Introduction
Emulsified isoflurane (EIso), which has clear
advantages in its convenience, low environmental pollution and
tissue toxicity, is widely used as a novel intravenous general
anesthetic (1,2). Compared with isoflurane (Iso)
inhalation, intravenous administration of EIso makes it easier to
control the depth of anesthesia and eliminates the requirement for
specific ventilatory circuits. In 1997, a previous study
demonstrated that Iso pre-treatment reduced myocardial infarct size
in an animal model, indicating pre-treatment as a promising
approach for limiting ischemia/reperfusion (I/R) (3). Recently, more focus was put into
researching the molecular mechanism of Iso protection from I/R.
Similar with Iso, pre-treatment with 8% EIso
demonstrated a myocardial protective effect on I/R injury in
rabbits, indicating that, despite the difference in form, both Iso
and EIso effectively protected against myocardial ischemia
(4). In the brain and heart,
injury caused by prolonged anoxia and ischemia was decreased by the
pre-treatment with Iso (5,6). In the lung, pre-treatment with EIso
was revealed to reduce lung injury induced by hepatic I/R by
decreasing the expression of tumor necrosis factor (TNF)-α and
downregulation of intercellular adhesion molecule (ICAM)-1
(7). It was also found that
pre-treatment with EIso caused cardioprotection against myocardial
I/R injury in rats by inhibiting apoptosis and stabilizing
mitochondria (8,9).
At present, it is widely accepted that I/R injury is
caused by reactive oxygen species (ROS) accumulation, pH
normalization and [Ca2+] rise, which leads to
mitochondrial destabilization and creates an ideal scenario for
mitochondrial permeability transition pore opening (10). Iso pre-treatment has been reported
as one of the most effective strategies by attenuating ROS level
upregulated by I/R, without a clear understanding of its molecular
mechanism. Notably, downregulation of tumor suppressor protein p53
by Iso pre-treatment indicates the association of Iso pre-treatment
with networks controlled by p53.
Tumor suppressor p53 is well-known for its role in
regulating apoptosis and the cell cycle in response to stress
signals. However, novel roles of p53 in regulating the respiratory
chain by transcriptionally regulating its downstream genes has
attracted recent attention. Previously, it was reported that p53
regulates mitochondrial respiration by directly targeting to its
downstream target gene and inducing the synthesis of cytochrome
c oxidase (SCO)2 (11). SCO2, which is
transcriptionally regulated by p53, serves a key role in
maintaining mitochondrial respiration. Downregulation of
SCO2 restored the impaired aerobic respiration,
indicating the regulatory role of p53 in mitochondrial respiration
in a SCO2-dependent manner (11). It has been previously showed that
p53/SCO2 signaling is activated by ROS generation to
promote mitochondrial oxygen consumption, resulting in
stabilization of the mitochondrial membrane (12).
As one of the numerous p53 target genes in an
unstressed condition, the cyclin-dependent kinase inhibitor, p21,
mediates the p53-dependent cell cycle G0/G1 phase arrest in
response to a variety of stress stimuli (13). Its role in regulating the cell
cycle was revealed to have protective effects to the kidney against
I/R injury in mice, suggesting that p21 may confer tolerance to I/R
injury (14). However, the
association between p53 and p21 under EIso treatment remains to be
elucidated.
In the present study, the effects of EIso treatment
on physiological processes of 16HBE cells, and the regulatory role
of p53 on cell proliferation and respiration were investigated. The
present study also investigated whether EIso treatment regulates
ROS level by affecting the regulatory activity of p53 on its
downstream genes associated with mitochondrial respiration.
Materials and methods
Cells culture and antibodies
Human lung bronchial epithelial cells (16HBE) were
stably transfected with short hairpin (sh) RNA constructs targeted
against p53 mRNA (16HBE-p53KD), SCO2 mRNA
(16HBE-SCO KD2) or scramble control (16HBE-SC).
Untreated cells constituted non-transfected 16HBE cells, and mock
cells constituted transfected 16HBE cells treated with fat
emulsion. All cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin, and 100 μg/ml streptomycin, and were cultured in
an atmosphere of 5% CO2 at 37°C.
Rabbit anti-actin (cat. no. ab179467), rabbit
anti-p53 (cat. no. ab179477), rabbit anti-p21 (ab7960), mouse
anti-SCO1 (cat. no. ab88658), rabbit
anti-SCO2 (cat. no. ab115877) and rabbit
anti-Tp53-induced glycolysis and apoptosis regulator (TIGAR; cat.
no. ab37910) primary antibodies were all purchased from Abcam
(Cambridge, MA, USA) and were used at a dilution of 1:1,000.
Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG H&L
(ab97040) and donkey anti-rabbit IgG H&L (ab7083) secondary
antibodies were also purchased from Abcam and were diluted to
1:5,000.
