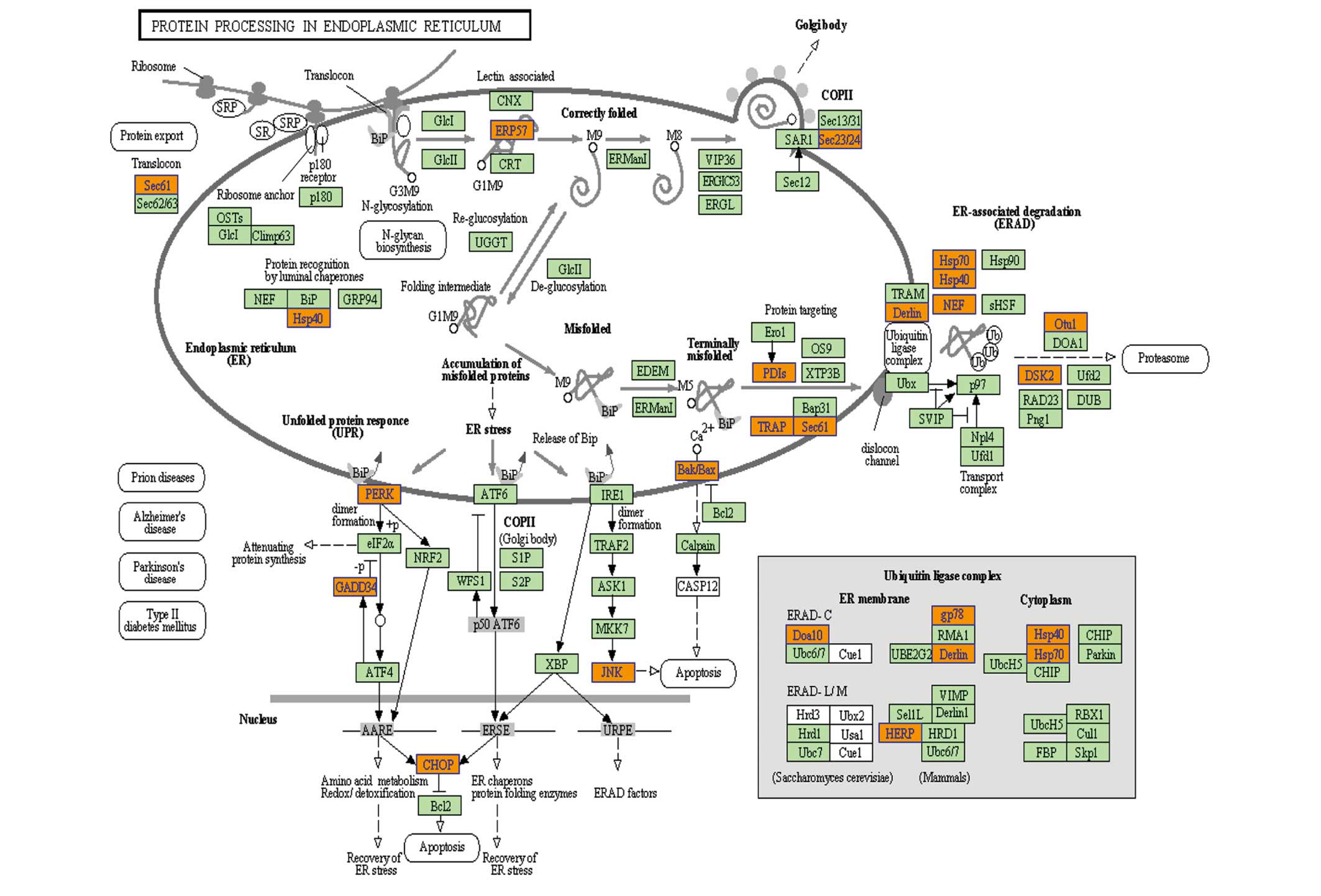
Subsequent pathway analysis of upregulated genes
revealed that protein processing in the endoplasmic reticulum (ER)
was the most significant KEGG pathway. Under normal physiological
conditions, the ER only transports correctly folded proteins to the
Golgi-apparatus, while misfolded proteins are extracted for
ubiquitin-dependent ER-associated degradation (ERAD) (25). However, insufficient degradation
leads to ER stress due to accumulation of misfolded proteins, and
the unfolded protein response (UPR) is then activated to maintain
the homeostasis of the ER (26).
