Introduction
Oxidative stress is important in various disease
processes, including in cancer, inflammation, cardiovascular
diseases, atherosclerosis, central nervous system disorders,
neurode-generative diseases, diabetes and respiratory diseases.
Almost all human organs can be damaged by oxidative stress
(1–5). In the cardiovascular system, reactive
oxygen species (ROS) induce the oxidation of low density
lipoprotein, cholesterol, cholesterol-derived species and protein
modifications, which can lead to foam cell formation,
atherosclerotic plaques and vascular thrombosis (6). Various studies have previously
demonstrated that cardiomyocyte damage induced by heart
ischemia/reperfusion is predominantly due to the generation of ROS
(7–9). Other studies also indicated that ROS
can damage the sarcoplasmic reticulum of cardiac cells, inducing
contractile dysfunction and Ca2+ release by modifying
the structure and function of cardiac proteins, which may be
important in the formation of myocardial ischemia/reperfusion
injury (9,10). Several investigations have
demonstrated that ROS induce cardiomyocyte apoptosis by activating
various signaling pathways, including mitogen-activated protein
kinase 14 (p38MAPK), MAPK 1 (also known as ERK1/2), MAPK 8 (also
known as JNK) and v-akt murine thymoma viral oncogene
homolog 1 (Akt1) signaling, which may contribute to the development
and progression of cardiac dysfunction and heart failure (11–13).
Additionally, angiotensin II stimulates ROS-mediated activation of
the transcription factor nuclear factor-κB, which is understood to
be involved in the induction of cardiac hypertrophy. ROS also
regulates the transcription of jun proto-oncogene, which influences
the expression of other genes in cardiac hypertrophy (14). In summary, oxidative stress
participates in a variety of pathological mechanisms associated
with cardiomyocyte diseases.
Numerous in vitro studies of oxidative stress
in cardiomyocytes have been performed using H9c2 cardiomyocytes.
H9c2 cells are a clonal cardiomyocyte cell line derived from
embryonic rat ventricles (15),
with a similar profile of signaling mechanisms to adult
cardiomyocytes. Under oxidative stress, H9c2 cardiomyocytes respond
in a similar manner to myocytes in primary cultures or isolated
heart experiments (16). H9c2
cells have been demonstrated to be a useful tool for the study of
the cellular mechanisms and signal transduction pathways of
cardiomyocytes (17–20).
H2O2-treated H9c2 cells have
been commonly used as an in vitro model for studying
oxidative stress in cardio-myocytes, and to evaluate the
cardioprotective effects of drugs against oxidative damage
(21–24). However, to the best of our
knowledge, H9c2 cells have not been previously used for
high-throughput drug screening. The current study used this model
to establish a cell-based screening assay in a high throughput
format. From a library of traditional Chinese medicine (TCM)
extracts, 17 primary hits were identified, 2 of which were further
validated as cardiopro-tective agents against oxidative damage. The
present study demonstrated the used of the
H2O2-induced cell damage model in a
high-throughput screening (HTS) assay, which may be established as
an efficient and low-cost HTS assay for the identification of
candidate drugs that reduce oxidative damage from large TCM
extract/chemical libraries.
Materials and methods
Cell culture
H9c2 cells (Cell Resource Centre of the Shanghai
Institutes for Biological Sciences, Chinese Academy of Science,
Shanghai, China) were maintained in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and
incubated at 37°C in a humid atmosphere of 5% CO2.
Following expansion, cells at passage 3 were used for all
experiments.
Cell counting kit (CCK)-8 assays
H9c2 cells were used to establish the cell model of
oxidative damage. H9c2 cells (100 µl/well) were seeded into
96-well plate at a density of 3.0×104 cells/ml and
incubated at 37°C, 5% CO2 overnight. The cells were then
treated with 100 µl 50 µmol/l
H2O2 (Shandong Siqiang Chemical Group Co.,
Ltd., Liaocheng, China) for 3 h. Following
H2O2 treatment, a CCK-8 assay kit (BestBio,
Shanghai, China) was used to detect cell viabilities according to
the manufacturer's instructions. In brief, following treatment with
H2O2, 10 µl CCK-8 solution was added
to each well. After 1–4 h incubation, cell viability was determined
by measuring the absorbance at 450 nm using a Flex Station 3
microplate spectrophotometer (Molecular Devices, LLC, Sunnyvale,
CA, USA).
For drug activity assays, 100 µl H9c2
cells/well were seeded into 96-well plates at a density of
3.0×104 cells/ml, and incubated overnight. Each plate
contained 8 negative and 8 positive control wells, and all cells,
excluding the positive controls, were treated with 100
µl/well H2O2 (50 µmol/l) for 3
h. Following H2O2 treatment, 0.1
µl/well dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis,
MO, USA) was added to the positive control wells, and 0.1
µl/well TCM extract samples were added to the all other
wells, excluding the negative controls. Cells were then incubated
for an additional 3 h. Cell viabilities were tested using the CCK-8
assay kit to assess drug activities.
Lactate dehydrogenase (LDH) activity,
malondialdehyde (MDA) content and superoxide dismutase (SOD)
activity assays
LDH, MDA and SOD were measured using the respective
assay kits (Beyotime Institute of Biotechnology, Haimen, China)
according to the manufacturer's instructions. Briefly, 1,000
µl/well H9c2 cells were seeded into 24-well plates at a
density of 3.0×104 cells/ml. Following a 3-h treatment
with 50 µmol/l H2O2, and a 3-h
incubation with 50 µmol/l quercetin and 25 µg/ml TCM
extracts, the cells were centrifuged at 400 × g for 5 min, and 120
µl of the supernatant was then transferred to a 96-well
plate for LDH activity determination. Subsequently, all cells were
lysed and centrifuged at 1,600 × g for 10 min, the supernatants
were then collected and stored at -80°C prior to MDA and SOD
detection.
For LDH assays, 60 µl work ing solution,
containing 20 µl lactic acid solution, 20 µl 1X INT
solution and 20 µl enzyme solution, was added to each sample
(total, 180 µl) for an additional 30 min incubation by
gently agitating at room temperature. The maximum LDH release of
target cells was determined by lysing target cells for 45 min and
subsequently measuring the LDH from the culture medium. Absorbance
values after the colorimetric reaction were measured at 490 nm with
a reference wavelength of 655 nm, using a Flex Station 3 microplate
spectrophotometer (Molecular Devices, LLC, Sunnyvale, CA, USA).
For MDA assays, 200 µl working solution was
added to 100 µl samples for an additional 15 min incubation
at 100°C, and were subsequently centrifuged at 1,000 × g for 10 min
after the samples cooled to room temperature. Subsequently, 200
µl supernatant was transferred to a 96-well plate for MDA
detection by Flex Station 3 microplate spectrophotometer at 532 nm
absorbance with a reference wavelength of 450 nm.
For SOD assays, 180 µl working solution was
added to 20 µl sample for an additional 30 min incubation at
37 °C. SOD activities were detected at 490 nm with a reference
wavelength of 600 nm, using the same microplate reader as
before.
Western blotting
The protein expression levels of the apop-totic
proteins, caspase-3, B-cell CLL/lymphoma 2 (Bcl-2),
Bcl-2-associated X protein (Bax), and the MAPK subfamily proteins,
p38, JNK and ERK1/2, were detected by western blotting. A total of
1.5×106 cells/ml/well were seeded into 6-well plates.
Following 3 h treatment with 12.5 µmol/l (for MAPK proteins)
or 50 µmol/l (for apoptotic proteins)
H2O2, cells were incubated with 25
µg/ml active extracts for 3 h. The cells were then rinsed
twice with ice-cold 1X phosphate-buffered saline (PBS) and
harvested under non-denaturing conditions by incubation at 4°C with
lysis buffer (Qiagen, Hilden, Germany) containing protease and
phosphorylase inhibitors for 10 min. The cell lysates were
centrifuged at 14,000 × g for 10 min at 4°C to remove insoluble
precipitates. The protein content in each sample was determined by
Bradford assay (Applygen Technologies, Inc., Beijing, China). Total
protein (50 µg) from cell culture samples was denatured and
separated by SDS-PAGE on 12% acrylamide gels, and subsequently
electrophoretically transferred to polyvinylidene fluoride
membranes. Nonspecific binding sites were blocked by incubation of
membranes in Tris-buffered saline with Tween (TBS-T) containing 5%
bovine serum albumin (BSA; Amresco, LLC, Solon, OH, USA) for 2 h.
The membranes were then incubated with primary antibodies against
rabbit anti-caspase-3 (cat. no. sc-7148; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), rabbit anti-Bcl-2 (cat. no. 2870S Cell
Signaling Technology, Inc., Danvers, MA, USA), rabbit anti-Bax
(cat. no. sc-526; Santa Cruz Biotechnology, Inc.), rabbit anti-p38
(cat. no. 9212; Cell Signaling Technology, Inc.), rabbit
anti-phospho (p)-p38 (cat. no. 4631; Cell Signaling Technology,
Inc.), rabbit-anti-JNK (cat. no. 9258; Cell Signaling Technology,
Inc.), rabbit anti-p-JNK (cat. no. 4671; Cell Signaling Technology,
Inc.), rabbit anti-ERK1/2 (cat. no. 9102S; Cell Signaling
Technology, Inc.), rabbit anti-p-ERK1/2 (cat. no. 9101S; Cell
Signaling Technology, Inc.) all at 1:1,000 dilution, overnight at
4°C. The membranes were washed with TBS-T and were subsequently
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (cat. no. 7074; 1:2,000; Cell Signaling
Technology, Inc.) for 2 h and visualized using a Chemi Doc XRS+
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
β-tubulin was used as a loading control.
Immunofluorescence assays for early
growth response-1 (Egr-1)
H9c2 cells (100 µl/well) at a density of
5.0×104 cells/ml were seeded into 96-well plates. The
cells were untreated (control) or incubated with 12.5 or 200
µmol/l H2O2 alone, or with 12.5
µmol/l H2O2 and 25 µg/ml active
extracts for 2 h. Subsequently, the cells were fixed with 4% (v/v)
formaldehyde (Amresco, LLC) in 1X PBS at room temperature for 15
min, then washed with 1X PBS 3 times, and blocked with 1% (w/v) BSA
(Amresco, LLC) in 1X PBS containing 0.3% (v/v) Triton X-100
(Amresco, LLC) at room temperature for 30 min. The primary antibody
against Egr-1 (cat. no. sc-110; 1:100; Santa Cruz Biotechnology,
Inc.) was incubated with the cells at 37°C for 2 h. Subsequently,
cells were washed with PBS and incubated with goat anti-rabbit
IgG-CruzFluor 488 (cat. no. sc-362262; 1:250; Santa Cruz
Biotechnology, Inc.) at 37°C for 1 h. Following 3 washes with 1X
PBS, cell nuclei were stained using Hoechst 33258 (Sigma-Aldrich)
at a final concentration of 2 µg/ml for 15 min. Fluorescent
images were captured using a DMI 4000B fluorescence microscope
(Leica Microsystems GmbH, Wetzlar, Germany).
