Introduction
Cyathus is a genus of fungi in the
Nidulariaceae family, which is collectively known as bird's nest
fungi (1). The Cyathus
genus produces high levels of cyathane-type diterpenoids with a
unique tricyclic skeleton (2–4).
Cyathane-type diterpenoids represent a group of natural products
with substantial diversity in chemical structure and bioactivities
(5–7). The identification of cyathin A3 and
allocyathin B3 from the liquid culture of Cyathus helenae in
1972 represents the first identification of cyathane-type
diterpenoids (2). There have been
continued investigations into these particular natural compounds,
leading to the purification and structural elucidation of a number
of cyathane-type diterpenoids from the Cyathus genus,
including C. helenae (2,8–10),
C. africanus (11), C.
striatius (12) and C.
earlei (13). However, until
now, reports on the bioactivities of these cyathane diterpenoids
remain limited, with the exception of a small number of studies on
the neurotrophic activities of striatoids A-F isolated from
cultures of the fungus Cyathus striatus (14), and identification of cyathuscavins
A, B and C as free-radical scavengers with DNA protective activity
(15).
In our previous study, eight cyathane-type
diterpenoids (cyathatriol, 11-O-acetylcyathatriol, cyathins
D-H and neosarcodonin O) were identified from the solid culture of
C. africanus (16). The
bioassay results firstly indicated that
11-O-acetylcyathatriol exhibits potent inhibitory effects
against nitric oxide (NO) secretion in lipopolysaccharide
(LPS)-activated macrophages. 11-O-acetylcyathatriol is a
cyathane diterpenoid with the highest yield from C.
africanus, and the previous bioassay results provide direct
evidence to support its possible anti-inflammatory property. In
order to confirm its anti-inflammatory effect and to clarify the
molecular mechanism, the in vitro and in vivo
anti-inflammatory activities, and the regulatory effect on the
inflammatory signal transduction pathway of
11-O-acetylcyathatriol were further investigated and
reported in the present study.
Materials and methods
Fungal material
The fungal strain was isolated from the fruiting
body of C. africanus, and identified by Professor Yuhui Chen
(Institute of Microbiology, Chinese Academy of Sciences, Beijing,
China). The fungus was deposited in the China General
Microbiological Culture Collection (CGMCC 5.1117).
Strain fermentation and isolation of
11-O-acetylcyathatriol
Strain fermentation of C. africanus was
performed, as described previously (16). The fermented substrate was
extracted using ethyl acetate (Tianjin Damao Chemical Reagent
Factory, Tianjin, China) by exhaustive maceration (5 l, three
times), and the solvent was then evaporated under reduced pressure
to obtain the crude extract (66.5 g). The residue was resuspensed
in 400 ml of water and partitioned with an equal volume of
CHCl3 (Tianjin Damao Chemical Reagent Factory) to yield
the CHCl3-soluble fraction (46 g). The
CHCl3-soluble fraction (30 g) was subjected to silica
gel column chromatography and eluted with a gradient of
n-hexane-ethyl acetate (v/v, 100:0–100:35) and
dichloromethane-acetone (v/v, 100:2-0:100; both Tianjin Damao
Chemical Reagent Factory) to yield eleven fractions (CA1-CA11).
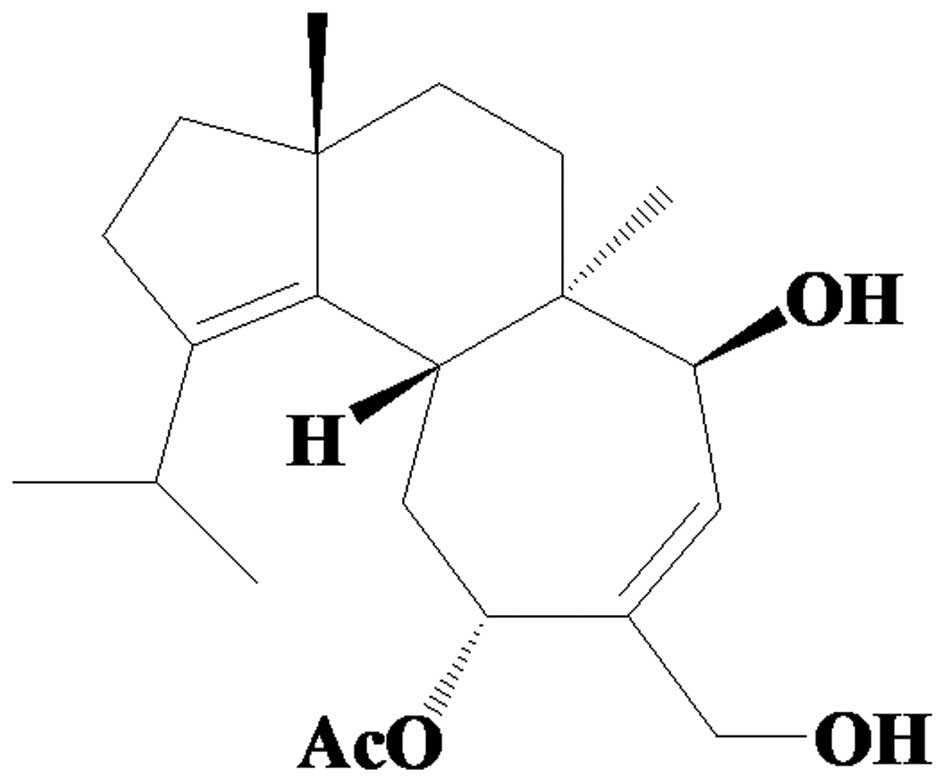
11-O-acetylcyathatriol (4.6 g; Fig. 1) was obtained from fraction CA9 by
recrystallization in methanol. The purity was determined by
normalization of the peak areas in high-performance liquid
chromatography (HPLC; LC-20A, Shimadzu Corporation, Kyoto, Japan),
and was found to be 99.3%, using an ultraviolet detector
(LT28-UV600; Agilent Technologies, Inc., Santa Clara, CA, USA).
