Introduction
Rhodiola rosea (R. rosea), a type of
adaptogen, belongs to the Crassulaceae plant family, of the
Sedoideae subfamily and Rhodiola genus. It has been
used as traditional medicine in Europe, Asia and Russia for
centuries (1). Greater than 20
compounds are present in the R. rosea root, including
salidroside (rhodioloside), rosavins and p-tyrosol, which are
understood to have important therapeutic activities (2). The commonly described functional
activities include, performance enhancement, fatigue reduction,
alleviation of depression symptoms, stimulation of the nervous
system and prevention of high altitude sickness (3,4).
R. rosea was previously demonstrated to have
immunostimulatory potential in rodents in vivo, and in human
peripheral blood mononuclear cells (PBMCs) in vitro
(5–10). Additionally, in vivo
administration of salidroside, the major component of R.
rosea, enhanced the proliferation of murine T cells and the
production of antibodies and cytokines, including interleukin
(IL)-2, IL-4 and interferon-γ (IFN-γ) (5). In vitro administration of the
aqueous extract of Rhodiola imbricate rhizome induced
increased expression levels of IL-1β in human PBMCs, and of
toll-like receptor-4 and granzyme-B in mouse splenocytes (10). Thus, R. rosea may
potentially be used to enhance cellular immunity under microgravity
conditions. However, it has not been previously reported whether
R. rosea has an in vivo immune-modulating effect in
humans (7,8). The effects of R. rosea on
cytokine production by human T cells and the differentiation of
regulatory T cells (Tregs) in vivo and in vitro is
currently unknown.
Spaceflight changes the immune system in various
ways. These include altered leukocyte distribution, altered serum
cytokine levels, reduced functions of natural killer cells,
granulocytes and monocytes, reduced leukocyte proliferation
following activation, decreased delayed-type hypersensitivity to
recall antigens, and latent viral reactivation (11–23).
A number of studies have investigated strategies to monitor the
immune system during spaceflight and to develop countermeasures. To
study whether R. rosea may enhance the functions of the
immune system during spaceflights, the effect of R. rosea
and its main component, salidroside, on human and mouse T cells was
examined in vitro and in vivo. Head-down bed rest
(HDBR) at −6° was used as a ground-based spaceflight model for the
study of human T cells in vivo, and hind limb unloading (HU)
was used as the in vivo mouse model.
Materials and methods
Ethical issues
The current study was approved by the Ethics
Committee of China Astronaut Research and Training Center. Written
consent was obtained from the subjects, who had been informed of
the risks and the experimental details.
Subjects
Fifteen male volunteers of age 26.63±4.03, height
171.8±3.0 cm and weight 63.6±6.2 kg (all presented as means ± SEM)
were recruited into the present study. All subjects were educated
to a junior high level or above. The subjects were healthy and
physically fit. Subjects with the following chronic or recent acute
illnesses were precluded from the study: Skeletal muscle diseases;
organic and functional diseases of psychiatry and neurology; and
sleep disorders. No subjects had a history of cancer, hepatitis or
any other relevant diseases, including autoimmune disorders. The
final selection was based on typical clinical results comprising
medical history, physical and psychological examination, complete
blood count, blood chemistry analysis and several other tests.
Eight subjects were randomly selected for the placebo group and
seven for the RR group. The subjects in the RR group received R.
rosea 0.5 g per day (prophylactic dose), twice a day from R1 to
R7, then 1.0 g per day (therapeutic dose), twice a day from R8 to
R45 during HDBR period. The dose of R. rosea was doubled for
therapeutic purposes as changes in muscles and bones began to be
significant following one week of bed rest. No medication, smoking,
alcohol or caffeinated drinks were permitted during the study.
Emergency medical surveillance and service were available
throughout bed rest protocol.
Head-down bed rest (HDBR) protocol
The entire study included 45 days of HDBR, 10 days
of adaptation prior to HDBR and 10 days of recovery following HDBR.
During HDBR, the subjects were in a flat, resting, head-down
position to −6° from horizontal. Lights were switched off between
22:00 and 06:00, with normal daylight illumination for the rest of
the day. All the subjects were housed in an air-conditioned
bedroom, maintained at 25±0.5°C with a relative humidity of 60–70%.
All dining, washing, urination and defecation activities were
carried out in a bedridden state. Changing position around the body
axis was permitted.
Human PBMC preparation
Sterile heparinized peripheral blood samples were
obtained from 12 healthy volunteers and the 15 test subjects prior
to (R-1), during (R15, R30 and R45) and following (R+9) the HDBR
protocol at 6:00 a.m. PBMCs were collected by Ficoll-Hypaque
density-gradient centrifugation.
Mice
Male C57BL/6 mice (age, 6–8 weeks old) were
purchased from Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China), and housed in a specific pathogen-free facility
at the Chinese Astronaut Research and Training Center (Beijing,
China). The mice were maintained under a 12-h light/dark cycle,
25±2°C and access to food and water ad libitum. All mice
were allowed to adapt to the environment for a minimum of 3 days
following shipment and prior to the onset of the experiment. The
experimental procedures used and the care of the animals were
approved by the ethics committee of the Chinese Astronaut Research
and Training Center.
Mouse hind limb unloading (HU) model
C57BL/6 mice at 8 weeks of age were randomly
assigned to four groups, with 3 mice in each group as follows:
Saline group; salidroside group; saline with HU group; and
salidroside with HU group. Mice in the HU groups were suspended by
their tails at a 30° head-down tilt with no load bearing on their
hind limbs, with unlimited access to food and water. Mice without
HU were housed individually in standard caging. The mice received
salidroside 50 mg/kg/day by intragastric administration for 28 days
prior to HU and for 14 days during HU. Immediately following the
HU, the mice were sacrificed by cervical dislocation and cells from
the spleens were collected for further experimentation.
Reagents
Salidroside was purchased from Sigma-Aldrich (St.