The library of TCM extracts
Each TCM herb (500 g) was soaked in water for 1 h
prior to extraction, and extraction was performed twice by boiling
in 10- and 8-fold volumes of water (v/v) for 2 h. Extracts were
filtered through gauze, and all crude extractions were combined and
concentrated to 500 ml. The concentrates were then separated on
macroporous resins (specification, Φ 5×60 cm; 1:1 weight ratio of
the concentrates; HaiGuang Chemical Co., Ltd., China) by successive
elution with water and different concentration gradients of ethanol
(20–95%), with 3 bed volumes (BV) of eluent volume at a flow rate
of 1 BV/h. Eight samples from each TCM herb were collected, and
concentrated at 70°C. Following freeze drying, 5 mg of each sample
was dissolved into 200 µl DMSO, then dispensed into 96-well
plates.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). Statistical analysis was performed using one-way
analysis of variance and Tukey's post-hoc tests using SPSS software
version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Creation and optimization of oxidative
damage cell model for HTS
To establish a stable HTS assay that generates
reliable outcomes, the present study optimized several factors that
may affect the assay results.
Sodium pyruvate-supplemented medium vs.
sodium pyruvate-free medium
Sodium pyruvate, a supplement in cell culture
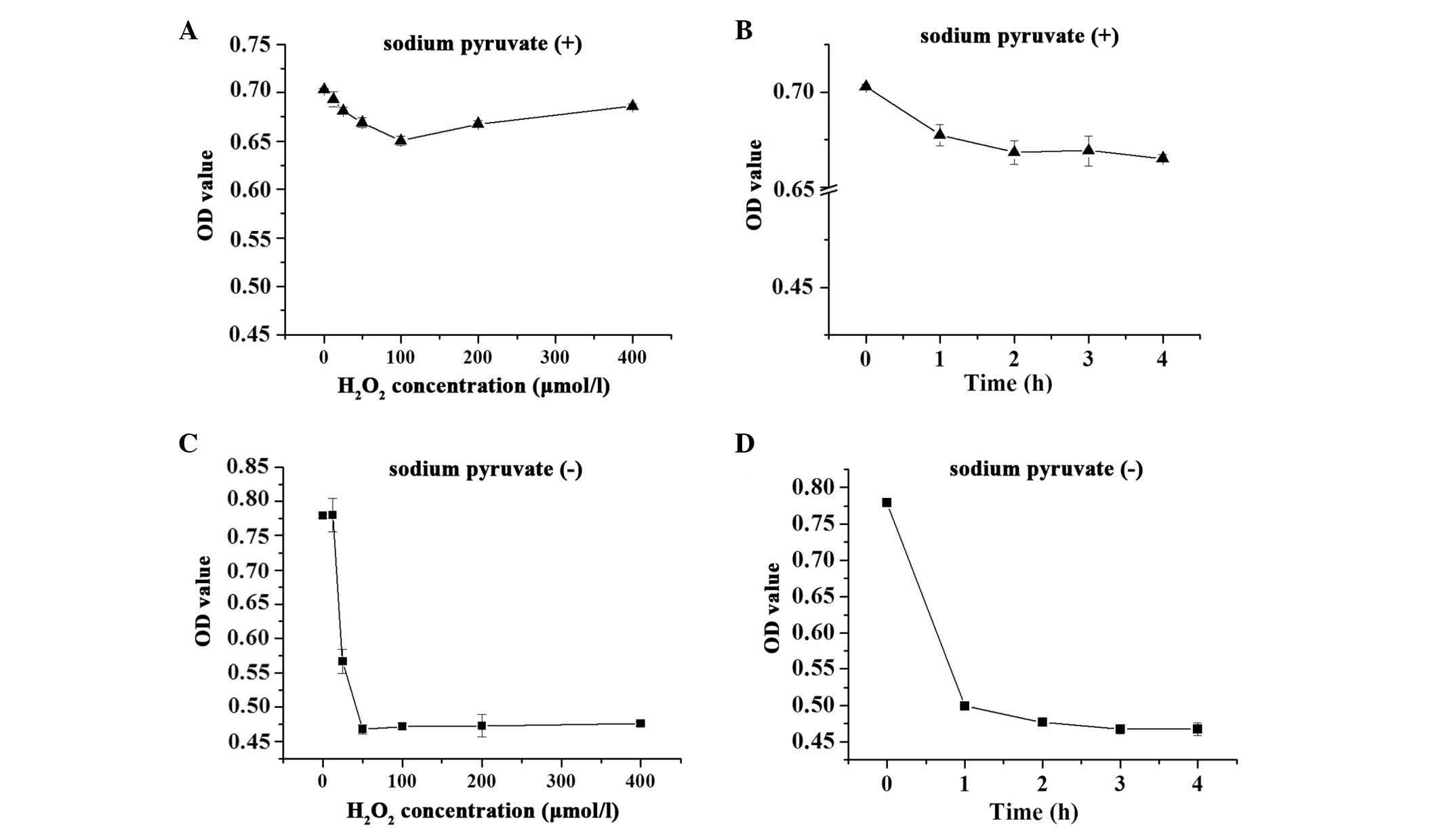
medium, may affect the screening assays. As demonstrated in
Fig. 1, when the H9c2 cells were
maintained in DMEM containing 110 mg/l sodium pyruvate, 12.5–200
µmol/l H2O2 induced a decrease in cell
viabilities (<7%; Fig. 1A and
B). However, H2O2 treatment of the cells
in sodium pyruvate-free DMEM resulted in a more marked decrease
(~40%) in cell viability (Fig. 1C and
D). Therefore, sodium pyruvate-free DMEM was used during the
oxidative damage model.
Concentration and incubation time
Optimization experiments were also performed to
determine the optimal working concentration and incubation time of
H2O2. H9c2 cells were exposed to varying
degrees of oxidative stress by treatment with
H2O2 for 0–4 h. The results demonstrated that
H2O2 reduced cell viability in a dose- and
time-dependent manner in the pyruvate-free groups, and exhibited an
almost 40% injury at 50 µmol/l for 3 h. Higher
concentrations or longer incubation time did not result in a more
significant change to the OD450 values (Fig. 1C and D).
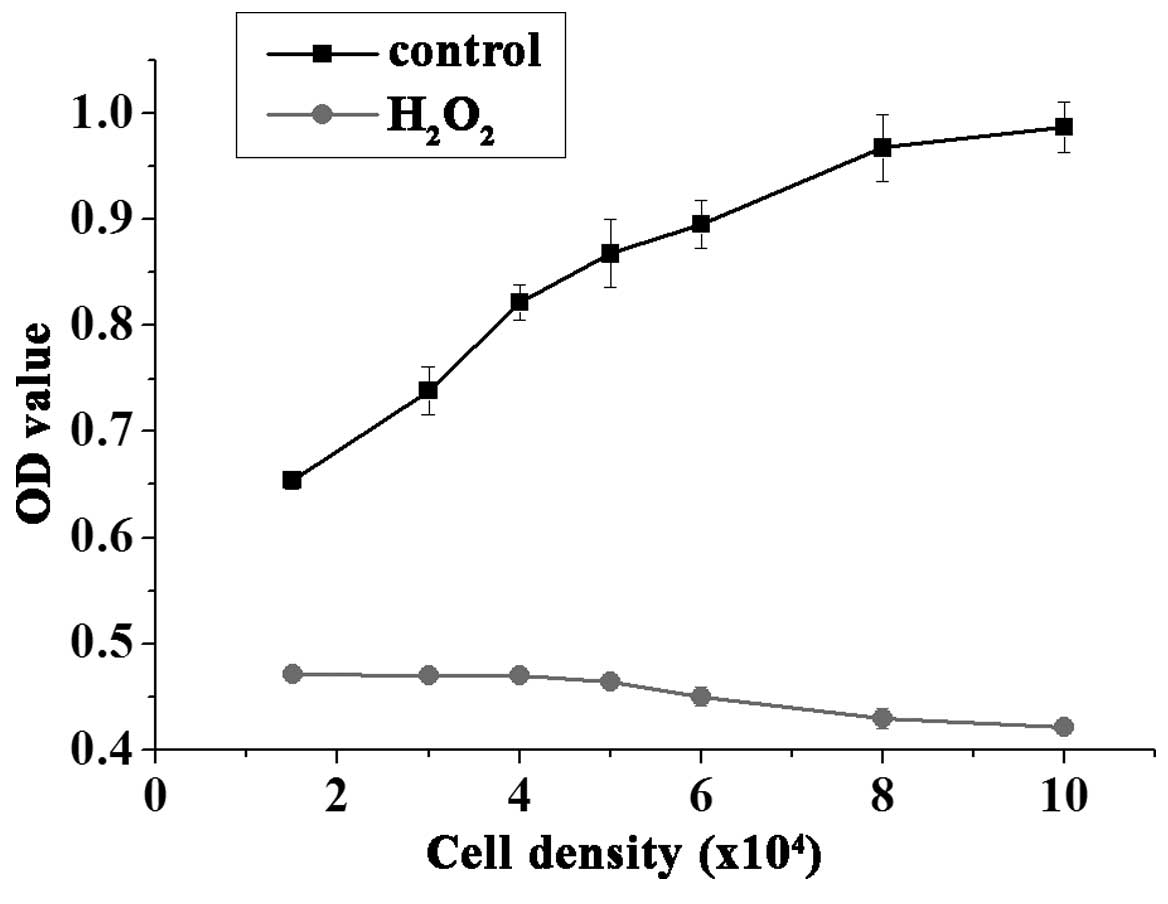
Cell density
A low number of cells per well may cause low
response values, however, a large cell number is not conducive to
cell growth, due to the contact inhibition. Thus, determining the
appropriate number of cells is essential for drug screening. As
demonstrated in Fig. 2,
1.5–4.0×104 cells/well treated with 50 µmol/l
H2O2 for 3 h exhibited consistent results,
whereas higher seeding densities exhibited reduced cell viability.
Thus, the current study used the cell density of 3.0×104
cells/well for the HTS assays.
