Introduction
Gastritis is a condition involving inflammation,
irritation and erosion, which occurs when the endogenous defense
mechanisms of the mucosal barrier cannot protect the organ. Alcohol
increases the production of reactive oxygen species (ROS) and
oxidative stress, and decreases the levels of antioxidant in a
number of cells and tissues, leading to gastric damage (1). ROS provoke severe changes at the
cellular level, which can lead to cell death resulting from the
marked reactivity. Increased ROS production leads to lipid
peroxidation by reacting with the double bonds in unsaturated fatty
acids, which causes the formation of multiple electrophilic
aldehyde species. These species are capable of forming adducts with
proteins, which leads to protein dysfunction (2). Oxidative stress occurs when ROS
production exceeds the capacity of the cellular antioxidant system,
or when the functioning of the antioxidant defense system is
insufficient to neutralize the oxidants (3). Ethanol causes severe oxidative stress
in gastric tissue, and a competent antioxidant defense system is
important to provide gastric protection (4). Previous studies have reported that
antioxidant enzymes protect against ethanol-induced gastric mucosal
injury (5–7).
Prostaglandins exert a gastroprotective effect
against gastric mucosal injury through the maintenance of gastric
mucus synthesis and secretion (8).
In particular, prostaglandin E2 (PGE2) is
important in the regulation of gastric mucus secretion (9). PGE2 has been shown to have
protective effects in various gastric injury models (10). However, ethanol reduces mucosal
PGE2 content (11).
According to the World Health Organization, >80%
of the world's population relies on medicinal herbs for their
primary healthcare requirements (12). The herbs used for traditional
medicine contain a wide range of substances, which are used to
prevent or treat various diseases (13). Syzygium aromaticum (SA), one
type of medicinal herb, has antioxidant activity (14), antifungal activity (15), a hypoglycemic effect (16), a bone-preserving effect (17), chemopreventive potential in lung
cancer (18), effects on the
immune response (19) and an
antiobesity effect (20). The
antioxidant properties of SA suggest that it may be a promising
candidate as an antigastritis or antiulcer agent. Therefore, in the
present study, whether SA water extract (SAWE) has gastroprotective
potential against ethanol-induced gastric mucosal injury in rats
was investigated.
Materials and methods
Preparation of SAWE
SAWE was prepared in K-herb Research Center of Korea
Institute of Oriental Medicine (Daejeon, Korea). The extraction and
high-performance liquid chromatography analysis were performed, as
described previously (21).
Ethanol-induced gastritis
Specific-pathogen-free male Sprague-Dawley rats,
(200–250 g; 6 weeks old) from Daehan Biolink Co., Ltd. (Chungbuk,
Korea) were acclimatized for 1 week prior to the start of the
investigation with evaluation of health status. The animals were
maintained in environmentally controlled rooms at 23±3°C under a
relative humidity of 50±10% with a 12 h light-dark cycle and 12–15
air changes/h, as previously described (22).
The present study was performed at the Korea
Institute of Oriental Medicine (Daejeon, Republic of Korea), and
the protocol was approved by the Institutional Animal Care and Use
Committee. All experimental procedures were performed in compliance
with the National Institute of Health Guidelines for the Care and
Use of Laboratory Animals (23),
and the National Animal Welfare Law of Korea (24).
Gastric lesions were induced via intragastric
administration of absolute ethanol, according to a previously
described method (25–27) with minor modification. A total of
35 rats were divided into five groups (n=7/group) and fasted for 18
h prior to the experiment. The rats in the control group were
orally administered with phosphate-buffered saline (PBS; 5 ml/kg
body weight) as the vehicle, and those in the absolute-ethanol
group were administered with absolute ethanol orally (5 ml/kg body
weight). The rats in the positive control group were administered
with cimetidine (100 mg/kg body weight) orally 2 h prior to the
administration of absolute ethanol for 3 days. Cimetidine was used
as a positive control drug as it has anti-inflammatory and
antioxidative activities, and is used widely in the treatment of
gastritis (28). The treatment
groups received SAWE (250 or 500 mg/kg body weight) 2 h prior to
the administration of absolute ethanol for 3 days.
At the end of the 3 days, the rats were sacrificed
with an overdose of 100 mg/kg pentobarbital, performed 24 h
following the final ethanol administration. The stomach was
removed, opened along the greater curvature and gently rinsed with
PBS. The stomach was stored at −70°C until biochemical
analysis.
Biochemical analysis
Biochemical analysis was performed using a
previously described method (29).
The stomach was cut into small sections and homogenized (1/10 w/v)
with tissue lysis/extraction reagent containing protease inhibitors
(Sigma-Aldrich, St. Louis, MO, USA). The homogenates were
centrifuged at 15,000 × g for 10 min at 4°C to precipitate the cell
debris, the protein concentration of the supernatant was determined
using a Protein Assay Dye Reagent Concentrate (Bio-Rad
Laboratories, Hercules, CA, USA), according to the manufacturer's
protocol. This homogenized sample was used to measure the levels of
malondialdehyde (MDA) and glutathione (GSH), and the activities of
catalase, glutathione-S-transferase (GST) and superoxide dismutase
(SOD). The protein concentrations were measured using Protein Assay
Reagent Concentrate (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), according to the manufacturer's protocol.