Preparation of EIso
The 8% EIso (v/v) was manufactured by Huarui
Pharmacy, Ltd. (Wuxi, China), according to the procedures described
previously (15,16). Briefly, liquid Iso was mixed with
30% Intralipid® (fat emulsion injection; Sino-Swed
Pharmaceutical Corp, Ltd., Beijing, China) at the final
concentration of 8% (v/v) in an ampoule. The sealed ampoule
containing mixture of liquid isoflurane and Intralipid®
was agitated vigorously on a vortex for 30 min until Iso was
solubilized into lipid emulsion. The stability of 8% EIso was at
least 6 months at room temperature.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression levels of certain genes were measured
by RT-qPCR. The total RNA was extracted from the cultured cells
using TRIzol reagent (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Complete extraction of RNA was
identified by running a MOPS denaturing agarose gel. The
first-strand cDNA was synthesized using random hexamer and the AMV
reverse transcriptase (Thermo Fisher Scientific, Inc.). For qPCR,
0.1 μl cDNA was used as a template in a 20 μl
reaction volume containing 20 pmol of each primer and 10 μl
SSO Fast EvaGreen Supermix (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) on an Applied Biosystems® 7500 Real-Time PCR
System (Thermo Fisher Scientific, Inc.). After 3 min initial
melting at 95°C, the mixture was amplified for a total of 40 cycles
with a two-step cycle process that began with melting at 95°C for
30 sec, and annealing and extension at 60°C for 1 min. The
nucleotide sequences of the PCR primers were as follows: β-actin,
forward, 5′-CGCAAAGACCTGTATGCCAA-3′ and reverse,
5′-CACACAGAGTACTTGCGCTC-3′; p53, forward,
5′-TGGCCATCTACAAGCAGTCA-3′ and reverse, 5′-GGTACAGTCAGAGCCAACCT-3′;
p21, forward, 5′-GGGCTGGGAGTAGTTGTCTT-3′ and reverse,
5′-ATTGTGGGAGGAGCTGTGAA-3′; SCO1, forward,
5′-ATTGCCCTGATGTCTGTCCA-3′ and reverse, 5′-CTCTTCTCTCGTGCCAGTCA-3′;
SCO2, forward, 5′-TCTTCATCACTGTGGACCCC-3′ and reverse,
5′-TTGGGGCCTGCATTGTAGTA-3′; TIGAR, forward,
5′-CTGGACCAGGTGAAAATGCG-3′ and reverse, 5′-ACTGGCTGCTAATCCTGGAA-3′
(Tsingke Biological Technology, Beijing, China).
Western blot analysis
Total protein was extracted using
ProteoPrep® Total Extraction Sample kit (cat. no.
PROT-TOT; Sigma-Aldrich, St. Louis, MO, USA) and the concentration
was determined using the QuantiPro™ BCA Assay kit (cat. no. QPBCA;
Sigma-Aldrich). The total proteins from the target cells were mixed
with the equal quantities of 2X sodium dodecyl sulfate (SDS) sample
buffer [10 mM Tris-HCl (pH 6.8), 0.05% SDS and 0.01% Bromophenol
Blue] and boiled at 100°C for 10 min. The proteins (50 μg)
were separated by 10% SDS-polyacrylamide gel electrophoresis and
were subsequently transferred onto nitrocellulose membranes. The
membranes were blocked for 1 h at room temperature with blocking
solution [5% bovine serum albumen in phosphate-buffered saline
(PBS) with Tween-20]. The membranes were then incubated overnight
at 4°C with the primary antibodies, followed by washing three times
with PBS containing 0.3% Tween-20 and incubation with the
HRP-conjugated secondary antibodies for 1 h at room temperature.
Immunoreactive proteins were visualized using enhanced
chemiluminescence detection (Pierce, Rockford, IL, USA).
Quantification of the band intensities was performed using the
ChemiDoc MP system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
with Image Lab 5 software.
Cell phase percentage assay
The cell cycle phase percentage was analyzed by flow
cytometry (17). Trypsinized cells
were washed with PBS and fixed in 75% ethanol. The cells were
subsequently incubated with 100 μg/ml RNase at 37°C for 30
min and stained with 50 μg/ml propidium iodide for 10 min at
room temperature. The cells were analyzed on a FACScan flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) with FCS
Express 5 software (De Novo Software, Glendale, CA, USA).
EdU incorporation assay
EdU is a thymidine analogue used to label
proliferating cells, which can incorporate into replicating DNA
when the cells are dividing (18).
The cells were assessed using Cell-LightTM EdU DNA cell
Proliferation kit (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Each assay was performed three
times.
Oxygen consumption
Treated or untreated cells were trypsinized and
resuspended at 106 cells/ml in DMEM. The oxygen
consumption was measured in a 1 ml volume stirred, sealed chamber
using a Clark-type oxygen micro-electrode (Unisense, Aarhus,
Denmark) at 37°C (11).
