Introduction
Obstructive nephropathy is a major cause of renal
failure, particularly in infants and children (1). Kidney fibrosis is an inevitable
outcome of progressive obstructive nephropathy (2). Obstructive nephropathy can be
congenital or acquired. Congenital obstructive nephropathy can be
easily identified by prenatal ultrasonography, and the number of
patients diagnosed with obstructive nephropathy has increased; the
prevalence is ~0.5% of fetuses, following the introduction of
prenatal ultrasonography as a screening method (3). Obstructive nephropathy in infants and
children results primarily from the consequence of congenital renal
maldevelopment, occurs during early kidney development and affects
renal morphogenesis, maturation and growth (4). In the most severe cases, this renal
injury will ultimately entail progressive renal tubular atrophy and
inevitable interstitial fibrosis with loss of nephrons (5).
Despite intense research, the lack of a
comprehensive understanding of the pathogenesis of renal scar
formation following injury remains a major obstacle in developing
effective therapeutic strategies. In the past several years,
epithelial-mesenchymal transition (EMT), a process by which fully
differentiated epithelial cells undergo transition to a fibroblast
phenotype, has emerged as an important pathway leading to the
generation of matrix-producing fibroblasts and myofibroblasts in
diseased kidneys (5). Under the
influence of cytokines, chemokines and other signaling molecules,
the cellular interactions that regulate the development of
interstitial fibrosis are complex (6). Proteomics studies facilitate the
direct understanding of the mechanisms of physiological and
pathological processes. Thus, the present study utilized 2-D
electrophoresis (2-DE) gel-based proteomics to analyze the protein
profiles of rat kidney tissues with experimentally induced neonatal
partial unilateral ureteral obstruction (PUUO). This was conducted
in order to provide insights into the pathogenesis of obstructive
nephropathy and develop a network of proteins that may be targeted
to prevent or reverse EMT in obstructive nephropathy.
Materials and methods
Experimental animals and surgical
procedures
This study included 26 Sprague Dawley (SD) rats
(Shengjing Hospital of China Medical University Experimental Animal
Center, Shenyang, China; age, 1 day; weight, 6–8 g), randomized
into four groups: PUUO1d, 1 day following PUUO (n=6), PUUO2d, 2
days following PUUO (n=8), PUUO5d, 5 days following PUUO (n=6) and
sham surgery (n=6).
After general anesthesia with isoflurane inhalation,
partial obstruction of the left ureter was conducted according to a
modified version of the technique demonstrated by Ulm and Miller
(7). Briefly, the left ureter was
exposed via an abdominal transperitoneal incision using a
microscope with ×4 magnification. The underlying psoas muscle was
split longitudinally to form a groove, into which the upper
one-third of the left ureter was embedded. The muscle edges were
closed by 8-0 sutures. In the sham group, a laparotomy was
performed and the left ureter was exposed and manipulated, but not
ligated. The incision was closed in a single layer. Subsequently,
the rats were placed in an animal facility with mother rats until
they recovered from the anesthetic.
At different time points (1, 2 and 5 days) the rats
were sacrificed using an overdose of 10% chloral hydrate (Aoxin
Chemical Co., Ltd., Yangzhou, China), and the left kidney was
excised, decapsulated, and the kidney volume, renal pelvis and the
thickness of renal parenchyma was measured immediately. Half of the
kidney was snap frozen in liquid nitrogen at −80°C. The other half
was fixed in 10% neutral-formalin, embedded in paraffin and
sectioned (4 µm) on a microtome for light microscopy.
No rats from the PUUO group or the sham group had a
wound infection or were eaten by mother rats prior to sacrifice.
The mother rats had free access to a standard rodent diet and were
housed with a 12-h light/dark cycle at 22°C and 55±5% relative
humidity. All experiments were conducted under the Shengjing
Hospital of China Medical University procedural and ethical
guidelines.
Double immunofluorescence staining
Double immunofluorescence labeling for podocalyxin,
ED-1, E-cadherin and α-smooth muscle actin (α-SMA) was performed on
paraffin-embedded kidney sections to label the podocytes,
macrophages, tubular epithelial cells and myofibroblasts in the
kidney. Sections (4 µm) pre-treated using heat-induced
antigen retrieval were stained with polyclonal rabbit
anti-podocalyxin (1:50; cat. no. ab205350; Abcam, Cambridge, MA,
USA), monoclonal mouse anti-ED-1 (1:50; cat. no. MAB1435; Chemicom,
Toronto, ON, Canada), polyclonal rabbit anti-E-cadherin (1:50; cat.