When misfolded protein stress exceeds the tolerance threshold of
the ER, and the UPR is insufficient for the maintenance of
homeostasis, cell apoptosis is activated, which is referred to as
ER stress-induced apoptosis (27,28).
PERK, IRE1 and ATF6 are three main sensor proteins located on the
ER membrane, which are activated as part of the UPR by dissociation
from GRP78 and relieve the stress through a series of pathways. As
illustrated in Fig. 3, a number of
upregulated genes were associated with the ERAD process, which
indicated that ER stress may have increased in NB4 cells after
knockdown of NS. Furthermore, prolonged ER stress is able to induce
apoptosis (29). However, further
studies are required to determine whether downregulation of NS may
directly activate apoptosis in NB4 cells. CHOP and JNK are key
mediators during ER stress-induced apoptosis (30). In the present study, PERK, CHOP and
JNK (MAPK9) were all upregulated. Therefore, it is indicated that
the p53-independent NS pathway may also be associated with
increases in ER stress and an imbalance of ER homeostasis.
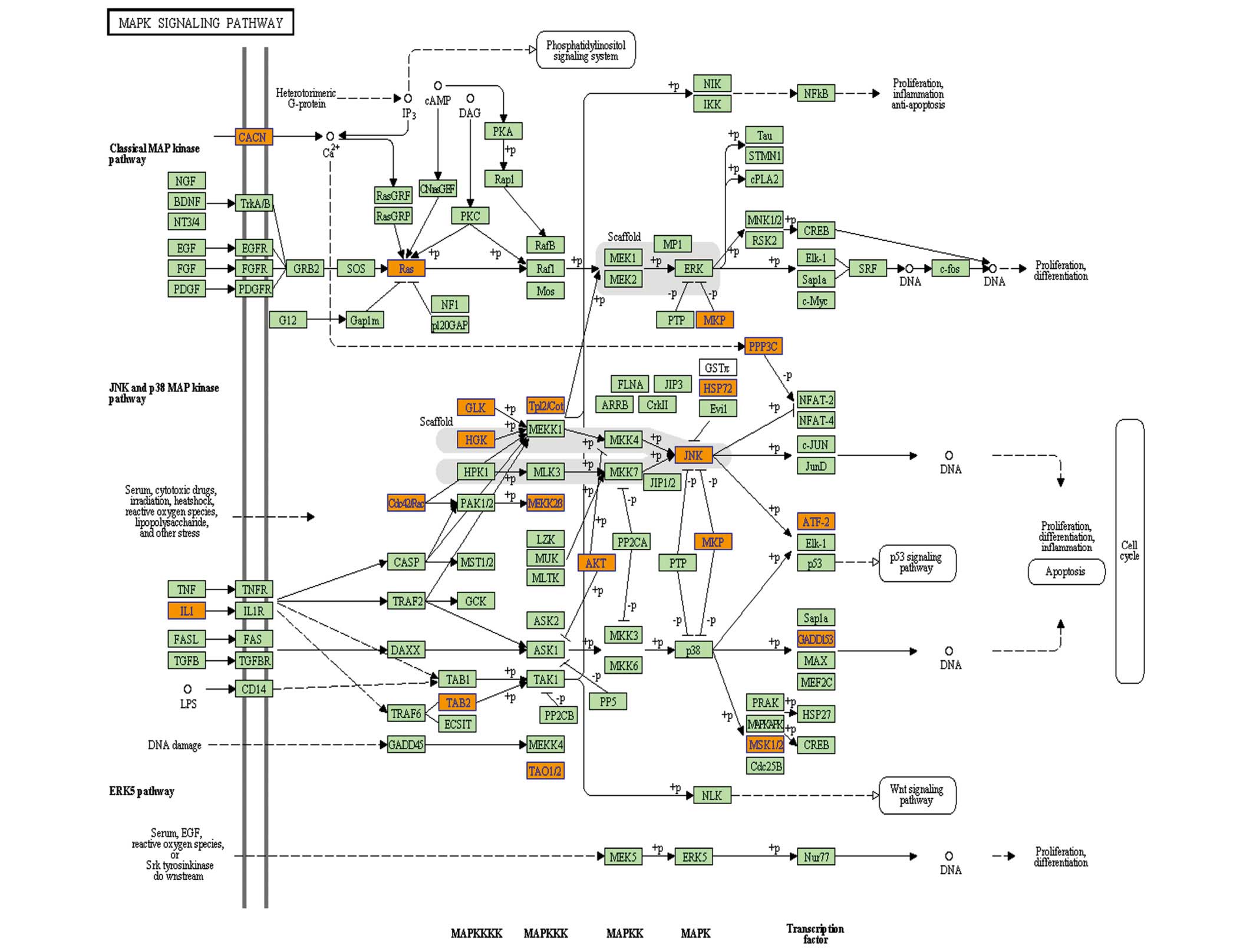
In addition, several of the upregulated genes were
enriched in the MAPK signaling pathway (Fig. 4), which was a similar finding to
previous observations in HL-60 cells (20). MAPK pathways mainly consist of the
JNK, the RAS/RAF/MEK/ERK2 and the p38MAPK pathways, while
activation of JNK and p38MAPK pathways is known to induce cell
apoptosis (31). In contrast to
the findings in HL-60 cells, only JNK(MAPK9) was upregulated in NB4
cells following NS knockdown. Therefore, activation of the JNK
pathway was another effect of NS knockdown in NB4 cells, indicating
that NS may exert its effects via the NS pathway.
Analysis of downregulated genes showed that mineral
absorption was most significant pathway. The key genes in this
pathway were metallothioneins (MTs). MT genes are a family of
cysteine-rich proteins, which are closely linked and mainly
comprise 11 MT-1 genes (including MT-1A, -B, -E - L and -X) and one
gene for other MT isoforms (MT-2A, MT-3 and MT-4) (32). MT-1 and MT-2 isoforms are widely
expressed in numerous cell types. MT-3 is associated with neuronal
cells; it is also called neuronal growth-inhibitory factor and
inhibits the outgrowth of neuronal cells (33). MT-4 is primarily expressed on
