Introduction
Stroke is the second leading cause of mortality
worldwide, and often results in physical and cognitive dysfunction,
and various other types of functional disorders (1,2).
Cognitive dysfunction is common following stroke, with an incidence
of up to 64% (3). Frequently
observed forms of cognitive impairment include learning and memory
disorders, attention deficits and other reductions in cognitive
ability that seriously restrict the functional rehabilitation of
stroke patients. Currently, the effect of cognitive impairment on
the quality of life and daily living of patients is greater than
the effect of physical dysfunction alone (4), and cognitive impairment increases the
economic burden of patients (5).
Stroke patients that develop cognitive disorders are more likely to
develop dementia (6). Thus, timely
treatment is critical for physical and mental function, and
comprehensive recovery and prevention of dementia for patients
following stroke.
Acupuncture is a popular treatment strategy in
traditional Chinese medicine (TCM), and has been accepted and
recognized as a therapy in China and Western countries (7). Acupuncture has a long history of use
in the treatment of mental disorders and diseases of the brain
(8). Acupuncture has been widely
used to clinically treat cognitive disorders in China and has
provided therapeutic benefits, and gained recognition from
professionals and the general public (9–11).
In TCM, two acupoints located on the Du meridian, Baihui (DU20; on
the anterior midline in front of boundary of the frontal and
parietal bones) and Shenting (DU24; on the anterior midline and the
central parietal bone) may be important in the nervous system. The
Shenting (DU24) acupoint is considered to be involved in the
improvement of human health, and Baihui (DU20) in the adjustment of
memory function. Thus, the Baihui (DU20) and Shenting (DU24)
acupoints have been commonly focused on to treat cognitive
disorders in China (12–14). Previous research by this group has
demonstrated that electroacupuncture (EA) at Baihui (DU20) and
Shenting (DU24) improves cognitive disorders following ischemic
stroke (15,16), however, the underlying mechanisms
remain to be elucidated. The present study aimed to further clarify
the mechanism by which acupuncture achieves therapeutic benefits to
cognitive dysfunction following ischemia/reperfusion (IR)-induced
stroke.
Brain tissue damage progresses due to a series of
complex pathophysiological changes following cerebral I/R injury.
Disruption of the blood brain barrier (BBB) (17) and formation of brain edema
(18) are considered important
pathological changes that occur following I/R injury. The BBB is an
important protective structure of the brain and facilitates
exchange of material between the blood and the brain tissue
(19). Alterations in the tight
junction organization in the BBB results in blood vessel
permeability and BBB breakdown following hypoxic ischemia (20), which allows white blood cells,
plasminogen plasma protein, intravascular fluid and other
substances to enter the brain (21). Inflammatory responses, brain edema
formation and disruption of brain tissue structure subsequently
occur, and eventually promote neuronal death or apoptosis (22,23).
The integrity of the structure and function of neurons is the
morphological basis of learning, memory and other cognitive
activities (24). Thus, neuronal
cell death or apoptosis may impair learning and memory. Matrix
metalloproteinase (MMP)-2/MMP-9 are important collagenases of the
MMP family and have previously been widely studied in acute
cerebral ischemia. MMPs are important in the breakdown of the BBB,
cerebral edema and inflammation, and pathophysiologic processes
involving angiogenesis following cerebral ischemia (25,26).
The present study hypothesized that MMP-2 and MMP-9 are also
closely associated with neural cell apoptosis, neuron regeneration
and the incidence of neural functional defects following cerebral
I/R injury (27).
The present study used a rat model of embolic middle
cerebral artery occlusion (MCAO) of focal cerebral I/R injury to
observe the effect of EA at the Baihui (DU20) and Shenting (DU24)
acupoints on learning and memory, and investigated the underlying
molecular mechanisms of I/R injury.
Materials and methods
Materials and reagents
MMP-2, MMP-9 and β-actin primary antibodies and
horseradish peroxidase (HRP)-conjugated secondary antibodies were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
2,3,5-Triphenyl tetrazolium chloride (TTC) and other chemicals used
were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless
otherwise stated.
Animals
Healthy adult male Sprague-Dawley rats (weight,
250–280 g; age, 3–4 months) were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China), housed under
pathogen-free conditions at 22°C with a 12 h light/dark cycle, and
received ad libitum access to food and water. All animal
procedures were conducted in accordance with international ethical
guidelines and the National Institutes of Health Guide for the Care
and Use of Laboratory Animals, and all experiments were approved by
the Institutional Animal Care and Use Committee of Fujian
University of Traditional Chinese Medicine (Fuzhou, China).
Establishing the cerebral I/R injury rat
model
MCAO was used to establish the rat model of cerebral
I/R injury. Rats were fasted for 24 h prior to surgery, and the
surgical procedures were performed as previously described by Longa
et al (28), with slight
modifications. Briefly, the rats were anesthetized by
intraperitoneal injection with 10% chloral hydrate (300 mg/kg). The
left common carotid artery (CCA), the left external carotid artery
(ECA) and the internal carotid artery (ICA) were carefully exposed
and isolated via a midline cervical incision. Nylon surgical thread
(~18–22 mm) was inserted into the ICA to block the left middle
cerebral artery (MCA) when the blunted distal end met resistance.
Reperfusion was achieved when the thread was removed after 2 h of
occlusion to restore blood supply to the MCA area. For rats in the
sham group, the left CCA, ECA and ICA were exposed, but no
ligations and occlusions were performed. The rectal temperature of
rats was monitored and the body temperature was maintained at 37°C
throughout the surgical procedures.
Upon recovery, the neurological deficit scores of
the rats were assessed and they were randomly divided into 2 groups
(n=20/group) as follows: Ischemia (MCAO) control group; and MCAO +
EA group. Following surgery, the rats recovered in pre-warmed
cages.
EA treatment
Following recovery from surgery (2 h after I/R), the
rats in the EA group received EA treatment for 7 days. Acupuncture
needles (0.3 mm in diameter) were inserted at a depth of 2 to 3 mm
into the skin at the Baihui (DU20) and Shenting (DU24) acupoints.
Stimulation was then generated using G6805 EA apparatus [Shanghai
Huayi (Group) Company Ltd., Shanghai, China] and the stimulation
parameters were set as follows: Waves of 1 and 20 Hz, and 1–3 mA
were delivered for 30 min once per day.
Neurological assessment
The neurological deficit score was assessed in a
single-blind manner, as previously described by Longa et al
(28). A score of 0 indicated no
neurological deficit was observed; score of 1 represented by a
failure to fully extend the right forepaw, indicated a mild
deficit; a score of 2 represented by circling to the right and a
score of 3 represented by falling to the right, indicated moderate
deficits; and a score of 4 was represented by failure to walk and
indicated a severe deficit. Rats scoring 0 or 4, exhibiting either
no or severe deficits, respectively, were excluded from the current
study.
Morris water maze
At day 3 following surgery, the spatial learning and
memory of rats was tested via the Morris water maze. The water maze
apparatus (Chinese Academy of Sciences, Beijing, China) consisted
of a tank (diameter, 120 cm; height, 50 cm) filled with water
(depth, 30 cm; temperature, 25±2°C). A circular escape platform,
measuring 6 cm in diameter and 28 cm in height, was submerged 2 cm
below the surface of the water. The tank was divided into 4 equal
quadrants. A video camera attached to a computer was placed above
the center of the tank for recording and analysis of the rats.