Production of lactate
Treated or untreated cells were trypsinized and
washed with PBS twice. A total of 106 cells were
resuspended in 100 μl ice-cold PBS, containing 3 mg/ml
glucose and 0, 0.28 or 0.56 mM EIso. Lactate production was
measured at 37°C for 30 min using the Lactate reagent kit (Trinity
Biotech Plc., Co Wicklow, Ireland) on a microplate reader (Synergy
2; BioTek Instruments, Inc., Winooski, VT, USA), according to the
manufacturer's protocol.
Measurement of intracellular ROS
To identify the intracellular ROS levels, treated or
untreated cells were harvested following any relevant treatment
after incubation with DMEM containing 1% FBS and 100 μM
DCFDA (freshly prepared) for 30 min in dark. The cells were washed
twice with PBS and treated with either dimethyl sulfoxide (0.1%)
alone or grape seed extract (GSE; 100 μg/ml) in PBS in the
dark. The increase in fluorescence was measured at an excitation
wavelength of 485 nm and emission wavelength of 538 nm using a
fluorescent plate reader (Synergy 2). The background fluorescence
of GSE (100 μg/ml) in the absence of DCFDA was also
adjusted.
Statistical analysis
Statistical analyses were performed using JMP 9
software (http://www.jmp.com/en_gb/home.html). Data were
analyzed by the Student's t-test and Fisher's exact test. P<0.05
was considered to indicate a statistically significant
difference.
Results
EIso increases the mRNA expression levels
of p21 and decreases the mRNA expression levels of TIGAR,
SCO1 and SCO2 in a p53-independent manner in
16HBE cells
To investigate the effects of EIso treatment on gene
expression in 16HBE cells, 0.28 or 0.56 mM EIso or mock were added
into medium. The mRNA expression levels of p53 and its downstream
genes were detected by RT-qPCR. The results revealed that, without
disturbance of the p53 mRNA level, p21 mRNA was upregulated
significantly and the mRNA expression levels of TIGAR,