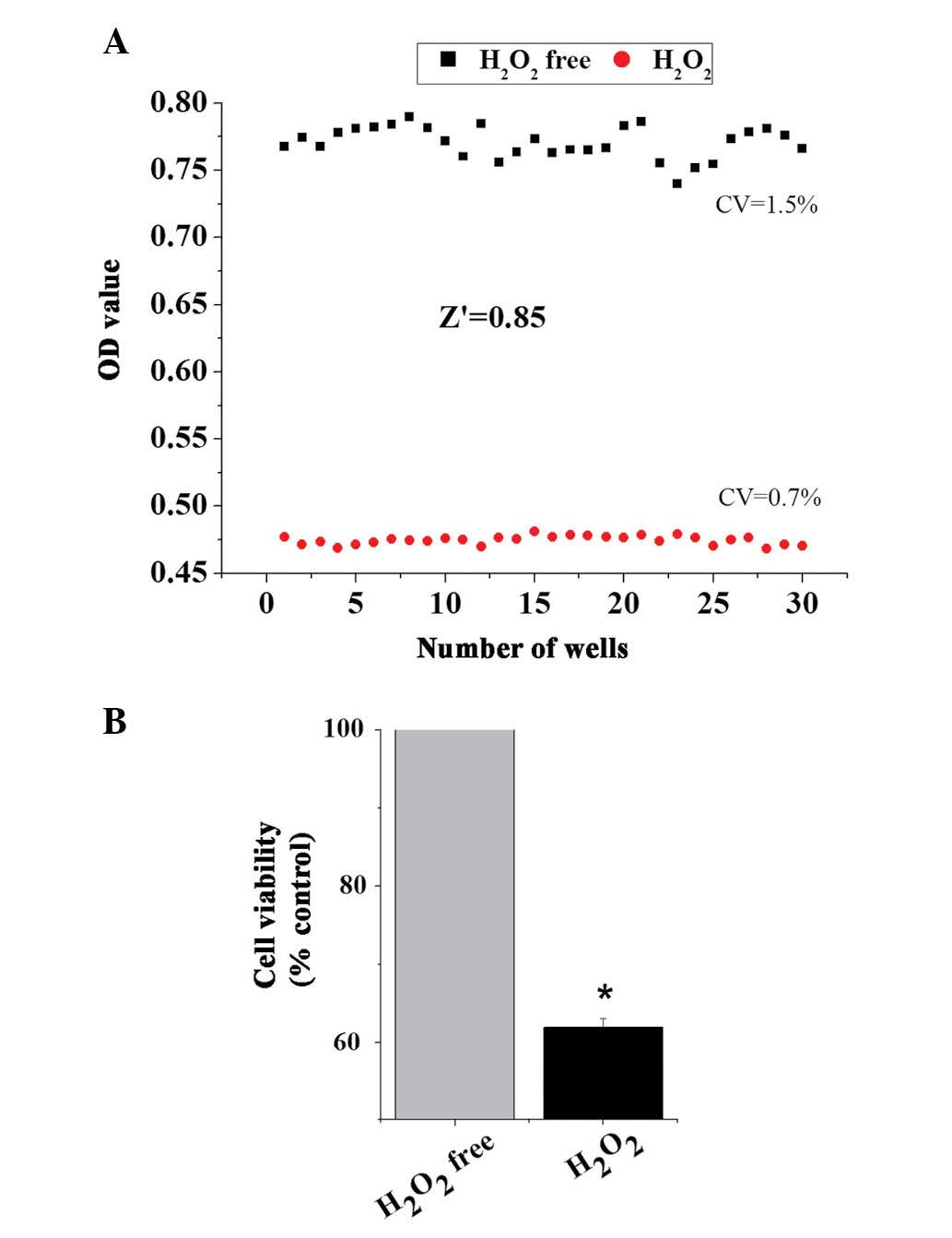
Variability and robustness of model
To assess whether the model of oxidative damage can
be applied to an HTS format, the present study applied the
optimized conditions to establish the
H2O2-induced cell damage model. The data of
the cell viabili-ties from 30 wells of positive control
(H2O2-free) and 30 wells of negative control
(H2O2-treated) were obtained to analyze
variability between wells and the robustness of the cell model of
oxidative stress using the Z′ factor, which is calculated from the
following formula: Z′=1-[3×(δc+
-δc−)⁄(µc+ -
µc−)]; δc+ =SD
of positive control; δc− =SD of negative
control; µc+= mean of positive
control; and µc− =mean of negative
control. Z′≥0.5 indicates the assay method can be performed
effectively in HTS (25). As
presented in Fig. 3, treatment of
H9c2 cells with 50 µmol/l H2O2 for 3 h
produced a Z′ value of ~0.85 and small critical values (CVs) (0.7%
for positive control and 1.5% for negative control). These results
demonstrated that there was an appropriate separation between the
SDs of the H2O2-induced cell damage model and
the untreated controls.
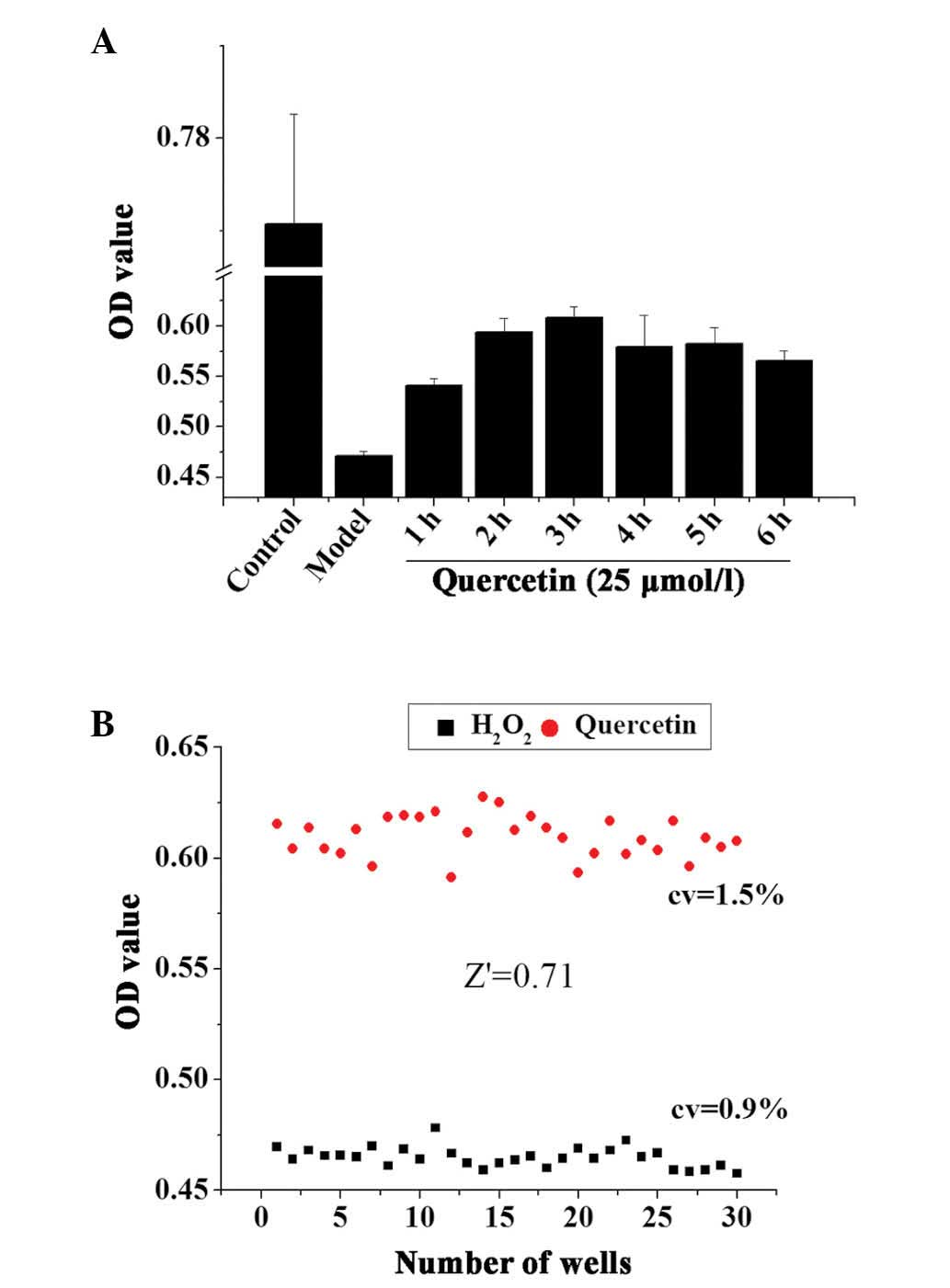
The incubation time of the TCM extracts may be an
important factor that affects the result of the assay. A positive
control drug, quercetin, was used to optimize the incubation time
of the cells with the drug following oxidative damage. Following 3
h treatment with 50 µmol/l H2O2, 25
µmol/l quercetin was added to H9c2 cells and incubated for
an additional 1–6 h. The results demonstrated that the optimal drug
incubation time following oxidative damage was 3 h (Fig. 4A).
The optimized incubation time was used to establish
the screening assays, and Z′ factor calculation validated whether
the assays were suitable for an HTS format. Quercetin-treated cells
(pretreated with 50 µmol/l H2O2 for 3
h) were used as the positive control and the cells treated with
H2O2 only as the negative control to further
investigate the robustness of the screening assays in an HTS
format. As demonstrated in Fig.
4B, quercetin increased the cell viabilities compared with 50
µmol/l H2O2 treatment. The Z′ factor
was 0.71, and the CVs of the positive and negative controls were
0.9 and 1.5%, respectively. This indicated that the
H2O2-induced cell damage model was suitable
for HTS assays.