These points served as the starting positions at which each rat was
lowered gently into the water, its head facing the wall of the
water maze. Morris water maze tasks include orientation, navigation
and space exploration trials. In the initial set of trials, each
rat was placed in the water at 4 locations equidistant from the
platform. If the rat arrived at the platform within the 90 sec time
restriction and remained on it for 3 sec, it was considered to have
successfully found the platform and was scored by the time taken.
When the rat was unable to find the platform within 90 sec, it was
placed on the platform for 10 sec and the time scored was 90 sec.
The computer recorded the time taken for each rat to identify the
safe platform, and each day the mean result of the time taken for
the 4 quadrants was assessed for each rat. The initial set of
trials was conducted over 5 days, with the experiment performed on
each rat once per day.
The second part of the experiment was performed on
day 7. This part assessed the ability of each rat to remember the
position of the platform by examining the time in which the rat
located the platform within the 90-sec time restriction. Following
all trials, the rats were dried thoroughly with a hair dryer and
returned to their cages.
Evaluation of infarct volume
At the end of experiments, rats were deeply
anesthetized using 10% chloral hydrate and euthanized
transcardially with 0.9% NaCl. The brains of all rats were removed
rapidly and dissected into six coronal blocks at a thickness of 2
mm/section and stained with 2% solution of TTC in
phosphate-buffered saline (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) at 37°C for 20 min. Subsequently, the brain
sections were fixed with 4% paraformaldehyde as previously
described (29). Normal tissue
stained deep red while infarct area exhibited a pale gray color due
to lack of stain uptake. Images of the stained slices were captured
with a Canon SX20 high-resolution digital camera (Canon, Inc.,
Tokyo, Japan), and the infarct volume was quantified with the Motic
Med 6.0 system (Motic China Group Co., Ltd., Xiamen, China). The
infarct volume was expressed as a percentage of the uninjured
contralateral hemisphere volume.
Transmission electron microscopy
(TEM)
Rats (n=6) were sacrificed via intraperitoneal
injection of chloral hydrate (300 mg/kg) and the left ventricle was
perfused with 200 ml of saline followed by 400 ml 4%
paraformaldehyde (pH 7.4). The brain was then post-fixed in
paraformaldehyde with 1% lanthanum nitrate tracer (LNT; Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) for ~24–48 h follow by
fixation in 3% glutaraldehyde-1.5% paraformaldehyde-1% LNT solution
for 24 h and conventional embedding for electron microscopy. Fixed
brains were dehydrated using a graded series of ethanol-1% LNT
solution of increasing concentrations, embedded in epoxy resin, and
cut into ultrathin sections (90 nm). The sections were mounted on
copper grids, stained in uranyl acetate and lead citrate and then
observed under TEM (H-7650; Hitachi Ltd, Tokyo, Japan).
Comprehensive observation at low magnification was performed,
followed by detailed observation of cell morphology, nuclei and
cellular organelles.
Western blotting
Total proteins were extracted from the left cerebral
hippocampal tissues and protein concentrations were determined by
bicinchoninic acid assay. Protein samples (50 µg) were
separated by electrophoresis on 12% SDS-PAGE gels, then transferred
onto polyvinylidene difluoride membranes in a Tris-glycine transfer
buffer. Following transfer, membranes were blocked for 2 h in 5%
nonfat dry milk at room temperature. Following blocking, protein
blots were detected with rabbit anti-MMP-2 (cat. no. 13132),
anti-MMP-9 (cat. no. 3852), and anti-β-actin (cat. no. 3700S)
antibodies (dilution, 1:1,000; Cell Signaling Technology, Inc.) at
4°C overnight followed by incubation with the appropriate
HRP-conjugated secondary antibody (cat. no. 14C10; Cell Signaling
Technology, Inc.) for 1 h at room temperature. Detected bands were
visualized using enhanced chemiluminescence and images were
captured using a ChemiDoc system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Western blots were repeated three times.
Statistical analysis
All data were analyzed using SPSS software (version
18.0; SPSS, Inc., Chicago, IL, USA). Quantitative data were
presented as mean ± standard deviation. Rank-sum testing was
performed on the neurological deficit score results. One-way
analysis of variance were used to assess statistical differences
between multiple groups. The homogeneity of variance was analyzed
using the least significant difference method and missing variance
using the Games-Howell method. P<0.05 was considered to indicate
a statistically significant difference.
Results
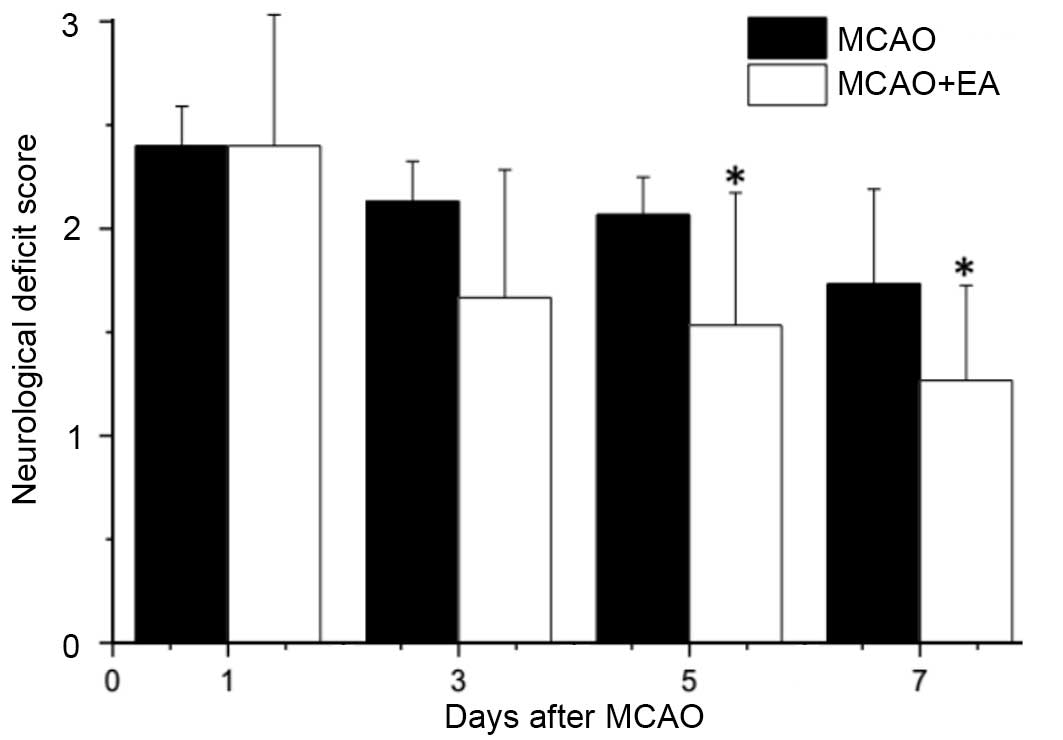
Effect of EA at Baihui (DU20) and
Shenting (DU24) acupoints on neurological deficits in I/R injured
rats
To investigate whether EA at the Baihui (DU20) and
Shenting (DU24) acupoints attenuate ischemic brain injury,
neurological scores were determined in rats at different time
points following stroke. Supporting the hypothesis of the current
study, rats in the sham group exhibited no manifestations of
neurological deficits (Fig. 1),
whereas all rats in the MCAO and MCAO + EA groups demonstrated
clear symptoms of cerebral injury compared with the sham rats.