SCO1 and SCO2 were significantly decreased,
indicating that changes in mRNA expression levels were irrelevant
to p53's transcriptional regulation (Fig. 1A). Western blot analysis of these
proteins further confirmed the downregulation by EIso treatment
(Fig. 1B). Knockdown of p53 mRNA
expression caused no changes in the effects of EIso treatment on
these gene expression levels, further confirming that these
processes were p53-independent (Fig.
1C and D). For further confirmation that the p53 DNA binding
activity was not affected by EIso treatment, ChIP was performed and
the quantity of responsive DNA element bound by p53 of each gene
were quantified by qPCR. Consistently, p53 DNA binding activities
at TIGAR, SCO1, and SCO2 were not inhibited
by EIso treatment (Fig. 1E).
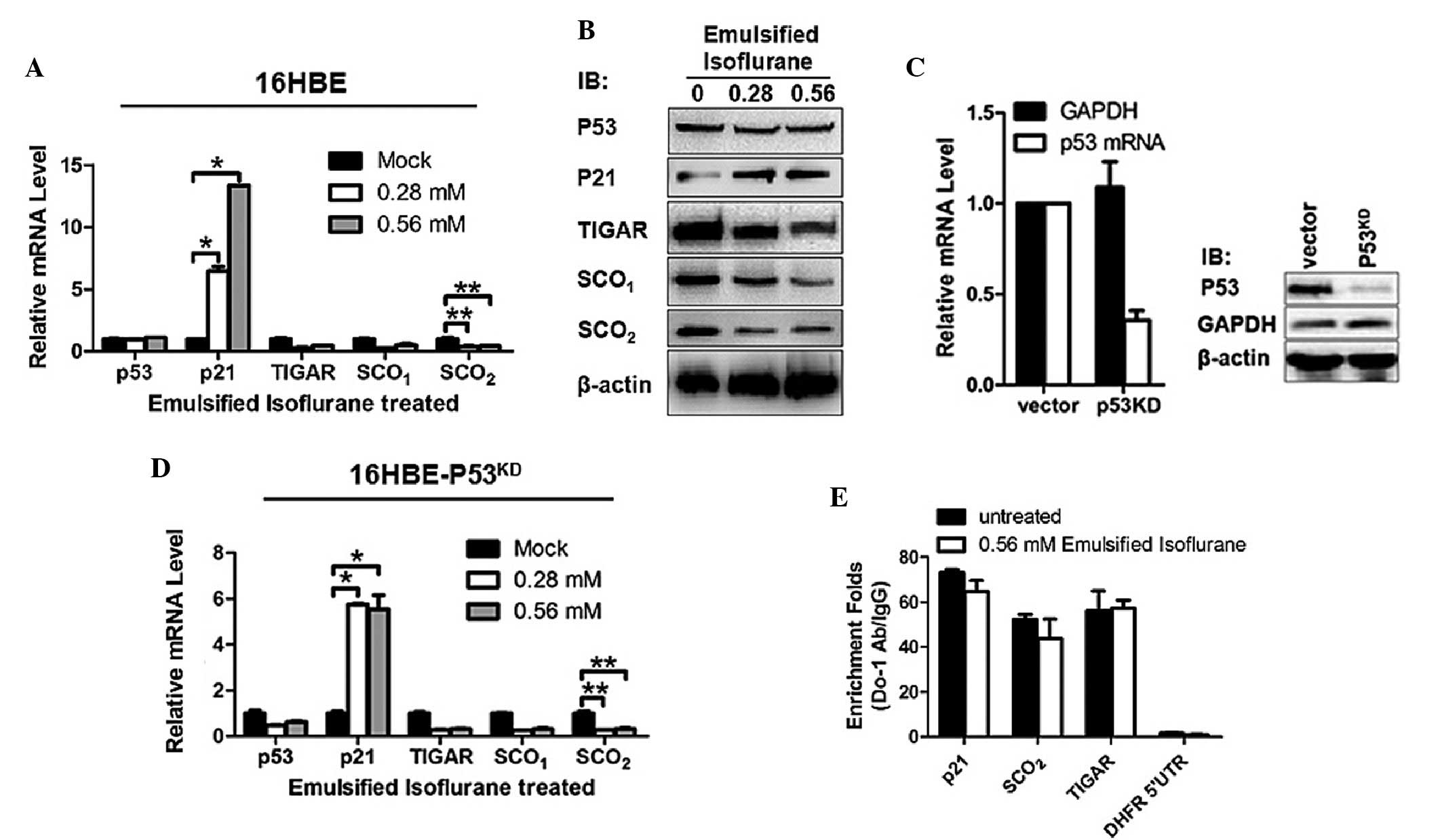 | Figure 1EIso treatment transcriptionally
increased the expression of p21 and decreased the expression levels
of TIGAR, SCO1 and SCO2. (A) RT-qPCR was
performed to assess the transcriptional expression levels of p53,
p21, TIGAR, SCO1 and SCO2 in EIso treated
16HBE cells. (B) Western blotting was performed to detect the
protein expression levels of p53, P21, TIGAR, SCO1 and
SCO2 in EIso treated 16HBE cells. β-actin was used as a
loading control. (C) The relative mRNA and protein expression
levels of p53 were determined in 16HBE cells stably transfected
with short hairpin RNA targeted to p53 mRNA. GAPDH and β-actin were
used as a loading controls for normalization. (D) The
transcriptional levels of p53, p21, TIGAR, SCO1 and
SCO2 were assessed in EIso treated
16HBE-p53KD cells. (E) Chromatin
immunoprecipitation-qPCR analysis was performed to identify the
binding of p53 to the promoter region of p21, SCO2 and
TIGAR. The data are presented as the mean ± standard deviation
(*P<0.05 and **P<0.01 compared with the
