Introduction
A monolayer of vascular endothelial cells (VECs)
acts as a physiological barrier between blood vessels and vascular
tissues; this monolayer maintains the integrity of the vascular
wall and the function of blood circulation. Ischemia/reperfusion
injury of important organs, including the heart, brain and kidneys,
causes severe damage to VECs and increases the production of
reactive oxygen species (ROS) (1).
ROS act as important intracellular messengers, inhibiting the
protein kinase B (AKT) and mitogen-activated protein kinase 1
signaling pathways, and directly inducing cell apoptosis (2,3). ROS
also affect the phosphatidylinositol 3-kinase (PI3K)-AKT signaling
pathway, which activates or inhibits downstream target proteins,
including B-cell lymphoma 2 (Bcl-2), Bcl-2 associated X protein
(Bax) and caspase-3, via phosphorylation, and regulates various
biological functions, including cell growth, proliferation,
adhesion and apoptosis (4).
Additionally, the changes to cell metabolism and apoptosis caused
by ROS are important factors in cardiovascular dysfunction
(5). Thus, the regulation of
ROS-associated pathways may be an important mechanism for the
protection of VECs.
Radix Astragali is the dried root of the leguminous
plant Astragalus membranaceus (Fischer) Bge. var.
mongolicus (Bge.) Hsiao. According to traditional Chinese
medicine, Radix Astragali demonstrates efficiency in tonifying Qi
to reinforce Yang, strengthening superficial resistance, promoting
urination to expel internal toxins/pus, promoting tissue
regeneration and improving the healing of sores; therefore, it is
an important and commonly used traditional Chinese medicine for
strengthening healthy energy and tonifying Qi (6). Astragalus polysaccharide (APS) is the
primary active ingredient of Radix Astragali and previous studies
have demonstrated it to have a variety of pharmacological effects.
APS reduces the damage to VECs caused by hypoxia/reoxygenation (HR)
and reperfusion injury of human cardiac microvascular endothelial
cells (HCMECs) (7,8). In particular, A-3, a component of
APS, may protect the function of VECs from damage induced by
paraoxon, which is associated with increased superoxide dismutase
(SOD) and decreased malondialdehyde (MDA) levels (9). Additionally, the combined use of
Radix Astragali and ligustrazine significantly protected VECs by
elevating nitric oxide (NO) release (10). Radix Astragali also inhibited
endothelial cell apoptosis induced by advanced glycation end
products via the downregulation of ROS levels (11). A previous investigation indicated
that APS may suppress HR-induced damage to HCMECs damage by
alleviating the oxidative stress caused by ROS and increasing NO
levels. Additionally, APS was demonstrated to activate the
PI3K-AKT-endothelial NO synthase (eNOS) signaling pathway, thus
promoting the proliferation and differentiation of endothelial
progenitor cells in the peripheral blood of patients with type 2
diabetes (12). APS also inhibited
the apoptosis of HCMECs induced by oxygen and glucose deprivation,
with the effects potentially mediated by changes in AKT
phosphorylation levels (13).
Additionally, it was previously demonstrated that APS potentially
protects HCMECs from HR injury via regulation of the PI3K-AKT
signaling pathway.
Thus, the present study aimed to investigate whether
APS protects HCMECs from HR-induced injury via inhibition of
ROS-induced oxidative stress and cell apoptosis, and if APS alters
the regulation of the PI3K-AKT pathway, using an HCMEC model of
HR-induced injury.
Materials and methods
Materials and cell culture
APS was purchased from Nanjing Zelang Medical
Technology Co., Ltd. (Nanjing, China). A bicinchoninic acid (BCA)
assay kit, caspase-3 assay kit, 2′,7′-dichlorofluorescin diacetate
(DCFH-DA) probe, Fura-2/AM probe and Hoechst apoptosis kit were
purchased from Beyotime Institute of Biotechnology (Shanghai,
China). Methylthiazolyl tetrazolium (MTT) was purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Rabbit
anti-human monoclonal antibodes: AKT (cat. no. 1063-1), Bax (cat.
no. 1017-1), Bcl-2 (cat. no. 1080-1), phosphorylated-AKT (p-AKT;
cat. no. 5508-1), PI3K (cat. no. 1683-1) and GAPDH (cat. no.
5632-1) were provided by Epitomics (Burlingame, CA, USA). SOD, MDA
and NO assay kits were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China; cat. nos. A001-1, A003-1,
and A012, respectively). Na2S2O4
was obtained from Aladdin Reagent (Shanghai) Co., Ltd. (Shanghai,
China).
HCMECs were purchased from ScienCell Research
Laboratories (Carlsbad, CA, USA). The cells were incubated with
rabbit anti-human polyclonal anti-factor VIII [cat. no. bs-0434R;
Shanghai Kemin Biotech Co., Ltd. (Shanghai, China)] and anti-CD31
(cat. no. BA1346; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) antibodies, and Dil-acetylated low-density lipoprotein
(Sciencell Research Laboratories) to confirm their endothelial
phenotype. The protocol and characterization were performed
according to a previous study (14). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% (v/v) fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.).
Cell grouping
HCMECs were divided into the following five
treatment groups: Control, HR, and APS-low (-L), -medium (-M) and
-high (-H). Cells in the control group were cultured without any
treatment. In the HR group, 200 μl cells
(1×105/ml) were incubated with 1 mM
Na2S2O4 for 4 h and cultured in
DMEM for a further 24 h. In the APS-L, -M and -H groups, cells were
pretreated with 25, 50 or 100 μg/ml APS, respectively, for
12 h. Cells (200 μl; 1×105/ml) were then
incubated with Na2S2O4 for 4 h and
cultured in DMEM for a further 24 h. The concentration range of APS
used was selected according to the results of a pilot study that
used a wider concentration range (data not presented).
Cell viability and apoptosis
Cells in the exponential growth phase were seeded in
96-well plates and cultured at 37°C and 5% CO2 for 24 h.
Following treatment, 20 μl MTT (5 mg/ml) was added to each
well. After 4 h, the culture media was discarded and replaced by
150 μl dimethyl sulfoxide. After 10 min incubation, the
absorbance was determined at 570 nm using an Infinite F200
microplate reader (Tecan Group, Ltd., Männedorf Switzerland). For
cell apoptosis measurements, 200 μl cells
(1×105/ml) were cultured in 6-well plates, incubated
with Hoechst and analyzed using a Hoechst apoptosis kit, according
to the manufacturer's protocol.
Intracellular ROS levels
Cells were treated as described. Following
treatment, 200 μl cells (1×105/ml) were washed
with phosphate-buffered saline (PBS; Beyotime Institute of
Biotechnology), then incubated with 20 μM DCFH-DA in PBS for
2 h. Subsequently, cells were examined with a
fluorospectrophotometer (SPEX Fluorolog-2; Horiba, Ltd., Kyoto,
Japan) at excitation and emission wavelengths of 340 and 520 nm,
respectively to measure the levels of intracellular ROS.