However, EA significantly improved neurological deficit scores
compared with the MCAO group (P<0.05; Fig. 1). These results suggested that EA
at the Baihui (DU20) and Shenting (DU24) acupoints provided
neuroprotective effects and promoted functional recovery in
cerebral I/R injured rats.
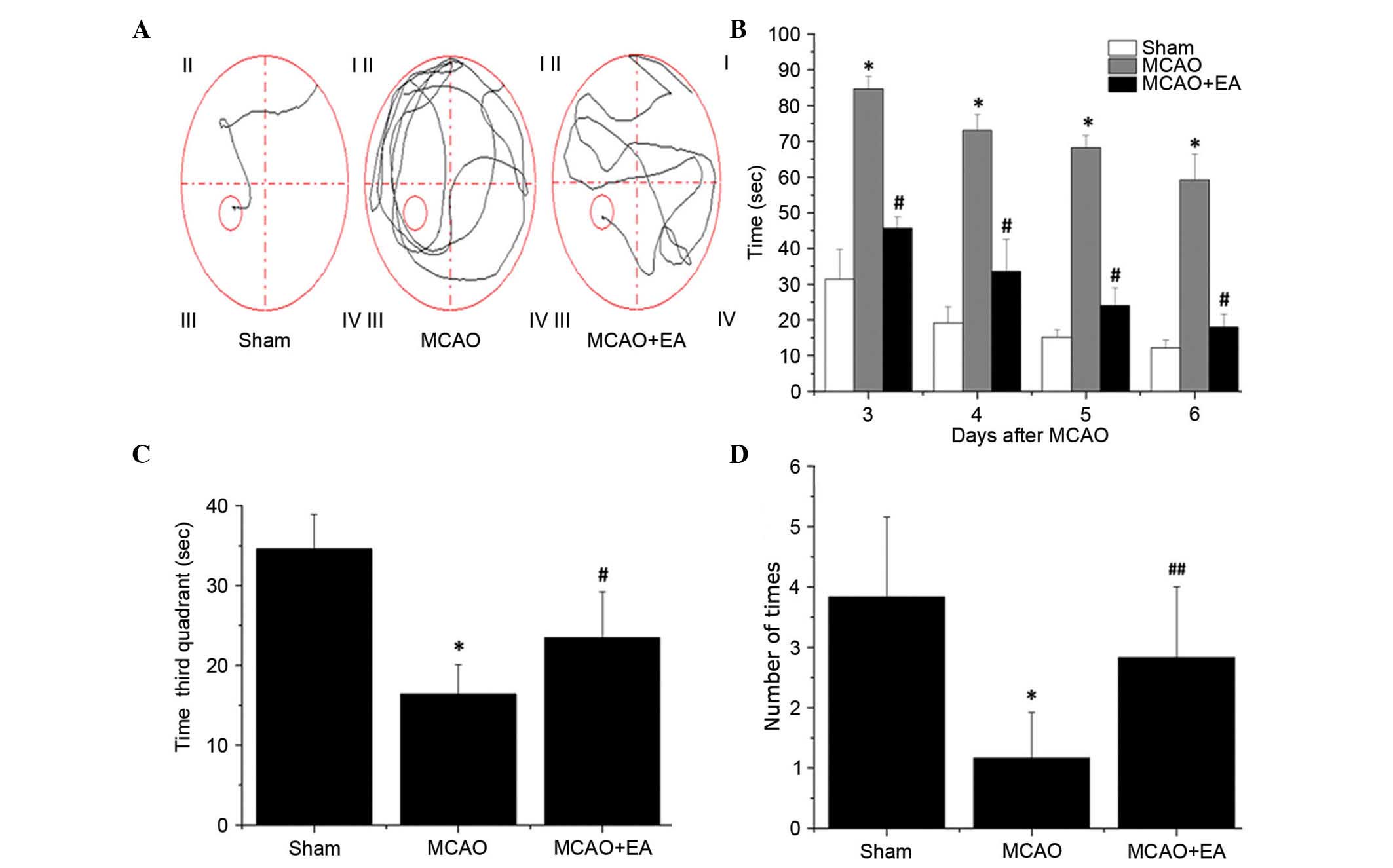
EA ameliorates cognitive impairment in
cerebral I/R injured rats
All rats were assessed in a Morris water maze on
days 3–7 following MCAO surgery. As shown in Fig. 2, the latency to reach the hidden
platform in the maze was significantly increased in the MCAO group
compared with sham rats (P<0.01), whereas the time taken in the
third quadrant for the rats to find the platform (within 90 sec) on
day 7 and the number of times that rats crossed the platform's
location was significantly decreased compared with rats in the sham
group (P<0.01; Fig. 2). This
indicates that cerebral I/R injury resulted in cognitive
impairment. However, EA significantly decreased the latency and
time taken in the third quadrant on day 7 (P<0.01), and
increased the number of platform crossings in the Morris water maze
(P<0.05) compared with the MCAO group (Fig. 2). These findings suggested that EA
at the Baihui (DU20) and Shenting (DU24) acupoints may ameliorate
cognitive impairment in cerebral I/R injured rats.