Reagents
RPMI-1640 medium and fetal bovine serum (FBS) were
obtained from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). The nitric oxide determination kit (Griess method), mouse
tumor necrosis factor (TNF)-α ELISA kit (cat. no. SEM024) and mouse
interleukin (IL)-6 ELISA kit (cat. no. SEM008), cell lysis buffer
and bicinchoninic acid (BCA) protein concentration assay kit were
products of Yantai Science and Biotechnology Co., Ltd. (Yantai,
China). Mouse anti-rabbit inducible nitric oxide synthase (iNOS)
polyclonal antibody (cat. no. 160862; Cayman Chemical Co., Ann
Arbor, MI, USA), mouse anti-rabbit cyclooxygenase-2 (COX-2)
polyclonal antibody (cat. no. 160106, Cayman Chemical Co.), goat
anti- rabbit phosphorylated (p) extracellular signal-regulated
kinase (ERK 1/2) polyclonal antibody (cat. no. AF1015; Affinity
Bio, Scoresby, VIC, Australia), goat anti-rabbit p-c-Jun N-terminal
kinase (p-JNK) polyclonal antibody (cat. no. AF3318; Affinity Bio),
goat anti-rabbit p-p38 polyclonal antibody (cat. no. AF3455;
Affinity Bio), goat anti-rabbit IκB-α polyclonal antibody (cat. no.
sc-371; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), goat
anti-rabbit β-actin polyclonal antibody (cat. no. sc-1616; Santa
Cruz Biotechnology, Inc.), horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG (H+L) (cat. no. S0001; Affinity Bio) were used
at a dilution of 1:1,000. MTT, LPS, hydrocortisone (cat. no. H4001;
purity ≥98% by HPLC) and other reagents were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Omeprazole enteric-coated
capsules (20 mg/capsule; lot. 613071024) was a product of Shandong
Luoxin Pharmaceutical Co., Ltd. (Linyi, China).
Culture of the RAW 264.7 cells
RAW 264.7 cells, obtained from American Type Culture
Collection (no. TIB-71) were cultured in RPMI-1640 medium
supplemented with 10% heat inactivated FBS at 37°C in a humidified
incubator. The medium was routinely replaced every 2 days. The RAW
264.7 cells were passaged by trypsinization [0.25% (w/v) trypsin;
Yantai Science and Biotechnology Co., Ltd.] until they attained
confluence.
Cell viability assay
The 11-O-acetylcyathatriol was dissolved in
100% cell culture grade dimethyl sulfoxide (Yantai Science and
Biotechnology Co., Ltd.) at a concentration of 50 mM. The stock
solution was stored at −20°C and diluted immediately prior to use.
The RAW 264.7 cells were adjusted to a density of
1×106/ml and then 200 µl was added into each well
of a 96-well plate. The cells were treated with
11-O-acetylcyathatriol at 0.39, 0.78, 1.56, 3.12, 6.25,
12.5, 25, 50 and 100 µM, and incubated at 37°C for 24 h. The
mitochondrial-dependent reduction of MTT to formazan was used to
measure cell viability (17). All
the experimental procedures were the same as those previously
reported (18).
Determination of NO
The RAW 264.7 cells were treated with 1 µg/ml
LPS, together with different concentrations (1.5625, 3.125, 6.25
and 12.5 µM) of 11-O-acetylcyathatriol at 37°C for 24
h. Subsequently, 100 µl of the culture supernatant was
removed, and the concentrations of nitrite were determined using an
NO determination kit, according to the manufacturer's protocol
(19).
Determination of levels of TNF-α and IL-6
cytokines
The RAW 264.7 cells were treated with 1 µg/ml
LPS, together with different concentrations (1.5625, 3.125, 6.25
and 12.5 µM) of 11-O-acetylcyathatriol at 37°C for 6
h. Following treatment, 100 µl of the culture supernatant
was removed and was used to determine the levels of TNF-α and IL-6
using respective ELISA assay kits, according to the manufacturer's
protocols (20).
Protein extraction
Following the indicated treatments, the medium was
removed and the cells were washed with phosphate-buffered saline
(PBS). The cells were lysed in 40 µl cell lysis buffer by
incubation at 4°C for 15 min, and then centrifuged at 13,400 × g
and 4°C for 10 min to obtain the total protein samples for analysis
of iNOS, COX-2, p-ERK1/2, p-JNK, p-p38 and IκB-α using western blot
analysis. Total protein concentrations were determined using the
BCA protein concentration assay kit, according to the
manufacturer's instructions (18,21).