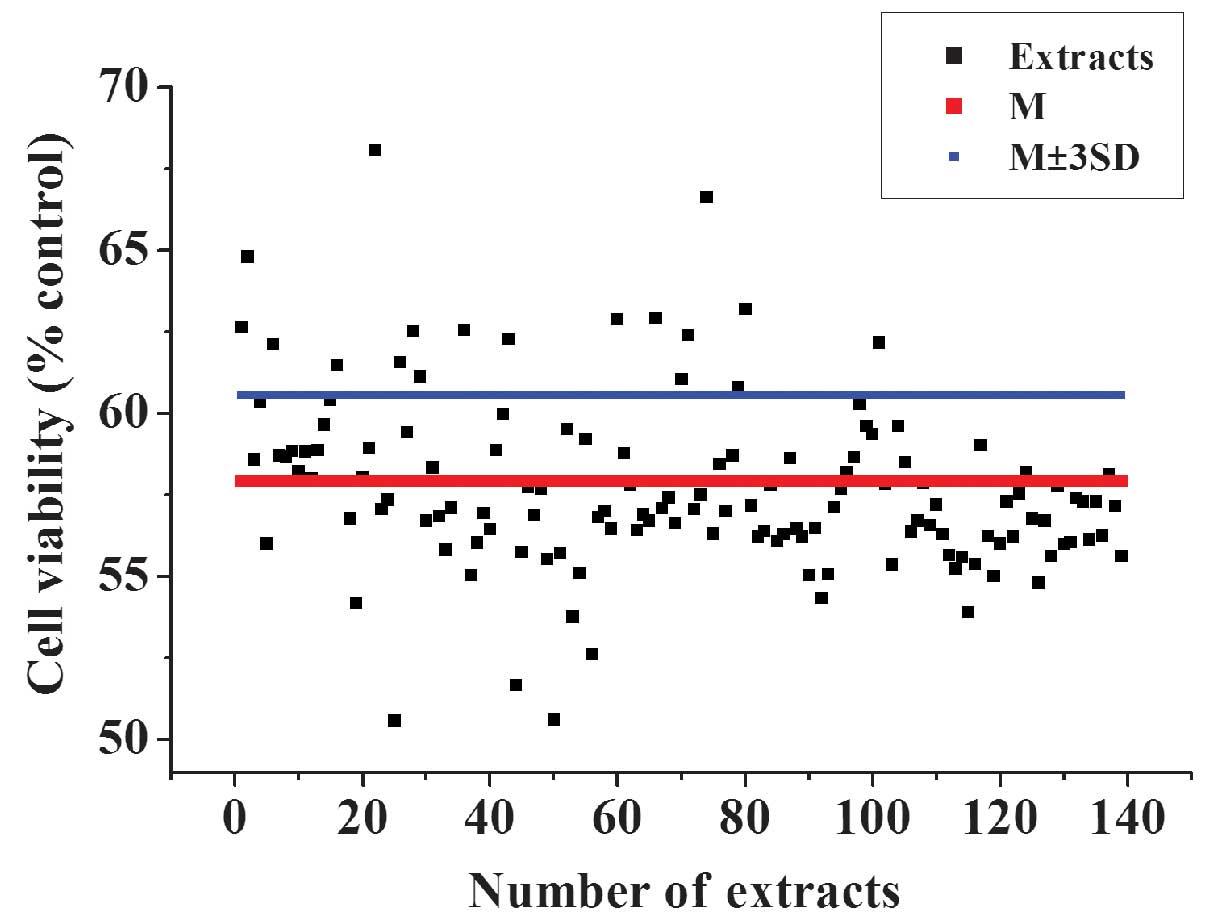
Identification of active extracts from
TCM
The optimized 96-well plate HTS system was then used
to identify extracts with antioxidative activity from a TCM
library. After a 3-h treatment with 50 µmol/l
H2O2, and a 3-h incubation with TCM extracts
at 37°C and 5% CO2, the cell viabilities were determined
using the CCK-8 kit and absorbance measured at 450 nm. In addition
to the extracts, 0.1 µl/well 25 mg/ml DMSO solution was
added to the cells. The final concentration of the samples in each
well was ~25 µg/ml. This concentration exhibited low
cytotoxic effects on the cells (data not shown). The extracts that
exhibited an OD450 value >the mean ± 3SD of the negative control
(H2O2-treated) were considered as potential
active samples. The top 17 hits from the primary screening were
selected for further validation (Fig.
5).
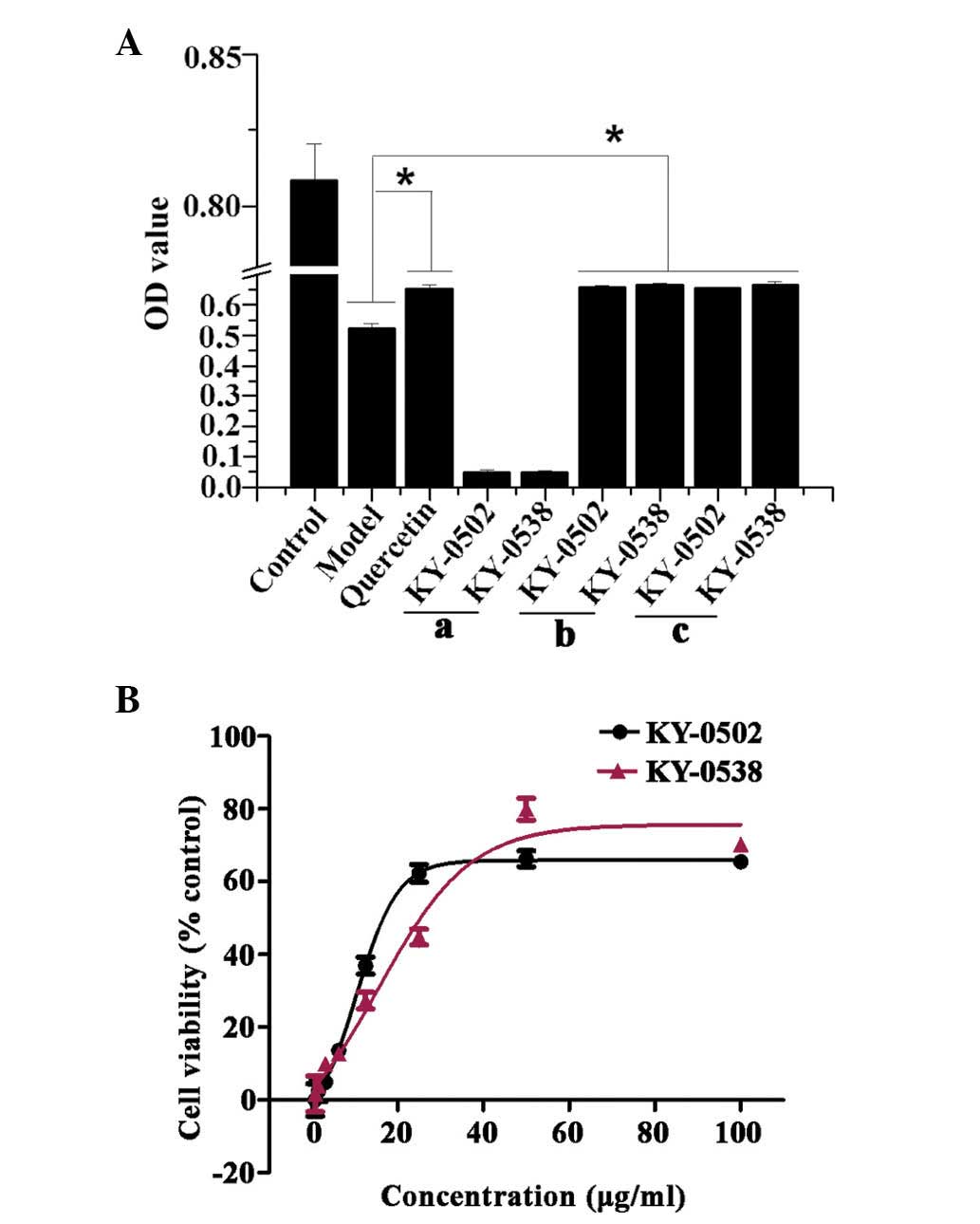
Validation of the primary hits
The increased OD450 values observed in the HTS assay
may be due to increased cell viabilities via protection of cells
from oxidative damage, reduced H2O2 toxicity
by a direct reaction with H2O2 in the culture
medium, or extract compounds themselves may absorb at 450 nm. In
order to exclude false positives, the current study further
validated the primary hits. To determine whether the extract
samples absorbed at 450 nm, 0.1 µl primary hit extracts were
directly added to the wells of a 96-well plate containing 100
µl pyruvate-free DMEM (no cells), and incubated for 3 h. The
results demonstrated that two extracts KY-0520 and KY-0538 had no
significant absorption at 450 nm (Fig.
6Aa). In order to test whether H2O2
directly reacts with the extracts in the culture medium, cells were
pretreated with 50 µmol/l H2O2 for 3
h, then the culture medium was replaced with pyruvate-free DMEM
containing 25 µg/ml primary hit and incubated for additional
3 h. The results indicated that KY-0520 and KY-0538 increased cell
viability compared with the H2O2-only
treatment when in the culture media with
H2O2, and when
H2O2/KY-0520 and KY-0538 treatments were
performed individually (Fig. 6Ab and
c). Taken together, the results indicate that activities of
KY-0520 and KY-0538 were due to antioxidative properties. The
present study additionally measured the cell viability following
treatment with KY-0520 and KY-0538 at different concentrations.
H9c2 cell viability was increased by KY-0520 and KY-0538 in a
concentration-dependent manner. The concentration-response curves
of KY-0520 and KY-0538 demonstrated that the compounds are active
between of 0.78 and 100 µg/ml (Fig. 6B), and the EC50 values
of KY-0520 and KY-0538 were 11.43 and 19.59 µg/ml,
respectively.
Characterization of the cardioprotective
activities of KY-0520 and KY-0538
The present study further investigated the
antioxidant activity of the TCM extracts. The results demonstrated
that H2O2-induced oxidative damage to H9c2
cells significantly increased LDH activity (P<0.0001; Fig. 7A) and MDA levels (P<0.001;
Fig. 7B), and decreased SOD
activity (P<0.001; Fig. 7C)
compared with the control. KY-0520 and KY-0538 treatment (25
µg/ml) significantly reduced the LDH activity (P<0.001;
Fig. 7A) and MDA levels
(P<0.01; Fig. 7B) compared with
H2O2-treated cells. Additionally, KY-0520 and
KY-0538 significantly increased the SOD activity levels compared
with H2O2-treated cells (P<0.05 and
P<0.001, respectively; Fig.
7C). These results suggest that KY-0520 and KY-053 prevent the
accumulation of free radicals and attenuate cardiomyocyte damage
induced by H2O2.