Intracellular Ca2+
measurements
Cells (200 μl; 8×103/ml) were
seeded onto 20-mm coverslips in 6-well plates and cultured for 48
h. Cells were then treated as described and cultured for a further
48 h. Following incubation with 1 μl Fura-2/AM and 499
μl Ca2+ solution (2 mM) for 30 min, cells were
washed three times with PBS. Cells were examined using the
fluorospectrophotometer at excitation wavelengths of 340 and 380
nm. The fluorescence intensity ratios at 340 and 380 nm were
analyzed to determine the intracellular Ca2+ levels.
Intracellular MDA, SOD and NO
measurements
Cells in the logarithmic growth phase were seeded in
6-well plates and cultured for 48 h. Cells (200 μl;
8×103/ml) were then treated as described and cultured
for a further 48 h. Cells were collected by centrifugation (1,000 ×
g for 10 min) and repeated freeze/thaw cycles were performed to
effuse the cellular contents. The supernatant was collected and
used to determine the expression of MDA, SOD and NO using assay
kits, according to the manufacturer's protocols.
Expression levels of PI3K/p-AKT, Bcl-2
and Bax
Cells in the logarithmic growth phase were seeded in
6-well plates and cultured for 48 h. The cells (200 μl;
8×103/ml) were then treated as described and cultured
for a further 48 h. The cells were collected and lysed (Beyotime
Institute of Biotechnology), and the lysates were centrifuged at
1,000 × g for 10 min to obtain the cellular protein. Protein
concentration was determined using the BCA kit. Equal protein
samples were loaded onto 10% SDS-PAGE gels (40 V for 4 h). The
separated proteins were transferred onto a nitrocellulose membrane
(Beyotime Institute of Biotechnology) and blocked (Beyotime
Institute of Biotechnology). The membrane was then incubated with
the PI3K, p-AKT, Bcl-2 and Bax primary rabbit anti-human monoclonal
antibodies (1:100) at 4°C overnight, followed by incubation with
goat anti-rabbit horseradish peroxidase-conjugated secondary
IgG(H+L) antibody [cat. no. A0208; Beyotime Institute of
Biotechnology (dilution, 1:500)]. Reactive protein was detected
with an enhanced chemiluminescence western blotting kit (Beyotime
Institute of Biotechnology) and Quantity One software version 4.6.2
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) on a ChemiDoc XRS
gel imaging system (Bio-Rad Laboratories, Inc.).
Caspase-3 activity
Cells in the logarithmic growth phase were seeded in
6-well plates and cultured for 48 h. Cells (200 μl;
8×103/ml) were then treated as described and cultured
for a further 48 h. Cells were collected via centrifugation (1,000
× g for 10 min), lysed and analyzed following the addition of the
substrates from the caspase-3 assay kit, according to the
manufacturer's protocol. Absorbance at 405 nm (Multiskan Spectrum;
Thermo Fisher Scientific, Inc.) was used to determine caspase-3
activity.
Statistical analysis
The paired Student's t-test was performed to
analyzed the data and SPSS software (version 17.0; SPSS, Inc.,
Chicago, IL, USA) was used. Data are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of APS on cell viability and
apoptosis
The current study investigated the effect of
treatment with Na2S2O4 and APS on
HCMEC viability (Fig. 1A) and
apoptosis (Fig. 1B and C).
Following Hoechst staining, apoptotic cells exhibited bright and
white fluorescence (Fig. 1C).
Compared with the control group (untreated HCMECs), treatment with
Na2S2O4 resulted in a significant
reduction in cell viability (P=0.003) and a significant increase in
the number of apoptotic cells (P= 0.001). By contrast, APS
treatment resulted in elevated cell viability and reduced apoptosis
in a concentration-dependent manner. At the middle and high
concentrations of APS, cell viability was significantly increased
compared with the HR group (P=0.009 and P=0.002, respectively;
Fig. 1A), possibly due to the
reduced apoptosis levels.
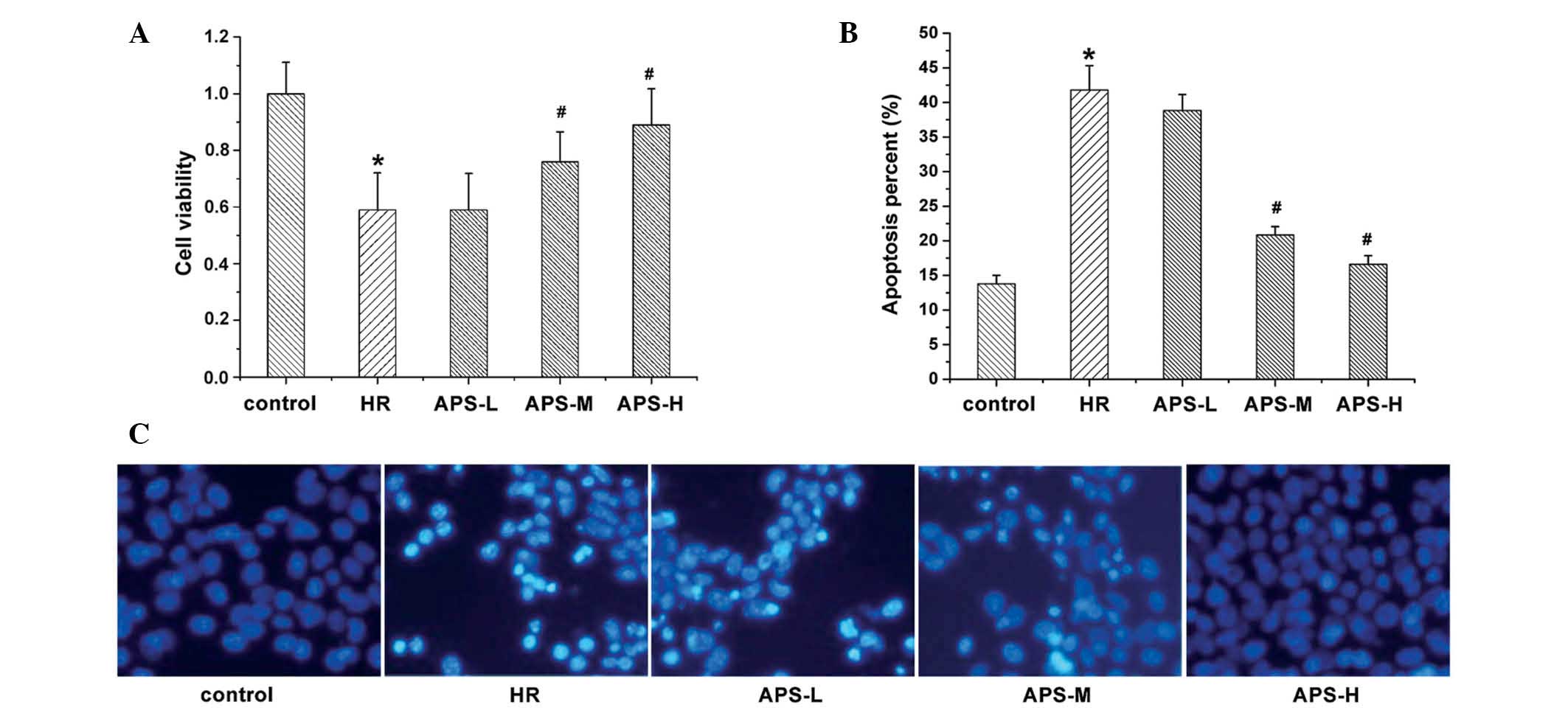 | Figure 1Cell viability and apoptosis of human
cardiac microvascular endothelial cells. (A) Cell viability was
determined using a methylthiazolyl tetrazolium assay following
treatment with Na2S2O4 (the HR
group), and 25, 50 or 100 μg/ml APS (APS-L, APS-M and APS-H
groups, respectively). Cells with no treatment served as the
control. (B and C) Apoptosis was measured under the same treatment
conditions (Hoechst staining; magnification, ×200). Data are
presented as the mean ± standard deviation (n=3).