To estimate lipid peroxidation, the content of MDA
was measured using a thiobarbituric acid-reactive substances assay
kit (BioAssay Systems, Hayward, CA, USA). The GSH content and the
activities of antioxidant enzymes, catalase, GST and SOD, were
measured using commercial kits (Cayman Chemical Company, Ann Arbor,
MI, USA), according to the manufacturer's protocols. The values for
the MDA and GSH contents are expressed as nmol/mg and
μmol/mg protein, respectively, and the activities of the
antioxidant enzymes are expressed as U/mg protein.
Measurement of PGE2
levels
The concentrations of PGE2 were measured
using a previously described method (27). The production of PGE2
was measured in the homogenates of the gastric tissue using an
immune-linked immunosorbent assay kit (Cayman Chemical Company),
according to the manufacturer's protocol.
Histopathology and periodic acid-Schiff
(PAS) histochemistry
The glandular face of the stomach was examined
histologically. The stomach tissues were preserved in 10%
buffered-formalin and processed for paraffin block preparation.
Sections measuring ~4 μm in thickness were stained with
hematoxylin (cat. no. MHS-16; Sigma-Aldrich) and eosin (cat. no.
HT110-1-32; Sigma-Aldrich) solution, and PAS (IMEB, Inc., San
Marcos, CA, USA) to estimate inflammation and mucus production,
respectively. The histopathological changes were assessed by
microscopy, according to the previously described criteria
(29).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. One-way analysis of variance was used to detect
significant differences between the control and treatment groups.
Dunnett's test was used for multiple comparisons. Statistical
analysis was performed using Systat software (version 10; Systat
Software Inc., San Jose, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of SAWE on lipid peroxidation and
GSH content in ethanol-induced gastritis
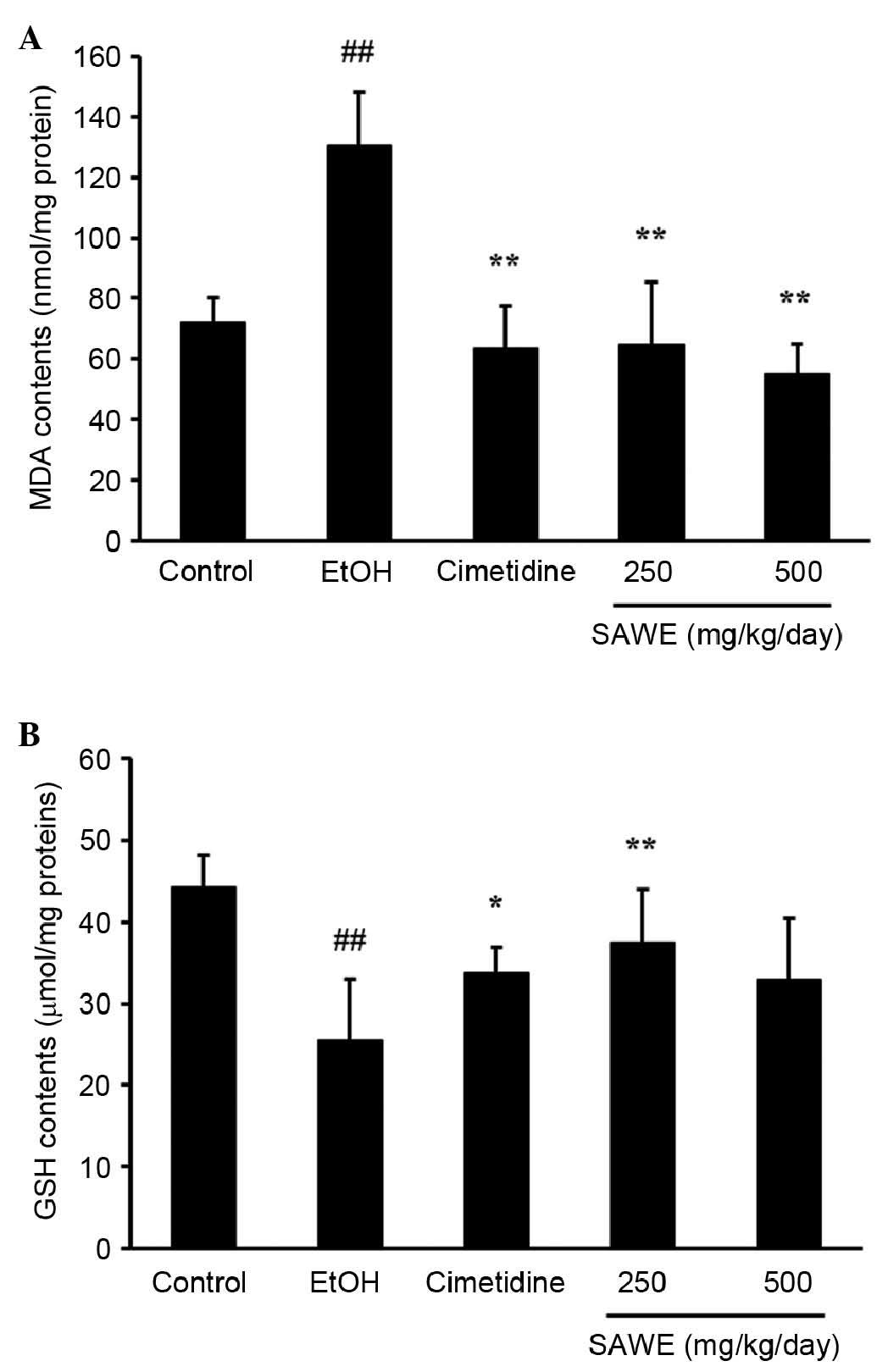
As shown in Fig.