no. ab53226; Abcam) and monoclonal mouse anti-α-SMA (1:50; cat. no.
ab7817; Abcam) overnight at 4°C. They were then incubated with
fluorescein isothiocyanate and rhodamine-conjugated goat anti-mouse
(cat. no. sc-3796) and goat anti-rabbit (cat. no. sc-3839)
secondary antibodies at 1:100 (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA). Negative control was performed by replacing
the primary antibody with phosphate-buffered saline. To stain the
nuclei, 4′,6-diamidino-2-phenylindole (Bioss, Inc., Beijing, China)
was used. The sections were examined by a fluorescence microscope
(Nikon CE1 Confocal Microscope, Nikon, Tokyo, Japan). Renal tissues
with positive double immunofluorescence staining were used for
future 2-DE. Negative tissues were abandoned.
2-DE screening
For 2-DE, the proteins from 6 kidney tissues from
each experimental group were pooled and a set of 12 gels were run,
with one pooled sample run in triplicate per group for
reproducibility. The tissue proteins were prepared with an acetone
precipitation method (8) and were
quantified using the 2D-Quant kit (GE Healthcare, Little Chalfont,
UK). Isoelectrofocusing (IEF) and sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were
performed according to the manufacturer's instructions of the Ettan
IPGphor IEF system (GE Healthcare). Specifically, 900 µg
total protein was mixed with hydration solution to a total volume
of 450 µl. Hydration and IEF electrophoresis were
automatically processed on the electrode plate of the IPGphor
horizontal electrophoresis system at 20°C. A homogeneous 12.5% gel
(0.75 mm) was used for the second phase vertical SDS-PAGE gel
electrophoresis. After electrophoresis, the gel was stained with
modified colloidal Coomassie Brilliant Blue (mcCBB) G-250 as
described previously (8).
Image acquisition and data analysis
The gels were scanned with a Power Look 2100XL image
scanner (Umax, Taipei, Taiwan). Spot detection, quantification and
matching involved the use of 2-D gel analysis software (Image
Master 2-D platinum 6.0, GE Healthcare) with the CBB-stained gels.
The relative volume of spots [% volume calculated as the spot
volume (the sum of the intensities of the pixel units within the
protein spot) normalized as a percentage of the total volume of all
the spots present in a gel] was obtained from 3 parallel
experiments. Spots with at least 2-fold difference in % volume,
indicated to be statistically significant (P<0.05), were defined
as differentially expressed proteins and were excised for further
analysis.
In-gel digestion and matrix-assisted
laser desorption/ionization time-of-flight mass spectrometry
(MALDI-TOF MS)
Spots were selected manually and destained in 50%
acetonitrile (ACN) in 25 mM ammonium bicarbonate buffer, dried in
the SpeedVac and re-hydrated in trypsin solution (15 µg/ml)
for 1 h at 4°C, followed by the addition of 5 ml 25 mM ammonium
bicarbonate buffer to immerse the gel fragments. After incubation
for 16 h at 37°C, the digested peptides were extracted with 5%
trifluoroacetic acid (TFA) and 2.5% TFA/50% ACN at 37°C for 1 h
separately. Tryptic peptides were finally dissolved in MALDI matrix
(5 mg/ml α-cyana-4-hydroxycinnamic acid in 0.1% TFA and 50% ACN),
and spotted onto 192-well stainless steel MALDI target plates
(8). The sample plate was placed
into a MALDI-TOF mass spectrometer (Applied Biosystems, Waltham,
MA, USA) for mass spectrometry to obtain peptide mass
fingerprinting. The MS and MS/MS spectra were subsequently searched
against the IPI.RAT. v3.83 database (http://www.ebi.ac.uk/IPI), with use of GPS (version
3.0; Applied Biosystems) and MASCOT (version 2.0; Matrix Science,
London, UK) database search algorithms. The following search
criteria were used: Trypsin specificity, cysteine
carbamidomethylation and methionine oxidation as variable
modifications, 2 trypsin miscleavage allowed, 0.2 Da MS tolerance
and 0.3 Da MS/MS tolerance. Protein identifications were accepted
with a Mowse score (9) ≥23 and
P<0.05. Differentially expressed proteins were investigated
using Gene Ontology (http://www.geneontology.org) for molecular function,
biological processes and cellular components.