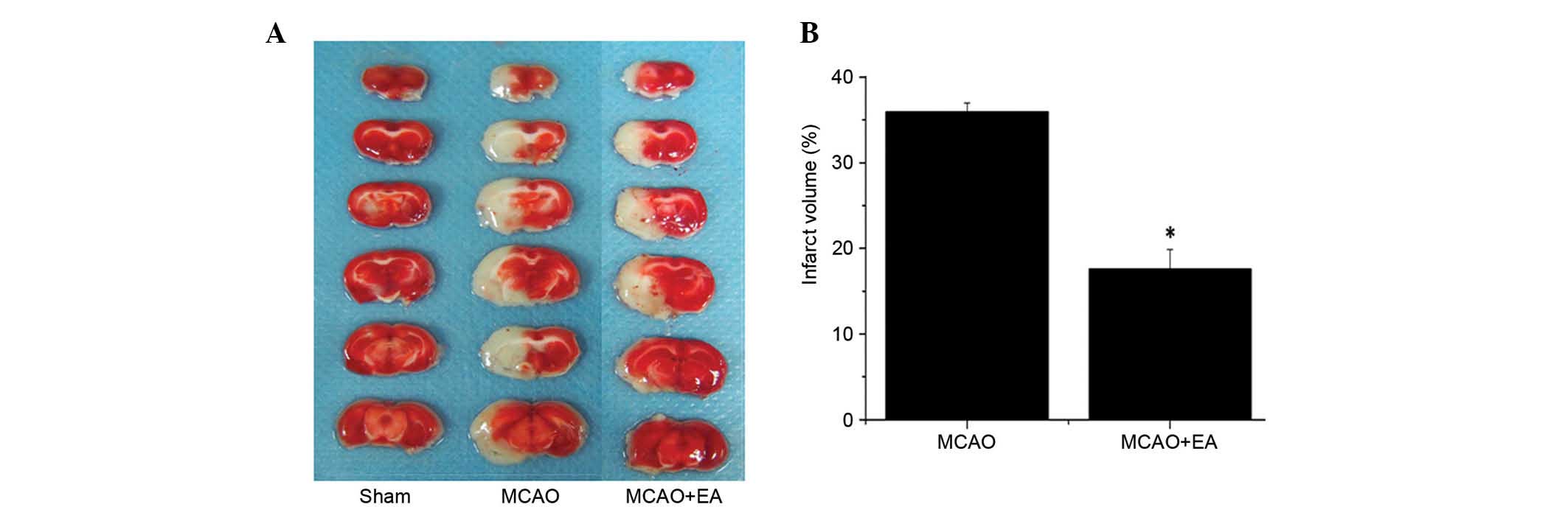
Effect of EA the Baihui (DU20) and
Shenting (DU24) acupoints on infarct volumes in cerebral I/R
injured rats
To evaluate the effect of EA on pathological damage,
the effect of EA on cerebral infarction was determined. Infarct
volume was measured using TTC staining. As demonstrated in Fig. 3, normal tissue stained deep red,
whereas the infarct area was stained pale white indicating a lack
of stain uptake. EA treatment significantly reduced cerebral
infarct volumes in cerebral I/R injured rats compared with the MCAO
group (P<0.01; Fig. 3). This
result indicates that EA at the Baihui (DU20) and Shenting (DU24)
acupoints may have therapeutic efficacy against cerebral I/R injury
by reducing secondary infarct expansion.
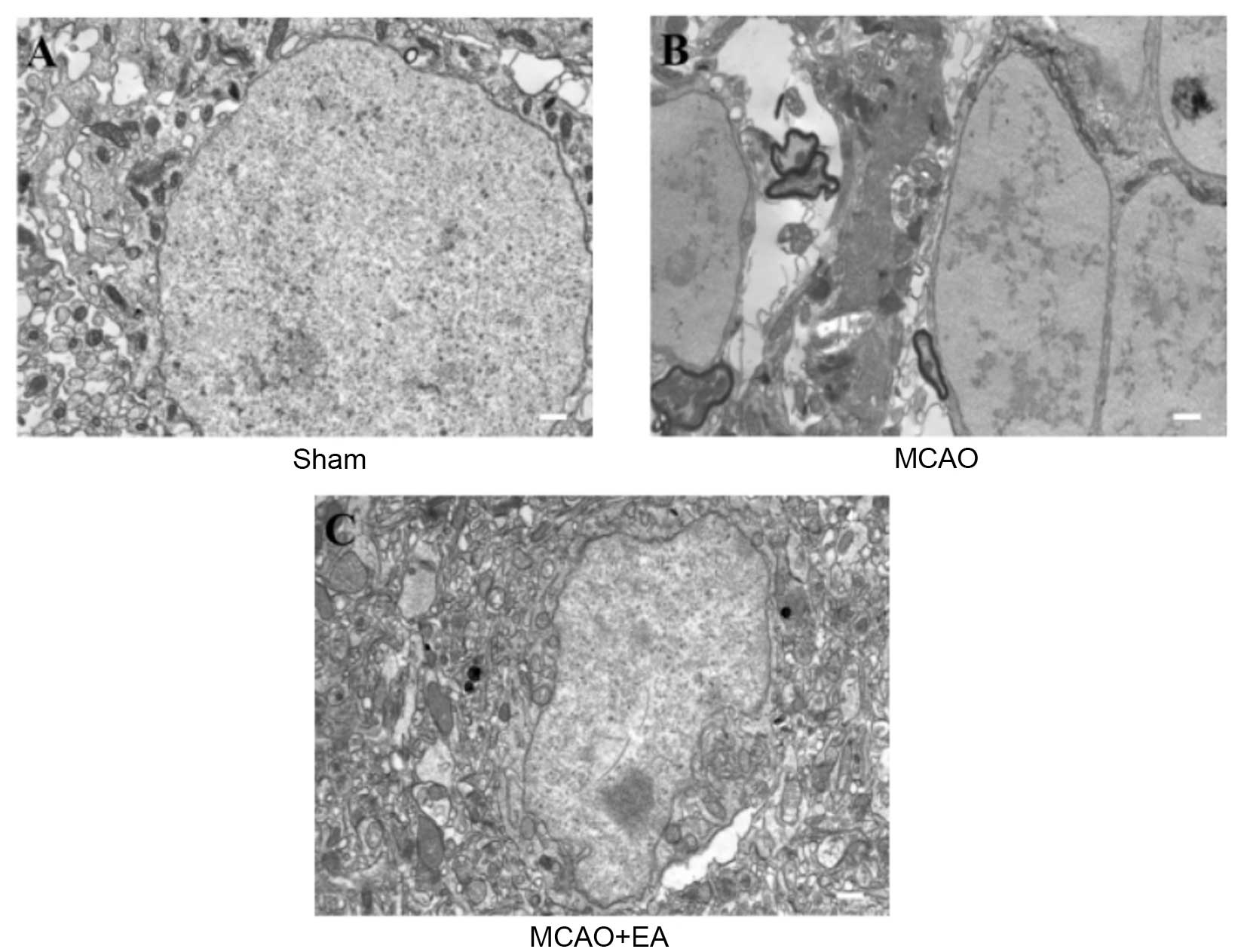
EA attenuates ultrastructural
characteristics of neuronal impairment in cerebral I/R injured
rats
To evaluate whether EA at the Baihui (DU20) and
Shenting (DU24) acupoints attenuates ultrastructural changes that
are characteristic of neuronal impairment, TEM was performed as
demonstrated in Fig. 4. In sham
rats, the neuronal cells were normal in appearance, with intact
cell membranes, normal nuclei, and uniform euchromatin
distribution, and nucleoli were observed in the nuclei. Abundant
mitochondria and rough endoplasmic reticulum were observed and
parts of the endoplasmic reticulum expanded the intracellular pool
shape (Fig. 4A). In the MCAO
group, neuronal vacuolar changes, rupture of cell membranes and
oncotic nuclei were observed. Extensive chromatin and organelle
loss, and mitochondrial swelling, mitochondrial cristae
disappearance and rough endoplasmic reticulum (Fig. 4B) were also present. In the EA
group, cell and nuclear membrane integrity was improved compared
with the MCAO group. The number of mitochondrial vacuoles was
markedly reduced and there was evidence of rough endoplasmic
reticulum expansion and degranulation (Fig. 4C). These results indicate that EA
at the Baihui (DU20) and Shenting (DU24) acupoints may attenuate
ultrastructural alterations that contribute to neuronal
impairment.