certain squamous epithelial cells (34). The differentially expressed MT
genes in the present study were mainly MT-1 genes, including MT-1A,
MT-1B, MT-1E, MT-1F, MT-1H, MT-1L and MT-1X. MTs participate in
zinc and copper homeostasis, protection against heavy metal
toxicity and oxidative damage. As zinc deficiency is associated
with oxidative stress (35),
downregulation of MTs may disturb the homeostasis of zinc and
copper, which increases oxidative stress in cells. Furthermore, as
MTs are rich in sulfhydryl and have a high anti-oxidant capacity,
they are able to effectively eliminate superoxide and hydroxyl
radicals (36). Therefore,
downregulation of MT expression may result in oxidative stress due
to the absence of anti-oxidants, which may be an underlying
mechanism for the imbalance of ER homeostasis indicated by the
present study.
In addition, MTs are closely linked with malignant
tumors. Metal-regulatory transcription factor-1 (MTF-1) is the only
known mediator of the metal responsiveness of MTs and is able to
regulate the expression of MTs (32,37,38).
MTF-1 is elevated in numerous tumor types, including lung, breast,
and cervical carcinoma-derived cell lines (39), which supports the link between MTs
and tumors. MTs have been shown to be distinctly elevated in
rapidly growing tissues such as the neonatal liver, suggesting that
MTs are important in cell proliferation (40,41).
Furthermore, increased expression of MTs is associated with drug
resistance (42,43), anti-apoptotic capacity (44,45),
breast cancer prognosis (46) and
differentiation of thyroid tumor cells (47). Thus, the DNA microarray data
analysis indicated that following a knockdown of NS in NB4 cells,
diminished proliferation and low tumorigenic capacity may occur due
to downregulation of MTs. This requires further verification in
future studies.
In the present study, a further significant pathway
with accumulation of downregulated genes was the peroxisome
pathway. Inhibition of the peroxisome pathway reduces the synthesis
of peroxisome, which is an intracellular organelle located in the
cytoplasm and contains an abundance of enzymes, including catalase,
oxidase and peroxidase. Therefore, decreased synthesis of
peroxisome reduces these enzymes, which may influence the redox
state of the cells or the ER, causing imbalance of ER
homeostasis.