Louis, MO, USA). The following monoclonal antibodies were used for
staining: Anti-human cluster of differentiation 4-peridinin
chlorophyll (CD4-PerCP) Cy5.5 (OKT4; BioLegend, Inc., San Diego,
CA, USA); anti-human CD25-phycoerythrin (PE; MEM-181; QuantoBio,
Beijing, China); anti-human forkhead box P3-Allophycocyanin
(Foxp3-APC; PCH101) and anti-mouse Foxp3-APC (FJK-16s; eBioscience,
Inc., San Diego, CA, USA); and anti-human IFN-γ-fluorescein
isothiocyanate (FITC; 4S.B3), anti-mouse CD4-FITC (H129.19),
anti-mouse CD25-PE (PC61) and anti-mouse IFN-γ-APC (XMG1.2; BD
Biosciences, San Jose, CA, USA). The following antibodies and
regents were purchased from BD Biosciences were used for cell
cultures: Anti-human CD3 (HIT3a); anti-human CD28 (CD28.2);
anti-mouse CD3 (145-2C11); anti-mouse CD28 (37.51); and protein
transport inhibitor (containing Brefelding A). Recombinant human
transforming growth factor (rhTGF)-β1 and rhIL-2 were purchased
from R&D Systems China Co., Ltd. (Shanghai, China). The mouse
IFN-γ enzyme linked immunosorbent assay (ELISA) kit was purchased
from eBioscience.
IFN-γ production
Human PBMCs were stimulated with 1 μg/ml
anti-CD3 and 1 μg/ml anti-CD28 for 2 days. Protein transport
inhibitor was added 4 hours prior to intracellular IFN-γ staining.
Mouse splenocytes were stimulated with 2 μg/ml anti-CD3 and
1 μg/ml anti-CD28 for 2 days. The supernatants were
collected and the concentrations of IFN-γ were measured using the
mouse IFN-γ ELISA kit.
Helper T cell differentiation
Human PBMCs were stimulated by anti-CD3 and
anti-CD28 under induced regulatory T cell (iTreg)-inducing
conditions (rhIL-2, 5 ng/ml; and rhTGF-β1, 10 ng/ml) with various
doses of salidroside (0, 10, 30, 50 and 100 μg/ml). After 5
days, the cells were collected and stained with CD4 and CD25
antibodies, then intracellular staining of Foxp3 was conducted
according to the manufacturer's protocols and the cells were
examined by FACSCalibur flow cytometry (BD Biosciences).
Mouse splenocytes were stimulated by anti-CD3 and
anti-CD28 under iTreg-inducing condition (rhIL-2, 2 ng/ml; and
rhTGF-β1, 1 ng/ml). iTreg-skewing cells were directly stimulated
for 3 days prior to intracellular Foxp3 staining.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from iTreg-skewing PBMCs using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacturer's protocol, and cDNA was
obtained using the FastQuant RT kit (Tiangen Biotech Co., Ltd.,
Beijing, China). RT-qPCR was performed using SYBR Green Supermix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) on an iCycler
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.), with
each sample in triplicate. The primers used for measurement were as
follows: Forward, 5′-TTCACCTGAGCCTAATAGTCC-3′ and reverse,
5′-CAAGTCTAAATCTGTGTCCTG-3′ for hypoxia inducible factor-1α
(HIF-1α); and forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′ for GAPDH. PCRs were performed for 40
cycles of 95°C for 20 sec, 56°C for 20 sec and 72°C for 20 sec. The
quantification was based on ΔΔCq calculations and were normalized
to GAPDH as the reference gene (24).
Statistical analysis
Statistical analysis of the results was performed
using Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). The
differences between the placebo group and RR group were analyzed by
repeated measures analysis of variance (ANOVA), with time and
treatment as two factors for repeated measures and were further
evaluated using Bonferroni correction. Unpaired or two-tail paired
t test was further used to evaluate the significance of the
differences. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of R. rosea on human T cells in
the HDBR model
To investigate the in vivo effects of R.
rosea on human T cells under simulated microgravity, placebo-
and RR-treated groups underwent a 45-day HDBR protocol (25). The subjects in the RR group
received Rhodiola rosea 0.50 g (prophylactic dose) twice a
day from R1 to R7, then 1.0 g (therapeutic dose) twice a day from
R8 to R45 during the HDBR period. As presented in Fig. 1A, the percentages of total T cells,
CD4+ and CD8+ T cells in the peripheral blood
of the placebo group did not change until 9 days following the
completion of bed rest (R+9), where the percentages were
significantly different to those at day R45 (Fig. 1A) (25). The changes included a significant
increase in the total T and CD4+ T cells and a decrease
in CD8+ T cells on R+9 compared with R45 (P=0.008,
P=0.013 and P=0.013, respectively). Consistently, an ~14% increase
in CD4:CD8 ratio was observed at R+9 compared with R45 (P=0.015,
Fig. 1A) (25). Compared with the placebo group, the
adaptogen RR-treated group exhibited a similar pattern of changes
in the T cell subsets (Fig.
1B).