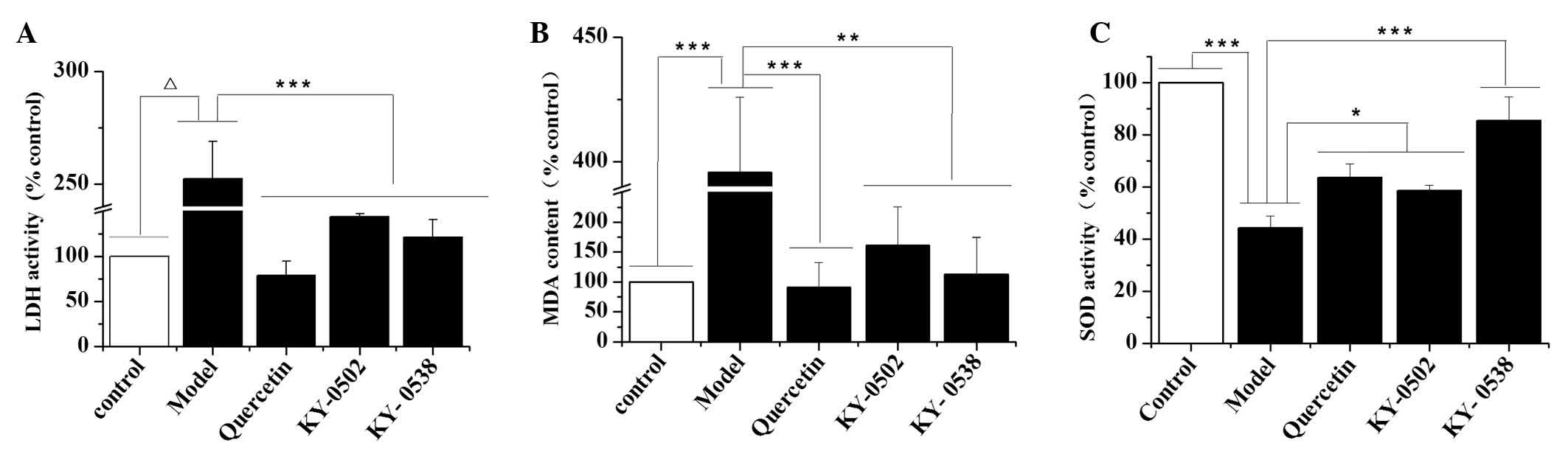 | Figure 7Effects of KY-0502 and KY-0538 on LDH
levels, MDA levels and SOD activity in
H2O2-treated H9c2 cells. H9c2 cells (1,000
µl/well) at the density of 3.0×104 cells/ml were
seeded into 24-well plates and randomly divided into five groups.
The cells were treated with dimethyl sulfoxide (control group), 50
µmol/l H2O2 (model group), 50
µmol/l H2O2 + 50 µmol/l
quercetin (quercetin group), 25 µg/ml KY-0502 (KY-0502
group) or 25 µg/ml KY-0538 (KY-0538 group). Subsequently,
the cells and medium were collected for LDH, MDA and SOD detection
using the corresponding assay kits. (A) LDH contents released from
the cells, (B) MDA contents of the cells and (C) SOD activities of
the cells. *P<0.05, **P<0.01,
***P<0.001 and ΔP<0.0001, comparisons
indicated by brackets. LDH, lactate dehydrogenase; MDA,
malondialdehyde; SOD, superoxide dismutase. |
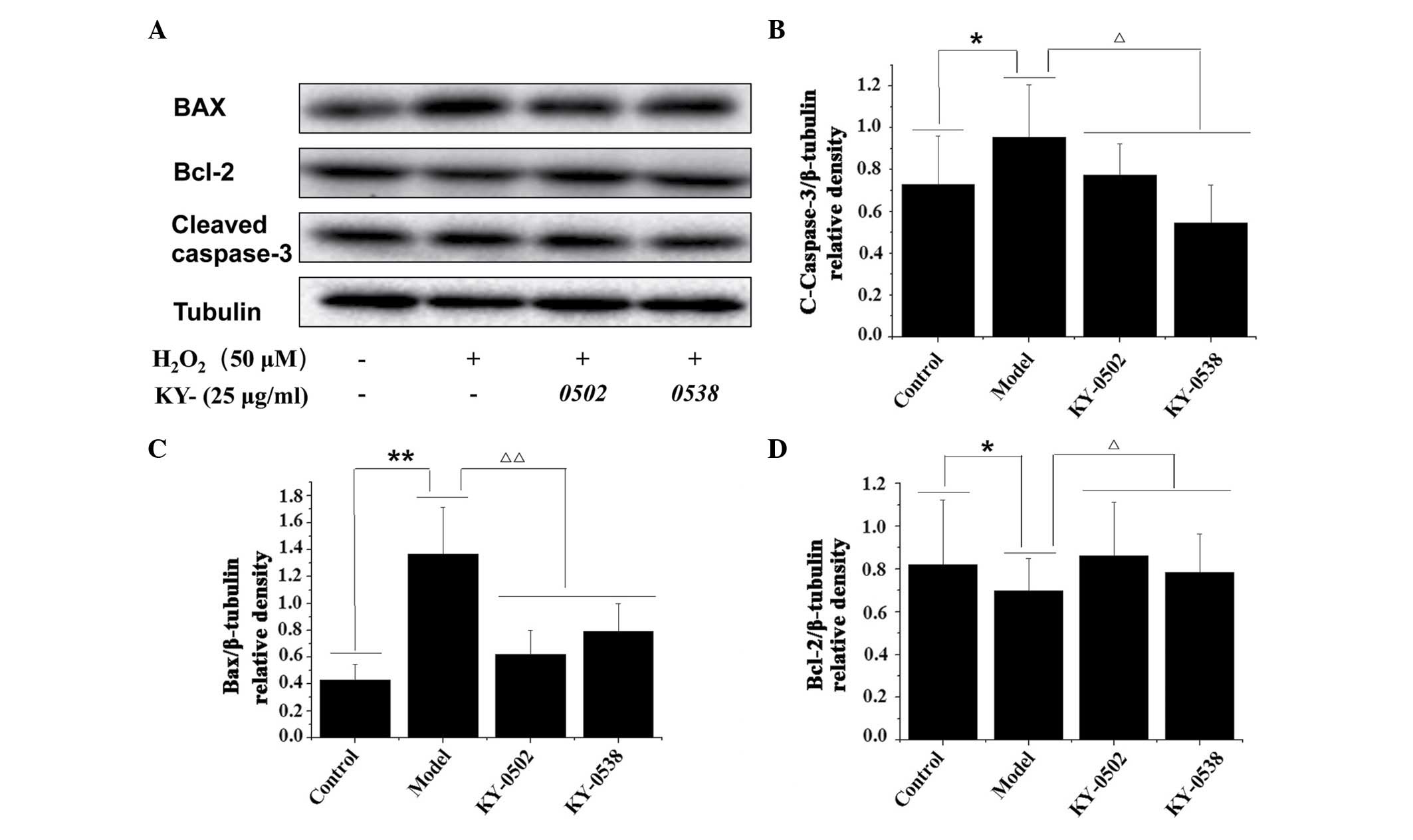
TCM extracts decrease
H2O2-induced apoptosis in H9c2 cells
Based on the cell viability and antioxidative
results, the current study further examined whether KY-0520 and
KY-0538 exhibited protective effects against
H2O2-induced cell apoptosis by western blot
analysis of apoptosis-associated proteins (Fig. 8A). As demonstrated in Fig. 8B and C, H2O2
treatment significantly increased the protein expression levels of
cleaved caspase-3 (1.31-fold increase; P<0.05;) and Bax
(3.19-fold increase; P<0.01) compared with the untreated
controls. KY-0520 and KY-0538 (25 µg/ml) significantly
decreased the protein expression levels of cleaved caspase-3
(P<0.05) and Bax (P<0.01) in H9c2 cells compared with
H2O2 treatment alone. Additionally, the
protein levels of Bcl-2, an anti-apoptotic protein, were decreased
following H2O2 treatment compared with
untreated controls (P<0.05), however, compared with
H2O2 treatment alone, Bcl-2 levels were
significantly increased following KY-0520 and KY-0538 treatment
(P<0.05; Fig. 8D). The results
indicated that the extracts inhibited apoptosis by regulation of
pro- and anti-apoptotic proteins in
H2O2-treated H9c2 cells.
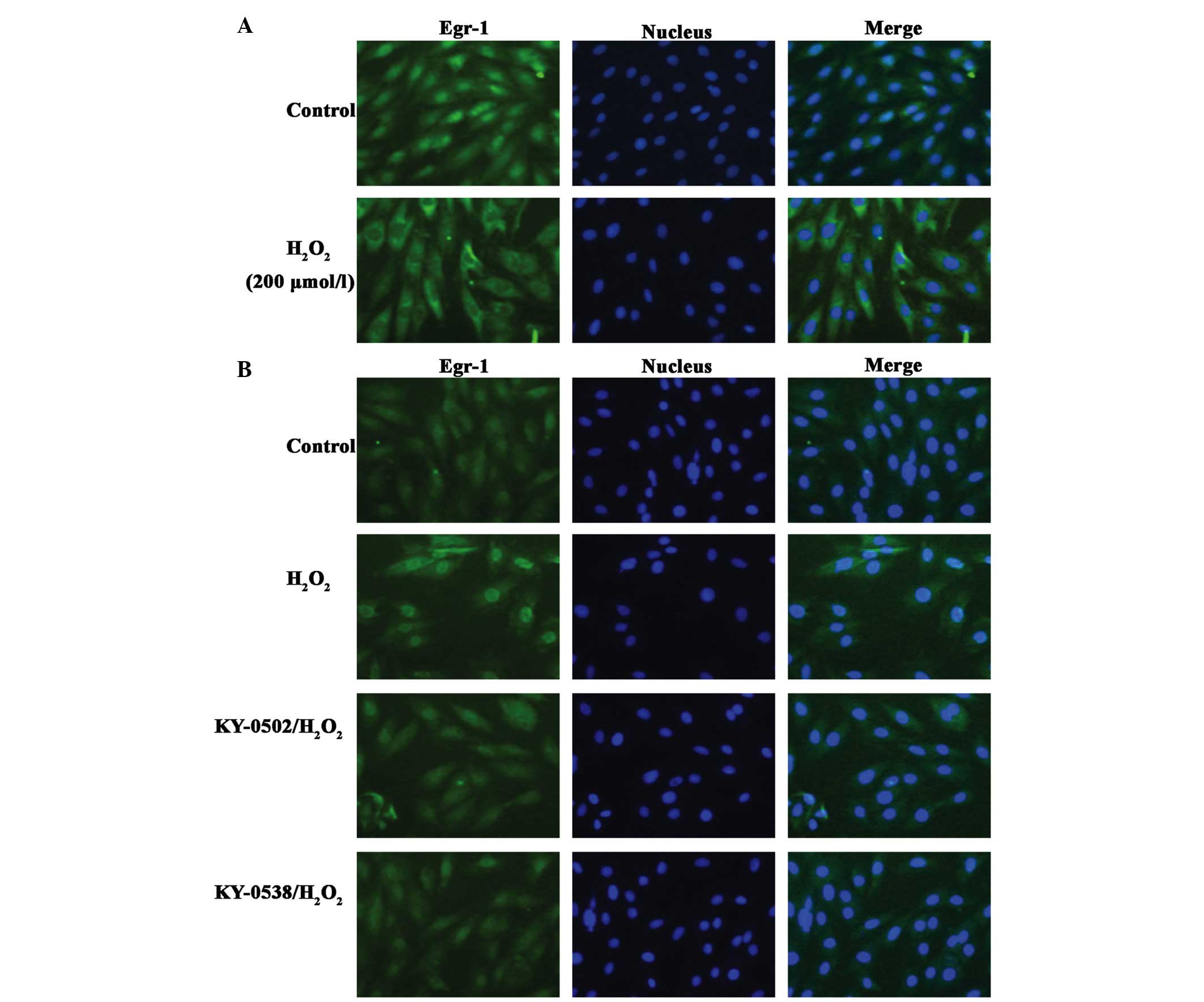
TCM extracts inhibit Egr-1 protein
accumulation in nucleus in H2O2-exposed H9c2
cells
It was previously demonstrated that following
exposure of H9c2 cells to 200 µmol/l
H2O2, Egr-1 is translocated from the
cytoplasm and accumulates in the nucleus (26). The present study treated H9c2 cells
with 200 µmol/l H2O2 for 2 h, however,
Egr-1 did not translocate from the cytoplasm to nucleus, it
accumulated in the cytoplasm and nuclear membrane (Fig. 9A). By contrast, at an
H2O2 concentration of 12.5 µmol/l,
Egr-1 nuclear staining was increased (Fig. 9B). KY-0520 and KY-0538 (25
µg/ml) markedly decreased Egr-1 immunostaining to near basal
levels, and caused Egr-1 redistribution in the nucleus and
cytoplasm (Fig. 9B). These results
suggested that KY-0520 and KY-0538 may protect H9c2 cells from
oxidative damage by regulating Egr-1 activity.