*P<0.01 vs. the control group; #P<0.01
vs. the HR group. HR, hypoxia/reoxygenation; APS, Astragalus
polysaccharide; -L, -low dose; -M, -medium dose; -H, -high
dose. |
Effect of APS on intracellular ROS
activity and Ca2+ concentration
Fig. 2 indicates
the ROS and Ca2+ levels in HCMEC treated with
Na2S2O4 (the HR group) and APS.
ROS (Fig. 2A) and Ca2+
(Fig. 2B) levels in the HR group
were significantly increased in comparison with the control group
(P=0.001). When cells were treated with the middle and high doses
of APS, the intracellular levels of ROS and Ca2+ were
significantly reduced compared with the HR group (APS-M, P=0.016
and APS-H, P= 0.004, and APS-M, P=0.027 and APS-H, P= 0.005,
respectively). Thus, APS effected ROS and Ca2+ levels in
a concentration-dependent manner.
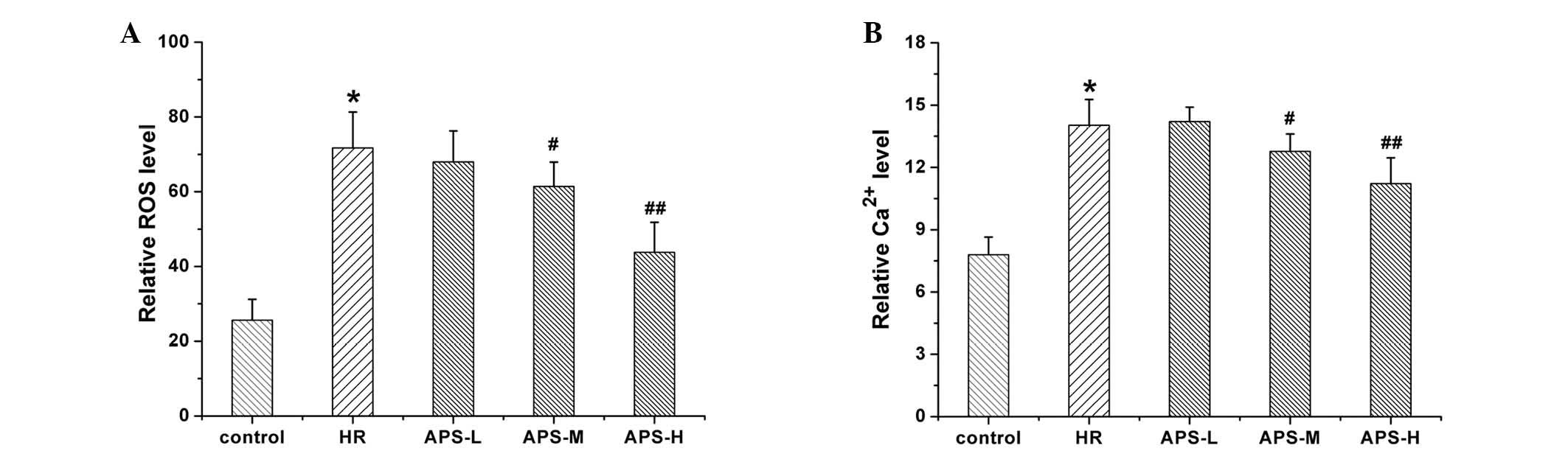 | Figure 2(A) ROS and (B) Ca2+ levels
in human cardiac microvascular endothelial cells following
treatment with Na2S2O4 (the HR
group) and 25, 50 or 100 μg/ml APS (APS-L, APS-M and APS-H
groups, respectively). Cells with no treatment served as the
control. Data are presented as the mean ± standard deviation (n=3).
*P<0.01 vs. the control group; #P<0.05,
##P<0.01 vs. the HR group. ROS, reactive oxygen
species; HR, hypoxia/reoxygenation group; APS, Astragalus
polysaccharide; -L, -low dose; -M, -medium dose; -H, -high
dose. |
Effect of APS on intracellular NO
content
Intracellular NO levels were measured following
treatment with Na2S2O4 (the HR
group) or APS (Fig. 3). NO levels
in the HR group were observed to be reduced compared with the
untreated cells (P=0.001). APS-L did not reverse the
Na2S2O4-induced decrease in ROS
levels in the HR group. However, following treatment with the
middle and high doses of APS, the NO level was significantly
elevated compared with the HR group (P=0.07 and P=0.002,
respectively), suggesting that APS can increase NO levels following
HR injury of HCMECs.