In addition, pathway analysis of downregulated genes
showed that the pathways involved were mostly associated with
biosubstance synthesis and metabolism, including sulfur metabolism,
RNA transport, N-glycan biosynthesis, fructose and mannose
metabolism, ribosome biogenesis in eukaryotes, glycosaminoglycan
biosynthesis and citrate cycle. This indicated that the
biosynthesis and metabolism of NB4 cells was reduced following
knockdown of NS expression (48,49).
In conclusion, gene expression profiling analysis of
NB4 cells following knockdown of NS revealed a large number of
differentially expressed genes. Pathway analysis indicated that ER
stress may increase in NB4 cells after NS inhibition, which may
cause an imbalance of ER homeostasis; furthermore, the JNK pathway
was activated. In addition, biosubstance synthesis and metabolism
in NB4 cells was reduced following NS knockdown. The present study
provided insight into the underlying mechanism of the
p53-independent NS signaling pathway, which may be utilized for the
development of novel treatments for p35-null/mutated cancer
types.
Acknowledgments
The present study was funded by the National Natural
Science Foundation of China (no. 81271911) and the Key Projects of
Medical Science and Technology of Henan Province (no.
201002006).
References
|
1
|
Tsai RY and McKay RD: A nucleolar
mechanism controlling cell proliferation in stem cells and cancer
cells. Genes Dev. 16:2991–3003. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu SJ, Cai ZW, Liu YJ, Dong MY, Sun LQ,
Hu GF, Wei YY and Lao WD: Role of nucleostemin in growth regulation
of gastric cancer, liver cancer and other malignancies. World J
Gastroenterol. 10:1246–1249. 2004.PubMed/NCBI
|
|
3
|
Uema N, Ooshio T, Harada K, Naito M, Naka
K, Hoshii T, Tadokoro Y, Ohta K, Ali MA, Katano M, et al: Abundant
nucleostemin expression supports the undifferentiated properties of
germ cell tumors. Am J Pathol. 183:592–603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu RL, Xu Y, Zhang ZH, Wang M, Sun JT, Qi
SY, Zhang Y and Li SZ: Expression of nucleostemin in prostate
cancer tissues and its clinical significance. Natl J Androl.
14:418–422. 2008.In Chinese.
|
|
5
|
Zhang GY, Yin L, Li SL, Xing WY, Zhao QM,
Le XP, Gao DL, Chen KS, Zhang YH and Zhang QX: Expression of
nucleostemin mRNA and protein in the esophageal squamous cell
carcinoma. Chin J Oncol. 30:125–128. 2008.In Chinese.
|
|
6
|
Guo Y, Liao YP, Zhang D, Xu LS, Li N, Guan
WJ and Liu CQ: In vitro study of nucleostemin as a potential
therapeutic target in human breast carcinoma SKBR-3 cells. Asian
Pac J Cancer Prev. 15:2291–2295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asadi MH, Derakhshani A and Mowla SJ:
Concomitant upregulation of nucleostemin and downregulation of Sox2
and Klf4 in gastric adenocarcinoma. Tumour Biol. 35:7177–7185.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kobayashi T, Masutomi K, Tamura K, Moriya
T, Yamasaki T, Fujiwara Y, Takahashi S, Yamamoto J and Tsuda H:
Nucleostemin expression in invasive breast cancer. BMC Cancer.
14:2152014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
You Y, Li X, Zheng J, Wu Y, He Y, Du W,
Zou P and Zhang M: Transcript level of nucleostemin in newly
diagnosed acute myeloid leukemia patients. Leuk Res. 37:1636–1641.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sijin L, Ziwei C, Yajun L, Meiyu D,
Hongwei Z, Guofa H, Siguo L, Hong G, Zhihong Z, Xiaolei L, et al:
The effect of knocking-down nucleostemin gene expression on the in
vitro proliferation and in vivo tumorigenesis of HeLa cells. J Exp
Clin Cancer Res. 23:529–538. 2004.PubMed/NCBI
|
|
11
|
Ma H and Pederson T: Depletion of the
nucleolar protein nucleostemin causes G1 cell cycle arrest via the
p53 pathway. Mol Biol Cell. 18:2630–2635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu RL, Zhang ZH, Zhao WM, Wang M, Qi SY,
Li J, Zhang Y, Li SZ and Xu Y: Expression of nucleostemin in
prostate cancer and its effect on the proliferation of PC-3 cells.
