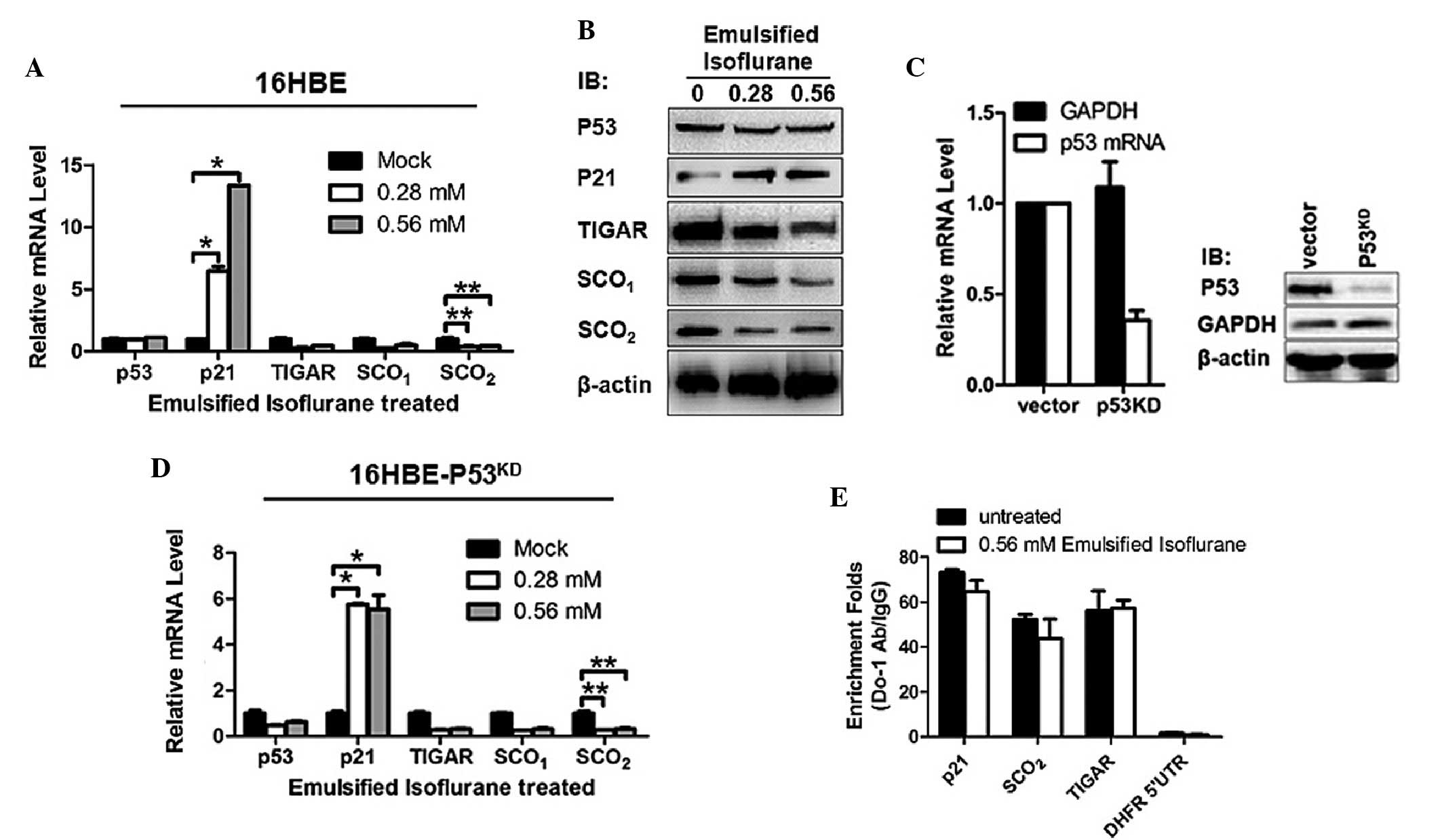
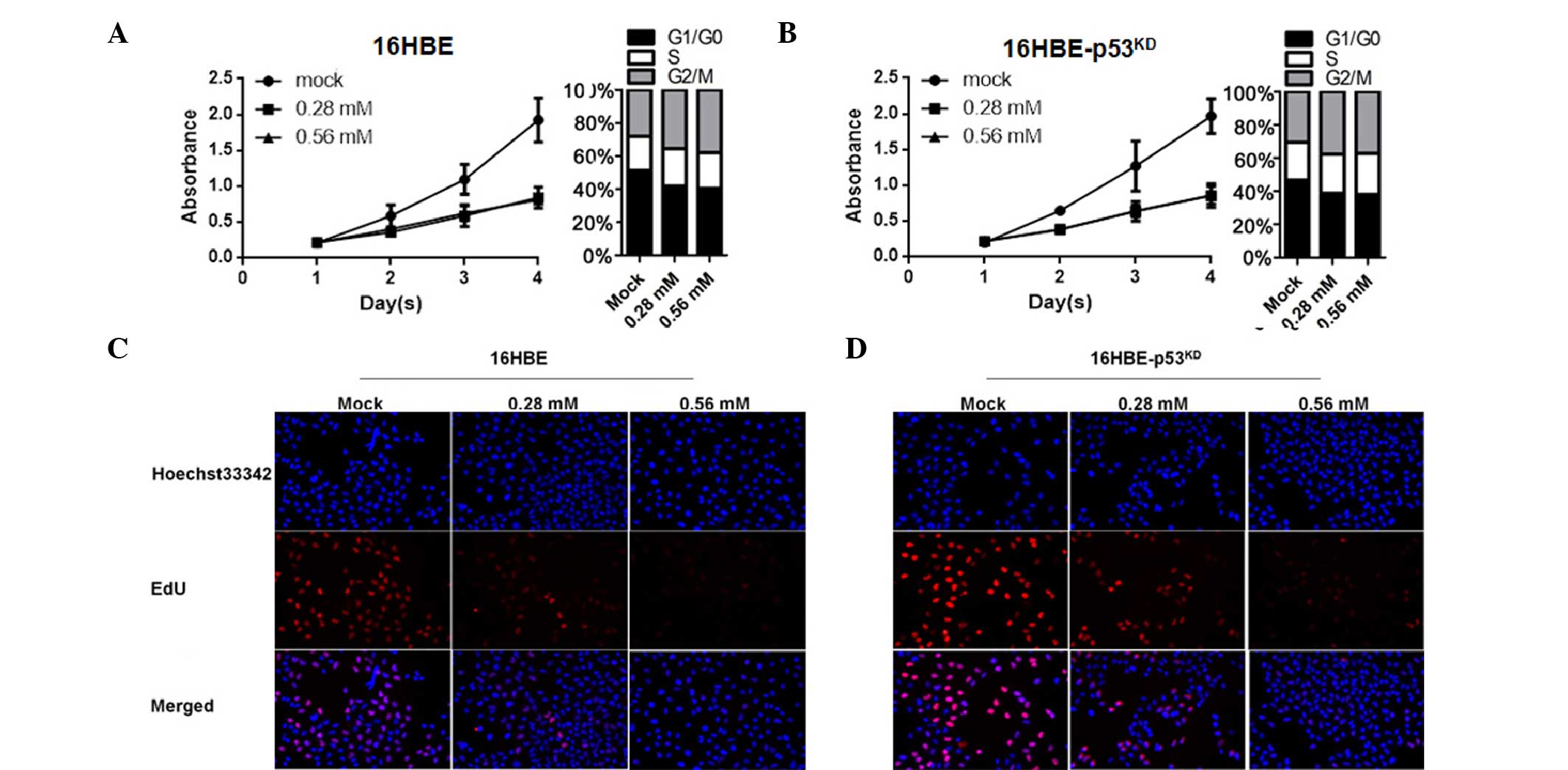
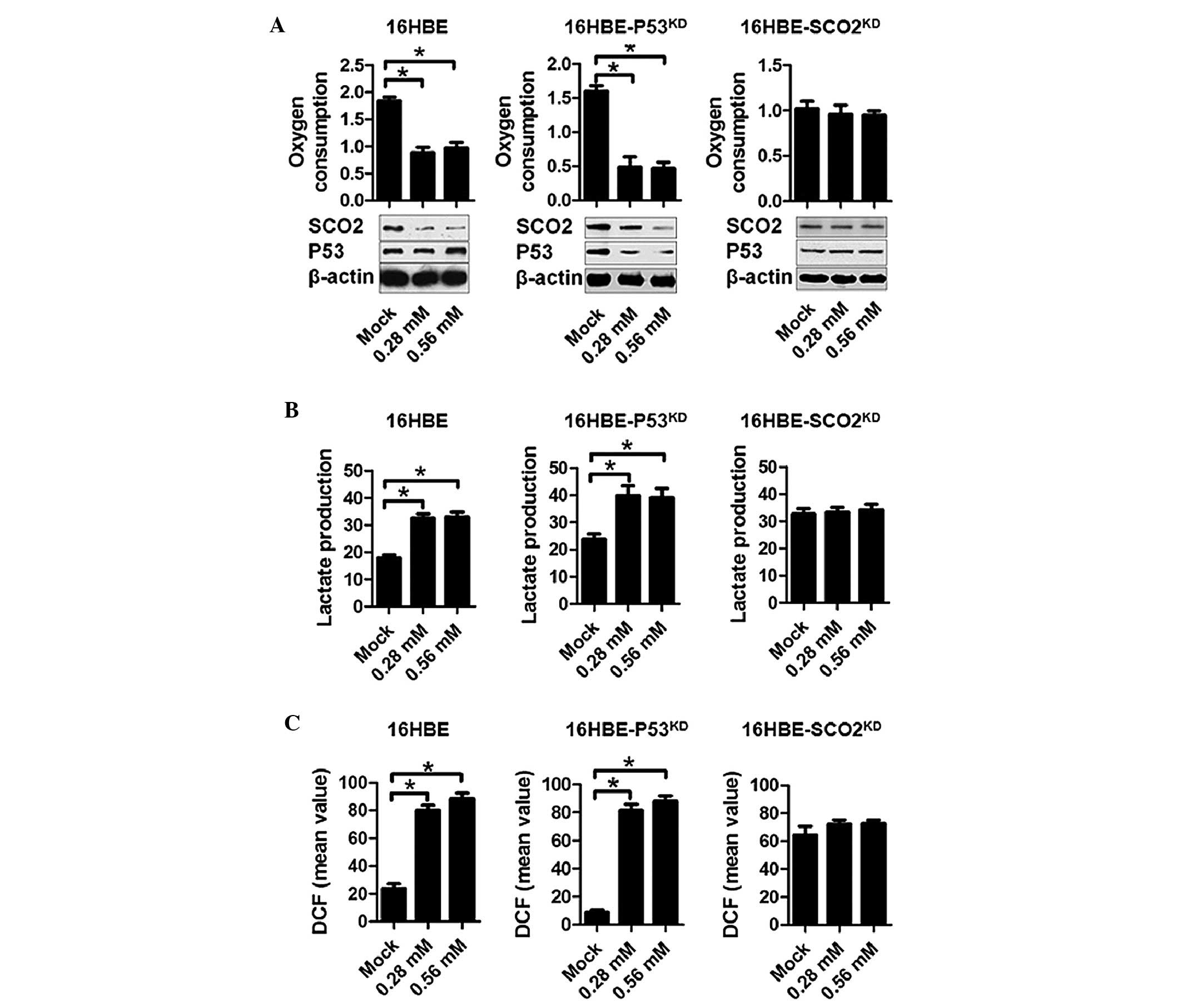
|
1
|
Lucchinetti E, Schaub MC and Zaugg M:
Emulsified intravenous versus evaporated inhaled isoflurane for
heart protection: Old wine in a new bottle or true innovation?
Anesth Analg. 106:1346–1349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu ZY and Liu J: Effects of emulsified
isoflurane on haemodynamics and cardiomyocyte apoptosis in rats
with myocardial ischaemia. Clin Exp Pharmacol Physiol. 36:776–783.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kersten JR, Schmeling TJ, Pagel PS, Gross
GJ and Warltier DC: Isoflurane mimics ischemic preconditioning via
activation of K (ATP) channels: Reduction of myocardial infarct
size with an acute memory phase. Anesthesiology. 87:361–370. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rao Y, Wang YL, Zhang WS and Liu J:
Emulsified isoflurane produces cardiac protection after
ischemia-reperfusion injury in rabbits. Anesth Analg.
106:1353–1359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schurr A, Reid KH, Tseng MT, West C and
Rigor BM: Adaptation of adult brain tissue to anoxia and hypoxia in
vitro. Brain Res. 374:244–248. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv X, Wang ZM, Huang SD, Song SH, Wu FX
and Yu WF: Emulsified isoflurane preconditioning reduces lung
injury induced by hepatic ischemia/reperfusion in rats. Int J Med
Sci. 8:353–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu ZY, Abbott GW, Fang YD, Huang YS and
Liu J: Emulsified isoflurane postconditioning produces
cardioprotection against myocardial ischemia-reperfusion injury in
rats. J Physiol Sci. 63:251–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu ZY, Peng XY, Liu F and Liu J:
Emulsified isoflurane protects rat heart in situ after regional
ischemia and reperfusion. Fundam Clin Pharmacol. 28:190–198. 2014.
View Article : Google Scholar
|
|
10
|
Di Lisa F and Bernardi P: Mitochondria and
ischemia-reperfusion injury of the heart: Fixing a hole. Cardiovasc
Res. 70:191–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matoba S, Kang JG, Patino WD, Wragg A,
Boehm M, Gavrilova O, Hurley PJ, Bunz F and Hwang PM: P53 regulates
mitochondrial respiration. Science. 312:1650–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura H, Matoba S, Iwai-Kanai E, Kimata
M, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Ariyoshi M, Mita Y,
et al: P53 promotes cardiac dysfunction in diabetic mellitus caused
by excessive mitochondrial respiration-mediated reactive oxygen
species generation and lipid accumulation. Circ Heart Fail.