1A, the concentration of MDA, an end product of lipid
peroxidation, was higher in the ethanol group (130.75±6.52 nmol/mg
protein; P<0.01), compared with the control group (72.05±3.17
nmol/mg protein). By contrast, the MDA content was significantly
lower, in a dose-dependent manner, in the groups treated with SAWE
at 250 (64.79±7.84 nmol/mg protein; P<0.01) or 500 mg/kg
(55.31±4.23 nmol/mg protein; P<0.01), compared with the ethanol
group. The positive control cimetidine-treated group also had a
lower MDA content (63.42±5.33 nmol/mg protein; P<0.01), compared
with the ethanol group.
GSH content was significantly lower in the ethanol
group (25.49±3.06 μmol/mg protein; P<0.01), compared with
the control group (44.32±1.80 μmol/mg protein; Fig. 1B). By contrast, the GSH contents
were higher in the groups treated with SAWE at 250 (37.43±2.93
μmol/mg protein; P<0.01) or 500 mg/kg (33.00±7.40 nmol/mg
protein), compared with the ethanol group. GSH content was
significantly lower in the cimetidine-treated positive control
group (33.80±1.42 μmol/mg protein; P<0.05), compared with
the ethanol group.
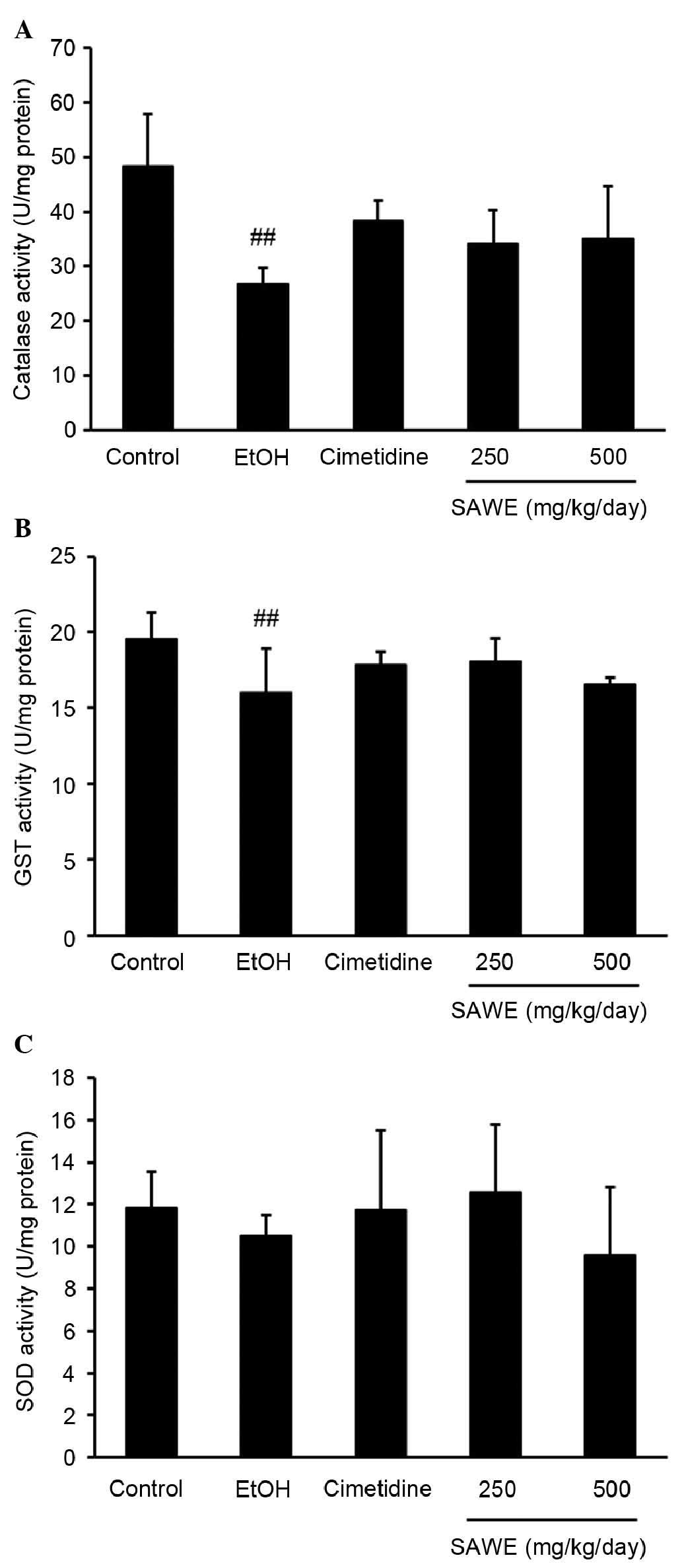
Effect of SAWE on the activities of
antioxidant enzymes in ethanol-induced gastritis
As shown in Fig.