Chin Med J (Engl). 121:299–304. 2008.
|
|
13
|
Seyed-Gogani N, Rahmati M, Zarghami N,
Asvadi-Kermani I, Hoseinpour-Feyzi MA and Moosavi MA: Nucleostemin
depletion induces post-g1 arrest apoptosis in chronic myelogenous
leukemia k562 cells. Adv Pharm Bull. 4:55–60. 2014.PubMed/NCBI
|
|
14
|
Amini S, Fathi F, Mobalegi J,
Sofimajidpour H and Ghadimi T: The expressions of stem cell
markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3,
Dppa4 and Esrrb in bladder, colon and prostate cancer and certain
cancer cell lines. Anat Cell Biol. 47:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beekman C, Nichane M, De Clercq S, Maetens
M, Floss T, Wurst W, Bellefroid E and Marine JC: Evolutionarily
conserved role of nucleostemin: Controlling proliferation of
stem/progenitor cells during early vertebrate development. Mol Cell
Biol. 26:9291–9301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jafarnejad SM, Mowla SJ and Matin MM:
Knocking-down the expression of nucleostemin significantly
decreases rate of proliferation of rat bone marrow stromal stem
cells in an apparently p53-independent manner. Cell Prolif.
41:28–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nikpour P, Mowla SJ, Jafarnejad SM,
Fischer U and Schulz WA: Differential effects of Nucleostemin
suppression on cell cycle arrest and apoptosis in the bladder
cancer cell lines 5637 and SW1710. Cell Prolif. 42:762–769. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lo D, Zhang Y, Dai MS, Sun XX, Zeng SX and
Lu H: Nucleostemin stabilizes ARF by inhibiting the ubiquitin
ligase ULF. Oncogene. 34:1688–1697. 2015. View Article : Google Scholar
|
|
19
|
Li X, Zhou J, Chen ZR and Chng WJ: P53
mutations in colorectal cancer-molecular pathogenesis and
pharmacological reactivation. World J Gastroenterol. 21:84–93.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun X, Jia Y, Wei Y, Liu S and Yue B: Gene
expression profiling of HL-60 cells following knockdown of
nucleostemin using DNA microarrays. Oncol Rep. 32:739–747.
2014.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Liu R, Zhang Z and Xu Y: Downregulation of
nucleostemin causes G1 cell cycle arrest via a p53-independent
pathway in prostate cancer PC-3 cells. Urol Int. 85:221–227. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zwolinska AK, Heagle Whiting A, Beekman C,
Sedivy JM and Marine JC: Suppression of Myc oncogenic activity by
nucleostemin haploinsufficiency. Oncogene. 31:3311–3321. 2012.
View Article : Google Scholar :
|
|
24
|
Paridaen JT, Janson E, Utami KH, Pereboom
TC, Essers PB, Rooijen C, Zivkovic D and MacInnes AW: The nucleolar
GTP-binding proteins Gnl2 and nucleostemin are required for retinal
neurogenesis in developing zebrafish. Dev Biol. 355:286–301. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stolz A and Wolf DH: Endoplasmic reticulum
associated protein degradation: A chaperone assisted journey to
hell. Biochim Biophys Acta. 1803:694–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Määttänen P, Gehring K, Bergeron JJ and
Thomas DY: Protein quality control in the ER: The recognition of
misfolded proteins. Semin Cell Dev Biol. 21:500–411. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu XL: The regulative mechanism of
organisms against stress injuries. Biofactors. 40:569–585. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu W, Hagiwara D, Morishita Y, Tochiya M,
Azuma Y, Suga H, Goto M, Banno R, Sugimura Y, Oyadomari S, et al:
Unfolded protein response in hupothalamic cultures of wild-type and
ATF6α-knockout mice. Neurosci Lett. 612:199–203. 2015. View Article : Google Scholar
|
|
29
|
Rao RV, Castro-Obregon S, Frankowski H,
Schuler M, Stoka V, Del RG, Bredesen DE and Ellerby HM: Coupling
endoplasmic reticulum stress to the cell death program. An
Apaf-1-independent intrinsic pathway. J Biol Chem. 277:21836–21842.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang ZX: Neuronal growth-inhibitory
factor (metallo-thionein-3): A unique metalloprotein. FEBS J.