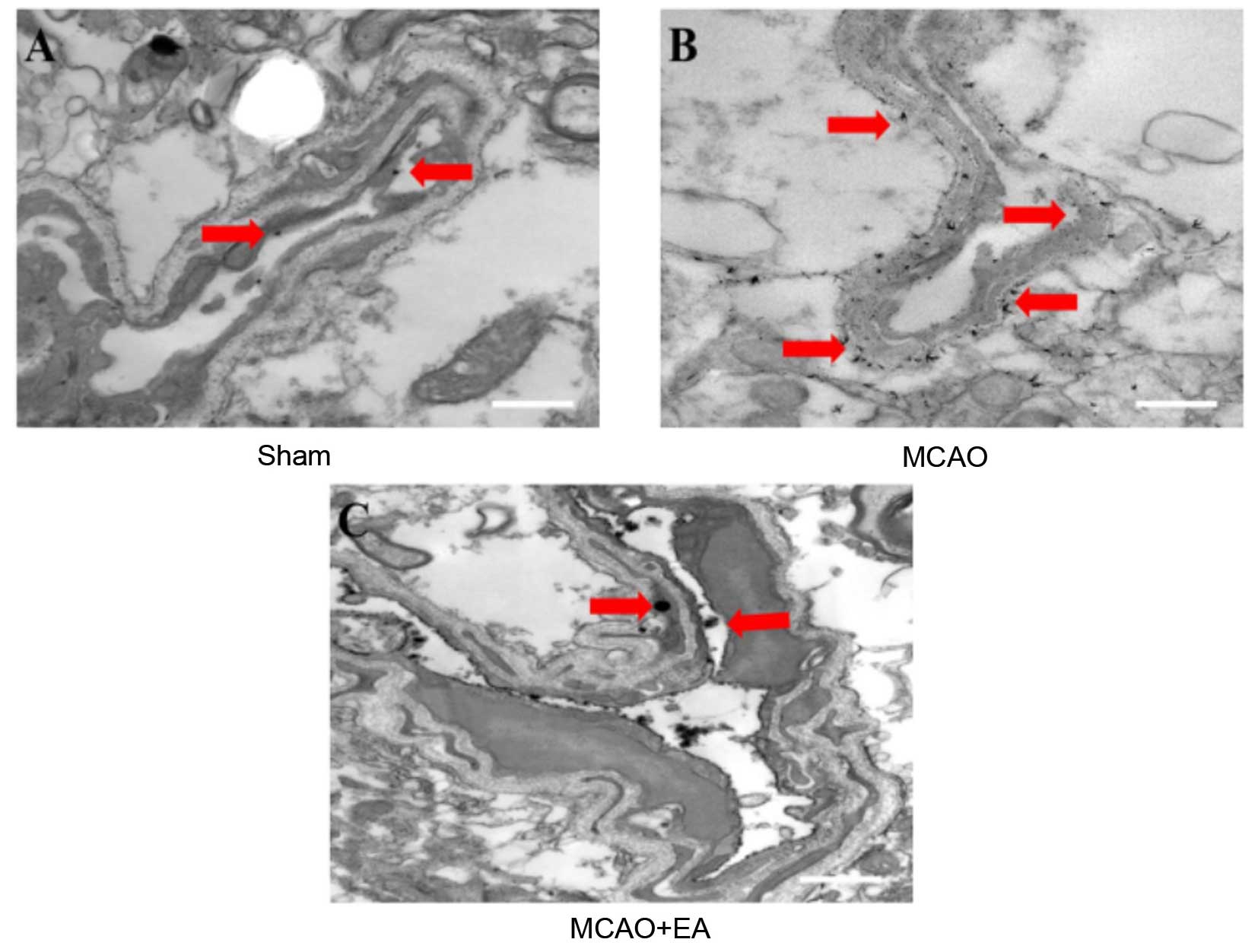
EA improves vascular ultrastructure in
cerebral I/R injured rats
LNT was utilized to evaluate whether EA at the
Baihui (DU20) and Shenting (DU24) acupoints improves vascular
ultrastructure in cerebral I/R injured rats, as demonstrated in
Fig. 5. Lanthanum particles were
observed within the lumen, in the sham-operated group (Fig. 5A). In the MCAO group, however,
lanthanum particles were dispersed inside and outside the
vasculature and clearly localized to the tight junctions (Fig. 5B). Compared with the MCAO group,
the number of lanthanum particles localized to the lumen was
reduced in the EA group (Fig. 5C).
This result suggests that EA at the Baihui (DU20) and Shenting
(DU24) acupoints improves vascular ultrastructure in the brain
tissues of cerebral I/R injured rats.
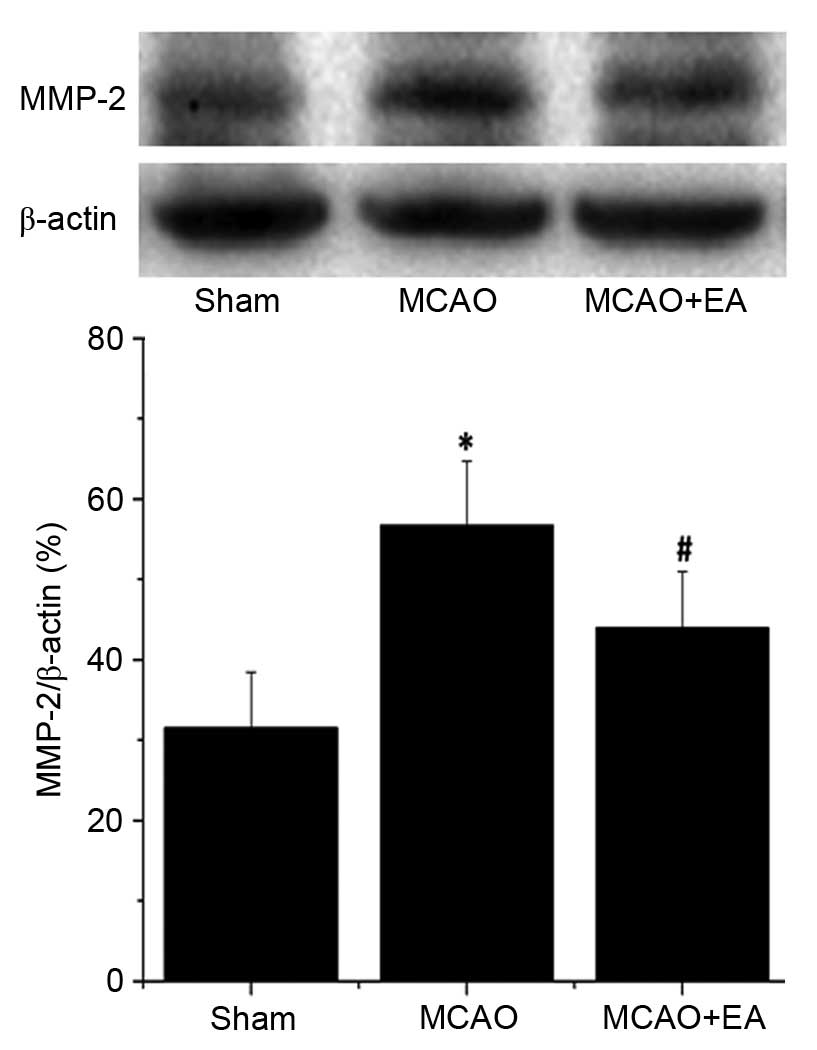
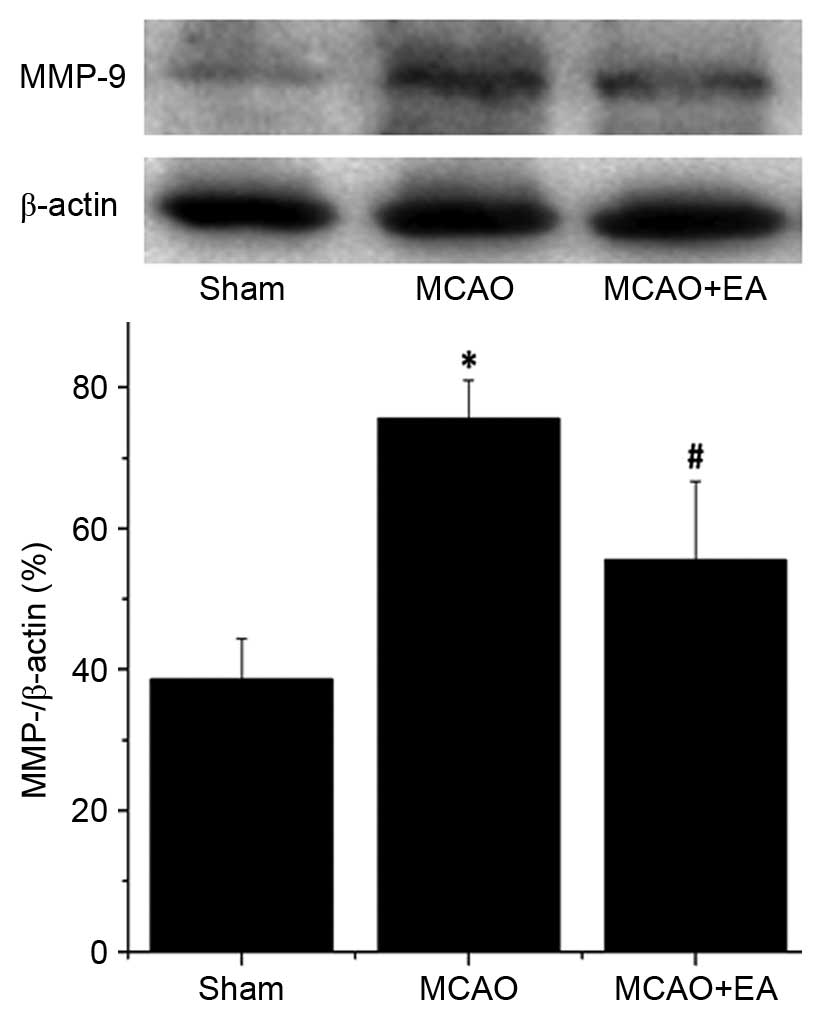
EA inhibits the expression of MMP-2 and
MMP-9 in the hippocampus following I/R injury
MMP-2 and MMP-9 are associated with cerebrovascular
disruption and neuronal damage, therefore, the effects of EA on the
protein expression levels of MMP-2 and MMP-9 in the hippocampus of
ischemic cerebral tissues were determined by western blotting. The
protein expression levels of MMP-2 (Fig. 6) and MMP-9 (Fig. 7) in the hippocampus were
significantly increased in the MCAO group compared with the sham
rats (P<0.01). Hippocampal expression levels of MMP-2 and MMP-9
were significantly decreased by EA treatment compared with the MCAO
group (P<0.01; Figs. 6 and
7), demonstrating that EA
significantly inhibited MMP-2 and MMP-9 expression following
cerebral I/R injury.
Discussion
Cognitive impairment following stroke is common and
negatively affects the life of patients (30). Acupuncture is one of most
frequently used treatment in TCM as it is simple and has limited
side effects, and has been used extensively in the treatment of
post-stroke onset of cognitive disorders (9–11).
Baihui (DU20) and Shenting (DU24) are important acupoints of the Du
meridian, which is often targeted to treat cognitive disorders in
China. Our previous research demonstrated that EA at the Baihui
(DU20) and Shenting (DU24) acupoints improves cognitive impairment
following ischemic stroke (15,16).
In the present study, a commonly used MCAO animal
model was used to accurately simulate pathological processes in
focal cerebral ischemia (31).
When performed successfully, development of cognitive dysfunction
is characteristic of this experimental model (32). After 7 days of EA at the Baihui
(DU20) and Shenting (DU24) acupoints, the neurological deficit
scores of the EA group were significantly decreased compared with
the MCAO model group, indicating that EA exerts a neuroprotective
effect.
To further investigate the neuroprotective effects
of EA, the Morris water maze test was used to determine learning
and memory abilities, and deficits in cerebral I/R injured rats.
The test is widely used in the study of animal cognition behavior,
and was designed to detect spatial learning and memory ability in