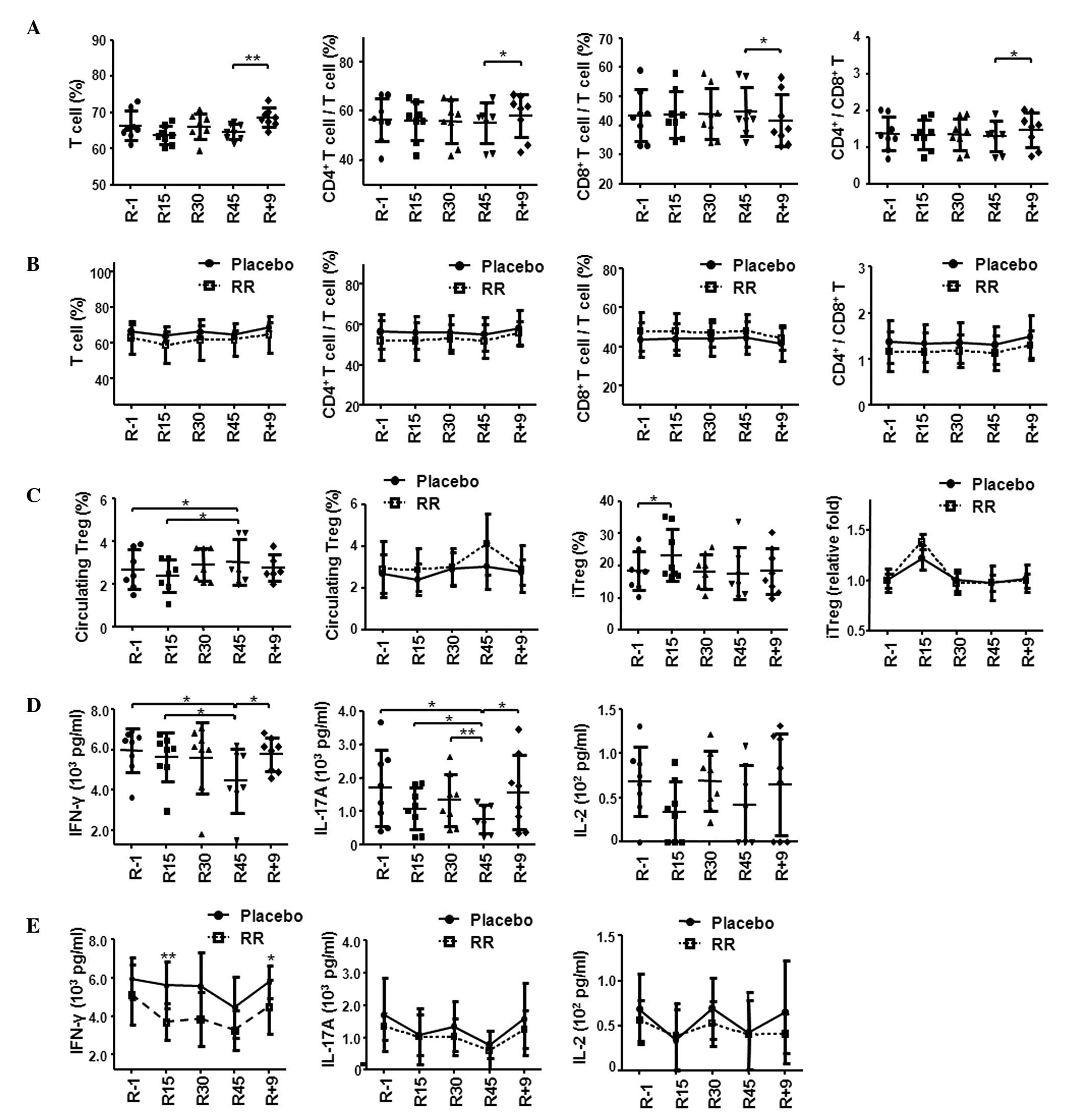 | Figure 1RR reduced the secretion of IFN-γ by
T cells but did not alter the differentiation of iTregs in 45-day
HDBR. (A) Analysis of total T cell, CD4+ T cell and
CD8+ T cell percentages within the placebo group. (B)
Comparison of total T cell, CD4+ T cell and
CD8+ T cell percentages between the placebo and RR
treated groups. (C) The changes in circulating Treg cells and iTreg
cells within the placebo group and between the placebo and RR
treated groups. (D) Changes to T cell-derived cytokines within the
placebo group. PBMCs were stimulated by anti-CD3 and anti-CD28
antibodies for 2 days. The supernatants were analyzed for cytokines
IFN-γ, IL-17A and IL-2. (E) The comparison of IFN-γ, IL-17A and
IL-2 secretion between the placebo and RR treated groups. Data are
presented as the mean ± standard deviation. The statistical
significance between any two time points within the placebo group
or between the placebo and RR-treated groups within any single time
point was calculated by two-tailed Student's t-test.
*P<0.05, **P<0.01. The placebo group is
indicated as a solid line and the RR-treated group as a dashed
line. RR, Rhodiola rosea; INF-γ, interferon-γ; iTregs,
induced T regulatory cells; HBDR, head-down bed rest; IL,
interleukin; CD, cluster of differentiation; PBMCs. peripheral
blood mononuclear cells; R, number of HDBR days. |
The percentage of circulating Treg cells
(CD4+ CD25+ Foxp3+
CD127−) in the HDBR PBMCs was observed to be increased
at R45 compared with R-1 (P=0.026) and had returned to the baseline
levels at R+9 (Fig. 1C) (25). Similar Treg changes were observed
in the group treated with RR (Fig.
1C). A late increase in circulatory Tregs was observed,
whereas, the percentage of iTregs was increased at R15 compared
with R-1, however, there was no change in iTreg levels at
R45(Fig. 1C) (25). The pattern of T cell levels in the
RR group was not significantly different to the placebo group
(Fig. 1C). The differentiation of
iTregs at various time points was induced by stimulation of PBMCs
with anti-CD3, anti-CD28 and TGF-β1 under 1g conditions for 3 days
(26).
The levels of cytokines produced by the activated T
cells were examined after stimulation of HDBR PBMCs with anti-CD3
and anti-CD28 under 1g conditions for 2 days. The levels of IFN-γ
and IL-17A in the placebo group exhibited a gradual decrease during
HDBR, reaching the lowest level at R45 (P=0.05, 0.003 vs. R1,
repeated measures ANOVA; 25.0%±26.2% and 53.8%±20.3%, respectively;
Fig. 1D) (25). Unlike the findings of previous
post-flight and HDBR studies (11,22,27),
the current study did not observe a decrease in IL-2 expression in
the placebo group (Fig. 1D)
(25). No consistent or
significant changes were observed in the production of IL-4 by T
cells (data not shown). In the RR group, the levels of IFN-γ
production by T cell were reduced upon anti-CD3 and anti-CD28
stimulation (R15). The decrease to IFN-γ levels was significantly
enhanced in the RR group compared with the placebo group at R15
(P=0.046, repeated measures ANOVA; Fig. 1E) (25). There was no difference in the
IL-17A and IL-2 levels between the placebo and the RR groups
(Fig. 1E).