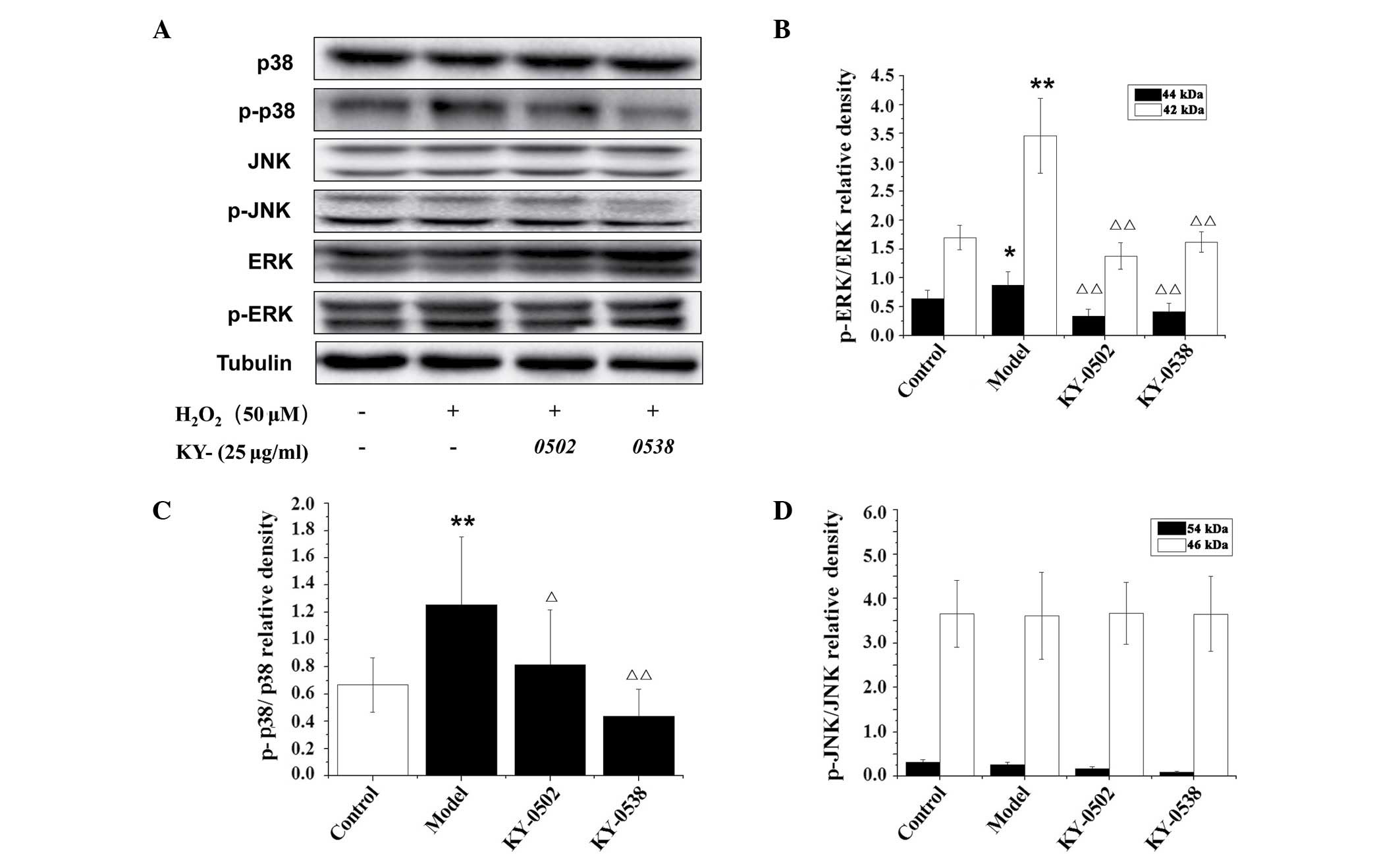
TCM extracts inhibit ERK1/2 and p38-MAPK
in H2O2-exposed H9c2 cells
The MAPK signaling pathways, including ERK1/2, p38
and JNK-MAPK, are critical for the regulation of apoptosis and
other cellular processes. Activation of the MAPK signaling pathways
is a characteristic feature of oxidant-induced apoptosis (27), and is well established in cardiac
myocytes (28). In the present
study, the protein levels of p-ERK1/2, p-JNK and p-p38 MAPK were
measured in H9c2 cells exposed to 12.5 µmol/l
H2O2, and the results are presented in
Fig. 10. Western blot analysis
demonstrated that the phosphorylation levels of p42- and p44-ERK
and p38 MAPK were significantly increased by
H2O2 (2.04-, 1.37- and 1.88-fold,
respectively) compared with untreated control cells (Fig. 10B and C). However the increased
phosphorylation of those kinases was reversed by 25 µg/ml
KY-0520 and KY-0538 after 3 h incubation. However, no significant
alterations in JNK phosphorylation were observed following 12.5
µmol/l H2O2 or 25 µg/ml active
extracts treatment (Fig. 10D).
These results indicate that KY-0520 and KY-0538 may regulate the
MAPK signaling pathway to protect against
H2O2-induced oxidative stress in H9c2
cells.
Discussion
Increasingly, studies indicate that ROS are
associated with the pathogenesis and progression of various
cardiovascular diseases. Sensitivity to oxidative stress is greater
in the heart compared with other organs due to lower levels of
antioxidant enzymes (29). The
pathogenesis of cardiac hypertrophy, developed by chronic
hypertrophy, is associated with ROS via regulation of the
intracellular pathways linked to MAPKs and
phosphatidylinositol-4,5-bisphosphate 3-kinase/Akt (30–32).
H2O2 is predominantly produced via the
dismutation of superoxide anions, it can also swiftly permeate the
cell membrane and react with intracellular metal ions to form toxic
hydroxyl radicals, which cause DNA damage (33). Previous studies demonstrated that
H2O2 is excessively produced during
cardiomyocyte apoptosis, leading to caspase-3 activation via
mitochondrial dysfunction and cytosolic release of mitochondrial
cytochrome c (34).
Numerous studies have investigated natural plant
compounds and TCM extracts for their antioxidant activities.
Silibinin (the major active component of silymarin extracted from
S. marianum) has been demonstrated to have antioxidative,
antitumor and anti-inflammatory properties (35). The volatile oil of Nardostachyos
Radix et Rhizoma (the root and rhizome of Nardostachys
jatamansi DC.) was reported to markedly suppress ROS formation
and dose-dependently increase glutathione levels in H9c2 cells
following oxidative injury (36).
Thus, novel antioxidant agents from natural plants and TCM may be
useful for the treatment of cardiac diseases.
Using H2O2 to treat H9c2 rat
myocardial cells, the present study established a cell model of
oxidative damage for HTS assay, and used the model to identify
cardioprotective agents from a library of TCM extracts. The actions
of the extract were determined by CCK-8 assay, which is based on
dehydrogenase activity detection in viable cells, and is widely
used for cell proliferation and cytotoxicity assays. The CCK-8
assay does not require washing or cell lysis, therefore,
variability is mini-mized. It has previously been successfully
applied in HTS studies as it is inexpensive and easy to operate
(37). Therefore, the present
study used the CCK-8 assay to evaluate the effect of TCM extracts
on the viability of oxidatively damaged cells. Two hits, KY-0520
and KY-0538, were further validated as cardioprotective agents, and
attenuated oxidative damage in a concentration-dependent manner
(EC50 values, ~11.43 and 19.59 µg/ml,
respectively).
The present study used 50 µmol/l
H2O2 to induce oxida-tive damage in the
model. Various studies have investigated the appropriate working
concentration of H2O2, however, results have
varied (21,38,39).
Sodium pyruvate is commonly supplemented in culture media, however,
this compound can nonenzymatically react with
H2O2, leading to liberation of
CO2, and the conversion of α-keto acid to carboxylic
acid (40,41). Therefore, the present study used
H2O2 diluted with pyruvate-free DMEM to
induce oxidative damage in our cell-based assays.
To establish the HTS assay model, two Z′ factors
were required to evaluate the screening method. One was used to
evaluate the robustness of the cell model, which indicates whether
the cell damage model was successfully established and suitable for
HTS. The other Z′ factor was used to evaluate the robustness of the
extract screening assay (Figs. 3
and 4). The majority of HTS assays
are based on specific targets. Compared with the HTS assays
designed to screen for drugs acting on specific targets, the cell
damage model has advantages and limitations. Cell-based screening
can directly evaluate the protective activities of drugs by
measuring cell viability, however, the direct targets of the drugs
and the signaling pathways involved are unclear. To understand the
mechanisms of action of the drugs, it is necessary to further
investigate the potential targets and signaling pathways. The
effects of the drug candidates identified in the present study may
be mediated by interaction with multiple targets and signaling
pathways. Therefore, the cell-protective functions of KY-0520 and
KY-0538 may be mediated by their antioxidant activity, and also via
interaction with other pathways. Other factors and pathways
associated with the effects of KY-0520 and KY-0538 may include
Egr-1. Immunofluorescence demonstrated the localization of Egr-1 to
be altered by KY-0520 and KY-0538 treatment under
H2O2-induced oxidative stress.
H2O2 is a strong oxidant that
markedly decreases cell viability and increases apoptosis. The
present study measured LDH activity to further investigate the
cardioprotective effect of KY-0520 and KY-0538. LDH assays are
widely used to quantify the level of LDH release. MDA levels and
SOD activity were also measured as indicators of oxidative damage
and myocardial function. KY-0520 and KY-0538 demonstrated
significant antioxidant activities and protective effects on
cardiomyocytes in vitro (Fig.