animal behavioral experiments (33–36).
The results of the directional navigation and spatial probe tests
demonstrated that rat behavior in the EA group was improved
compared with the MCAO group. Thus, this evidence indicated that EA
at the Baihui (DU20) and Shenting (DU24) acupoints improve the
learning and memory ability of MCAO rats, a result that was
consistent with previous research (15).
However, learning and memory disorders are complex.
Acupuncture has been previously demonstrated to improve learning
and memory dysfunction, and current research suggests that the
effect of acupuncture treatment following cerebral ischemia may be
mediated by promotion of cholinergic neural transmission,
facilitating dopaminergic synaptic transmission and enhancing
neurotrophin signaling (37,38).
However, the specific mechanisms remain to be elucidated. The
present research considers that behavioral change occurs as a
result of the pathological effects that occur following ischemic
insult, and used TTC staining to measure the cerebral infarction
volume of rats to measure this pathological effect. The findings of
the current study demonstrated that cerebral infarction volume was
decreased in the MCAO group, indicating that EA at the Baihui
(DU20) and Shenting (DU24) acupoints affects pathological
progression in brain tissue following cerebral ischemia. The
macrostructural changes result from smaller tissue alterations that
occur at the microstructural level. Based on results from LNT
experiments, increased lanthanum particles were visible inside and
outside of the blood vessels in MCAO rats, which illustrates that
vascular permeability has been modified and the BBB has been
compromised. However, cerebral ultrastructural morphology was
improved in animals treated with EA compared with MCAO group. These
results indicate that EA at the Baihui (DU20) and Shenting (DU24)
acupoints may improve the outcome of pathological progression
following cerebral ischemia.
The BBB provides a protective barrier between the
periphery and the central nervous system (CNS), and as such, is
important for the protection of CNS tissue (17). Following cerebral ischemia,
vascular permeability is altered and the BBB damaged, which results
in brain edema, inflammation and harmful pathological changes
within affected brain areas (39).
Extensive CNS tissue damage, neuronal necrosis and apoptosis
subsequently occur over time (22,40).
The brain is comprised of neurons and glial cells (41), and the structural and functional
integrity of neurons is considered the morphological basis of
learning and memory (24), thus,
neuronal damage leads to learning and memory dysfunction. The TEM
results of the current study demonstrate that the morphology and
extent of neuronal damage in the EA group was markedly improved
compared with the MCAO group. These findings indicate that EA at
the Baihui (DU20) and Shenting (DU24) acupoints may reduce the
effects on learning and memory following cerebral ischemia via
improving neuronal ultrastructure and morphology.