Effect of R. rosea on human T cells in
vitro
To investigate whether the suppressive effect of
R. rosea on T cell function may also be observed in
vitro under 1g conditions, normal human PBMCs were treated with
salidroside, the main component of R. rosea, and stimulated
with anti-CD3 and anti-CD28 monoclonal antibodies. In contrast to
the in vivo results obtained from the HDBR experiment, no
significant alterations of IFN-γ production was observed between
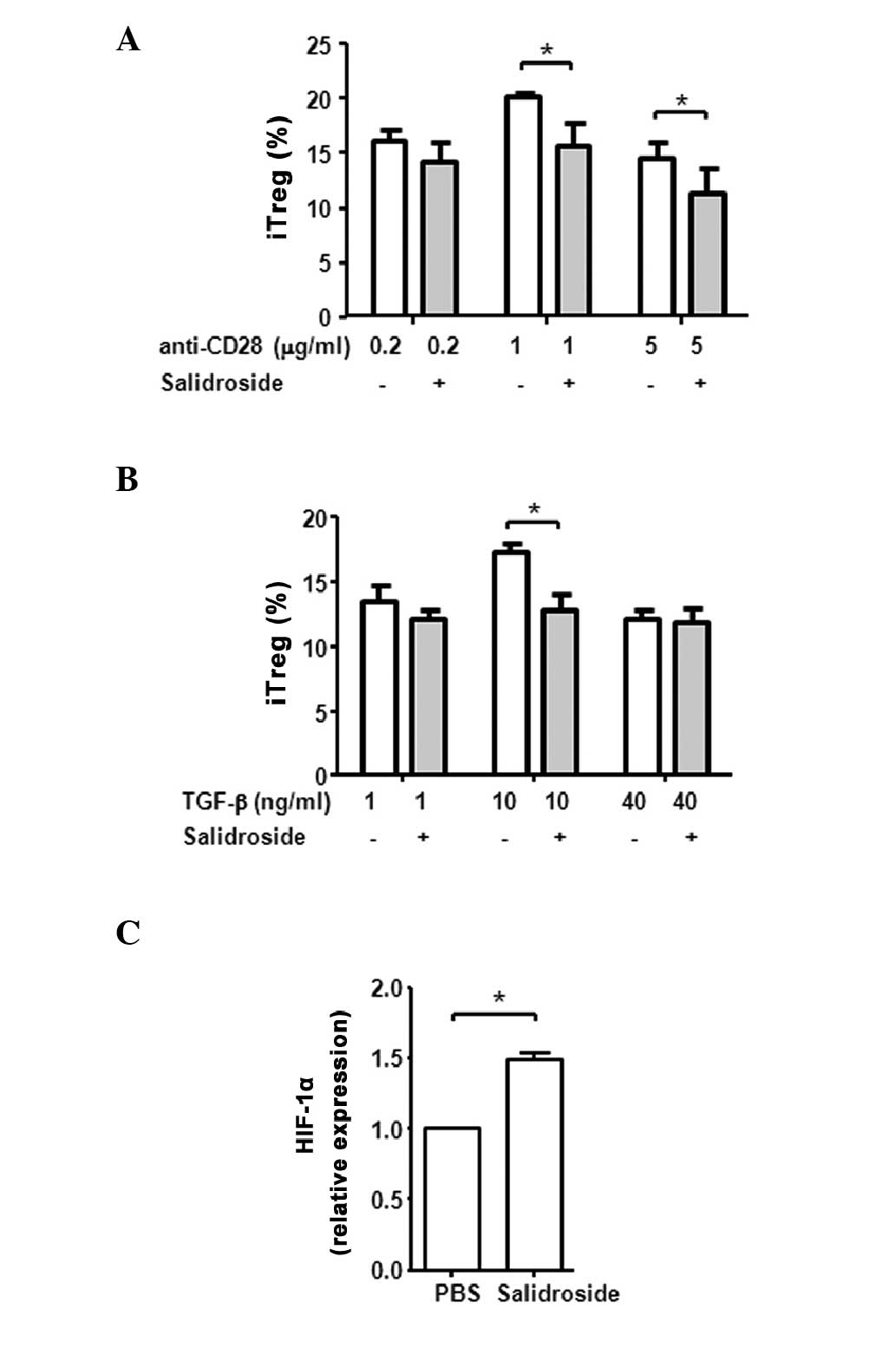
salidroside-treated or untreated PBMCs (Fig. 2A). Notably, a significant decrease
in iTreg cell differentiation was observed in the cells treated
with salidroside (Fig. 2B). The
inhibition of iTreg differentiation by salidroside was
dose-dependent, as an increase in salidroside concentration
resulted in a further decrease to the iTreg cell percentage, with
the lowest percentage of T cells observed following 100
μg/ml salidroside treatment (P=0.019 vs. 0 μg/ml;
Fig. 2B). These results suggest
that, in contrast to the in vivo effect, salidroside may
have a direct and suppressive impact on regulatory T cell
differentiation in vitro.
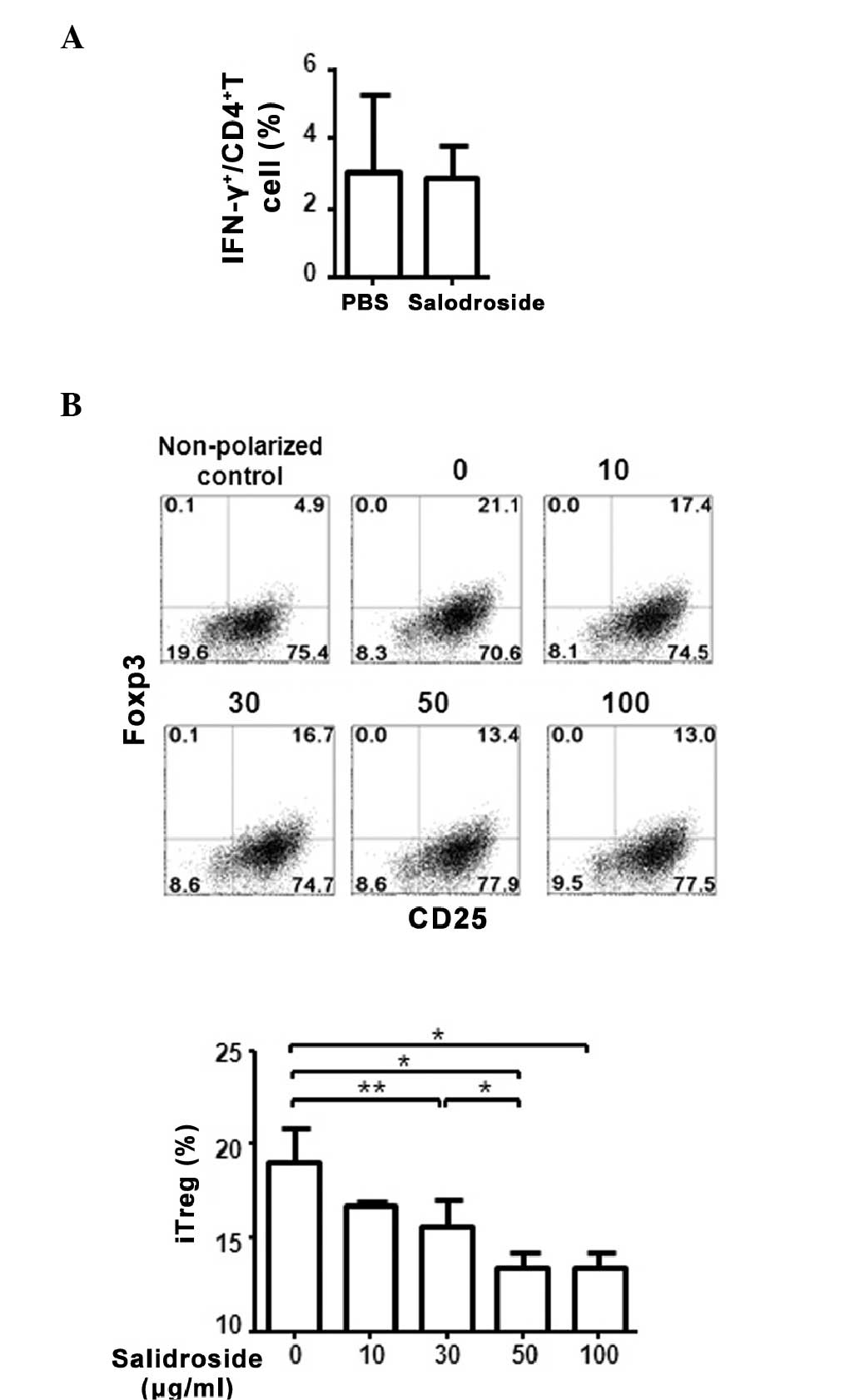 | Figure 2Salidroside treatment of human PBMCs
in vitro suppressed the differentiation of iTreg cells. (A)
Human PBMCs were stimulated with anti-CD3 and anti-CD28 antibodies
for 2 days in the presence or absence of 50 μg/ml
salidroside. The intracellular level of IFN-γ in CD4+ T
cells was measured by flow cytometry. The experiment was performed
3 times using different blood donors. (B) The suppression of iTreg
differentiation by salidroside is dose-dependent. Human PBMCs were
cultured in regulatory T cell-induction media with increasing
concentrations of salidroside. Following 5 days of treatment, cells
were stained with anti-CD4, anti-CD25, and anti-Foxp3 antibodies.
The flow cytometry results were shown in the left panel with the
numbers indicating the amount of salidroside (μg/ml). Data
are presented as the mean ± standard deviation.
*P<0.05, **P<0.01, comparisons between
the two subsets by unpaired Student t-test. PBMC, peripheral blood