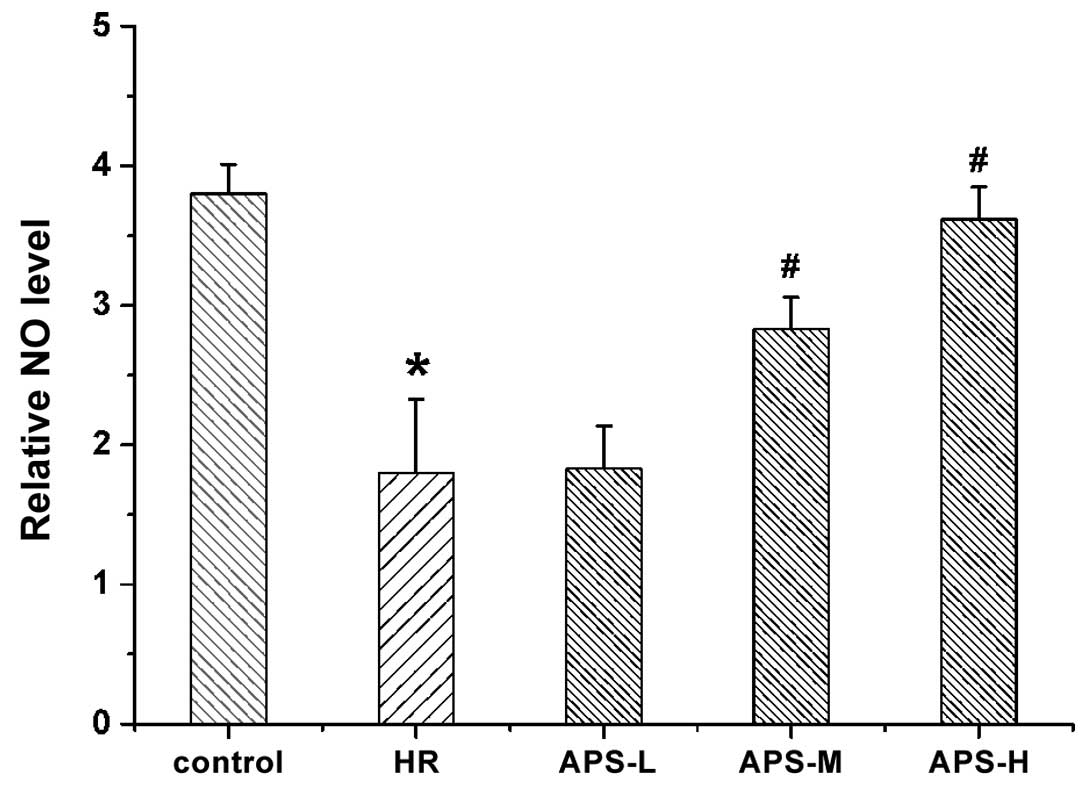 | Figure 3NO levels in human cardiac
microvascular endothelial cells following treatment with
Na2S2O4 (the HR group) and 25, 50
or 100 μg/ml APS (APS-L, APS-M and APS-H groups,
respectively). Cells with no treatment served as the control. Data
are presented as the mean ± standard deviation (n = 3).
*P<0.01 vs. the control group, #P<0.01
vs. the HR group. NO, nitric oxide; HR, hypoxia/reoxygenation; APS,
Astragalus polysaccharide; -L, -low dose; -M, -medium dose; -H,
-high dose. |
Effect of APS on intracellular MDA
content and SOD activity
MDA and SOD levels in HCMECs treated with
Na2S2O4 (the HR group) and various
concentrations of APS are presented in Table I. Compared with the untreated
cells, the MDA concentration in the HR group was significantly
increased by ~1.4 fold (P=0.001). However, compared with the HR
group, the level of MDA was significantly decreased when cells were
pretreated with the three doses of APS (P=0.008, P=0.005 and
P=0.003, respectively). As for SOD, the levels were significantly
reduced in the HR group compared with the control group (P=0.001).
Additionally, compared with the HR group, APS pretreatment
significantly increased the levels of SOD in a
concentration-dependent manner (P=0.008, P=0.002 and P=0.002,
respectively).
 | Table IMDA and SOD levels in human cardiac
microvascular endothelial cells treated with
Na2S2O4 and various concentrations
of APS. |
Table I
MDA and SOD levels in human cardiac
microvascular endothelial cells treated with
Na2S2O4 and various concentrations
of APS.
| Group | MDA, mM/mg
protein | SOD, U/l
protein |
|---|
| Control | 1.80±0.03 | 19.10±0.67 |
| HR | 4.26±0.04a | 9.14±0.41a |
| APS-L | 3.90±0.03b | 11.81±0.26b |
| APS-M | 3.20±0.04b | 16.05±0.33b |
| APS-H | 2.79±0.05b | 17.99± 0.32b |
Effect of APS on PI3K/p-AKT protein
expression levels
Western blotting with qualitative and quantitative
analysis was performed to evaluate the expression of PI3K/p-AKT in
HCMECs following treatment with
Na2S2O4 (the HR group) and various
concentrations of APS. As presented in Fig. 4, similar levels of AKT were
observed in all groups, thus, AKT was used as the internal control
of quantification of p-AKT levels. Reduced levels of PI3K and p-AKT
were detected in the HR group compared with the control group
(P=0.001), suggesting the downregulation of PI3K levels and reduced
phosphorylation of AKT. Following APS pretreatment at all three
doses, the PI3K and p-AKT levels were significantly upregulated in
a dose-dependent manner compared with the HR group (PI3K: APS-L,
P=0.007; APS-M, P=0.001; and APS-H, P=0.001 and p-AKT: P=0.009,
P=0.001 and P= 0.001).