Western blot analysis
Total proteins (30 µg) were subjected to
appropriate SDS-PAGE and then transferred onto nitrocellulose
membranes (Pall Corporation, Port Washington, NY, USA). The
membranes were blocked with 5% skim milk in Tris-buffered saline
with 0.05% (v/v) Tween-20 (TBS-T; Yantai Science and Biotechnology
Co., Ltd.) at room temperature for 1 h. Following washing with
TBS-T, the membranes were incubated with the respective primary
antibody solutions at 4°C overnight. The membranes were washed with
TBS-T and then incubated in HRP-conjugated secondary antibody
solution for 1 h at room temperature. The membranes were washed
with TBS-T and detected using enhanced chemiluminescence (Beyotime
Institute of Biotechnology, Shanghai, China). Images were exposed
to X-ray films (Kodak, Rochester, NY, USA) and collected, and the
respective bands were quantitated by densitometric analysis using
DigDoc100 software. The densitometric data of iNOS, COX-2,
p-ERK1/2, p-JNK, p-p38 and IκB-α were normalized on the basis of
the levels of β-actin (18,21).
Animal grouping
Male Sprague-Dawley rats weighing 180–220 g were
purchased from the Experimental Animal Center of Shandong Luye
Pharmaceutical Co., Ltd. [Yantai, China; animal care and use
committee approval no. SCXK (Lu) 20090009]. All experimental
procedures performed in the present study were performed in
accordance with the guidelines for the care and use of laboratory
animals, and were approved by the Ethical Committee of Yantai
University (Yantai, China; approval no. 201408002; 14th Aug, 2014).
A total of 36 rats were randomly divided into six groups: Normal
control group, ethanol model group, omeprazole group (positive
control group), low dose 11-O-acetylcyathatriol group (5
mg/kg), middle dose 11-O-acetylcyathatriol group (10 mg/kg),
high dose 11-O-acetylcyathatriol group (15 mg/kg).
Ethanol-induced gastric injury
The rats in the normal control group and ethanol
model group were administered with 5 mg/ml sodium carboxymethyl
cellulose (1 ml/100 g body weight; intragastrically). The rats in
the positive control group were administered with omeprazole (5
mg/kg/day). The 11-O-acetylcyathatriol treatment groups were
administered with respective doses of 11-O-acetylcyathatriol
(low dose, 5 mg/kg/day; middle dose, 10 mg/kg/day; high dose, 15
mg/kg/day). The drugs were suspended in 5 mg/ml sodium
carboxymethyl cellulose and administered intragastrically for 10
days (1 ml/100 g body weight, once each day). At 2 h following the
final administration, the ethanol model group and the treatment
groups were administered with 75% ethanol (1 ml/200 g
intragastrically) to induce gastric injury, and specimens were
collected after 1 h.
Specimen collection and handling
Following intraperitoneal anesthesia with 10%
chloral hydrate (0.35 ml/100 g), an incision was made along the
greater curvature of the stomach following ligation of the cardia
and pylorus of the stomach. The stomach was rinsed with normal
saline, and pathological changes in the gastric mucosa were
examined and recorded using the gastric ulcer index (UI), which was
calculated using the Guth method (22). The stomach was fixed with 10%
formaldehyde (Yantai Science and Biotechnology Co., Ltd.), and the
same area of the gastric mucosa was then embedded in paraffin
(Yantai Science and Biotechnology Co., Ltd.), sectioned (size, 5
µm) using a microtome and stained with hematoxylin and eosin
(Yantai Science and Biotechnology Co., Ltd.). The pathological
features were determined by analysis under a microscope (MM-40;
Nikon Corporation, Tokyo, Japan). The animals were sacrificed by
air embolism.
UI score calculation
Damage ≤1 mm, including erosion point, was assigned
a score of 1. Injury ≤2 mm was assigned a score of 2. Injury ≤3 mm
was assigned a score of 3. Injury ≤4 mm was assigned a score of 4,
and damage >4 mm was assigned a score of 5. The scores were
added together to obtain the UI. The ulcer inhibition rate was
calculated as follows: Ulcer inhibition rate (%) = 100% × (UI of
model group - UI of drug treatment group) / UI of model group.
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). The results are expressed as the mean ±
standard deviation. Statistical comparisons were conducted using a
post hoc test subsequent to one-way analysis of variance and
P<0.05 was considered to indicate a statistically significant
difference.
Results and Discussion
The RAW 264.7 cells were treated with different
concentrations of 11-O-acetylcyathatriol for 24 h, and the
cell viability was then assessed using the MTT method. As shown in
Fig. 2A,
11-O-acetylcyathatriol did not show any significant
cytotoxic effects at concentrations between 0.39 and 25 µM
against the RAW 264.7 cells.