mononuclear cell; iTreg, induced regulatory T cell; CD, cluster of
differentiation; INF-γ, interferon-γ; Foxp3, forkhead box P3. |
As the signaling pathways downstream of CD28 and the
TGF-β1 receptor are crucial to iTreg differentiation, the
concentration of anti-CD28 antibody and TGF-β1 were titrated to
examine whether salidroside increased the effects of these two
pathways. As presented in Fig. 3A
and B, the optimal concentration for induction of iTreg
differentiation in the absence of salidroside was 1 μg/ml
for anti-CD28 antibody and 10 ng/ml for TGF-β1. Compared with cells
that received anti-CD28 or TGF-β1 only, salidroside treatment
significantly reduced iTreg differentiation (P=0.046 and P=0.016,
respectively). Increasing the concentrations of anti-CD28 or TGF-β1
did not abolish the suppressive effect of salidroside. The
suppression of Treg differentiation in vitro was not due to
the inhibition of T cell survival or proliferation by salidroside
treatment (data not shown).
R. rosea, specifically, salidroside, was
previously reported to increase HIF-1α expression and its nuclear
translocation in cardiomyocytes, fibroblasts, kidney and liver
cells (28–30). HIF-1α was also previously observed
to suppress Treg differentiation by promoting the glycolytic
activity of T cells and by binding Foxp3 to promote its proteasomal
degradation (31,32). Thus, the current study examined
whether the treatment of salidroside alters the expression of
HIF-1α in T cells when cultured in regulatory T cell inducing
conditions. As presented in Fig.
3C, the HIF-1α mRNA levels in PBMCs were significantly
increased by salidroside in vitro, compared with PBS treated
cells (P=0.010).
These data demonstrate that salidroside has a
significant effect on iTred differentiation, however, the effects
are different in vitro and in vivo. Salidroside
directly suppressed the differentiation of iTregs in vitro
under 1g conditions. However, when R. rosea was administered
in vivo during the HDBR model, it did not significantly
alter iTreg differentiation, though the actual T cell
differentiation assay was also performed in vitro under 1g
condition.