7). Furthermore, previous studies have demonstrated that
H2O2 can decrease the Bcl-2/Bax ratio and
increase the level of cleaved caspase-3, therefore inducing
apoptosis (42,43). Western blot analysis demonstrated
that KY-0520 and KY-0538 regulate the Bcl-2/Bax ratio in
H2O2-exposed H9c2 cells, and decrease the
H2O2-induced cleaved caspase-3 activation
(Fig. 8). These effects may
contribute to the antioxidant activity of KY-0520 and KY-0538 and
their protection against oxidative stress.
Egr-1 is a transcription factor encoded by an
immediate early gene (44). Egr-1
is weakly expressed under normal conditions, and its expression is
activated by various environmental stimuli associated with injury
and stress, including growth factors, cytokines, T cell receptor
ligation, hormones, thrombin, shear stress and mechanical forces,
neurotransmitters, ultraviolet light, ROS, ischemia/reperfusion and
hypoxia (45–52). Egr-1 mRNA is expressed following
cardioplegic arrest and reperfusion in human hearts, and in rat
hearts subjected to cold cardioplegia for 40 min followed by 40 min
reperfusion. The expression of Egr-1 and the downstream effects on
transcription are tightly controlled, and cell-specific
upregulation induced by processes such as hypoxia and ischemia, has
been previously linked to multiple aspects of cardiovascular
injury. Egr-1 regulates cell growth and proliferation (53,54),
and positively modulates inflammation, thrombosis and apoptosis, by
direct and indirect mechanisms (45,55).
It was previously reported that targeting rodent Egr-1 selectively
reduced the infarct size following myocardial ischemia/reperfusion.
The mechanisms reported to be involved were associated with the
attenuation of intercellular adhesion molecule 1-dependent
inflammation and inhibition of other Egr-1-dependent molecules,
including tumor necrosis factor-α (TNF-α), vascular cell adhesion
molecule-1 (VCAM-1), tissue factor (TF), plasminogen activator
inhibitor type 1, and p53. Inhibition of functional TF, VCAM-1 and
TNF-α has been previously demonstrated to reduce the infarct size
in experimental models of myocardial ischemia/reperfusion. The
transcription of the pro-apoptotic factor, p53, is inhibited by
Egr-1, with an associated reduction in apoptosis and infarct size
(56). A previous study
demonstrated that Egr-1 represses transcription from the
calsequestrin (CSQ) promoter, resulting in reduced expression of
CSQ, which is a major calcium storage protein critical for normal
cardiac function (57).
Additionally, overexpression of Egr-1 directly induced caspase
activation and apoptosis in human cardiac fibroblast cultures in
vitro (58). These studies
indicate that inhibition of Egr-1 activity may be
cardioprotective.
The findings of the present study were consistent
with a previous study that demonstrated that
H2O2 induces the translocation of Egr-1 from
the cytoplasm to nucleus, thus promoting the accumulation of Egr-1
in the nuclei of H9c2 cells (Fig.
9B) (26). However, in
contrast to the previous study, a high concentration of
H2O2 (200 µmol/l) resulted in high
levels of Egr-1 in the cytoplasm and nuclear membrane, rather than
accumulation in the nucleus (Fig.
9) (26). The different
regulatory mechanisms of Egr-1 under different concentrations of
H2O2 are not clear. KY-0520 and KY-0538
effectively reversed the translocation of Egr-1 from the cytoplasm
to the nucleus induced by 12.5 µmol/l
H2O2 (Fig.
9). The cardio-protective activities of KY-0520 and KY-0538
appear to be associated with inhibition of Egr-1 activity and ROS
scavenging.
Egr-1 expression is upregulated in response to
cardiac ischemia/reperfusion stress (59). Additionally, a previous study
demonstrated that Egr-1 mRNA expression in H9c2 cells was
upregulated by H2O2 in vitro, and that
the upregulation was dependent on MEK/ERK and JNK signaling
(26,60). ERK1/2 is a component of the
classical MAPK pathway that was previously demonstrated to be
directly activated by high levels of ROS (including xanthine
oxidase-derived H2O2) leading to
transcription of Egr-1 (60–62).
Thus, the present study investigated whether these pathways are
modified during H2O2-induced oxidative
stress. Consistent with previous studies (26,63),
the western blot analysis of the present study indicated that the
phosphorylation levels of ERK1/2 and p38-MAPK kinase were increased
following 12.5 µmol/l H2O2 treatment
(Fig. 10). Therefore, it is
speculated that ERK1/2 and p38-MAPK may be important upstream
regulators that mediated Egr-1 modulation during cardiomyocyte
oxidative stress. The results of the present study indicated that
the antioxidative effects of KY-0520 and KY-0538 may be mediated by
suppression of the ERK1/2, p38-MAPK/Egr-1 signaling pathways in
H2O2-induced oxidative stress.
In summary, the hits from the HTS assays may
generate novel drugs that have the potential to be used as
therapeutics for cardiomyocyte diseases, including heart
ischemia/reperfusion, cardiac hypertrophy, cardiac dysfunction and
heart failure. The present study established and validated a
H2O2-induced cell damage model for use in
HTS, however, further mechanistic research is required to
understand the effects of the identified hits. Further
investigation of the activity of the hits will be performed using
primary cardiomyocyte cells or appropriate animal models.
Acknowledgments
The authors of the present study thank the Natural
Products Research Department, State Key Laboratory of New-tech for
the Chinese Medicine Pharmaceutical Process for providing the TCM
extracts library. The current work was supported by the Ministry of
Science and Technology of China (no. 2013zx0942203).
References
|
1
|
Galli F, Piroddi M, Annetti C, Aisa C,
Floridi E and Floridi A: Oxidative stress and reactive oxygen
species. Contrib Nephrol. 149:240–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pham-Huy LA, He H and Pham-Huy C: Free
radicals, antioxidants in disease and health. International journal
of biomedical science: Int J Biomed Sc. 4:89–96. 2008.
|
|
3
|
Geronikaki AA and Gavalas AM: Antioxidants
and inflammatory disease: Synthetic and natural antioxidants with
anti-inflammatory activity. Comb Chem High Throughput Screen.
9:425–442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo RF and Ward PA: Role of oxidants in
lung injury during sepsis. Antioxid Redox Signal. 9:1991–2002.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gilgun-Sherki Y, Melamed E and Offen D:
Oxidative stress induced-neurodegenerative diseases: The need for
antioxidants that penetrate the blood brain barrier.
Neuropharmacology. 40:959–975. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar SV, Saritha G and Fareedullah M:
Role of antioxidants and oxidative stress in cardiovascular
diseases. Ann Biol Res. 3:158–175. 2010.
|
|
7
|
Venardos KM, Perkins A, Headrick J and
Kaye DM: Myocardial ischemia-reperfusion injury, antioxidant enzyme
systems, and selenium: A review. Cur Med Chem. 14:1539–1549. 2007.
View Article : Google Scholar
|
|
8
|
Zhao ZQ: Oxidative stress-elicited
myocardial apoptosis during reperfusion. Curr Opin Pharmacol.
4:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saini HK, Machackova J and Dhalla NS: Role
of reactive oxygen species in ischemic preconditioning of
subcellular organelles in the heart. Antioxid Redox Signal.
6:393–404. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zima AV and Blatter LA: Redox regulation
of cardiac calcium channels and transporters. Cardiovasc Res.
71:310–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cesselli D, Jakoniuk I, Barlucchi L,
Beltrami AP, Hintze TH, Nadal-Ginard B, Kajstura J, Leri A and
Anversa P: Oxidative stress-mediated cardiac cell death is a major
determinant of ventricular dysfunction and failure in dog dilated
cardiomyopathy. Circ Res. 89:279–286. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwon SH, Pimentel DR, Remondino A, Sawyer
DB and Colucci WS: H2O2 regulates cardiac
myocyte phenotype via concentration-dependent activation of
distinct kinase pathways. J Mol Cell Cardiol. 35:615–621. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rayment NB, Haven AJ, Madden B, Murday A,
Trickey R, Shipley M, Davies MJ and Katz DR: Myocyte loss in
chronic heart failure. J Pathol. 188:213–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giordano FJ: Oxygen, oxidative stress,
hypoxia, and heart failure. J Clin Invest. 115:500–508. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimes BW and Brandt BL: Properties of a
clonal muscle cell line from rat heart. Exp Cell Res. 98:367–381.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su CY, Chong KY, Edelstein K, Lille S,
Khardori R and Lai CC: Constitutive hsp70 attenuates hydrogen
peroxide-induced membrane lipid peroxidation. Biochem Biophys Res
Commun. 265:279–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park ES, Kang JC, Kang DH, Jang YC, Yi KY,
Chung HJ, Park JS, Kim B, Feng ZP and Shin HS: 5-AIQ inhibits
H2O2-induced apoptosis through reactive
oxygen species scavenging and Akt/GSK-3β signaling pathway in H9c2
cardiomyocytes. Toxicol Appl Pharmacol. 268:90–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aggeli IK, Gaitanaki C and Beis I:
Involvement of JNKs and p38-MAPK/MSK1 pathways in
H2O2-induced upregulation of heme oxygenase-1
mRNA in H9c2 cells. Cell Signal. 18:1801–1812. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka H, Sakurai K, Takahashi K and
Fujimoto Y: Requirement of intracellular free thiols for hydrogen
peroxide-induced hypertrophy in cardiomyocytes. J Cell Biochem.
89:944–955. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu S, Zhu H, Wei X, Zhang C, Jiang L, Liu
Y, Luo Q and Xiao X: Oxidative stress-mediated up-regulation of
myocardial ischemic preconditioning up-regulated protein 1 gene
expression in H9c2 cardiomyocytes is regulated by cyclic
AMP-response element binding protein. Radic Biol Med. 49:580–586.
2010. View Article : Google Scholar
|
|
21
|
Law CH, Li JM, Chou HC, Chen YH and Chan
HL: Hyaluronic acid-dependent protection in H9C2 cardiomyocytes: A
cell model of heart ischemia-reperfusion injury and treatment.
Toxicology. 303:54–71. 2013. View Article : Google Scholar
|
|
22
|
Diestel A, Drescher C, Miera O, Berger F
and Schmitt KR: Hypothermia protects H9c2 cardiomyocytes from
H2O2 induced apoptosis. Cryobiology.
62:53–61. 2011. View Article : Google Scholar
|
|
23
|
Eguchi M, Liu Y, Shin EJ and Sweeney G:
Leptin protects H9c2 rat cardiomyocytes from
H2O2-induced apoptosis. FEBS J.