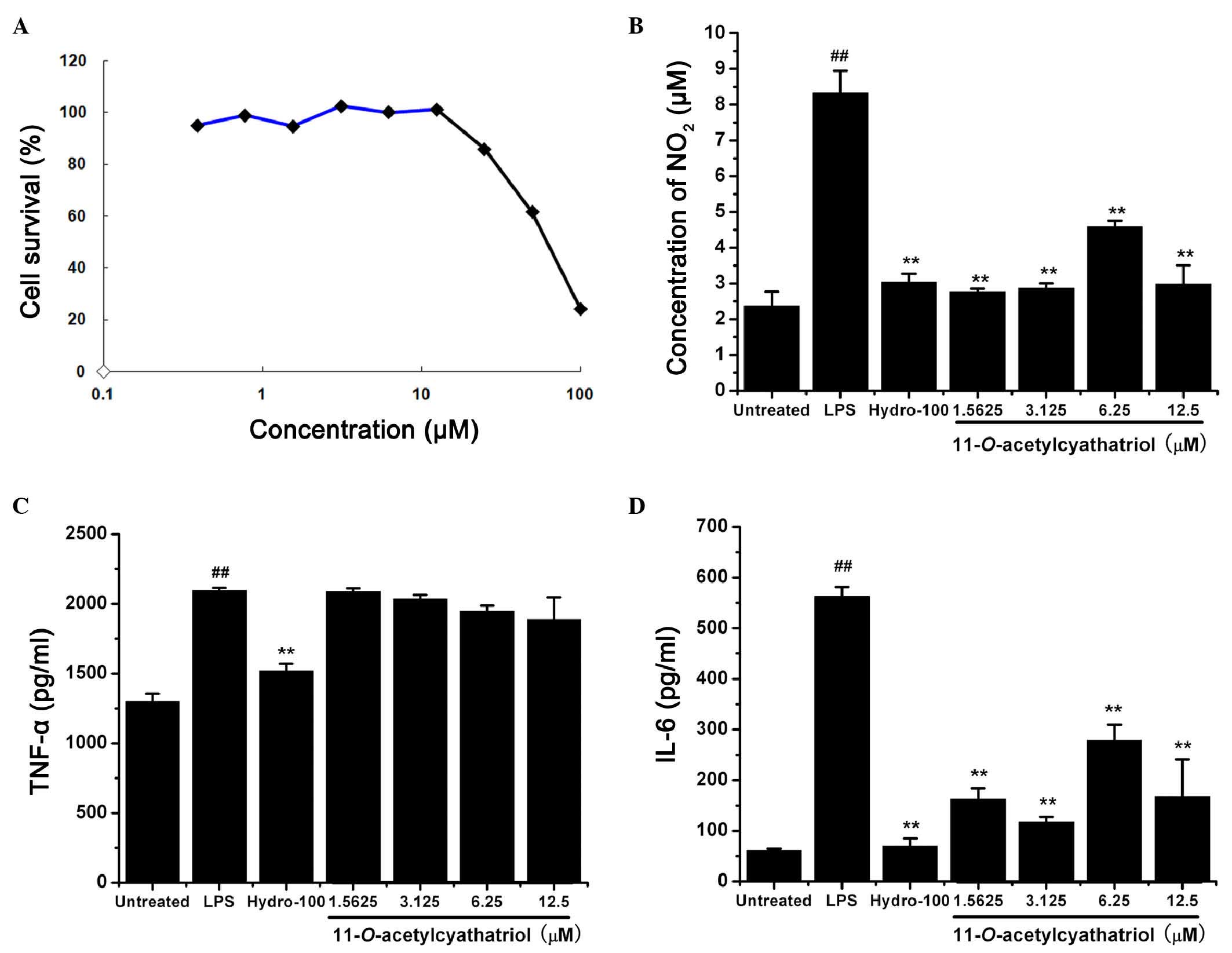 | Figure 2Cytotoxic effects of
11-O-acetylcyathatriol against RAW 264.7 cells and its
effects on TNF-α, IL-6 and NO. (A) Cytotoxicity of
11-O-acetylcyathatriol against RAW 264.7 cells. Cells were
treated with 11-O-acetylcyathatriol at concentrations of
0.39, 0.78, 1.56, 3.125, 6.25, 12.5, 25, 50 and 100 µM for
24 h. The cell viability was evaluated using an MTT assay. (B)
Effects of 11-O-acetylcyathatriol on NO secretion. RAW 264.7
cells were treated with 1 µg/ml LPS with either indicated
concentrations of 11-O-acetylcyathatriol (1.5625, 3.125,
6.25 and 12.5 µM) or hydrocortisone (100 µM) for 24
h. Culture supernatant (100 µl) was removed to determine the
concentration of NO2−. The experiment was
performed in triplicate, and the results are expressed as the mean
± standard deviation from three separate experiments (n=3).
**P<0.01, vs. LPS group; ##P<0.01, vs.
untreated group. Effects of 11-O-acetylcyathatriol on the
the release of (C) TNF-α and (D) IL-6. The RAW 264.7 cells were
treated with 1 µg/ml of LPS with either the indicated
concentrations of 11-O-acetylcyathatriol (1.5625, 3.125,
6.25 and 12.5 µM) or hydrocortisone (100 µM) for 6 h.
Culture supernatant (100 µl) was removed to determine the
levels of TNF-α and IL-6 using respective ELISA assay kits,
according to the manufacturer's protocols. The experiment was
performed in triplicate and the results are expressed as the mean ±
standard deviation from three separate experiments (n=3).