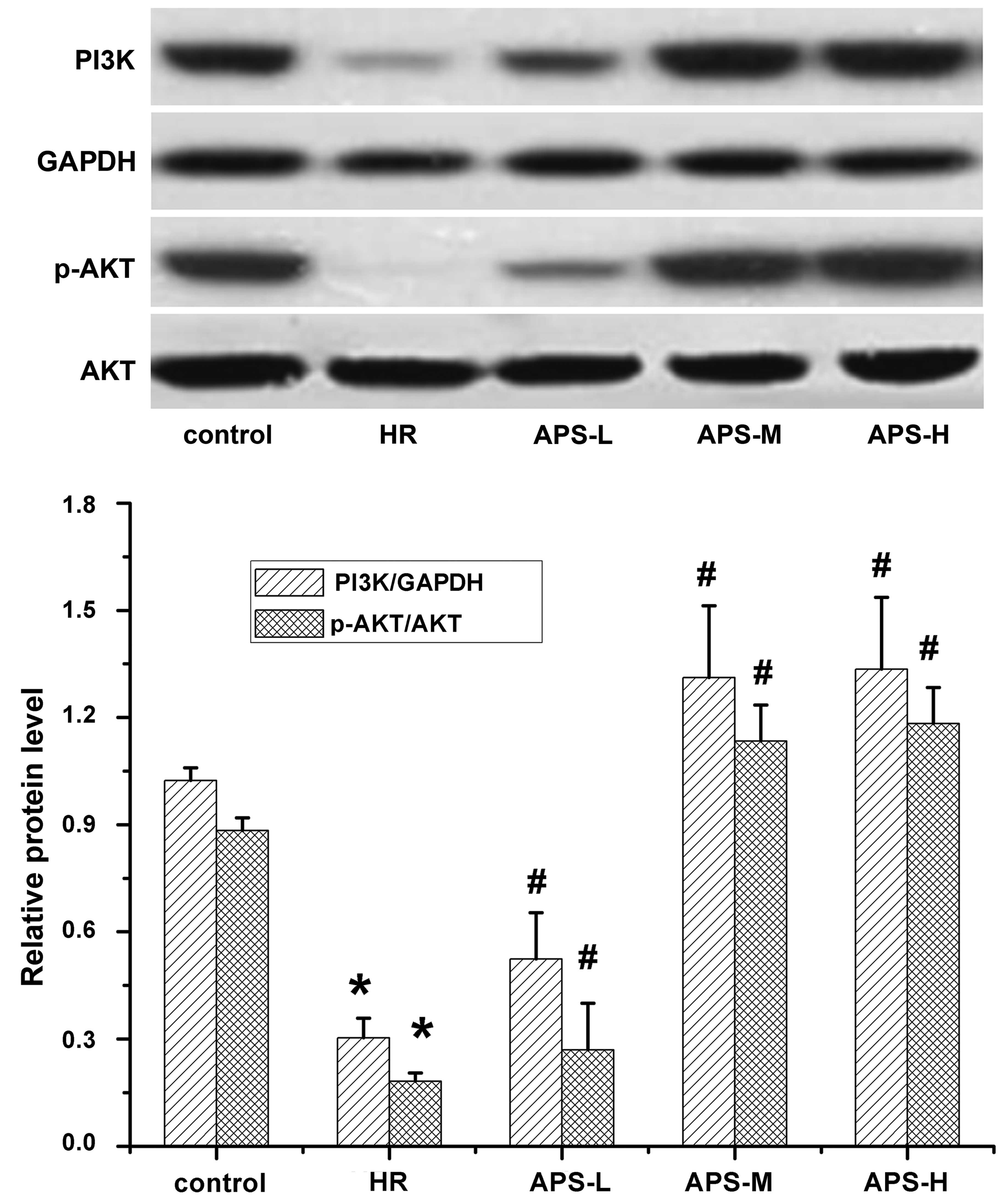 | Figure 4Western blot analysis of PI3K/p-AKT
expression levels in human cardiac microvascular endothelial cells
treated with Na2S2O4 (the HR
group) and 25, 50 or 100 μg/ml APS (APS-L, APS-M and APS-H
groups, respectively). Cells with no treatment served as the
control. GAPDH was used as the internal control. Data are presented
as the mean ± standard deviation (n=3). *P<0.01 vs.
the control group, #P<0.01 vs. the HR group. PI3K,
phosphatidylinositol 3-kinase; p-AKT, phosphorylated-protein kinase
B; HR, hypoxia/reoxygenation; APS, Astragalus polysaccharide; -L,
-low dose; -M, -medium dose; -H, -high dose. |
Effect of APS on Bcl-2 and Bax protein
expression levels
The expression levels of Bcl-2 and Bax in HCMECs
were determined by western blotting with qualitative and
quantitative analysis (Fig. 5).
Compared with control cells, the expression of Bcl-2 in the HR
group was significantly reduced (P=0.003). However, compared with
the HR group, the middle and high doses of APS significantly
increased the Bcl-2 expression levels (P=0.008 and P=0.002,
respectively). As for Bax, its expression was significantly
increased in the HR group (P=0.001). Following APS preconditioning,
the high expression of Bax induced by odium dithionite was
decreased by APS at all the three doses to a relatively low level
(all P=0.001).
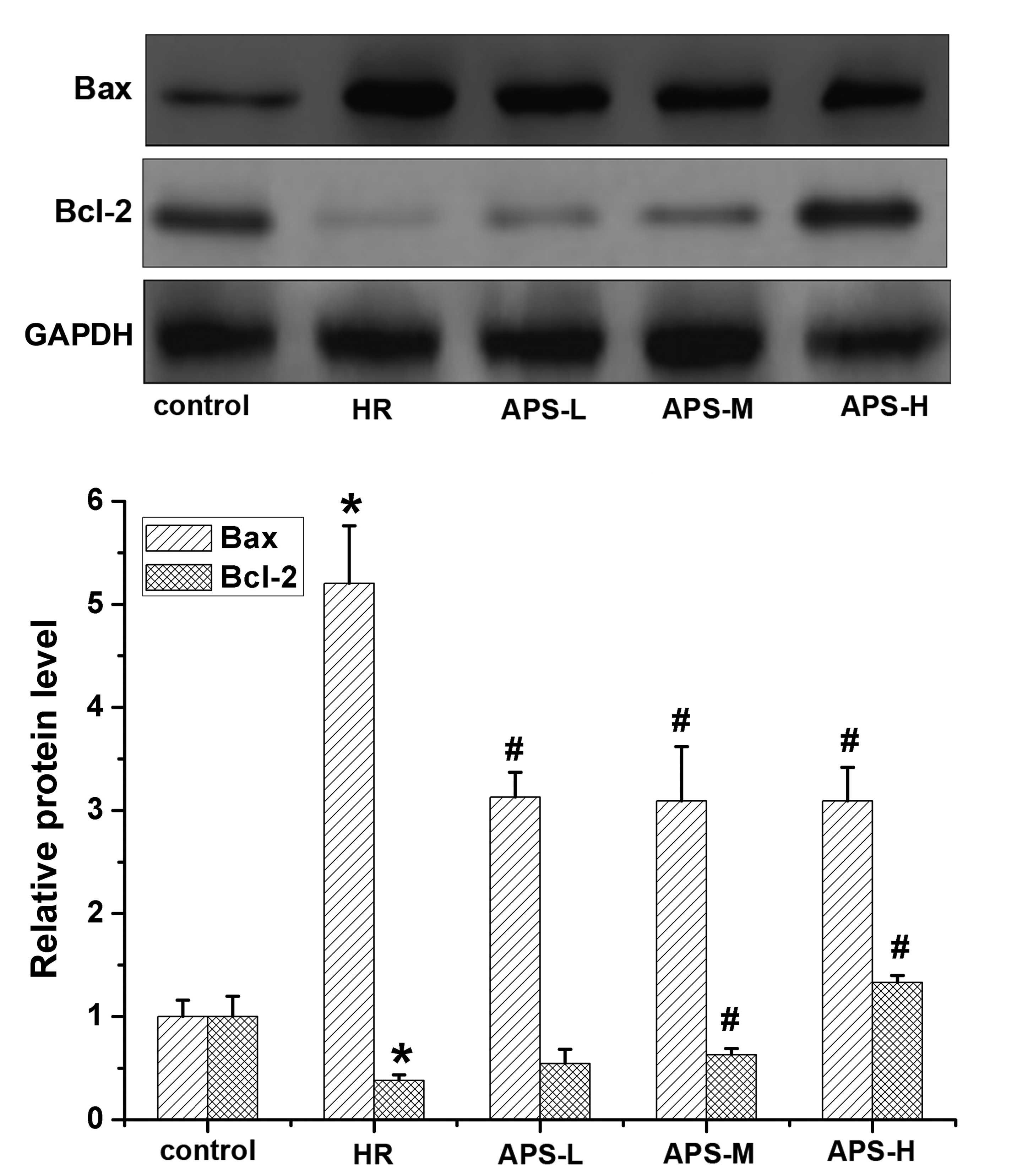 | Figure 5Western blot analysis of Bcl-2 and
Bax expression levels in human cardiac microvascular endothelial
cells treated with Na2S2O4 (the HR
group) and 25, 50 or 100 μg/ml APS (APS-L, APS-M and APS-H
groups, respectively). Cells with no treatment served as the
control. GAPDH was used as the internal control. Data are presented
as the mean ± standard deviation (n=3). *P<0.01 vs.