mock/untreated cells). EIso, emulsified isoflurane; KD, knockdown;
RT, reverse transcription; qPCR, quantitative polymerase chain
reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Ig,
immunoglobulin; SCO, synthesis of cytochrome c oxidase; TIGAR,
Tp53-induced glycolysis and apoptosis regulator. |
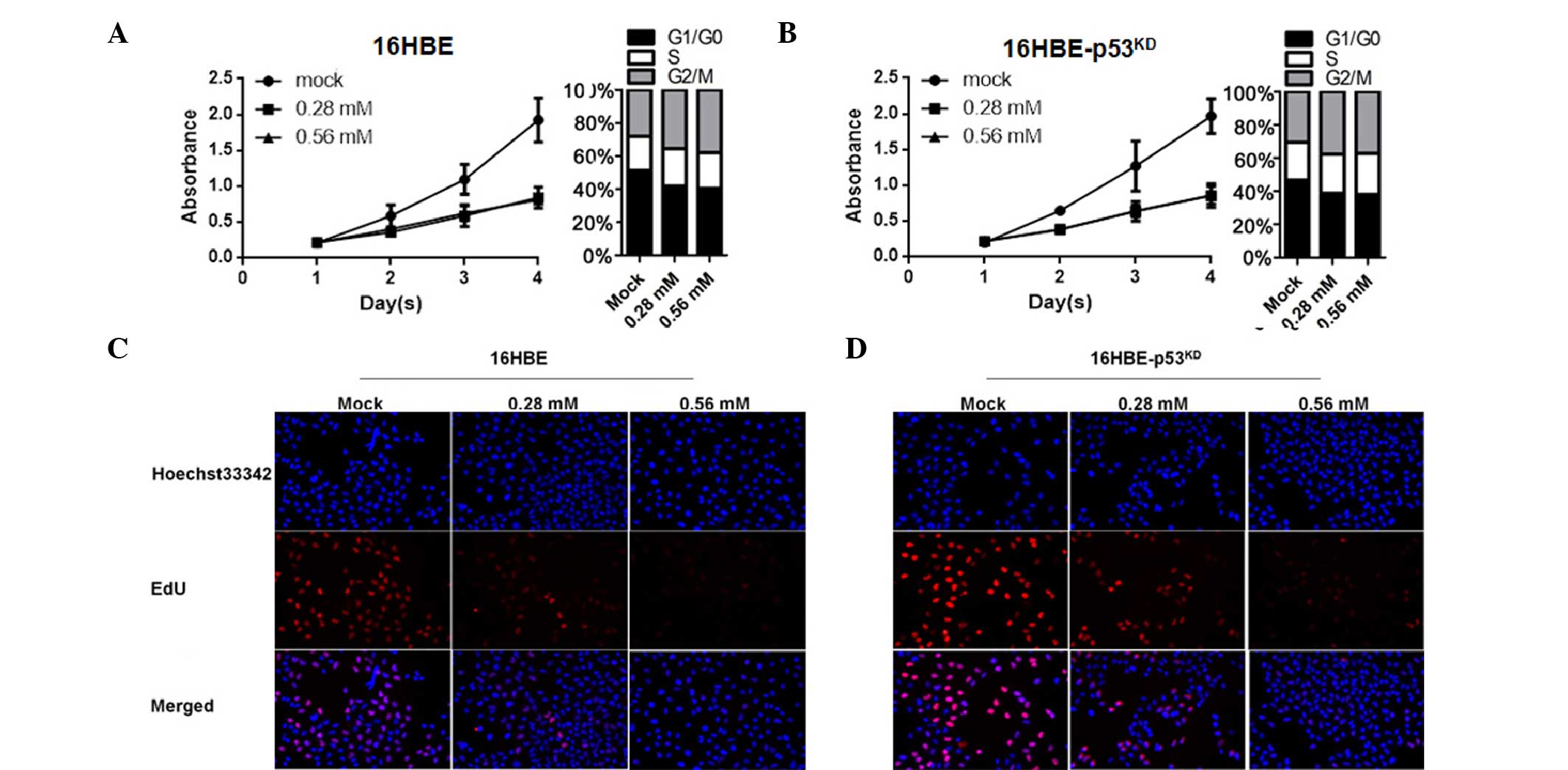
EIso treatment inhibits the cell cycle
and thus inhibits cell proliferation by upregulating p21
The expression of p21 serves an important role in
cell cycle arrest by inhibiting cyclin-dependent kinase activities.
Considering the effect of p21 on cell cycle arrest, the present
study next investigated the effect of EIso on the cell cycle by
upregulating p21. Compared with the mock group, EIso-treated 16HBE
cells exhibited a reduction in cell proliferation by cell cycle
inhibition at the G0/G1 stage (Fig.
2A). Consistent with previous results (Fig. 1D and E), no change occurred in
16HBE cells with reduced levels of p53 (Fig. 2B). The EdU incorporation assay was
performed to detect whether EIso treatment influenced the number of
proliferating cells. The results demonstrated that the number of
EdU-positive cells in the EIso-treated group was reduced compared
with the mock group (Fig. 2C and
D).
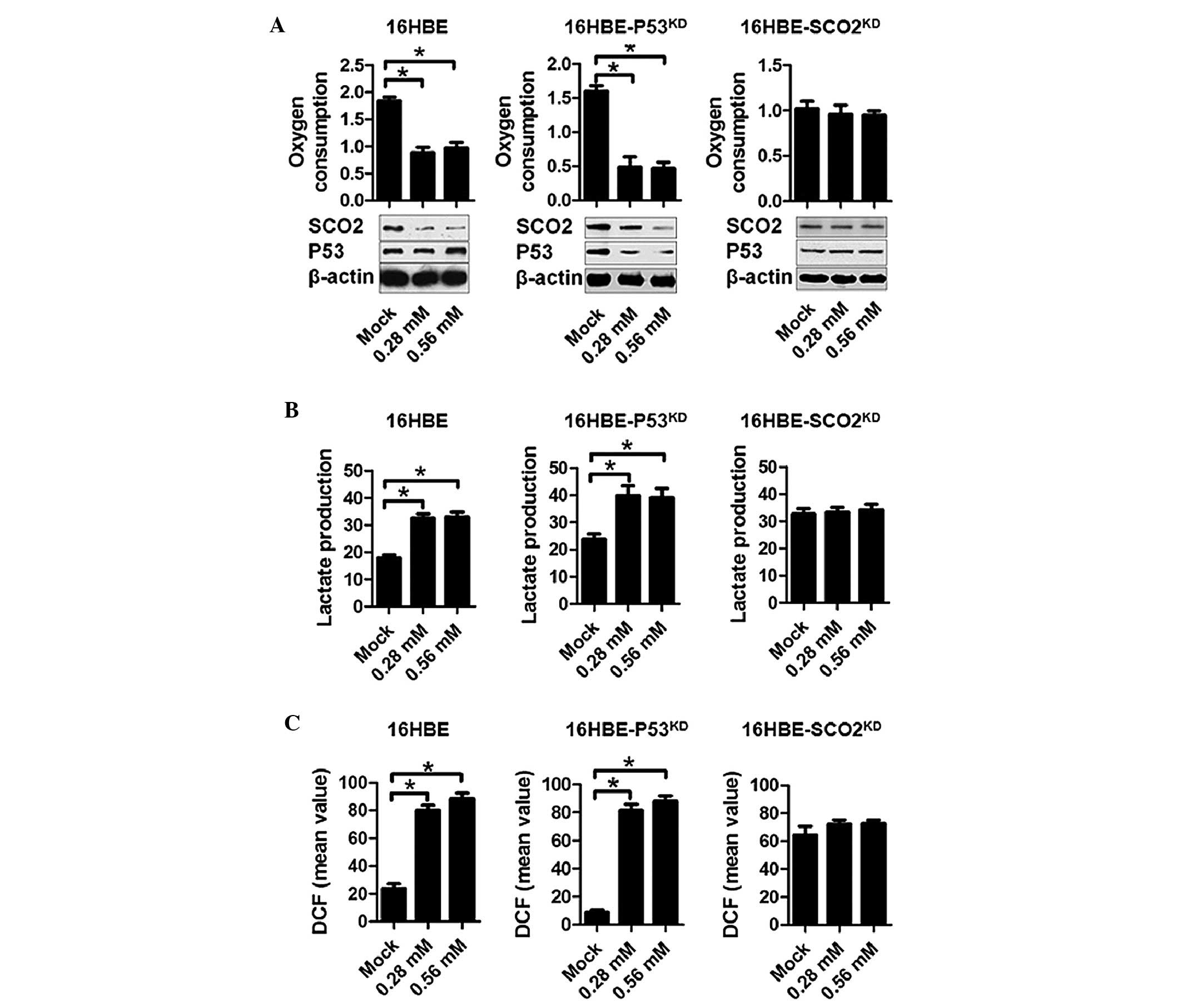
EIso treatment inhibits respiration,
promotes glycolysis and sensitizes cells to oxidative damage
As one of the essential proteins for the assembly of
the mitochondrial cytochrome c oxidase, SCO2
serves a critical role in the metabolic centre of eukaryotic oxygen
consumption (19,20). Since SCO2 levels were
reduced by EIso treatment, the present study assessed the oxygen
consumption and lactate production in EIso-treated s16HBE cells. To
create a stable, respiration-inhibited cell line, shRNA targeting
SCO2 mRNA was transfected into 16HBE cells
(16HBE-SCO2KD). The RT-qPCR and