**P<0.01, vs. LPS group; ##P<0.01, vs.
untreated group. LPS, lipopolysaccharide; NO, nitric oxide; TNF-α,
tumor necrosis factor-α; IL-6, interleukin-6; Hydro,
hydrocortisone. |
The RAW 264.7 cells were treated with 1 µg/ml
LPS with or without indicated concentrations of
11-O-acetylcyathatriol (1.5625, 3.125, 6.25 and 12.5
µM) or the positive control, hydrocortisone (100 µM),
for 24 h. The concentrations of NO2 in the supernatant
were then measured as an indicator of NO production. As shown in
Fig. 2B,
11-O-acetylcyathatriol significantly suppressed the
LPS-induced production of NO, and its inhibitory activity was
equally potent, compared with the positive control of
hydrocortisone, a commonly used anti-inflammatory drug. The RAW
264.7 cells were also treated with 1 µg/ml LPS with or
without indicated concentrations of 11-O-acetylcyathatriol
(1.5625, 3.125, 6.25 and 12.5 µM) or the positive control,
hydrocortisone (100 µM), for 6 h. The levels of the
pro-inflammatory TNF-α and IL-6 cytokines in the supernatant were
detected using respective ELISA kits. As shown in Fig. 2C and D,
11-O-acetylcyathatriol did not exhibit significant
inhibition of LPS-induced TNF-α release, but markedly inhibited
IL-6 release. These results showed that
11-O-acetylcyathatriol significantly inhibited certain
inflammatory mediators, including NO and IL-6, induced by LPS in
RAW 264.7 macrophage cells, and this ability may, to a certain
extent, underlie its anti-inflammatory activities.
As the excessive production of NO is always
associated with high protein expression levels of iNOS, the protein
levels of iNOS and COX-2 were detected using western blot analysis.
As shown in Fig. 3A, the
LPS-induced high protein expression levels of iNOS and COX-2 were
completely inhibited by the high dose of
11-O-acetylcyathatriol (100 µM). Mitogen-activated
protein kinase (MAPK) cascades have been shown to be important in
the transduction of extracellular signals to cellular responses. In
mammalian cells, three MAPK families have been clearly
characterized: Classical MAPK, also known as ERK, JNK/MAPK
(JNK/SAPK) and p38 kinase. The MAPK pathways relay, amplify and
integrate signals from a diverse range of stimuli, and elicit
appropriate physiological responses, including cellular
proliferation, differentiation, inflammatory responses and
apoptosis, in mammalian cells. The effect of
11-O-acetylcyathatriol on the phosphorylation of the ERK1/2,