MMP-2 and MMP-9 are important collagenases in the
MMP family that degrade extracellular matrix, are involved in the
breakdown of the BBB, promote expansion of cerebral infarct volume
and other pathological processes that follow cerebral I/R injury
(42). In the present study, MMP-2
and MMP-9 expression levels were consistent with TTC and LNT
results, which is indicative of an association between the
integrity of the BBB and cerebral ischemia, and cerebral infarction
volume and MMP-2 and MMP-9 expression. In addition, evidence from
previous studies suggests that MMP-2 and MMP-9 also involved in
axon growth and regeneration, myelin formation during neurogenesis,
and neuronal apoptosis (43,44).
MMP-2 may participate in acute neuron injury and delayed repair
mechanisms, and MMP-9 is associated with increased lesion volume
expansion and a decline in neurological function (45). In the current study, the changes to
protein expression levels of MMP-2 and MMP-9 in the hippocampus
were consistent with reduced behavioral performance in rats,
indicating that EA at the Baihui (DU20) and Shenting (DU24)
acupoints may improve learning and memory function via inhibiting
protein expression of MMP-2 and MMP-9.
The present study selected only a single time point
to intervene, and only one test to assess learning and memory
function. Certain previous research suggest that the water maze is
not suitable for study of experimental stroke as the result of test
may be closely associated with the learning and memory function of
rats (46). TEM was performed to
detect breakdown of the BBB, however, the presence and structure of
tight junctions and other associated proteins was not assessed.
Therefore, the interpretation of the data is restricted by these
limitations in the study design. However, the overall pattern in
the data remains clear and limitations will be addressed and
improved in subsequent studies.
In conclusion, the results of the present study
indicate that EA at the Baihui (DU20) and Shenting (DU24) acupoints
may improve the learning and memory ability of rats following
cerebral ischemia. The mechanism of the therapeutic effects of EA
may be associated with improved brain ultrastructure and
morphological integrity, and reduced expression of MMP-2 and MMP-9
proteins in the hippocampus, thus reducing brain tissue damage and
improving function following cerebral I/R.
Acknowledgments
The present study was sponsored by the International
S&T Cooperation Program of China (grant no. 2011DFG33240) and
the Mechanism of Acupuncture to Improve Cognitive Function (grant
no. X2012004; collaborative). The authors would like to thank
Clarity Manuscript Consultants for their help in editing this
manuscript.
References
|
1
|
Donnan GA, Fisher M, Macleod M and Davis
SM: Stroke. Lancet. 371:1612–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miniño AM, Murphy SL, Xu J and Kochanek
KD: Deaths: Final data for 2008. Natl Vital Stat Rep. 59:1–126.
2011.
|
|
3
|
Hachinski V, Iadecola C, Petersen RC,
Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino
JG, Kalaria RN, et al: National institute of neurological disorders
and stroke-canadian stroke network vascular cognitive impairment
harmonization standards. Stroke. 37:2220–2241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JH, Kim BJ, Bae HJ, Lee J, Lee J, Han
MK, O KY, Park SH, Kang Y, Yu KH and Lee BC: Impact of post-stroke
cognitive impairment with no dementia on health-related quality of
life. J Stroke. 15:49–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ovbiagele B, Goldstein LB, Higashida RT,
Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl
S, Sacco RL, et al: Forecasting the future of stroke in the United
States: A policy statement from the American heart association and
American stroke association. Stroke. 44:2361–2375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pendlebury ST and Rothwell PM: Prevalence,
incidence and factors associated with pre-stroke and post-stroke
dementia: A systematic review and meta-analysis. Lancet Neurol.
8:1006–1018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SK and Bae H: Acupuncture and immune
modulation. Auton Neurosci. 157:38–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jittiwat J and Wattanathorn J: Ginger
pharmacopuncture improves cognitive impairment and oxidative stress
following cerebral ischemia. J Acupunct Meridian Stud. 5:295–300.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang GC, Fu WB, Xu NG, Liu JH, Zhu XP,
Liang ZH, Huang YF and Chen YF: Meta analysis of the curative
effect of acupuncture on post-stroke depression. J Tradit Chin Med.
32:6–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao H, Wang Y, Chang D, Zhou L and Liu J:
Acupuncture for vascular mild cognitive impairment: A systematic
review of randomised controlled trials. Acupunct Med. 31:368–374.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng Y, Bai L, Ren Y, Chen S, Wang H,
Zhang W and Tian J: FMRI connectivity analysis of acupuncture
effects on the whole brain network in mild cognitive impairment
patients. Magn Reson Imaging. 30:672–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Zhao L, Yang S, Chen Z, Li Y,
Peng X, Yang Y and Zhu M: Clinical observation on effect of scalp
electroacupuncture for mild cognitive impairment. J Tradit Chin
Med. 33:46–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin YW and Hsieh CL: Electroacupuncture at
baihui acupoint (GV20) reverses behavior deficit and long-term
potentiation through N-methyl-d-aspartate and transient receptor
potential vanilloid subtype 1 receptors in middle cerebral artery
occlusion rats. J Integr Neurosci. 9:269–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang WW, Xie CL, Lu L and Zheng GQ: A
systematic review and meta-analysis of baihui (GV20)-based scalp
acupuncture in experimental ischemic stroke. Sci Rep.
4:39812014.PubMed/NCBI
|
|
15
|
Feng X, Yang S, Liu J, Huang J, Peng J,
Lin J, Tao J and Chen L: Electroacupuncture ameliorates cognitive
impairment through inhibition of NF-κB-mediated neuronal cell
apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med
Rep. 7:1516–1522. 2013.PubMed/NCBI
|
|
16
|
Yang S, Ye H, Huang J, Tao J, Jiang C, Lin
Z, Zheng G and Chen L: The synergistic effect of acupuncture and
computer-based cognitive training on post-stroke cognitive
dysfunction: A study protocol for a randomized controlled trial of
2×2 factorial design. BMC Complement Altern Med. 14:2902014.
View Article : Google Scholar
|
|
17
|
Jin R, Song Z, Yu S, Piazza A, Nanda A,
Penninger JM, Granger DN and Li G: Phosphatidylinositol-3-kinase
gamma plays a central role in blood-brain barrier dysfunction in
acute experimental stroke. Stroke. 42:2033–2044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balami JS, Chen RL, Grunwald IQ and Buchan
AM: Neurological complications of acute ischaemic stroke. Lancet
Neurol. 10:357–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abbott NJ, Patabendige AA, Dolman DE,
Yusof SR and Begley DJ: Structure and function of the blood-brain
barrier. Neurobiol Dis. 37:13–25. 2010. View Article : Google Scholar
|
|
20
|
Krueger M, Härtig W, Reichenbach A,
Bechmann I and Michalski D: Blood-brain barrier breakdown after
embolic stroke in rats occurs without ultrastructural evidence for
disrupting tight junctions. PLoS One. 8:e564192013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bao Dang Q, Lapergue B, Tran-Dinh A,
Diallo D, Moreno JA, Mazighi M, Romero IA, Weksler B, Michel JB,
Amarenco P and Meilhac O: High-density lipoproteins limit
neutrophil-induced damage to the blood-brain barrier in vitro. J
Cereb Blood Flow Metab. 33:575–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spatz M: Past and recent BBB studies with
particular emphasis on changes in ischemic brain edema: Dedicated
to the memory of Dr. Igor Klatzo. Acta Neurochir Suppl. 106:21–27.