semi-quantitative western blotting were performed to confirm the
disruption of SCO2 expression (data not shown). In 16HBE
and 16HBE-p53KD, EIso treatment exhibited a marked
reduction in oxygen consumption compared with the mock goup.
However, this effect was abolished by SCO2 knockdown in
16HBE-SCO2KD cells, indicating that the
effect of EIso treatment was functioning by a cessation of
oxidative phosphorylation and a compensatory increase in lactate
production (Fig. 3A and B). 16HBE
and 16HBE-P53KD, but not 16HBE-SCO2KD cells, demonstrated a
bioenergetic balance on respiration and glycolysis, as evidenced by
a negative association between oxygen consumption and lactate
production (Fig. 3A and B). These
results suggested that the sensitivity of EIso treated 16HBE cells
to oxygen was caused by generation of ROS. To further examine this
possibility, intracellular ROS levels were measured using the
hydrogen peroxide-sensitive dye dichlorofluorescein (DCF). Compared
with the mock group, 16HBE and 16HBE-p53KD cells
exhibited notably increased levels of ROS, as indicated by DCF
staining intensity. No detectable change was observed in the
16HBE-SCO2KD cells (Fig. 3C).
Discussion
Ischemia followed by reperfusion injury causes a
number of clinical disorders to the lung, including lung
dysfunction syndrome and failure. This process may cause I/R injury
directly to the lung and indirectly by causing remote organ injury,
including gut and liver I/R injury (21). The activation of inflammatory
reactions may occur via various signaling pathways that culminate
in the activation of nuclear factor (NF)-κB and upregulation of
TNF-α and ICAM-1 (22). It is
reported that 1.5 h hepatic ischemia followed by 4 h reperfusion
caused irreversible lung damage, with a significant increasing
trend of NF-κB translocation, and an increase in TNF-α, and ICAM-1
transcriptional levels in the lung tissue (7).