Effect of R. rosea on murine T cells in
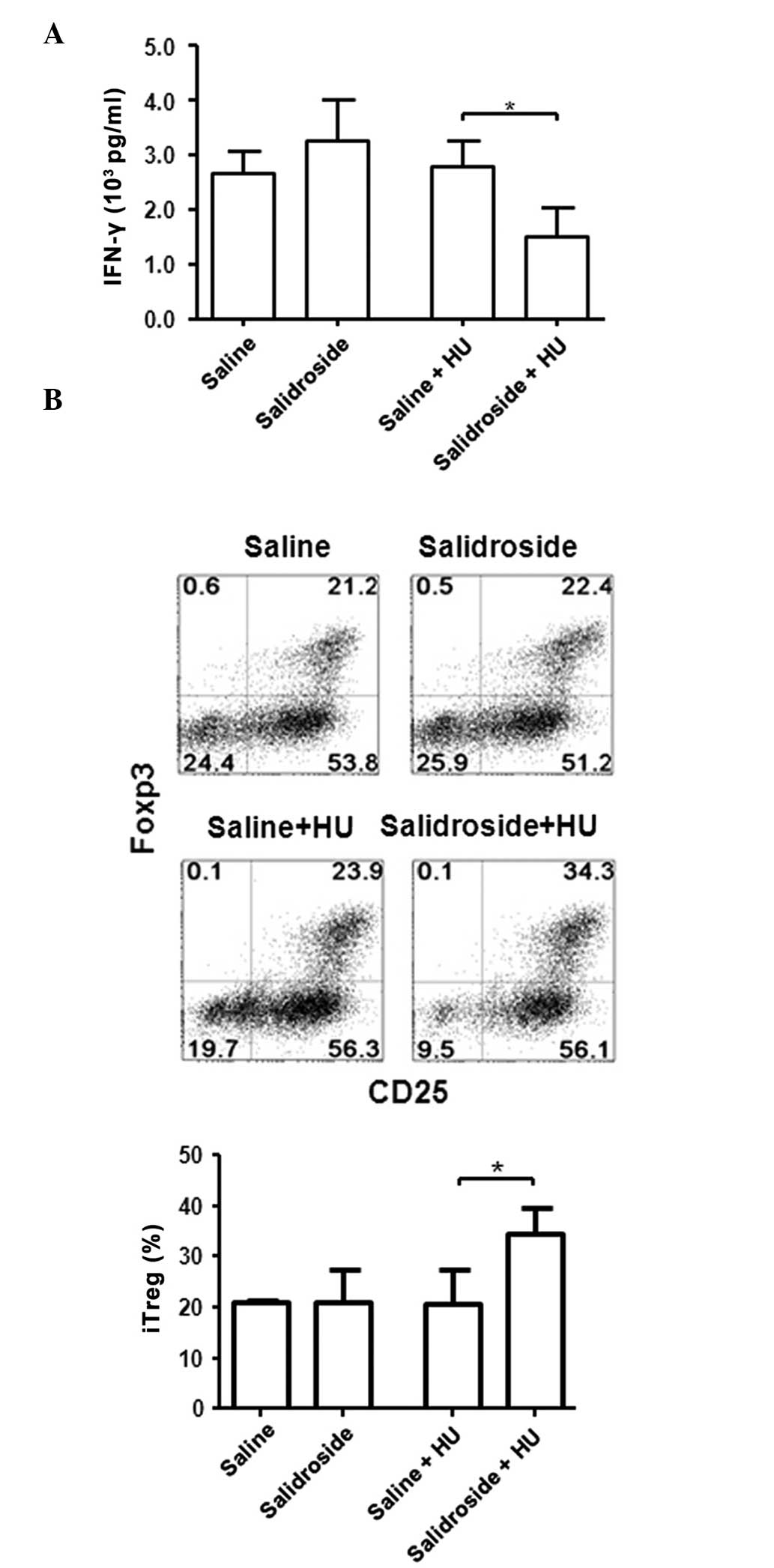
HU model
To investigate whether similar differences are also
observed in the murine system, a 14-day HU mouse model was used.
Mice received salidroside at 50 mg/kg/day by intragastric
administration for 28 days prior to HU and 14 days following HU.
The mice were subsequently sacrificed and splenic T cells were
cultured under various conditions at 1g. The control groups
included saline treatment with and without HU and salidroside
treatment without HU. There was no difference in the levels of
IFN-γ in T cells following anti-CD3 and anti-CD28 stimulation
between the saline with and without HU groups, and in the
salidroside without HU group (Fig.
4A). However, the salidroside with HU group exhibited a
significant reduction in IFN-γ production by T cells, compared with
the saline with HU group (P=0.032), a similar pattern to the
results obtained from human HDBR samples (Fig. 4A). The differentiation of iTregs
was also similar among saline groups with or without HU, and the
salidroside group without HU. However, the salidroside with HU
group exhibited a significant increase in the level of iTreg
differentiation (Fig. 4B).
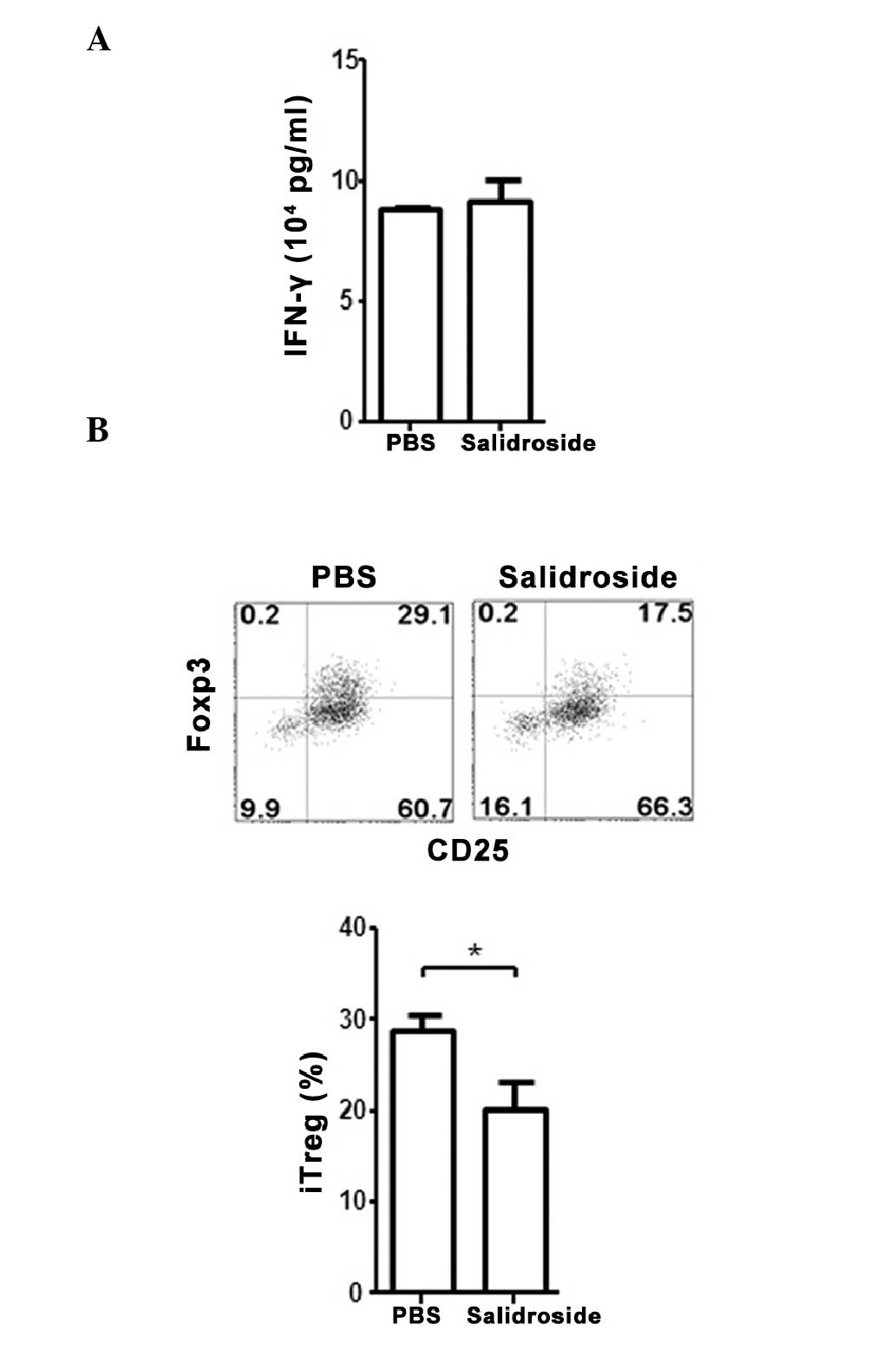
Effect of R. rosea on murine T cells in
vitro
The direct effect of salidroside on murine T cells
in vitro was also investigated. Similar to human PBMCs,
mouse splenic T cells treated with salidroside showed no
significant difference in the levels of IFN-γ, however, compared
with PBS treatment, a significant decrease in iTreg differentiation
was observed (P=0.034; Fig. 5A and
B).