JNK, p38 proteins were detected using western blot analysis. As
shown in Fig. 3B,
11-O-acetylcyathatriol treatment markedly inhibited the
phosphorylation of the p38 protein induced by LPS, but showed only
weak suppression of the phosphorylation of the ERK1/2 and JNK
proteins. The activation of NF-κB always results from the
phosphorylation and degradation of the IκB-α protein. Therefore,
the effect of 11-O-acetylcyathatriol on LPS-induced IκB-α
protein degradation was investigated using western blot analysis.
As shown in Fig. 3C,
11-O-acetylcyathatriol had only a weak inhibitory effect on
the IκB-α protein degradation induced by LPS.
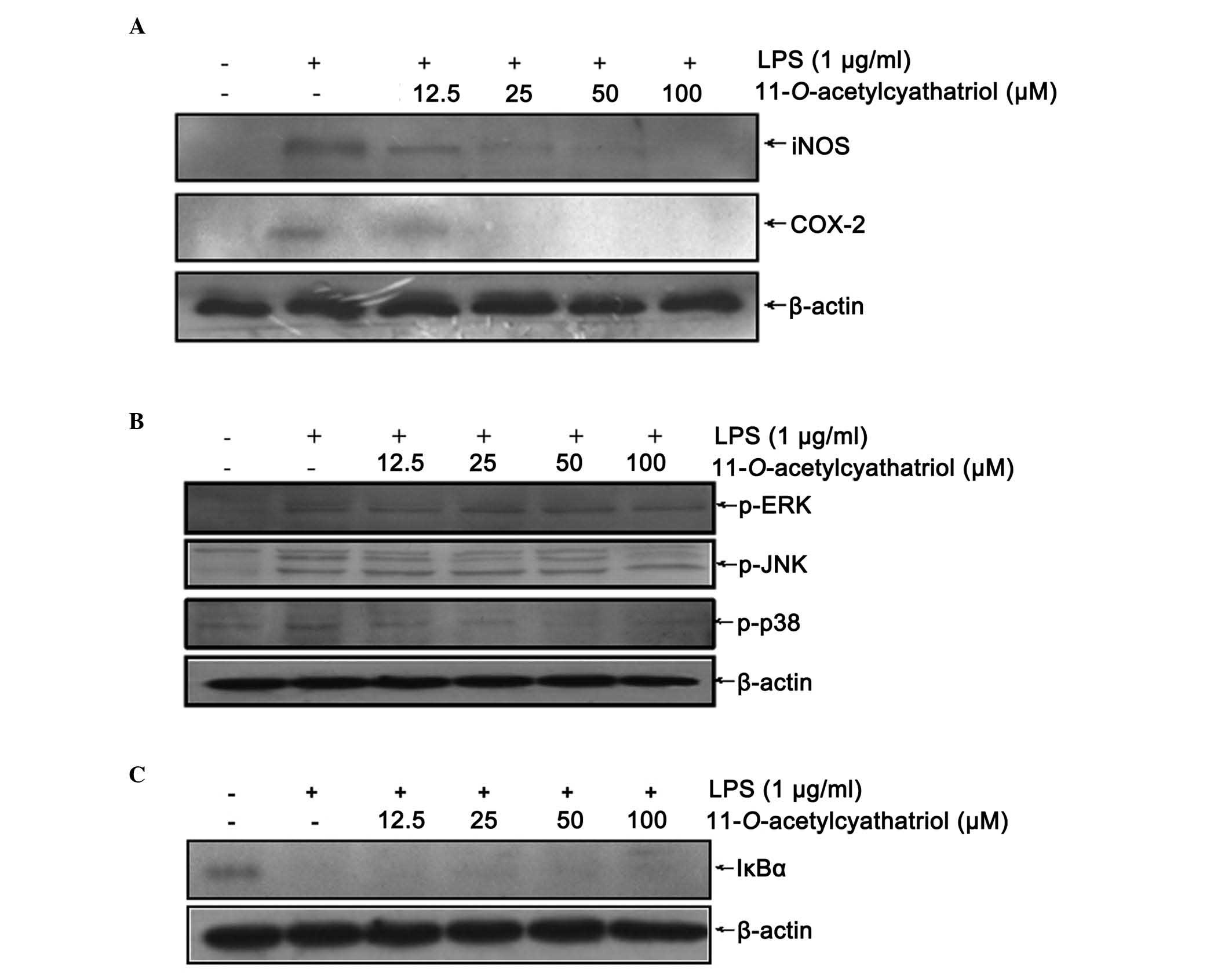 | Figure 3Effects of
11-O-acetylcyathatriol on the protein expression levels of
iNOS and COX-2. The RAW 264.7 cells were treated by 1 µg/ml
of LPS with indicated concentrations of
11-O-acetylcyathatriol (12.5, 25, 50 and 100 µM) for
24 h, and the expression levels of (A) iNOS and COX-2 were detected
using western blot analysis. (B) Effects of
11-O-acetylcyathatriol on the phosphorylation of ERK1/2, JNK
and p38 proteins. RAW 264.7 cells were treated with 1 µg/ml
LPS with 11-O-acetylcyathatriol (12.5, 25, 50 and 100
µM) for 30 min, and the protein expression levels of
p-ERK1/2, p-JNK and p-p38 were detected using western blot
analysis. (C) Effects of 11-O-acetylcyathatriol on the
protein degradation of IκB-α. RAW 264.7 cells were treated with 1
µg/ml of LPS with 11-O-acetylcyathatriol (12.5, 25,
50 and 100 µM) for 10 min, and the protein expression of
IκB-α was detected using western blot analysis. iNOS, inducible
nitric oxide synthase; COX-2, cyclooxygenase-2; LPS,
lipopolysaccharide; ERK, extracellular signal-regulated kinase;
JNK, c-Jun N-terminal kinase; IκB-α, inhibitor of nuclear
factor-κB-α; p-, phosphorylated. |
As is well known, high doses of alcohol are rapidly
absorbed in the stomach, directly damaging the gastric mucosa
epithelial cells and destroying the gastric mucosa barrier. The
local production of increased numbers of inflammatory mediators can
cause leukocyte infiltration and an increase in gastric acid
secretion, inducing gastric injury, gastritis and gastric ulcer
formation. The effect of 11-O-acetylcyathatriol on
alcohol-induced gastric mucosa injury in rats were further
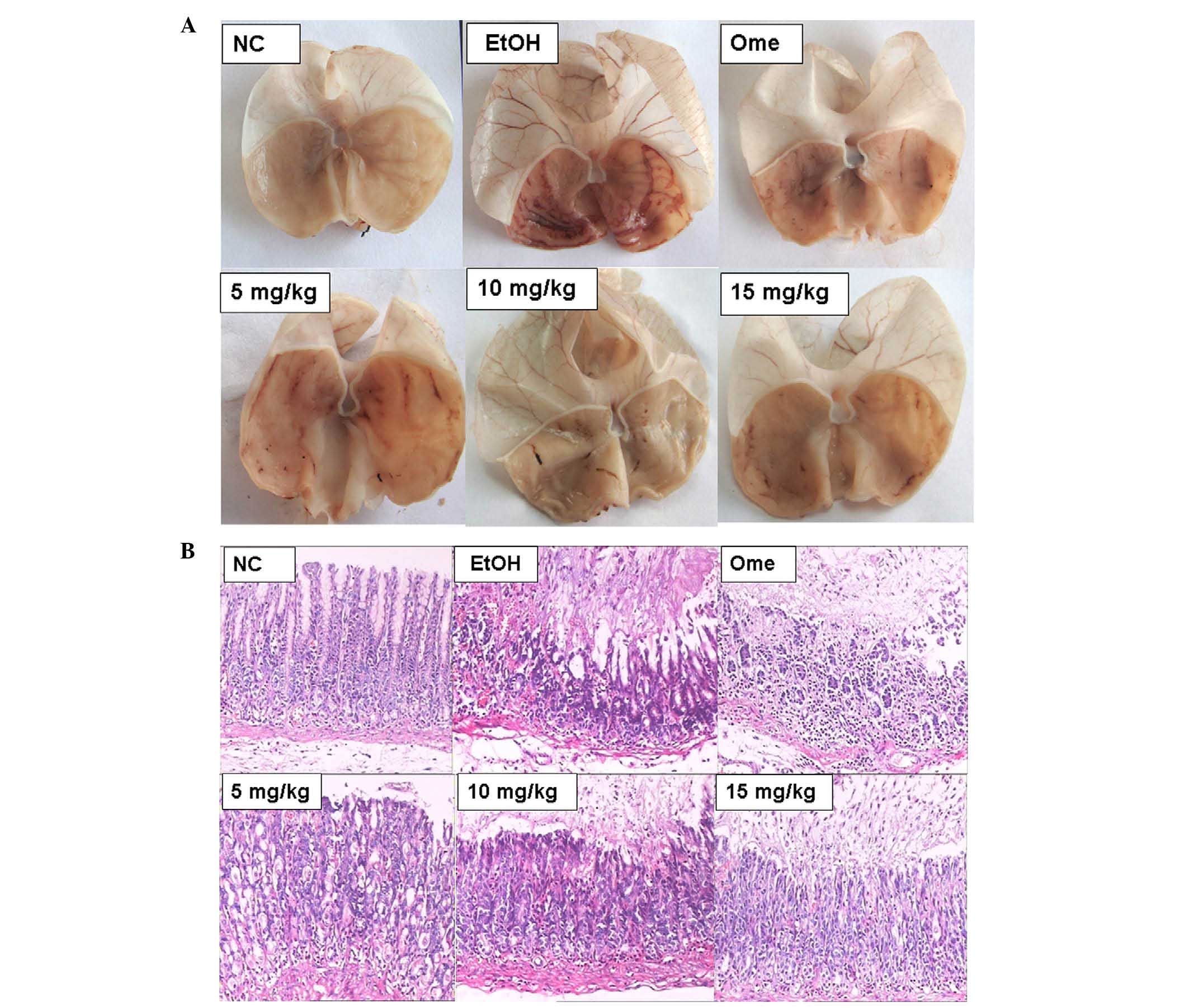
investigated in the present study. As shown in Table I, gastric mucosa injury in the rats
of the model group (UI score, 63.00±11.08) was significantly