EIso has been focussed on due to its effects on
eliminating the requirement for specific ventilator circuits, and
providing rapid anesthetic induction and recovery (2). Additionally, EIso pre-treatment has
been found to attenuate oxidative stress and prevent I/R injury.
Wang et al (23) reported
that EIso pre-treatment protects isolated rat Kupffer cells against
I/R induced injury by decreasing the production of ROS. It is also
been reported that EIso pre-treatment caused a significant effect
on attenuating inflammation and oxidative-caused damage (24). Notably, EIso pre-treatment markedly
decreased I/R-induced lung injury in rats, which prompted the
present study to investigate whether it is the same in human lung
cells.
The results of the present study revealed that EIso
pre-treatment markedly attenuated the mRNA and protein expression
levels of TIGAR, SCO1 and SCO2, and
stimulated the expression of p21, which all are the direct
downstream target genes of p53 in 16HBE cells. As a result of the
downregulation of p21, the cell cycle was arrested at G0/G1 phase
and cell proliferation was significantly inhibited compared with
the untreated group. Considering the important roles of TIGAR,
SCO1 and SCO2 in respiration, downregulation
of these proteins prompted the present study to detect the
respiration-associated processes. Consistently, EIso treatment
decreased the oxygen consumption in 16HBE cells and promoted the
production of lactate and the levels of ROS. To the best of our
knowledge, this is the first study providing evidence treatment of
16HBE cells with EIso inhibits cell proliferation by arresting the
cell cycle and inhibits respiration by transcriptionally
downregulating respiration chain-associated genes, including TIGAR,
SCO1 and SCO2 in a p53-independent
manner.
ROS accumulate during ischemia and increase rapidly
in the process of reperfusion. The existence of ROS causes membrane
lipid peroxidation, changes in protein structure or function, and
irreversible oxidative damage to the genome (25–27).
The findings that overexpression of antioxidant enzymes at or prior
to reperfusion contributes to significant protection from I/R
injury in numerous models (28),
indicating the damages of EIso treatment to treated cells via the
inhibition of respiration. In the present study, it was found that
EIso treatment increased ROS accumulation in 16HBE cells by
downregulation respiration-associated genes, including TIGAR,
SCO1 and SCO2. Whether the accumulated ROS
causes DNA damage or not following treatment with EIso in different
oxygen concentrations was subsequently investigated, and revealed
that in high oxygen concentration >20% EIso treatment promoted
cell apoptosis (data not shown). This result indicated that the
protective effect of EIso treatment in 16HBE cells may be dependent
on low oxygen concentration.
Acknowledgments
The authors would like to thank Professor Ming Chen
(Life Science and Technology College, Sichuan University, Sichuan,
China) for his excellent technical assistance.
References
|
1
|
Lucchinetti E, Schaub MC and Zaugg M:
Emulsified intravenous versus evaporated inhaled isoflurane for
heart protection: Old wine in a new bottle or true innovation?
Anesth Analg. 106:1346–1349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu ZY and Liu J: Effects of emulsified
isoflurane on haemodynamics and cardiomyocyte apoptosis in rats
with myocardial ischaemia. Clin Exp Pharmacol Physiol. 36:776–783.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kersten JR, Schmeling TJ, Pagel PS, Gross
GJ and Warltier DC: Isoflurane mimics ischemic preconditioning via
activation of K (ATP) channels: Reduction of myocardial infarct
size with an acute memory phase. Anesthesiology. 87:361–370. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rao Y, Wang YL, Zhang WS and Liu J:
Emulsified isoflurane produces cardiac protection after
ischemia-reperfusion injury in rabbits. Anesth Analg.
106:1353–1359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schurr A, Reid KH, Tseng MT, West C and
Rigor BM: Adaptation of adult brain tissue to anoxia and hypoxia in
vitro. Brain Res. 374:244–248. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv X, Wang ZM, Huang SD, Song SH, Wu FX
and Yu WF: Emulsified isoflurane preconditioning reduces lung
injury induced by hepatic ischemia/reperfusion in rats. Int J Med
Sci. 8:353–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu ZY, Abbott GW, Fang YD, Huang YS and
Liu J: Emulsified isoflurane postconditioning produces
cardioprotection against myocardial ischemia-reperfusion injury in
rats. J Physiol Sci. 63:251–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu ZY, Peng XY, Liu F and Liu J:
Emulsified isoflurane protects rat heart in situ after regional
ischemia and reperfusion. Fundam Clin Pharmacol. 28:190–198. 2014.