the control group; #P<0.01 vs. the HR group. Bax,
Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; HR,
hypoxia/reoxygenation; APS, Astragalus polysaccharide; -L, -low
dose; -M, -medium dose; -H, -high dose. |
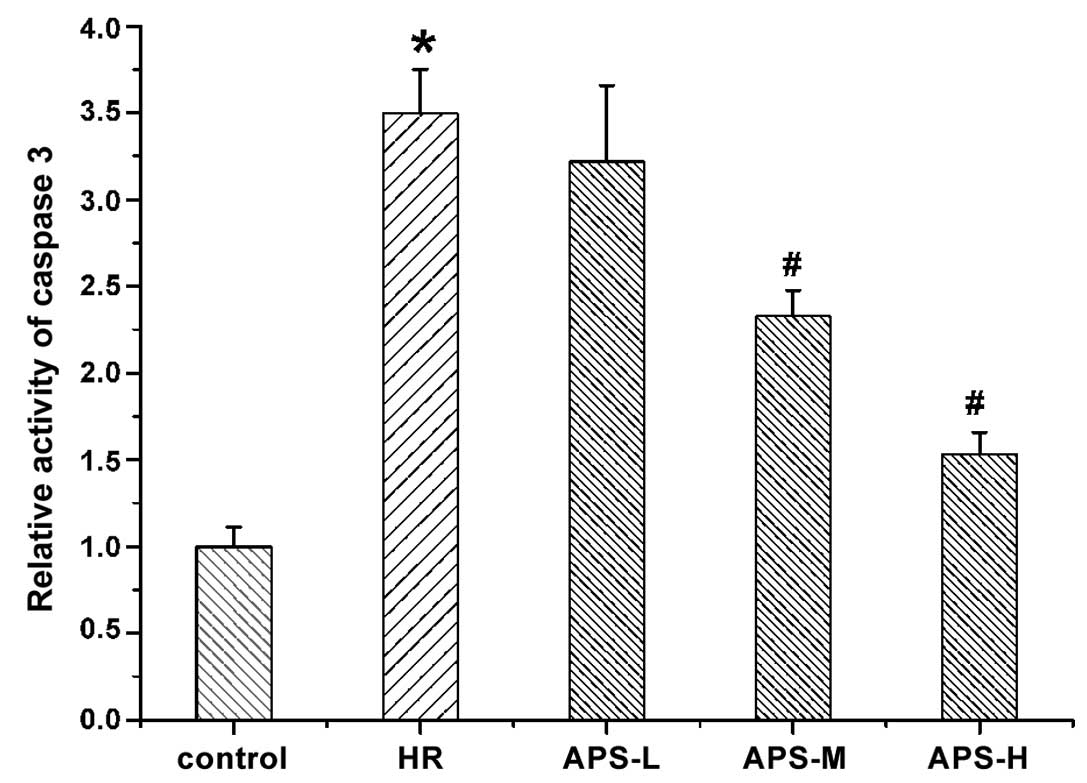
Effect of APS on caspase-3 activity
The activity of caspase-3 in HCMEC was determined
using a caspase-3 assay kit and the results are presented in
Fig. 6. The activity of caspase-3
was significantly increased by
Na2S2O4 treatment (the HR group)
compared with the control cells (P=0.001). Notably, the middle and
high doses of APS significantly reduced the high activity of
caspase-3 induced by Na2S2O4
compared with the HR group in a concentration-dependent manner
(P=0.003 and P=0.001, respectively).
Discussion
The vascular endothelium is composed of a monolayer
of endothelial cells that secrete a variety of vasoactive
substances via autocrine and paracrine mechanisms, targeting
various cell types, including vascular smooth muscle cells and
peripheral white blood cells. Therefore, the vascular endothelium
not only serves as a physiological barrier, it is also important in
antithrombosis and inhibition of inflammation of the vascular wall.
HR-induced injury damages the vascular endothelium and, thus,
impairs the function of VECs. Using MTT and Hoechst assays, the
current study demonstrated that HCMECs are protected by APS,
particularly at high doses, following HR-induced injury.
Free radicals are important in the endothelial
injury induced by HR. ROS produced by HR stimuli penetrate the
cellular membrane causing lipid peroxidation and cellular damage.
The stable internal and external environments of the vessels are
disrupted by ROS, resulting in VEC injury. ROS-induced endothelial
injury is associated with elevated levels of intracellular free
Ca2+, an important second messenger in cells. An excess
of Ca2+ promotes the hydrolysis of phospholipase into
noxious substances, including fatty acid and leukotriene, which are
harmful to cells and promote the decomposition of cytoskeletal
components, leading to cellular damage. Dysregulation of
Ca2+ levels in VECs changes the expression levels of
eNOS and induces cell apoptosis (15). Additionally, increases of
Ca2+ levels exceeding the normal threshold in cells
facilitates the accumulation of ROS. ROS accumulation reduces the
activity of eNOS, which catalyzes NO production under normal
physiological conditions. NO is a vascular protective factor
produced by endothelial cells. During oxidative stress, the
majority of superoxide anions inhibit the biological activity of
eNOS, thus reducing the production and activity of NO. The
decreased NO level and biological activity initiates vasodilatation
and damage to the function of VECs. The activation of AKT in VECs
may increase the release of NO and maintain the integrity of the
functional layer of VECs (16).
A previous report demonstrated that Radix Astragali
protects endothelial cells from apoptosis via the inhibition of ROS
(10). Zhu et al (17) observed that 10–50 μg/ml APS
significantly inhibited ROS production induced by tumor necrosis
factor-α in HCMECs. Additionally, APS was demonstrated to
ameliorate diabetes in palmitate-induced KKAy diabetic mice via the
ROS pathway (18). The current
study investigated the detailed mechanisms involved in the
protection of HCMECs from HR by APS. It was observed that APS
protected HCMECs from HR-induced injury by significantly decreasing
the levels of ROS and Ca2+, and enhancing the levels of
NO. It was also demonstrated that APS was able to protect VECs from
HR-induced injury via regulating the levels of vasoactive
substances and oxidizing materials, including ROS.