275:3136–3144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park ES, Kang JC, Jang YC, Park JS, Jang
SY, Kim DE, Kim B and Shin HS: Cardioprotective effects of
rhamnetin in H9c2 cardiomyoblast cells under
H2O2-induced apoptosis. J Ethnopharmacol.
153:552–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang JH, Chung TD and Oldenburg KR: A
simple statistical parameter for use in evaluation and validation
of high throughput screening assays. J Biomol Screen. 4:67–73.
1999. View Article : Google Scholar
|
|
26
|
Aggeli IK, Beis I and Gaitanaki C: ERKs
and JNKs mediate hydrogen peroxide-induced Egr-1 expression and
nuclear accumulation in H9c2 cells. Physiol Res. 59:443–454.
2010.
|
|
27
|
Ryter SW, Kim HP, Hoetzel A, Park JW,
Nakahira K, Wang X and Choi AM: Mechanisms of cell death in
oxidative stress. Antioxid Redox Signal. 9:49–89. 2007. View Article : Google Scholar
|
|
28
|
Clerk A, Michael A and Sugden PH:
Stimulation of multiple mitogen-activated protein kinase
sub-families by oxidative stress and phosphorylation of the small
heat shock protein, HSP25/27, in neonatal ventricular myocytes.
Biochem J. 333:581–589. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Di Meo S, Venditti P and De Leo T: Tissue
protection against oxidative stress. Experientia. 52:786–794. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bogoyevitch MA: Signalling via
stress-activated mitogen-activated protein kinases in the
cardiovascular system. Cardiovasc Res. 45:826–842. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ravingerová T, Barancík M and Strnisková
M: Mitogen-activated protein kinases: A new therapeutic target in
cardiac pathology. Mol Cell Biochem. 247:127–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y: Mitogen-activated protein kinases
in heart development and diseases. Circulation. 116:1413–1423.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao Z, Huang K and Xu H: Protective
effects of flavonoids in the roots of Scutellaria baicalensis
Georgi against hydrogen peroxide-induced oxidative stress in
HS-SY5Y cells. Pharmacol Res. 43:173–178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park C, So HS, Shin CH, Baek SH, Moon BS,
Shin SH, Lee HS, Lee DW and Park R: Quercetin protects the hydrogen
peroxide-induced apoptosis via inhibition of mitochondrial
dysfunction in H9c2 cardiomyoblast cells. Biochem Pharmacol.
66:1287–1295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anestopoulos I, Kavo A, Tentes I,
Kortsaris A, Panayiotidis M, Lazou A and Pappa A: Silibinin
protects H9c2 cardiac cells from oxidative stress and inhibits
phenylephrine-induced hypertrophy: Potential mechanisms. J Nutr
Biochem. 24:586–594. 2013. View Article : Google Scholar
|
|
36
|
Maiwulanjiang M, Chen J, Xin G, Gong AG,
Miernisha A, Du CY, Lau KM, Lee PS, Chen J, Dong TT, et al: The
volatile oil of Nardostachyos Radix et Rhizoma inhibits the
oxidative stress-induced cell injury via reactive oxygen species
scavenging and Akt activation in H9c2 cardiomyocyte. J
Ethnopharmacol. 153:491–498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen B, Mao R, Wang H and She JX: Cell
line and drug-dependent effect of ERBB3 on cancer cell
proliferation, chemosensitivity, and multidrug actions. Int J High
Throughput Screen. 1:49–55. 2010.
|
|
38
|
Li H, Deng Z, Liu R, Loewen S and Tsao R:
Carotenoid compositions of coloured tomato cultivars and
contribution to antioxidant activities and protection against
H2O2-induced cell death in H9c2. Food Chem.
136:878–888. 2013. View Article : Google Scholar
|
|
39
|
Woo SM, Min KJ, Kim S, Park JW, Kim DE,
Chun KS, Kim YH, Lee TJ, Kim SH, Choi YH, et al: Silibinin induces
apoptosis of HT29 colon carcinoma cells through early growth
response-1 (EGR-1)-mediated non-steroidal anti-inflammatory
drug-activated gene-1 (NAG-1) up-regulation. Chem Biol Interact.
211:36–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
O'Donnell-Tormey J, Nathan CF, Lanks K,
DeBoer CJ and de la Harpe J: Secretion of pyruvate. An antioxidant
defense of mammalian cells. J Exp Med. 165:500–514. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Holleman AF: Notice sur l'action de l'eau
oxygénée sur les acides α-cétoniques et sur les dicétones 1. 2.
Recl Trav Chim Pays-Bas Belg. 23:169–172. 1904.In French.
View Article : Google Scholar
|
|
42
|
Dorn GW II: Apoptotic and non-apoptotic
programmed cardiomyocyte death in ventricular remodelling.
Cardiovasc Res. 81:465–473. 2009. View Article : Google Scholar :
|
|
43
|
Shih PH, Yeh CT and Yen GC: Anthocyanins
induce the activation of phase II enzymes through the antioxidant
response element pathway against oxidative stress-induced
apoptosis. J Agric Food Chem. 55:9427–9435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sukhatme VP, Cao XM, Chang LC, Tsai-Morris
CH, Stamenkovich D, Ferreira PC, Cohen DR, Edwards SA, Shows TB,
Curran T, et al: A zinc finger-encoding gene coregulated with c-fos
during growth and differentiation, and after cellular
depolarization. Cell. 53:37–43. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan SF, Fujita T, Lu J, Okada K, Shan Zou
Y, Mackman N, Pinsky DJ and Stern DM: Egr-1, a master switch
coordinating upregulation of divergent gene families underlying
ischemic stress. Nat Med. 6:1355–1361. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gaggioli C, Deckert M, Robert G, Abbe P,
Batoz M, Ehrengruber MU, Ortonne JP, Ballotti R and Tartare-Deckert
S: HGF induces fibronectin matrix synthesis in melanoma cells
through MAP kinase-dependent signaling pathway and induction of
Egr-1. Oncogene. 24:1423–1433. 2005. View Article : Google Scholar
|
|
47
|
Guha M, O'Connell MA, Pawlinski R, Hollis
A, McGovern P, Yan SF, Stern D and Mackman N: Lipopolysaccharide
activation of the MEK-ERK1/2 pathway in human monocytic cells
mediates tissue factor and tumor necrosis factor alpha expression
by inducing Elk-1 phosphorylation and Egr-1 expression. Blood.
98:1429–1439. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hjoberg J, Le L, Imrich A, Subramaniam V,
Mathew SI, Vallone J, Haley KJ, Green FH, Shore SA and Silverman
ES: Induction of early growth-response factor 1 by platelet-derived
growth factor in human airway smooth muscle. Am J Physiol Lung Cell
Mol Physiol. 286:L817–L825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li CJ, Ning W, Matthay MA,
Feghali-Bostwick CA and Choi AM: MAPK pathway mediates
EGR-1-HSP70-dependent cigarette smoke-induced chemokine production.
Am J Physiol Lung Cell Mol Physiol. 292:L1297–L1303. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kaufmann K and Thiel G: Epidermal growth
factor and thrombin induced proliferation of immortalized human
keratinocytes is coupled to the synthesis of Egr-1, a zinc finger
transcriptional regulator. J Cell Biochem. 85:381–391. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rössler OG and Thiel G: Thrombin induces
Egr-1 expression in fibroblasts involving elevation of the
intracellular Ca2+ concentration, phosphorylation of ERK
and activation of ternary complex factor. Mol Biol. 10:402009.
|
|
52
|
Lohoff M, Giaisi M, Köhler R, Casper B,
Krammer PH and Li-Weber M: Early growth response protein-1 (Egr-1)
is preferentially expressed in T helper type 2 (Th2) cells and is
involved in acute transcription of the Th2 cytokine interleukin-4.
J Biol Chem. 285:1643–1652. 2010. View Article : Google Scholar :
|
|
53
|
Gashler A and Sukhatme VP: Early growth
response protein 1 (Egr-1): Prototype of a zinc-finger family of
transcription factors. Prog Nucleic Acid Res Mol Biol. 50:191–224.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu C, Rangnekar VM, Adamson E and Mercola
D: Suppression of growth and transformation and induction of
apoptosis by EGR-1. Cancer Gene Ther. 5:3–28. 1998.PubMed/NCBI
|
|
55
|
Thiel G and Cibelli G: Regulation of life
and death by the zinc finger transcription factor Egr-1. J Cell
Physiol. 193:287–292. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bhindi R, Fahmy RG, McMahon AC, Khachigian
LM and Lowe HC: Intracoronary delivery of DNAzymes targeting human
EGR-1 reduces infarct size following myocardial ischaemia
reperfusion. J Pathol. 227:157–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kasneci A, Kemeny-Suss NM, Komarova SV and
Chalifour LE: Egr-1 negatively regulates calsequestrin expression
and calcium dynamics in ventricular cells. Cardiovasc Res.
1:695–702. 2009.
|
|
58
|
Zins K, Pomyje J, Hofer E, Abraham D,
Lucas T and Aharinejad S: Egr-1 upregulates Siva-1 expression and
induces cardiac fibroblast apoptosis. Int J Mol Sci. 15:1538–1553.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Aebert H, Cornelius T, Ehr T, Holmer SR,
Birnbaum DE, Riegger GA and Schunkert H: Expression of immediate
early genes after cardioplegic arrest and reperfusion. Ann Thorac
Surg. 63:1669–1675. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen CA, Chen TS and Chen HC:
Extracellular signal-regulated kinase plays a proapoptotic role in
podocytes after reactive oxygen species treatment and inhibition of
integrin-extracellular matrix interaction. Exp Biol Med (Maywood).
237:777–783. 2012. View Article : Google Scholar
|
|
61
|
Hartney T, Birari R, Venkataraman S,
Villegas L, Martinez M, Black SM, Stenmark KR and Nozik-Grayck E:
Xanthine oxidase-derived ROS upregulate Egr-1 via ERK1/2 in PA
smooth muscle cells; model to test impact of extracellular ROS in
chronic hypoxia. PLoS One. 6:e275312011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Iyoda T, Zhang F, Sun L, Hao F,
Schmitz-Peiffer C, Xu X and Cui MZ: Lysophosphatidic acid induces
early growth response-1 (Egr-1) protein expression via protein
kinase Cδ-regulated extracellular signal-regulated kinase (ERK) and
c-Jun N-terminal kinase (JNK) activation in vascular smooth muscle
cells. J Biol Chem. 287:22635–22642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang C, Dostanic S, Servant N and
Chalifour LE: Egr-1 negatively regulates expression of the
sodium-calcium exchanger-1 in cardiomyocytes in vitro and in vivo.
Cardiovasc Res. 65:187–194. 2005. View Article : Google Scholar
|