View Article : Google Scholar
|
|
10
|
Di Lisa F and Bernardi P: Mitochondria and
ischemia-reperfusion injury of the heart: Fixing a hole. Cardiovasc
Res. 70:191–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matoba S, Kang JG, Patino WD, Wragg A,
Boehm M, Gavrilova O, Hurley PJ, Bunz F and Hwang PM: P53 regulates
mitochondrial respiration. Science. 312:1650–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura H, Matoba S, Iwai-Kanai E, Kimata
M, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Ariyoshi M, Mita Y,
et al: P53 promotes cardiac dysfunction in diabetic mellitus caused
by excessive mitochondrial respiration-mediated reactive oxygen
species generation and lipid accumulation. Circ Heart Fail.
5:106–115. 2012. View Article : Google Scholar
|
|
13
|
Rodriguez R and Meuth M: Chk1 and p21
cooperate to prevent apoptosis during DNA replication fork stress.
Mol Biol Cell. 17:402–412. 2006. View Article : Google Scholar :
|
|
14
|
Nishioka S, Nakano D, Kitada K, Sofue T,
Ohsaki H, Moriwaki K, Hara T, Ohmori K, Kohno M and Nishiyama A:
The cyclin-dependent kinase inhibitor p21 is essential for the
beneficial effects of renal ischemic preconditioning on renal
ischemia/reperfusion injury in mice. Kidney Int. 85:871–879. 2014.
View Article : Google Scholar
|
|
15
|
Yang XL, Ma HX, Yang ZB, Liu AJ, Luo NF,
Zhang WS, Wang L, Jiang XH, Li J and Liu J: Comparison of minimum
alveolar concentration between intravenous isoflurane lipid
emulsion and inhaled isoflurane in dogs. Anesthesiology.
104:482–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou JX, Luo NF, Liang XM and Liu J: The
efficacy and safety of intravenous emulsified isoflurane in rats.
Anesth Analg. 102:129–134. 2006. View Article : Google Scholar
|
|
17
|
Ou YC, Yang CR, Cheng CL, Raung SL, Hung
YY and Chen CJ: Indomethacin induces apoptosis in 786-O renal cell
carcinoma cells by activating mitogen-activated protein kinasesand
AKT. Eur J Pharmacol. 563:49–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chehrehasa F, Meedeniya AC, Dwyer P,
Abrahamsen G and Mackay-Sim A: EdU, a new thymidine analogue for
labelling proliferating cells in the nervous system. J Neurosci
Methods. 177:122–130. 2009. View Article : Google Scholar
|
|
19
|
Papadopoulou LC, Sue CM, Davidson MM,
Tanji K, Nishino I, Sadlock JE, Krishna S, Walker W, Selby J,
Glerum DM, et al: Fatal infantile cardioencephalomyopathy with COX
deficiency and mutations in SCO2, a COX assembly gene. Nat Genet.
23:333–337. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leary SC, Cobine PA, Kaufm an BA, Guercin
GH, Mattman A, Palaty J, Lockitch G, Winge DR, Rustin P, Horvath R
and Shoubridge EA: The human cytochrome c oxidase assembly factors
SCO1 and SCO2 have regulatory roles in the maintenance of cellular
copper homeostasis. Cell Metab. 5:9–20. 2007. View Article : Google Scholar
|
|
21
|
Hato S, Urakami A, Yamano T, Uemura T, Ota
T, Hirai R and Shimizu N: Attenuation of liver and lung injury
after hepatic ischemia and reperfusion by a cytokine-suppressive
agent, FR167653. Eur Surg Res. 33:202–209. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okaya T, Holthaus R, Kato A and Lentsch
AB: Involvement of the neuropeptide substance P in lung
inflammation induced by hepatic ischemia/reperfusion. Inflamm Res.
53:257–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Lv H, Song S, Shen X, Yang L and
Yu W: Emulsified isoflurane preconditioning protects isolated rat
Kupffer cells against hypoxia/reoxygenation-induced injury. Int J
Med Sci. 10:286–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin Z, Lv E, Zhan L, Xing X, Jiang J and
Zhang M: Intravenous pretreatment with emulsified isoflurane
preconditioning protects kidneys against ischemia/reperfusion
injury in rats. BMC Anesthesiol. 14:282014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kowaltowski AJ and Vercesi AE:
Mitochondrial damage induced by conditions of oxidative stress.
Free Radic Biol Med. 26:463–471. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robin E, Guzy RD, Loor G, Iwase H, Waypa
GB, Marks JD, Hoek TL and Schumacker PT: Oxidant stress during
simulated ischemia primes cardiomyocytes for cell death during
reperfusion. J Biol Chem. 282:19133–19143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zweier JL, Flaherty JT and Weisfeldt ML:
Direct measurement of free radical generation following reperfusion
of ischemic myocardium. Proc Natl Acad Sci USA. 84:1404–1407. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Z, Siu B, Ho YS, Vincent R, Chua CC,
Hamdy RC and Chua BH: Overexpression of MnSOD protects against
myocardial ischemia/reperfusion injury in transgenic mice. J Mol
Cell Cardiol. 30:2281–2289. 1998. View Article : Google Scholar
|