Discussion
Various studies have previously reported that R.
rosea has anti-stress and immunostimulatory activities
(3–10). Thus, the current study investigated
whether R. rosea may improve the function of the immune
system during spaceflight. R. rosea exhibited differential
effects in vitro and in vivo. The administration of
R. rosea in vivo decreased the production of IFN-γ by human
T cells following simulated microgravity (HDBR). The treatment with
R. rosea in vitro, however, did not change the production of
IFN-γ by T cells. Similarly, the differentiation of iTregs was not
altered in R. rosea-treated human or mouse cells following
microgravity simulation, whereas, iTreg differentiation was
significantly decreased when R. rosea was directly added
into the T cell culture. These differences suggest that R.
rosea may have a direct suppressive effect on regulatory T cell
differentiation in vitro and may have an indirect impact on
regulatory T cell differentiation by the production of Th1 type
cytokines under microgravity conditions in vivo. It is
possible that the differences in doses and durations of R.
rosea treatments in vitro and in vivo may account
for the different effects demonstrated. This may be difficult to
confirm as T cells cultured in vitro for >40 days may
need multiple rounds of T cell receptor-mediated activation and the
presence of cytokines to promote cell survival. It is also possible
that the different modulatory effects that were observed following
R. rosea treatment occurred as a result of the different
experimental conditions used for the in vivo microgravity
model and the in vitro 1g model. However, this is unlikely
as PBMCs derived from humans/mice with or without microgravity were
eventually cultured in the same culture conditions as the in
vitro experiment (anti-CD3 and anti-CD28 with or without
TGF-β1). In addition, mice receiving saline and R. rosea
under 1g conditions exhibited similar levels of IFN-γ production
and iTreg differentiation (Fig.
3). This further suggests that R. rosea may have
differential modulatory functions on T cells directly (in
vitro) and indirectly under microgravity (in vivo).
Regarding the direct suppressive effect of R.
rosea on iTregs, these data suggest a casual link between R.
rosea-promoted HIF-1α transcription in T cells and a reduction
in iTreg cell differentiation. Whether R. rosea may alter
HIF-1α transcription in vivo is awaiting further
investigation.
Collectively, the results of the present study
obtained from human and mouse T cells indicate that R. rosea
has a direct negative impact on the differentiation of regulatory T
cells in vitro. Thus, the increase in Treg differentiation
and decrease in IFN-γ production by R. rosea in vivo under
microgravity conditions is probably due to the effect of R.
rosea on cells other than T cells. Whether they are antigen
presenting cells or even cells from neuronal pathways remains
unclear. The results of the current study do not support an
immunostimulatory effect of R. rosea and suggest that R.
rosea may not improve T cell immunity under microgravity in
vivo.
Acknowledgments
The current work was supported by grants from the
Natural Basic Research Program of China (2011CB711000), the
National Natural Science Foundation of China (31270935 and
81471525, Q.G.; 31171144 and 81272177, X.C.), Beijing Natural
Science Foundation (5152010, Q.G.) and the Opening Foundation of
the State Key Laboratory of Space Medicine Fundamentals and
Application, China Astronaut Research and Training Center
(SMFA12K08).
Abbreviations:
|
HDBR
|
head-down bed rest
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
IFN-γ
|
interferon-γ
|
|
IL
|
interleukin
|
|
Treg
|
regulatory T cell
|
|
iTreg
|
induced regulatory T cell
|
|
R. rosea
|
Rhodiola rosea
|
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
HU
|
hind limb unloading
|
|
TGF-β1
|
transforming growth factor β1
|
References
|
1
|
Ishaque S, Shamseer L, Bukutu C and Vohra
S: Rhodiola rosea for physical and mental fatigue: A systematic
review. BMC Complement Altern Med. 12:702012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brekhman II and Dardymov IV: New
substances of plant origin which increase nonspecific resistance.
Annu Rev Pharmacol. 9:419–430. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petkov VD, Yonkov D, Mosharoff A,
Kambourova T, Alova L, Petkov VV and Todorov I: Effects of alcohol
aqueous extract from Rhodiola rosea L. roots on learning and
memory. Acta Physiol Pharmacol Bulg. 12:3–16. 1986.PubMed/NCBI
|
|
4
|
Lee Y, Jung JC, Jang S, Kim J, Ali Z, Khan
IA and Oh S: Anti-inflammatory and neuroprotective effects of
constituents isolated from Rhodiola rosea. Evid Based Complement
Alternat Med. 2013:5140492013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guan S, He J, Guo W, Wei J, Lu J and Deng
X: Adjuvant effects of salidroside from Rhodiola rosea L. on the
immune responses to ovalbumin in mice. Immunopharmacol
Immunotoxicol. 33:738–743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mishra KP, Chanda S, Shukla K and Ganju L:
Adjuvant effect of aqueous extract of Rhodiola imbricata rhizome on
the immune responses to tetanus toxoid and ovalbumin in rats.