2A, catalase activity was significantly lower in the ethanol
group (26.78±1.31 U/mg protein; P<0.01), compared with the
control group (48.39±4.26 U/mg protein; P<0.01). By contrast, no
significant differences in catalase activity were observed in the
groups treated with SAWE at 250 (34.09±2.77 U/mg protein) or 500
mg/kg (35.04±4.32 U/mg protein) or with cimetidine (38.39±1.48 U/mg
protein). However, the GST activity was significantly lower in the
ethanol group (16.03±1.27 U/mg protein), compared with that in the
control group (19.53±0.78 U/mg protein; P<0.01; Fig. 2B). The administration of SAWE at
250 or 500 mg/kg or cimetidine had no significant effect on GST
activity, compared with ethanol treatment. SOD activity did not
differ significantly between any groups (Fig. 2C).
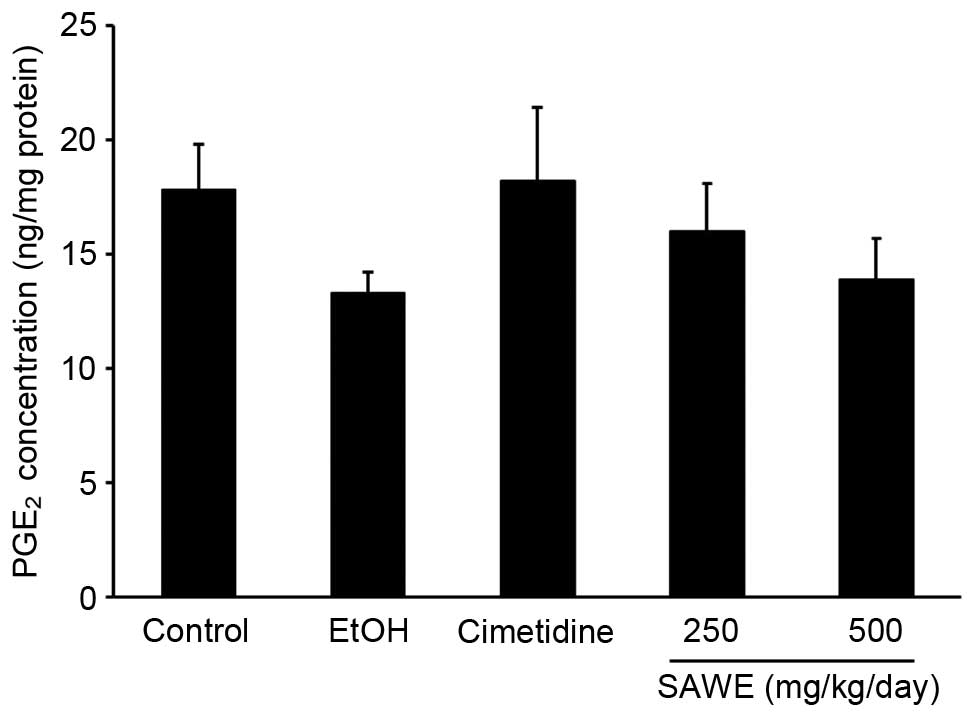
Effects of SAWE on the production of
PGE2
The production of PGE2 was lower in the
ethanol group (13.30±0.88 ng/mg protein), compared with the control
group (17.81±1.98 ng/mg protein; Fig.
3). No significant difference in the production of
PGE2 was observed in the cimetidine-treated positive
control group (18.18±3.23 ng/mg protein), compared with the ethanol
group. SAWE treatment at a dose of 250 (15.98±2.06 ng/mg protein)
or 500 mg/kg (13.88±1.79 ng/mg protein) had no significant effects
on the production of PGE2, compared with the ethanol
treatment group.
PAS staining evaluation of gastric
lesions
The PAS staining was higher in the gastric mucosa of
the ethanol group, compared with the control group, indicating an
increase in glycoprotein content in the gastric mucosa (Fig. 4). By contrast, the SAWE (250 or 500
mg/kg) and cimetidine-treated groups exhibited normal levels of
mucin in the glandular tissue of the stomach, as shown by the
increase in magenta staining in the mucosal cell layer, compared
with ethanol treatment.