Excessive accumulation of ROS directly results in
lipid peroxidation of the cell membrane, with MDA produced as the
typical by-product. The levels of MDA are associated with the
severity of oxidative stress experienced by cells. SOD is an
endogenous antioxidative enzyme that breaks down intracellular ROS
when cells are exposed to an external stress. Thus, changes to the
levels of intracellular MDA and SOD can be used to indirectly
reflect the degree of cellular oxidative damage. It was previously
reported that APS treatment reduced ROS and MDA levels, and
increased the expression levels of SOD in EA.hy926 cells with
bronchopulmonary dysplasia (19).
Similarly, the present study observed that APS treatment decreased
the MDA levels and increased the SOD levels in HCMECs with HR
injury.
The PI3K/AKT signaling pathway regulates various
biological functions of cells, including cell growth, proliferation
and adhesion. Intracellular accumulation of ROS inhibits the
PI3K/AKT signaling pathway and induces cell apoptosis (2,3).
Specifically, the activation of PI3K results in the recruitment and
phosphorylation of AKT, and affects the target proteins of this
pathway, including Bcl-2, Bax and caspase-3, via a signaling
cascade (4). The upregulation of
PI3K/AKT signaling may inhibit the apoptosis of HCMECs and the
endothelial dysfunction induced by HR (20). Activation of the PI3K/AKT pathway
may also prevent HR-induced apoptosis of myocardium microvascular
endothelial cells (21). It was
previously indicated that the PI3K/AKT signaling pathway is
important in the regulation of endothelial cell apoptosis, thus,
APS may exert cytoprotective effects via regulation of PI3K/AKT
signaling. Cao et al (22)
observed that APS inhibits the apoptosis of myocardial cells and
reduces heart failure in a doxorubicin-induced mouse model via the
suppression of AKT activity and the reduction of ROS levels. Ye
et al (23) demonstrated
that the proliferation of MDA-MB-468 breast cancer cells was
arrested by regulating AKT phosphorylation at Thr308 and Ser473.
Additionally, extracts of Radix Astragali attenuated
cytokine-induced keratinocyte damage via the intracellular ROS
level and the PI3K/AKT pathway (24). Astragaloside was previously
demonstrated to inhibit myocardial cell apoptosis induced by
doxorubicin via a reduction in ROS levels, which was associated
with the PI3K/AKT signaling pathway (25). In the current study, APS attenuated
HR-induced HCMEC damage via upregulation of PI3K expression and
increased phosphorylation of AKT. This suggests that APS protects
HCMECs from HR injury through regulation of the PI3K/AKT signaling
pathway.
The accumulation of ROS enhances apoptosis in
endothelial cells (26). The Bcl-2
protein family is important in the process of apoptosis. In
particular, Bcl-2 is the major anti-apoptotic protein. It binds to
the pro-apoptotic protein, Bax, forming heterodimers in the outer
mitochondrial membrane; this reduces the release of caspase from
the mitochondria, leading to inhibition of cell apoptosis. Caspases
are essential proteins in cell apoptosis; in particular, caspase-3
is the crucial effector and a focal point of the apoptosis pathway.
The apoptosis of HCMECs induced by HR was associated with decreased
expression levels of Bcl-2, and increased expression levels of Bax
and activated caspase-3 (27).
Xiao et al (28) reported
that treatment with 100–200 μg/ml APS decreased the
apoptosis of HL-60 cells by inhibiting the activity of caspase-3.
In the current study, it was observed that APS protected HCMECs
from HR-induced injury by upregulating Bcl-2 expression levels,
downregulating Bax expression levels and inhibiting caspase-3
activity. Additionally, the current study demonstrated that HCMEC
protection by APS was concentration-dependent. The higher dose of
APS was associated with the greatest change in ROS,
Ca2+, NO, MDA, SOD, PI3K/AKT, Bcl-2 and Bax levels, as
well as caspase-3 activity.
Notably, the lack of antagonist or agonist use to
intervene in key signaling mechanisms was a limitation of the
present study, and should be taken into consideration when
developing protocols for future studies.
In conclusion, APS protected HCMECs from HR-induced
injury by reducing the levels of ROS, Ca2+, MDA and Bax,
increasing the levels of NO, SOD, Bcl-2 and PI3K, enhancing the
phosphorylation of AKT, and inhibiting the activity of caspase-3.
Furthermore, APS acted in a concentration-dependent manner,
providing greater protection at higher doses. These results may
provide an insight into the mechanisms associated with HR-induced
injury of HCMECs and the protective effect of APS. The findings of
the current study may serve as a guideline for the clinical
application of APS and the treatment of HR-induced injury.
Acknowledgments
The authors are grateful for the financial support
from the National Natural Science Foundation of China (nos.
81102573 and 81273692).
References
|
1
|
Mangge H, Becker K, Fuchs D and Gostner
JM: Antioxidants, inflammation and cardiovascular disease. World J
Cardiol. 6:462–477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao G, Cai H, Cai B and Tu S: Effect of
5-hydroxymethylfurfural derived from processed Cornus officinalis
on the prevention of high glucose-induced oxidative stress in human
umbilical vein endothelial cells and its mechanism. Food Chem.
140:273–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Wang Z, Zuo G, Li B, Zhang J,
Tian N and Chen S: Low shear stress induces human vascular
endothelial cell apoptosis by activating Akt signal and increasing
reactive oxygen species. Nan Fang Yi Ke Da Xue Xue Bao. 33:313–317.
2013.PubMed/NCBI
|
|
4
|
Hu L, Sun Y and Hu J: Catalpol inhibits
apoptosis in hydrogen peroxide-induced endothelium by activating
the PI3K/Akt signaling pathway and modulating expression of Bcl-2
and Bax. Eur J Pharmacol. 628:155–163. 2010. View Article : Google Scholar
|
|
5
|
Ginter E, Simko V and Panakova V:
Antioxidants in health and disease. Bratisl Lek Listy. 115:603–606.
2014.
|
|
6
|
Zhang X, Xu X and Wang N: Progress of
studies on protective mechanism of Radix Astragali in vascular
endothelial cells. Chinese Pharm J. 48:1526–1530. 2013.
|
|
7
|
Hai-Yan Z, Yong-Hong G, Zhi-Yao W, Bing X,
Ai-Ming W, Yan-Wei X, Bei L, Li-Xia L and Li-Xin C: Astragalus
polysaccharide suppresses the expression of adhesion molecules
through the regulation of the p38 MAPK signaling pathway in human
cardiac microvascular endothelial cells after ischemia-reperfusion
injury. Evid Based Complement Alternat Med. 2013:2804932013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu B, Zhu H, Gao Y, Liu B, Zhu L and Chen
L: Influences of Astragalus polysaccharides on genetic
transcription of P-selectin and E-selectin in human cardiac
microvascular endothelial cells after ischemia-reperfusion injury.