277:29112010. View Article : Google Scholar
|
|
33
|
Vašák M and Meloni G: Chemistry and
biology of mammalian metallothioneins. J Biol Inorg Chem.
16:1067–1078. 2011. View Article : Google Scholar
|
|
34
|
Eide DJ: The oxidative stress of zinc
deficiency. Metallomics. 3:1124–1129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miura T, Muraoka S and Ogiso T:
Antioxidant activity of metallothionein compared with reduced
glutathione. Life Sci. 60:301–309. 1997. View Article : Google Scholar
|
|
36
|
Ghoshal K and Jacob ST: Regulation of
metallothionein gene expression. Prog Nucleic Acid Res Mol Biol.
66:357–384. 2001. View Article : Google Scholar
|
|
37
|
Günes C, Heuchel R, Georgiev O, Müller KH,
Lichtlen P, Bluthmann H, Marino S, Aguzzi A and Schaffner W:
Embryonic lethality and liver degeneration in mice lacking the
metal-responsive transcriptional activator MTF-1. EMBO J.
17:2846–2854. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Klassen RB, Crenshaw K, Kozyraki R,
Verroust PJ, Tio L, Atrian S, Allen PL and Hammond TG: Megalin
mediates renal uptake of heavy metal metallothionein complexes. Am
J Physiol Renal Physiol. 287:F393–F403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi Y, Amin K, Sato BG, Samuelsson SJ,
Sambucetti L, Haroon ZA, Laderoute K and Murphy BJ: The
metal-responsive transcription factor-1 protein is elevated in
human tumors. Cancer Biol Ther. 9:469–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cherian MG and Apostolova MD: Nuclear
localization of metallothionein during cell proliferation and
differentiation. Cell Mol Biol (Noisy-le-grand). 46:347–356.
2000.
|
|
41
|
Ogra Y and Suzuki KT: Nuclear trafficking
of metallothionein: Possible mechanisms and current knowledge. Cell
Mol Biol (Noisy-le-grand). 46:357–365. 2000.
|
|
42
|
Knipp M: Metallothioneins and platinum
(II) anti-tumor compounds. Curr Med Chem. 16:522–537. 2009.
View Article : Google Scholar
|
|
43
|
Boulikas T and Vougiouka M: Cisplatin and
platinum drugs at the molecular level (Review). Oncol Rep.
10:1663–1682. 2003.PubMed/NCBI
|
|
44
|
McGee HM, Woods GM, Bennett B and Chung
RS: The two faces of metallothionein in carcinogenesis:
Photoprotection against UVR-induced cancer and promotion of tumour
survival. Photochem Photobiol Sci. 9:586–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dutsch-Wicherek M, Sikora J and
Tomaszewska R: The possible biological role of metallothionein in
apoptosis. Front Biosci. 13:4029–4038. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gomulkiewicz A, Podhorska-Okolow M, Szulc
R, Smorag Z, Wojnar A, Zabel M and Dziegiel P: Correlation between
metallothionein (MT) expression and selected prognostic factors in
ductal breast cancers. Folia Histochem Cytobiol. 48:242–248. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Królicka A, Kobierzycki C, Puła B,
Podhorska-Okołów M, Piotrowska A, Rzeszutko M, Rzeszutko W,
Rabczyński J, Domosławski P, Wojtczak B, et al: Comparison of
metallo-thionein (MT) and Ki-67 antigen expression in benign and
malignant thyroid tumours. Anticancer Res. 30:4945–4949. 2010.
|
|
48
|
Kishton RJ and Rathmell JC: Novel
therapeutic targets of tumor metabolism. Cancer J. 21:62–69. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gottfried E, Kreutz M and Mackensen A:
Tumor metabolism as modulator of immune response and tumor
progression. Semin Cancer Biol. 22:335–341. 2012. View Article : Google Scholar : PubMed/NCBI
|