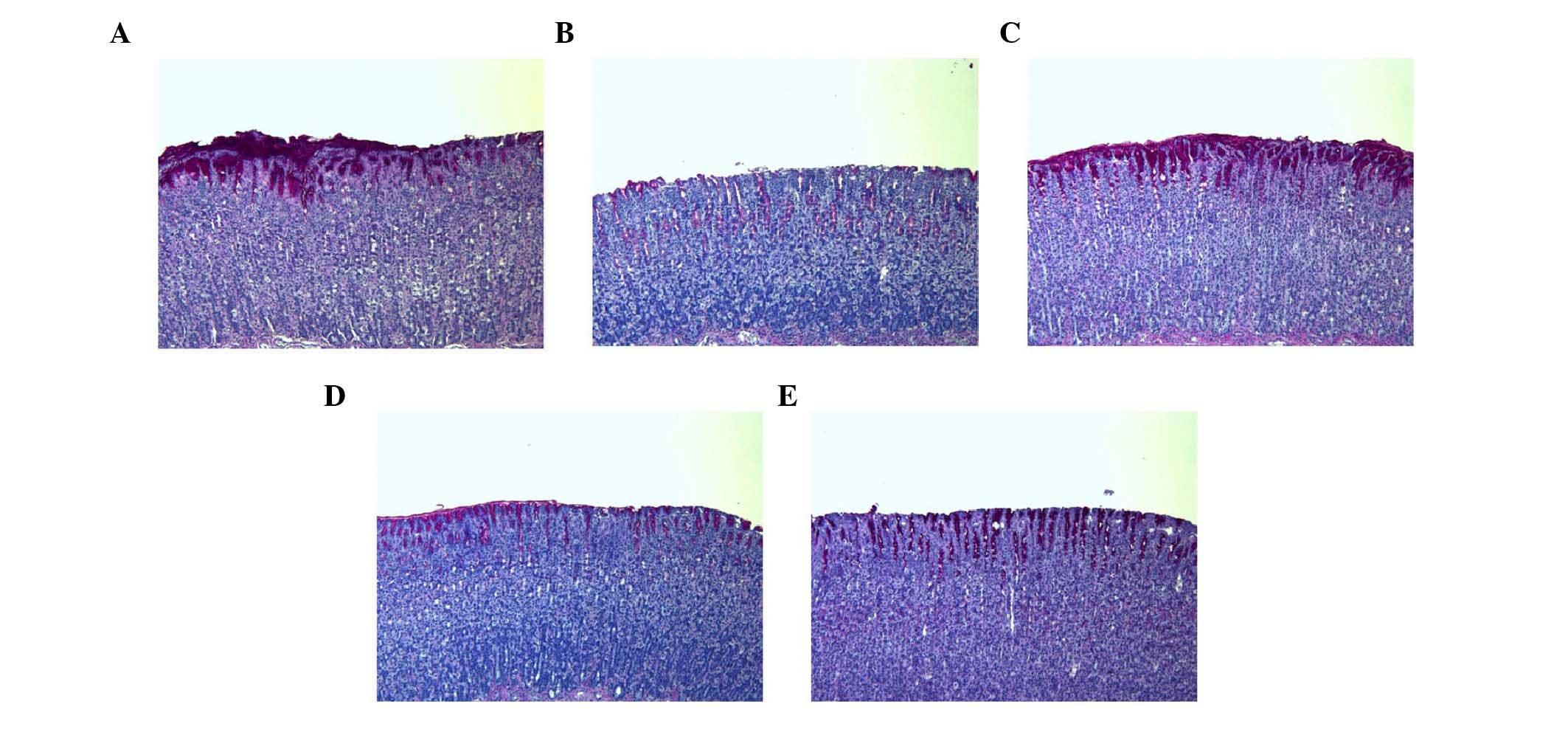
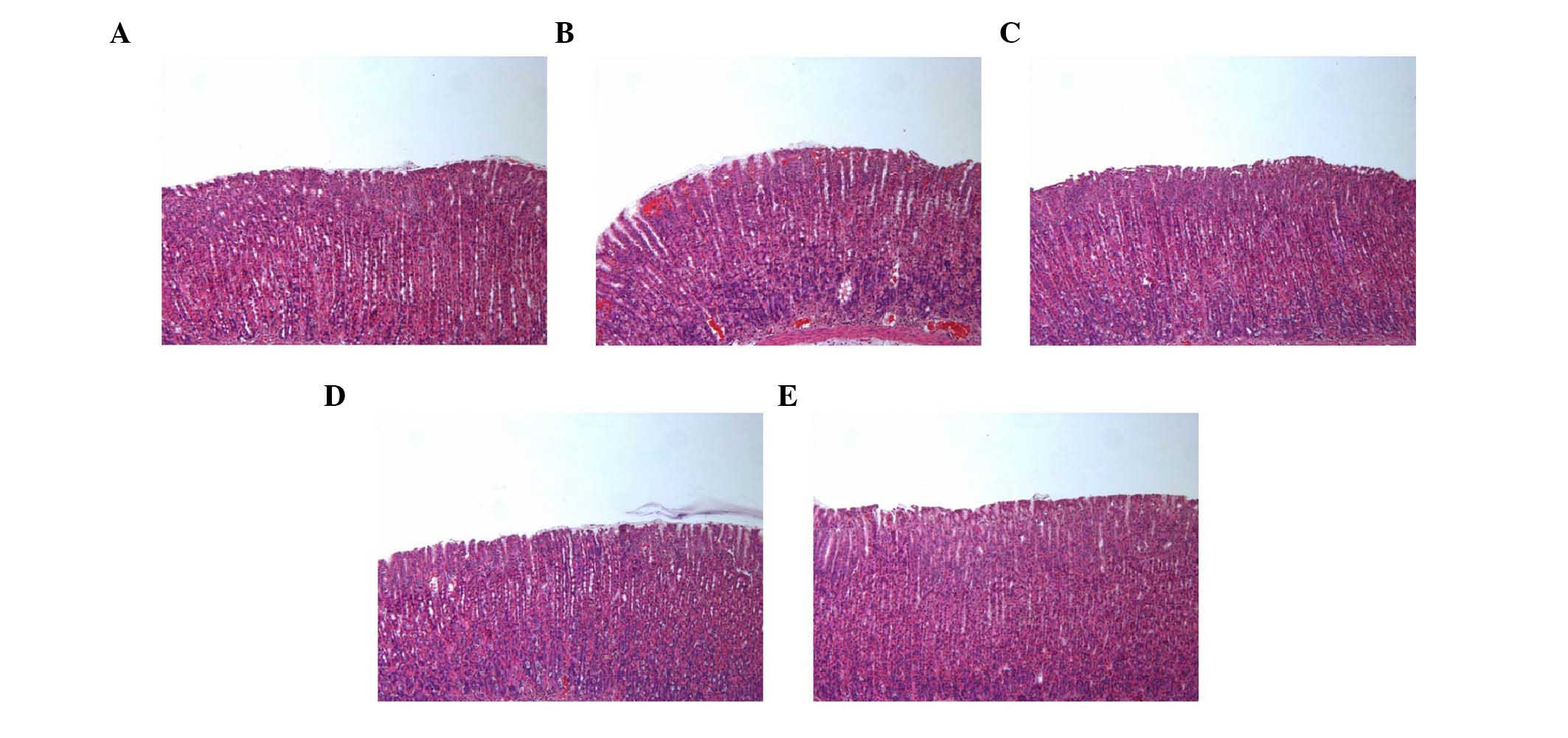
Histological evaluation of gastric
lesions
In the control group, normal histological structure
of the gastric mucosa was observed (Fig. 5). By contrast, the ethanol group
showed inflammatory cell infiltration in the mucosa and submucosa.