2010. View Article : Google Scholar
|
|
23
|
Yang D, Li SY, Yeung CM, Chang RC, So KF,
Wong D and Lo AC: Lycium barbarum extracts protect the brain from
blood-brain barrier disruption and cerebral edema in experimental
stroke. PLoS One. 7:e335962012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kasai H, Fukuda M, Watanabe S,
Hayashi-Takagi A and Noguchi J: Structural dynamics of dendritic
spines in memory and cognition. Trends Neurosci. 33:121–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barr TL, Latour LL, Lee KY, Schaewe TJ,
Luby M, Chang GS, El-Zammar Z, Alam S, Hallenbeck JM, Kidwell CS
and Warach S: Blood-brain barrier disruption in humans is
independently associated with increased matrix metalloproteinase-9.
Stroke. 41:e123–e128. 2010. View Article : Google Scholar
|
|
26
|
Jin R, Yang G and Li G: Molecular insights
and therapeutic targets for blood-brain barrier disruption in
ischemic stroke: Critical role of matrix metalloproteinases and
tissue-type plasminogen activator. Neurobiol Dis. 38:376–385. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen W, Hartman R, Ayer R, Marcantonio S,
Kamper J, Tang J and Zhang JH: Matrix metalloproteinases inhibition
provides neuroprotection against hypoxia-ischemia in the developing
brain. J Neurochem. 111:726–736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tao J, Chen B, Gao Y, Yang S, Huang J,
Jiang X, Wu Y, Peng J, Hong Z and Chen L: Electroacupuncture
enhances hippocampal NSCs proliferation in cerebral
ischemia-reperfusion injured rats via activation of notch signaling
pathway. Int J Neurosci. 124:204–212. 2014. View Article : Google Scholar
|
|
30
|
Shim H: Vascular cognitive impairment and
post-stroke cognitive deficits. Curr Neurol Neurosci Rep.
14:4182014. View Article : Google Scholar
|
|
31
|
Fu YK, Chang CJ, Chen KY, Hwang LC, Wu KH,
Chang KW, Jan ML, Chen CC and Chang CH: Imaging of regional
metabolic activity by (18) F-FDG/PET in rats with transient
cerebral ischemia. Appl Radiat Isot. 67:1743–1747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boyko M, Kutz R, Gruenbaum BF, Cohen H,
Kozlovsky N, Gruenbaum SE, Shapira Y and Zlotnik A: The influence
of aging on poststroke depression using a rat model via middle
cerebral artery occlusion. Cogn Affect Behav Neurosci. 13:847–859.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang ZZ, Zhang Y, Liu YQ, Zhao N, Zhang
YZ, Yuan L, An L, Li J, Wang XY, Qin JJ, et al: RNA
interference-mediated phosphodiesterase 4D splice variants
knock-down in the prefrontal cortex produces antidepressant-like
and cognition-enhancing effects. Br J Pharmacol. 168:1001–1014.
2013. View Article : Google Scholar :
|
|
34
|
de Souza Silva MA, Lenz B, Rotter A,
Biermann T, Peters O, Ramirez A, Jessen F, Maier W, Hüll M,
Schröder J, et al: Neurokinin3 receptor as a target to predict and
improve learning and memory in the aged organism. Proc Natl Acad
Sci USA. 110:15097–15102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Langdon KD, Granter-Button S, Harley CW,
Moody-Corbett F, Peeling J and Corbett D: Cognitive rehabilitation
reduces cognitive impairment and normalizes hippocampal CA1
architecture in a rat model of vascular dementia. J Cereb Blood
Flow Metab. 33:872–879. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
D'Intino G, Paradisi M, Fernandez M,
Giuliani A, Aloe L, Giardino L and Calzà L: Cognitive deficit
associated with cholinergic and nerve growth factor down-regulation
in experimental allergic encephalomyelitis in rats. Proc Natl Acad
Sci USA. 102:3070–3075. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leung MC, Yip KK, Ho YS, Siu FK, Li WC and
Garner B: Mechanisms underlying the effect of acupuncture on
cognitive improvement: A systematic review of animal studies. J
Neuroimmune Pharmacol. 9:492–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Greggio S, de Paula S, de Oliveira IM,
Trindade C, Rosa RM, Henriques JA and DaCosta JC: NAP prevents
acute cerebral oxidative stress and protects against long-term
brain injury and cognitive impairment in a model of neonatal
hypoxia-ischemia. Neurobiol Dis. 44:152–159. 2011.PubMed/NCBI
|
|
39
|
Pop V, Sorensen DW, Kamper JE, Ajao DO,
Murphy MP, Head E, Hartman RE and Badaut J: Early brain injury
alters the blood-brain barrier phenotype in parallel with β-amyloid
and cognitive changes in adulthood. J Cereb Blood Flow Metab.
33:205–214. 2013. View Article : Google Scholar
|
|
40
|
Pillai DR, Shanbhag NC, Dittmar MS,
Bogdahn U and Schlachetzki F: Neurovascular protection by targeting
early blood-brain barrier disruption with neurotrophic factors
after ischemia-reperfusion in rats. J Cereb Blood Flow Metab.
33:557–566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: Uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar
|
|
42
|
Leonardo CC, Eakin AK, Ajmo JM, Collier
LA, Pennypacker KR, Strongin AY and Gottschall PE: Delayed
administration of a matrix metalloproteinase inhibitor limits
progressive brain injury after hypoxia-ischemia in the neonatal
rat. J Neuroinflammation. 5:342008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Manso H, Krug T, Sobral J, Albergaria I,
Gaspar G, Ferro JM, Oliveira SA and Vicente AM: Variants of the
matrix metallopro-teinase-2 but not the matrix metalloproteinase-9
genes significantly influence functional outcome after stroke. BMC
Med Genet. 11:402010. View Article : Google Scholar
|
|
44
|
Wei W, Zhang W, Huang Y, Li Y, Zhu G, Chen
F and Li J: The therapeutic effect of (DL)-3-n-butylphthalide in
rats with chronic cerebral hypoperfusion through downregulation of
amyloid precursor protein and matrix metalloproteinase-2. J Int Med
Res. 40:967–975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Batra A, Latour LL, Ruetzler CA,
Hallenbeck JM, Spatz M, Warach S and Henning EC: Increased plasma
and tissue MMP levels are associated with BCSFB and BBB disruption
evident on post-contrast flair after experimental stroke. J Cereb
Blood Flow Metab. 30:1188–1199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu HS, Shen H, Harvey BK, Castillo P, Lu
H, Yang Y and Wang Y: Post-treatment with amphetamine enhances
reinnervation of the ipsilateral side cortex in stroke rats.
Neuroimage. 56:280–289. 2011. View Article : Google Scholar : PubMed/NCBI
|