higher, compared with that in the normal control group (UI score,
0; P<0.01). Omeprazole treatment significantly decreased injury
in model group (UI score, 39.00±8.25; P<0.01).
11-O-acetylcyathatriol treat ment (5, 10 and 15 mg/kg)
exerted significant protective effects on the alcohol-induced
gastric mucosa injury, in a dose-dependent manner, compared with
the model group (P<0.01). The ulcer inhibitory rates were 53.70,
73.28 and 77.25%, respectively. As shown in the images in Fig. 4A and B, the severity of gastric
mucosal hyperemia, edema, cellular degeneration and necrosis in the
11-O-acetylcyathatriol-treatment groups were markedly lower,
compared with those in the model group.
 | Table IGastric mucosal UI and ulcer
inhibition rate. |
Table I
Gastric mucosal UI and ulcer
inhibition rate.
| Group | Number of
cases | UI | Ulcer inhibition
rate (%) |
|---|
| Normal control | 6 | 0 | 0 |
| Ethanol model | 6 | 63.00±11.08 | – |
| Omeprazole | 6 |
39.00±8.25a | 38.10 |
| 5 mg/kg | 6 |
29.17±12.61a | 53.70 |
| 10 mg/kg | 6 |
16.83±6.97a | 73.28 |
| 15 mg/kg | 6 |
14.33±5.72a | 77.25 |
In the present study, the possible anti-inflammatory
molecular mechanisms of 11-O-acetylcyathatriol, a natural
cyathane diterpenoid identified from the medicinal fungus
Cyathus africanus, were examined. The
11-O-acetylcyathatriol compound was observed to
significantly inhibit various LPS-induced responses of the
macrophages, including the overproduction of NO and the release of
the pro-inflammatory, cytokine IL-6. Further investigations
indicated that 11-O-acetylcyathatriol downregulated the high
protein expression levels of iNOS and COX-2, which also inhibited
the activation of the MAPK/p-38 signaling transduction pathway.
This is the first direct evidence, to the best of our knowledge, to
demonstrate the anti-inflammatory effect and molecular mechanism of
11-O-acetylcyathatriol. Taken together, these results may
provide the theoretical basis for the use of cyathane diterpenoids,
or the use of medicinal plants containing similar constituents and
their analogues, in the treatment of inflammatory diseases.
Acknowledgments
This study was supported by the State Key Laboratory
of Mycology, Institute of Microbiology, Chinese Academy of Sciences
(Shanghai, China), the Taishan Scholar Project to Fenghua Fu, and
the Undergraduate Scientific and Technological Innovation Project
of Yantai University (Yantai, China; grant no. 131404).