The administration of SAWE (250 or 500 mg/kg) or cimetidine
attenuated the loss of epithelial cells and evidence of hemorrhage
in the stomach area.
Discussion
Several medicinal herbs, including Aloe vera,
Curcuma longa and Glycyrrhiza glabra, have been
reported to possess antiulcer activities (30), and certain phytochemicals in
medicinal herbs, including gallic acid, glycyrrhizinic acid,
phenolic compounds and flavonoids, have been shown to have
gastroprotective effects by regulating gastric mucus secretion or
through antioxidant activity (31). Among these, eugenol, gallic acid
and ellagic acid, which are abundant constituents of SAWE, have
antigastric effects and antioxidant activities (32–34).
Based on the previous studies, the present study hypothesized that
SAWE, which contains these bioactive components, has a preventive
effect against gastric injury.
The administration of ethanol has long been used as
a reproducible method to induce gastric injury in experimental
animals (35). Ethanol-induced
gastric damage is characterized by hemorrhage, mucosal edema,
inflammatory cell infiltration and loss of epithelial cells
(36). Therefore, the present
study, investigated whether SAWE has a protective effect against
ethanol-induced gastric injury in rats. The administration of
ethanol induced severe gastric lesions, as shown by inflammatory
cell infiltration and loss of epithelial cells. By contrast, the
administration of SAWE (250 or 500 mg/kg) attenuated the gastric
injury induced by ethanol.
ROS, including superoxide anions, hydrogen peroxide,
hydroxyl radicals, and lipid peroxidation are important in the
pathogenesis of gastric mucosal injury (4,7,37).
MDA is the final product of lipid peroxidation and is used as an
estimate of lipid peroxidation levels (38). Lipid peroxidation is caused by an
imbalance between antioxidant defense systems and oxidative damage,
which affects cell membranes. MDA content was higher in the ethanol
group, compared with the SAWE-treated groups (250 or 500 mg/kg) and
cimetidine-treated group. These results suggested that SAWE had
protective effects against ethanol-induced gastric injury by
inhibiting lipid peroxidation.
GSH, an endogenous antioxidant, reacts with
peroxides and toxic oxygen radicals, including hydroxyl ions and
singlet oxygen, to protect cells from damage (39). The present study found that the GSH
content was significantly lower in the ethanol group, compared with
the control group. By contrast, GSH contents were higher in the
SAWE-treated groups (250 or 500 mg/kg) and cimetidine-treated
group, compared with the ethanol group. These findings indicated
that pretreatment with SAWE protected the gastric mucosa from
ethanol-induced gastric injury by increasing the GSH content.
The important cellular antioxidant enzymes,
including catalase, GST, and SOD, contribute to the gastric
oxidative-antioxidative balance. Decreases in the activities of
these enzymes in the gastric mucosa of rats exposed to ethanol
leads to the accumulation of ROS and, consequently, to an increase
in MDA levels (40). In the
present study, ethanol decreased the activities of catalase and
GST, suggesting the importance of these enzymes in the pathogenesis
of gastric injury. There were no significant changes in the
activities of catalase or GST in the low-dose SAWE-treated group
(250 mg/kg) or the cimetidine-treated group, compared with the
ethanol group. No alterations in SOD activity were observed in any
groups. These results suggested that SAWE enhanced the cellular
antioxidant system, which may provide protection against
ethanol-induced gastric injury.
PGs are key molecules, which activate ulcer-healing
mechanisms and are synthesized in the gastric mucosal cells by
cyclooxygenases. PGs stimulate the secretion of bicarbonates and
mucus, promote ulcer healing and inhibit the secretion of gastric
acid. PGE2, one of the major PGs of the gastric mucosa,
can inhibit the secretion of gastric acid (41). The ethanol-induced depletion of
gastric mucus has been described previously (42), and this may be caused by an
inhibitory effect on the gastric production of PGE2
(43). In the present study, the
production of PGE2 was reduced in the ethanol group,
whereas the concentration of PGE2 was higher in the rats
treated with SAWE at 250 mg/kg/day, compared with the concentration
in the ethanol group. The secretion of mucus observed in the
SAWE-treated group may have been attributed to the increased
PGE2 production observed in the gastric mucosa.
In conclusion, SAWE had a protective effect against
ethanol-induced gastric injury by improving antioxidative status
and increasing PGE2 production, and by suppressing
inflammatory cell infiltration and loss of epithelial cells in the
gastric mucosa. These findings suggested that SAWE has potential
for further development as a treatment against alcohol-induced
gastric injury.