5:106–115. 2012. View Article : Google Scholar
|
|
13
|
Rodriguez R and Meuth M: Chk1 and p21
cooperate to prevent apoptosis during DNA replication fork stress.
Mol Biol Cell. 17:402–412. 2006. View Article : Google Scholar :
|
|
14
|
Nishioka S, Nakano D, Kitada K, Sofue T,
Ohsaki H, Moriwaki K, Hara T, Ohmori K, Kohno M and Nishiyama A:
The cyclin-dependent kinase inhibitor p21 is essential for the
beneficial effects of renal ischemic preconditioning on renal
ischemia/reperfusion injury in mice. Kidney Int. 85:871–879. 2014.
View Article : Google Scholar
|
|
15
|
Yang XL, Ma HX, Yang ZB, Liu AJ, Luo NF,
Zhang WS, Wang L, Jiang XH, Li J and Liu J: Comparison of minimum
alveolar concentration between intravenous isoflurane lipid
emulsion and inhaled isoflurane in dogs. Anesthesiology.
104:482–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou JX, Luo NF, Liang XM and Liu J: The
efficacy and safety of intravenous emulsified isoflurane in rats.
Anesth Analg. 102:129–134. 2006. View Article : Google Scholar
|
|
17
|
Ou YC, Yang CR, Cheng CL, Raung SL, Hung
YY and Chen CJ: Indomethacin induces apoptosis in 786-O renal cell
carcinoma cells by activating mitogen-activated protein kinasesand
AKT. Eur J Pharmacol. 563:49–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chehrehasa F, Meedeniya AC, Dwyer P,
Abrahamsen G and Mackay-Sim A: EdU, a new thymidine analogue for
labelling proliferating cells in the nervous system. J Neurosci
Methods. 177:122–130. 2009. View Article : Google Scholar
|
|
19
|
Papadopoulou LC, Sue CM, Davidson MM,
Tanji K, Nishino I, Sadlock JE, Krishna S, Walker W, Selby J,
Glerum DM, et al: Fatal infantile cardioencephalomyopathy with COX
deficiency and mutations in SCO2, a COX assembly gene. Nat Genet.
23:333–337. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leary SC, Cobine PA, Kaufm an BA, Guercin
GH, Mattman A, Palaty J, Lockitch G, Winge DR, Rustin P, Horvath R
and Shoubridge EA: The human cytochrome c oxidase assembly factors
SCO1 and SCO2 have regulatory roles in the maintenance of cellular
copper homeostasis. Cell Metab. 5:9–20. 2007. View Article : Google Scholar
|
|
21
|
Hato S, Urakami A, Yamano T, Uemura T, Ota
T, Hirai R and Shimizu N: Attenuation of liver and lung injury
after hepatic ischemia and reperfusion by a cytokine-suppressive
agent, FR167653. Eur Surg Res. 33:202–209. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okaya T, Holthaus R, Kato A and Lentsch
AB: Involvement of the neuropeptide substance P in lung
inflammation induced by hepatic ischemia/reperfusion. Inflamm Res.
53:257–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Lv H, Song S, Shen X, Yang L and
Yu W: Emulsified isoflurane preconditioning protects isolated rat
Kupffer cells against hypoxia/reoxygenation-induced injury. Int J
Med Sci. 10:286–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin Z, Lv E, Zhan L, Xing X, Jiang J and
Zhang M: Intravenous pretreatment with emulsified isoflurane
preconditioning protects kidneys against ischemia/reperfusion
injury in rats. BMC Anesthesiol. 14:282014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kowaltowski AJ and Vercesi AE:
Mitochondrial damage induced by conditions of oxidative stress.
Free Radic Biol Med. 26:463–471. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robin E, Guzy RD, Loor G, Iwase H, Waypa
GB, Marks JD, Hoek TL and Schumacker PT: Oxidant stress during
simulated ischemia primes cardiomyocytes for cell death during
reperfusion. J Biol Chem. 282:19133–19143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zweier JL, Flaherty JT and Weisfeldt ML:
Direct measurement of free radical generation following reperfusion
of ischemic myocardium. Proc Natl Acad Sci USA. 84:1404–1407. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Z, Siu B, Ho YS, Vincent R, Chua CC,
Hamdy RC and Chua BH: Overexpression of MnSOD protects against
myocardial ischemia/reperfusion injury in transgenic mice. J Mol
Cell Cardiol. 30:2281–2289. 1998. View Article : Google Scholar
|