Immunopharmacol Immunotoxicol. 32:141–146. 2010. View Article : Google Scholar
|
|
7
|
Li HX, Sze SC, Tong Y and Ng TB:
Production of Th1- and Th2-dependent cytokines induced by the
Chinese medicine herb, Rhodiola algida, on human peripheral blood
monocytes. J Ethnopharmacol. 123:257–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mishra KP, Padwad YS, Jain M, Karan D,
Ganju L and Sawhney RC: Aqueous extract of Rhodiola imbricata
rhizome stimulates proinflammatory mediators via phosphorylated
IkappaB and transcription factor nuclear factor-kappaB.
Immunopharmacol Immunotoxicol. 28:201–212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Ding Y, Zhou J, Sun X and Wang S:
The in vitro and in vivo antiviral effects of salidroside from
Rhodiola rosea L. against coxsackievirus B3. Phytomedicine.
16:146–155. 2009. View Article : Google Scholar
|
|
10
|
Mishra KP, Ganju L, Chanda S, Karan D and
Sawhney RC: Aqueous extract of Rhodiola imbricata rhizome
stimulates Toll-like receptor 4, granzyme-B and Th1 cytokines in
vitro. Immunobiology. 214:27–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crucian BE, Stowe RP, Pierson DL and Sams
CF: Immune system dysregulation following short- vs long-duration
spaceflight. Aviat Space Environ Med. 79:835–843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sonnenfeld G and Shearer WT: Immune
function during space flight. Nutrition. 8:899–903. 2002.
View Article : Google Scholar
|
|
13
|
Buravkova LB, Rykova MP, Grigorieva V and
Antropova EN: Cell interactions in microgravity: Cytotoxic effects
of natural killer cells in vitro. J Gravit Physiol. 11:177–180.
2004.
|
|
14
|
Kaur I, Simons ER, Castro VA, Mark Ott C
and Pierson DL: Changes in neutrophil functions in astronauts.
Brain Behav Immun. 18:443–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pierson DL, Stowe RP, Phillips TM, Lugg DJ
and Mehta SK: Epstein-Barr virus shedding by astronauts during
space flight. Brain Behav Immun. 19:235–242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crucian BE, Stowe RP, Mehta SK, Yetman DL,
Leal MJ, Quiriarte HD, Pierson DL and Sams CF: Immune status,
latent viral reactivation, and stress during long-duration
head-down bed rest. Aviat Space Environ Med. 80(Suppl): A37–A44.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaur I, Simons ER, Castro VA, Ott CM and
Pierson DL: Changes in monocyte functions of astronauts. Brain
Behav Immun. 19:547–554. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaur I, Simons ER, Kapadia AS, Ott CM and
Pierson DL: Effect of spaceflight on ability of monocytes to
respond to endotoxins of gram-negative bacteria. Clin Vaccine
Immunol. 15:1523–1528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stowe RP, Sams CF and Pierson DL: Effects
of mission duration on neuroimmune responses in astronauts. Aviat
Space Environ Med. 74:1281–1284. 2003.PubMed/NCBI
|
|
20
|
Baqai FP, Gridley DS, Slater JM, Luo-Owen
X, Stodieck LS, Ferguson V, Chapes SK and Pecaut MJ: Effects of
spaceflight on innate immune function and antioxidant gene
expression. J Appl Physiol. 106:1935–1942. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gridley DS, Slater JM, Luo-Owen X, Rizvi
A, Chapes SK, Stodieck LS, Ferguson VL and Pecaut MJ: Spaceflight
effects on T lymphocyte distribution, function and gene expression.
J Appl Physiol (1985). 106:194–202. 2009. View Article : Google Scholar
|
|
22
|
Kelsen J, Bartels LE, Dige A, Hvas CL,
Frings-Meuthen P, Boehme G, Thomsen MK, Fenger-Grøn M and Dahlerup
JF: 21 Days head-down bed rest induces weakening of cell-mediated
immunity - Some spaceflight findings confirmed in a ground-based
analog. Cytokine. 59:403–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guéguinou N, Huin-Schohn C, Bascove M,
Bueb JL, Tschirhart E, Legrand-Frossi C and Frippiat JP: Could
spaceflight–associated immune system weakening preclude the
expansion of human presence beyond Earth's orbit? J Leukoc Biol.
86:1027–1038. 2009. View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2 (Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Xu X, Tan C, Li P, Zhang S, Pang X, Liu H,
Li L, Sun X, Zhang Y, Wu H, et al: Changes of cytokines during a
spaceflight analog-a 45-day head-down bed rest. PLoS One.
8:e774012013. View Article : Google Scholar
|
|
26
|
Tauber S, Hauschild S, Paulsen K, Gutewort
A, Raig C, Hürlimann E, Biskup J, Philpot C, Lier H, Engelmann F,
et al: Signal transduction in primary human T lymphocytes in
altered gravity during parabolic flight and clinostat experiments.
Cell Physiol Biochem. 35:1034–1051. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crucian BE, Cubbage ML and Sams CF:
Altered cytokine production by specific human peripheral blood cell
subsets immediately following space flight. J Interferon Cytokine
Res. 20:547–556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Liu A, Hou R, Zhang J, Jia X,
Jiang W and Chen J: Salidroside protects cardiomyocyte against
hypoxia-induced death: A HIF-1-alpha-activated and VEGF-mediated
pathway. Eur J Pharmacol. 607:6–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng KY, Guo AJ, Bi CW, Zhu KY, Chan GK,
Fu Q, Xu SL, Zhan JY, Lau DT, Dong TT, et al: The extract of
Rhodiolae Crenulatae Radix et Rhizoma induces the accumulation of
HIF-1alpha via blocking the degradation pathway in cultured kidney
fibroblasts. Planta Med. 77:894–899. 2011. View Article : Google Scholar
|
|
30
|
Zheng KY, Zhang ZX, Guo AJ, Bi CW, Zhu KY,
Xu SL, Zhan JY, Lau DT, Dong TT, Choi RC and Tsim KW: Salidroside
stimulates the accumulation of HIF-1α protein resulted in the
induction of EPO expression: A signaling via blocking the
degradation pathway in kidney and liver cells. Eur J Pharmacol.
679:34–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dang EV, Barbi J, Yang HY, Jinasena D, Yu
H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al: Control of
T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell.
146:772–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi LZ, Wang R, Huang G, Vogel P, Neale G,
Green DR and Chi H: HIF1alpha-dependent glycolytic pathway
orchestrates a metabolic checkpoint for the differentiation of TH17
and Treg cells. J Exp Med. 208:1367–1376. 2011. View Article : Google Scholar : PubMed/NCBI
|