Acknowledgments
This study was supported by a grant 'Evaluation of
Herb-Herb Interaction by Array Methods funded by the Korea
Institute of Oriental Medicine' from the Korea Institute of
Oriental Medicine (grant no. K12271).
References
|
1
|
Oliveira CP, Kassab P, Lopasso FP, Souza
HP, Janiszewski M, Laurindo FR, Iriya K and Laudanna AA: Protective
effect of ascorbic acid in experimental gastric cancer: Reduction
of oxidative stress. World J Gastroenterol. 9:446–448. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cederbaum AI: Introduction-serial review:
Alcohol, oxidative stress and cell injury. Free Radic Biol Med.
31:1524–1526. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasaki M and Joh T: Oxidative stress and
ischemia-reperfusion injury in gastrointestinal tract and
antioxidant, protective agents. J Clin Biochem Nutr. 40:1–12. 2007.
View Article : Google Scholar
|
|
4
|
La Casa C, Villegas I, Alarcón de la
Lastra C, Motilva V and Martín Calero MJ: Evidence for protective
and antioxidant properties of rutin, a natural flavone, against
ethanol induced gastric lesions. J Ethnopharmacol. 71:45–53. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moghadamtousi SZ, Rouhollahi E, Karimian
H, Fadaeinasab M, Abdulla MA and Kadir HA: Gastroprotective
activity of Annona muricata leaves against ethanol-induced gastric
injury in rats via Hsp70/Bax involvement. Drug Des Devel Ther.
8:2099–2110. 2014.PubMed/NCBI
|
|
6
|
Alvarez-Suarez JM, Dekanski D, Ristić S,
Radonjić NV, Petronijević ND, Giampieri F, Astolfi P,
González-Paramás AM, Santos-Buelga C, Tulipani S, et al: Strawberry
polyphenols attenuate ethanol-induced gastric lesions in rats by
activation of antioxidant enzymes and attenuation of MDA increase.
PLoS One. 6:e258782011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kahraman A, Erkasap N, Köken T, Serteser
M, Aktepe F and Erkasap S: The antioxidative and antihistaminic
properties of quercetin in ethanol-induced gastric lesions.
Toxicology. 183:133–142. 2003. View Article : Google Scholar
|
|
8
|
Ishihara K, Kuwata H, Ohara S, Okabe H and
Hotta K: Changes of rat gastric mucus glycoproteins in
cytoprotection: Influences of prostaglandin derivatives. Digestion.
39:162–171. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hawkey CJ and Rampton DS: Prostaglandins
and the gastrointestinal mucosa: Are they important in its
function, disease, or treatment? Gastroenterology. 89:1162–1188.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeuchi K, Kato S and Tanaka A:
Gastrointestinal cytoprotection by prostaglandin E and EP receptor
subtypes. Nihon Yakurigaku Zasshi. 117:274–282. 2001.In Japanese.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao W, Zhu F, Shen W, Fu A, Zheng L, Yan
Z, Zhao L and Fu G: Protective effects of DIDS against
ethanol-induced gastric mucosal injury in rats. Acta Biochim
Biophys Sin (Shanghai). 41:301–308. 2009. View Article : Google Scholar
|
|
12
|
World Health Organization: General
guidelines for methodologies on research and evaluation of
traditional medicine. World Health Organization; Geneva: 2000
|
|
13
|
Duraipandiyan V, Ayyanar M and Ignacimuthu
S: Antimicrobial activity of some ethnomedicinal plants used by
Paliyar tribe from Tamil Nadu, India. BMC Complement Altern Med.
6:352006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaieb K, Zmantar T, Ksouri R, Hajlaoui H,
Mahdouani K, Abdelly C and Bakhrouf A: Antioxidant properties of
the essential oil of Eugenia caryophyllata and its antifungal
activity against a large number of clinical Candida species.
Mycoses. 50:403–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pinto E, Vale-Silva L, Cavaleiro C and
Salgueiro L: Antifungal activity of the clove essential oil from
Syzygium aromaticum on Candida, aspergillus and dermatophyte
species. J Med Microbiol. 58:1454–1462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuroda M, Mimaki Y, Ohtomo T, Yamada J,
Nishiyama T, Mae T, Kishida H and Kawada T: Hypoglycemic effects of
clove (Syzygium aromaticum flower buds) on genetically diabetic
KK-Ay mice and identification of the active ingredients. J Nat Med.
66:394–399. 2012. View Article : Google Scholar
|
|
17
|
Karmakar S, Choudhury M, Das AS, Maiti A,
Majumdar S and Mitra C: Clove (Syzygium aromaticum Linn) extract
rich in eugenol and eugenol derivatives shows bone-preserving
efficacy. Nat Prod Res. 26:500–509. 2012. View Article : Google Scholar
|
|
18
|
Banerjee S, Panda CK and Das S: Clove
(Syzygium aromaticum L.), a potential chemopreventive agent for
lung cancer. Carcinogenesis. 27:1645–1654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Halder S, Mehta AK, Mediratta PK and
Sharma KK: Essential oil of clove (Eugenia caryophyllata) augments
the humoral immune response but decreases cell mediated immunity.