References
|
1
|
Poinar G Jr: Bird's nest fungi
(Nidulariales: Nidulariaceae) in baltic and dominican amber. Fungal
Biol. 118:325–329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ayer WA and Taube H: Metabolites of
Cyathus helenae, Cyathin A3 and allocyathin B3, members of a new
group of diterpenoids. Tetrahedron Lett. 13:1917–1920. 1972.
View Article : Google Scholar
|
|
3
|
Shi XW, Liu L, Gao JM and Zhang AL:
Cyathane diterpenes from Chinese mushroom Sarcodon scabrosus and
their neurite outgrowth-promoting activity. Eur J Med Chem.
46:3112–3117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma BJ and Liu JK: A new bitter diterpenoid
from Sarcodon scabrosus. J Basic Microbiol. 45:328–330. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen JW, Ruan Y and Ma BJ: Diterpenoids of
macromycetes. J Basic Microbiol. 49:242–255. 2009. View Article : Google Scholar
|
|
6
|
Wrighf DL and Whitehead CR: Recent
progress on the synthesis of cyathane type diterpenes. Org Prep
Proced Int. 32:307–330. 2000. View Article : Google Scholar
|
|
7
|
Krzyczkowshi W: The structure, medicinal
properties and biosynthesis of cyathane diterpenoids.
Biotechnologia. 1:146–147. 2008.
|
|
8
|
Ayer WA, Browne LM, Mercer JR, et al:
Metabolites of bird's nest fungi. Part 8. Some minor metabolites of
Cyathus helenae and some correlations among the cyathins. Can J
Chem. 56:717–721. 1978. View
Article : Google Scholar
|
|
9
|
Ayer WA and Carstens LL: Diterpenoid
metabolites of Cyathus helenae, Cyathin B3 and cyathin C3. Can J
Chem. 51:3157–3160. 1973. View
Article : Google Scholar
|
|
10
|
Ayer WA and Taube H: Metabolites of
Cyathus helenae. A new class of diterpenoids. Can J Chem.
51:3842–3854. 1973. View
Article : Google Scholar
|
|
11
|
Ayer WA, Yoshida T and van Schie DMJ:
Metabolites of bird's nest fungi. Part 9. Diterpenoid metabolites
of Cyathus africanus Brodie. Can J Chem. 56:2113–2120. 1978.
View Article : Google Scholar
|
|
12
|
Hecht HJ and Striatin ABC: Novel
diterpenoid antibiotics from Cyathus striatus; x-ray crystal
structure of striatin A. J Chem Soc Chem Commun. 15:665–666. 1978.
View Article : Google Scholar
|
|
13
|
Ayer WA: Metabolites of bird's nest fungi.
Part 11. Diterpenoid metabolites of Cyathus earlei. Can J Chem.
57:3332–3337. 1979. View
Article : Google Scholar
|
|
14
|
Bai R, Zhang CC, Yin X, Wei J and Gao JM:
Striatoids A-F, cyathane diterpenoids with neurotrophic activity
from cultures of the fungus Cyathus striatus. J Nat Prod.
78:783–788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang HS, Kim KR, Jun EM, Park SH, Lee TS,
Suh JW and Kim JP: Cyathuscavins A, B and C, new free radical
scavengers with DNA protection activity from the Basidiomycete
Cyathus stercoreus. Bioorg Med Chem Lett. 18:4047–4050. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han J, Chen Y, Bao L, Yang X, Liu D, Li S,
Zhao F and Liu H: Anti-inflammatory and cytotoxic cyathane
diterpenoids from the medicinal fungus Cyathus africanus.
Fitoterapia. 84:22–31. 2013. View Article : Google Scholar
|
|
17
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival. Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao F, Chen L, Zhang M, Bi C, Li L, Zhang
Q, Shi C, Li M, Zhou S and Kong L: Inhibition of
lipopolysaccharide-induced iNOS and COX-2 expression by indole
alkaloid, 3-(hydroxymethyl)-6,7-di-hydroindolo
[2,3-a]quinolizin-(12H)-one, via NF-κB inactivation in RAW 264.7
macrophages. Planta Med. 79:782–787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishihara T, Kohno K, Ushio S, Iwaki K,
Ikeda M and Kurimoto M: Tryptanthrin inhibits nitric oxide and
prostaglandin E (2) synthesis by murine macrophages. Eur J
Pharmacol. 407:197–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao F, Xu H, He EQ, Jiang YT and Liu K:
Inhibitory effects of sesquiterpenes from Saussurea lappa on the
overproduction of nitric oxide and TNF-alpha release in
LPS-activated macrophages. J Asian Nat Prod Res. 10:1045–1053.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao F, Gao Z, Jiao W, Chen L, Chen L and
Yao X: In vitro anti-inflammatory effects of beta-carboline
alkaloids, isolated from Picrasma quassioides, through inhibition
of the iNOS pathway. Planta Med. 78:1906–1911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeon WY, Lee MY, Shin IS, Lim HS and Shin
HK: Protective effects of the traditional herbal formula oryeongsan
water extract on ethanol-induced acute gastric mucosal injury in
rats. Evid Based Complement Alternat Med. 2012:4381912012.
View Article : Google Scholar : PubMed/NCBI
|