J Beijing Univ Tradit Chin Med. 34:177–180. 2011.
|
|
9
|
Yin Y, Li P, Lu G, Liang J and Zhao F:
Protective effects of APS-A3 on blood vessel endothelium function
induced by paraoxon. Lishizhen Med Mater Med Res. 22:583–585.
2011.
|
|
10
|
Li T, Chen L, Li Q, Zhou Y, Tang H and
Cheng S: The protective effects of tetramethylpyrazine (TMP)
combined with Astragalus polysaccharides (APS) on vascular
endothelial cells (VECs). China J Tradit Chin Med Pharm.
26:2672–2675. 2011.
|
|
11
|
Zhong Y, Cheng C, Huang H, Li A, Liu B and
Liu S: Astragalus polysaccharides protects advanced glycation
end-products induced endothelial cells apoptosis. Clin Med Eng.
21:18–20. 2014.
|
|
12
|
Xu H, Wu Q, Xie X and Kong D: Effect of
Astragalus polysaccharides on peripheral endothelial progenitor
cells via PI3K/Akt/eNOS signal pathway in patients with type 2
diabetes. J Clin Rehabilitative Tissue Eng Res. 15:4272–4276.
2011.
|
|
13
|
Wang S, Feng Y, Wang L, Wang Y, Xu D and
Ruan K: The effect of polysaccharide from Radix Astragali on cells
survival against oxygen glucose deprivation and phosphorylation of
Akt. Pharm Biotechnol. 18:288–290. 2011.
|
|
14
|
Zhu H, Chen L and Zhu L: Effect of
astragalus polysaccharides on expression of ICAM-1 and VCAM-1 in
human cardiac microvascular endothelial cells after hypoxia and
reoxygenation. Liaoning J Tradit Chinese Med. 35:293–295. 2008.
|
|
15
|
Suriyo T, Watcharasit P, Thiantanawat A
and Satayavivad J: Arsenite promotes apoptosis and dysfunction in
microvascular endothelial cells via an alteration of intracellular
calcium homeostasis. Toxicol In Vitro. 26:386–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukai Y, Shimokawa H, Matoba T, Hiroki J,
Kunihiro I, Fujiki T and Takeshita A: Acute vasodilator effects of
HMG-CoA reductase inhibitors: Involvement of PI3-kinase/Akt pathway
and Kv channels. J Cardiovasc Pharmacol. 42:118–124. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu YP, Shen T, Lin YJ, Chen BD, Ruan Y,
Cao Y, Qiao Y, Man Y, Wang S and Li J: Astragalus polysaccharides
suppress ICAM-1 and VCAM-1 expression in TNF-α-treated human
vascular endothelial cells by blocking NF-ĸB activation. Acta
Pharmacol Sin. 34:1036–1042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu M, Qin J, Hao Y, Liu M, Luo J, Luo T
and Wei L: Astragalus polysaccharide suppresses skeletal muscle
myostatin expression in diabetes: Involvement of ROS-ERK and NF-ĸB
pathways. Oxid Med Cell Longev. 2013:7824972013. View Article : Google Scholar
|
|
19
|
Huang WM, Liang YQ, Tang LJ, Ding Y and
Wang XH: Antioxidant and anti-inflammatory effects of Astragalus
polysaccharide on EA.hy926 cells. Exp Ther Med. 6:199–203.
2013.PubMed/NCBI
|
|
20
|
Zuo H, Liao D, Lin L, Zhang R and Li X:
Resveratrol attenuates hypoxia-reperfusion injury induced rat
myocardium microvascular endothelial cell dysfunction through
upregulating PI3K/Akt/SVV pathways. Zhonghua Xin Xue Guan Bing Za
Zhi. 42:670–674. 2014.In Chinese. PubMed/NCBI
|
|
21
|
Su C, Xia T, Ren S, Qing S, Jing D, Lian
H, Bin Q, Yuan Z and Xiang Z: Effect of diazoxide preconditioning
on cultured rat myocardium microvascular endothelial cells against
apoptosis and relation of PI3K/Akt pathway. Balkan Med J. 31:83–87.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao Y, Ruan Y, Shen T, Huang X, Li M, Yu
W, Zhu Y, Man Y, Wang S and Li J: Astragalus polysaccharide
suppresses doxorubicin-induced cardiotoxicity by regulating the
PI3k/Akt and p38MAPK pathways. Oxid Med Cell Longev.
2014:6742192014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye MN, Chen HF, Zhou RJ and Liao MJ:
Effects of Astragalus polysaccharide on proliferation and Akt
phosphorylation of the basal-like breast cancer cell line. Zhong Xi
Yi Jie He Xue Bao. 9:1339–1346. 2011.In Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim BH, Oh I, Kim JH, Jeon JE, Jeon B,
Shin J and Kim TY: Anti-inflammatory activity of compounds isolated
from Astragalus sinicus L. in cytokine-induced keratinocytes and
skin. Exp Mol Med. 46:e872014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jia Y, Zuo D, Li Z, Liu H, Dai Z, Cai J,
Pang L and Wu Y: Astragaloside IV inhibits doxorubicin-induced
cardiomyocyte apoptosis mediated by mitochondrial apoptotic pathway
via activating the PI3K/Akt pathway. Chem Pharm Bull (Tokyo).
62:45–53. 2014. View Article : Google Scholar
|
|
26
|
Ge GH, Dou HJ, Yang SS, Ma JW, Cheng WB,
Qiao ZY, Hou YM and Fang WY: Glucagon-like peptide-1 protects
against cardiac microvascular endothelial cells injured by high
glucose. Asian Pac J Trop Med. 8:73–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Velotta JB, Kimura N, Chang SH, Chung J,
Itoh S, Rothbard J, Yang PC, Steinman L, Robbins RC and Fischbein
MP: αB-crystallin improves murine cardiac function and attenuates
apoptosis in human endothelial cells exposed to
ischemia-reperfusion. Ann Thorac Surg. 91:1907–1913. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao B, Xu Y, He H, Jiang QL, Li SY, Shu
HY, Liang EY, Yi ZS, Ye JY, Huang LF, et al: Anti-apoptotic effect
of Astragalus polysaccharide on myeloid cells. Zhongguo Shi Yan Xue
Ye Xue Za Zhi. 21:1243–1247. 2013.In Chinese. PubMed/NCBI
|