Phytother Res. 25:1254–1256. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung CH, Ahn J, Jeon TI, Kim TW and Ha TY:
Syzygium aromaticum ethanol extract reduces high-fat diet-induced
obesity in mice through downregulation of adipogenic and lipogenic
gene expression. Exp Ther Med. 4:409–414. 2012.PubMed/NCBI
|
|
21
|
Kim JH, Seo CS, Kim SS and Ha H:
Simultaneous determination of gallic acid, ellagic acid, and
eugenol in Syzygium aromaticum and verification of chemical
antagonistic effect by the combination with Curcuma aromatica using
regression analysis. J Anal Methods Chem. 2013:3752942013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeon WY, Shin IS, Shin HK and Lee MY:
Gastroprotective effect of the traditional herbal medicine,
Sipjeondaebo-tang water extract, against ethanol-induced gastric
mucosal injury. BMC Complement Altern Med. 14:3732014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the care and use of laboratory animals. 8th
edition. National Academies Press (US); Washington (DC): 2011
|
|
24
|
Ministry of Food and Drug Safety:
Laboratory Animal Act, Act No. 11987. 2013
|
|
25
|
Robert A, Nezamis JE, Lancaster C and
Hanchar AJ: Cytoprotection by prostaglandins in rats. Prevention of
gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl,
and thermal injury. Gastroenterology. 77:433–443. 1979.PubMed/NCBI
|
|
26
|
Ishida K, Kojima R, Tsuboi M, Tsuda Y and
Ito M: Effects of artichoke leaf extract on acute gastric mucosal
injury in rats. Biol Pharm Bull. 33:223–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee MY, Shin IS, Jeon WY, Seo CS, Ha H,
Huh JI and Shin HK: Protective effect of Bojungikki-tang, a
traditional herbal formula, against alcohol-induced gastric injury
in rats. J Ethnopharmacol. 142:346–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hernández-Muñoz R and Montiel-Ruíz F:
Reversion by histamine H2-receptor antagonists of plasma membrane
alterations in ethanol-induced gastritis. Dig Dis Sci.
41:2156–2165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin IS, Jeon WY, Shin HK, Cha SW and Lee
MY: Banhabaekchulchunma-tang, a traditional herbal formula
attenuates absolute ethanol-induced gastric injury by enhancing the
antioxidant status. BMC Complement Altern Med. 13:1702013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rai N, Kumar N, Gautam P and Rawat P:
Herbal plants as potent candidate for anti-ulcer drug development.
Environ Conserv J. 13:187–189. 2012.
|
|
31
|
Sen S, Chakraborty R, De B and Mazumder J:
Plants and phytochemicals for peptic ulcer: An overview. Pharmacogn
Rev. 3:2702009.
|
|
32
|
Jung J, Lee JH, Bae KH and Jeong CS:
Anti-gastric actions of eugenol and cinnamic acid isolated from
Cinnamomi Ramulus. Yakugaku Zasshi. 131:1103–1110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abdelwahab SI: Protective mechanism of
gallic acid and its novel derivative against ethanol-induced
gastric ulcerogenesis: Involvement of immunomodulation markers,
Hsp70 and Bcl-2-associated X protein. Int Immunopharmacol.
16:296–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iino T, Tashima K, Umeda M, Ogawa Y,
Takeeda M, Takata K and Takeuchi K: Effect of ellagic acid on
gastric damage induced in ischemic rat stomachs following ammonia
or reperfusion. Life Sci. 70:1139–1150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qodriyah HM and Asmadi AY: Eurycoma
longifolia in Radix for the treatment of ethanol-induced gastric
lesion in rats. Pak J Biol Sci. 16:1815–1818. 2013. View Article : Google Scholar
|
|
36
|
Guslandi M: Effect of ethanol on the
gastric mucosa. Dig Dis. 5:21–32. 1987. View Article : Google Scholar
|
|
37
|
Mizui T, Sato H, Hirose F and Doteuchi M:
Effect of antiperoxidative drugs on damage induced by ethanol in
rats. Life Sci. 41:755–763. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dursun H, Bilici M, Albayrak F, Ozturk C,
Saglam MB, Alp HH and Suleyman H: Antiulcer activity of fluvoxamine
in rats and its effect on oxidant and antioxidant parameters in
stomach tissue. BMC Gastroenterol. 9:362009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cadirci E, Suleyman H, Aksoy H, Halici Z,
Ozgen U, Koc A and Ozturk N: Effects of Onosma armeniacum root
extract on ethanol-induced oxidative stress in stomach tissue of
rats. Chem Biol Interact. 170:40–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Olaleye SB and Farombi EO: Attenuation of
indomethacin- and HCl/ethanol-induced oxidative gastric mucosa
damage in rats by kolaviron, a natural biflavonoid of Garcinia kola
seed. Phytother Res. 20:14–20. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adhikary B, Yadav SK, Roy K, Bandyopadhyay
SK and Chattopadhyay S: Black tea and theaflavins assist healing of
indomethacin-induced gastric ulceration in mice by antioxidative
action. Evid Based Complement Alternat Med. 2011:5465602011.
View Article : Google Scholar
|
|
42
|
Slomiany A, Morita M, Sano S, Piotrowski
J, Skrodzka D and Slomiany BL: Effect of ethanol on gastric mucus
glycoprotein synthesis, translocation, transport, glycosylation,
and secretion. Alcohol Clin Exp Res. 21:417–423. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsukada H, Zielenski J, Mizuta K, Slomiany
BL and Slomiany A: Prostaglandin protection against ethanol-induced
gastric injury: Regulatory effect on the mucus glycoprotein
metabolism. Digestion. 36:201–212. 1987. View Article : Google Scholar : PubMed/NCBI
|