Immunoblot analysis
Protein was isolated using the isolation kit from
Beyotime Institute of Biotechnology (Haimen, China) according to
the manufacturer's instructions, and was quantified using the
2D-Quant kit. In total, 50 µg protein extract from rats was
separated by 12% SDS-PAGE and then transferred with Tris-HCl
methanol (20 mM Tris, 150 mM glycine and 20% methanol) onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA) in a trans-blot electrophoresis transfer cell (Bio-Rad,
Hercules, CA, USA). Membranes were probed with antibodies against
electron transfer flavoprotein, β polypeptide (ETFB; polyclonal
goat anti-rat; 1:250; cat. no. ab104944; Abcam Ltd., Hong Kong,
China) or actin (1:2,000; polyclonal goat anti-rat; cat. no.
sc-1616; Santa Cruz Biotechnology, Inc.). All immunoblots were run
at least in triplicate. Visualization of the antigen-antibody
complexes was conducted using enhanced chemiluminescence reagents
(Pierce Biotechnology, Rockford, IL, USA). Detected bands were
quantified by ImageJ2x software (version 2.1.4.7; National
Institutes of Health, Bethesda, MA, USA). The relative density of
each protein was calculated by dividing the optical density value
of each protein by that of the loading control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific Inc., Waltham, MA, USA),
according to the manufacturer's instructions. cDNA was synthesized
from 3 µg RNA using the Takara RNA PCR kit (Takara, Bio
Inc., Otsu, Japan). qPCR amplifications were performed in
triplicate on a Light Cycler (Roche Applied Science, Penzberg,
Germany) with the following primers: Sense:
5′ATGAGCCTCGCTATGCCACAC3′ and antisense: 5′ACCTTGGAGGTCAGGTCCACAC3′
for ETFB; and sense: 5′GGAGATTACTGCCCTGGCTCCTA3′ and antisense:
5′GACTCATCGTACTCCTGCTTGCTG3′ for β-actin. The house keeping gene
β-actin was used as an endogenous control. The amplification
profile involved 5 sec of denaturation at 95°C and 20 sec of
annealing at 64°C for 45 cycles. The relative mRNA levels for each
sample were calculated using the 2−ΔΔCq method (10).
Statistical analysis
All statistical analyses were performed using SPSS
version 18.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation (% volume of spots for 2-DE analysis,
and relative density of bands on immunoblot analysis). Two-tailed
Student's t-test was employed for analyzing significant differences
compared with the sham group. A normality test and homogeneity of
variance from the PUUO and sham group was analyzed using two-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Morphological changes of the obstructed
kidney
By gross anatomy, the appearance of rat kidney in
the PUUO1d group was not enlarged, and the renal pelvis was not
dilated. By gross anatomy, there was no visible hydronephrosis. In
the PUUO2d group, the hyperemia kidney appeared as increased kidney
volume, visible hydronephrosis and dilated renal pelvis. The damage
was increasingly severe with the persistence of the obstruction and
hydronephrosis, and a thin cortex were observed in the kidneys from
the PUUO5d group. The kidneys from rats in the of sham surgery
group showed normal morphology.
Double immunofluorescence staining
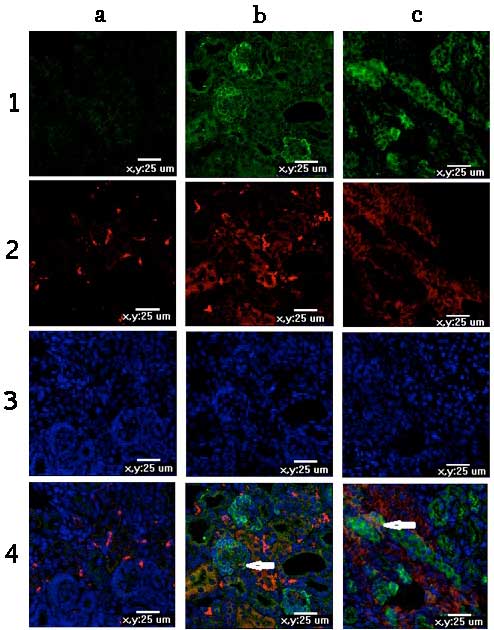
Podocalyxin is the hallmark of podocytes, while ED-1
is the hallmark of macrophages. As presented in Fig. 1, positive double labeling for
podocalyxin and ED-1 on day 2 after obstruction (Fig. 1B) revealed that podocytes took on
mesenchymal characteristics. Similarly, E cadherin is the marker of
tubular epithelial cell, α-SMA is the marker of myofibroblasts, and
the positive double labeling for them indicated that phenotypic
changes in tubular epithelial cells occurred on day 5 after PUUO
(Fig. 1C). The phenotypic changes
in podocytes induced by PUUO occurred earlier compared with the
tubular epithelial cells. Thus, in the PUUO2d group, kidneys from
the two rats that did not exhibit phenotypic changes were not
included in the rest of the experiments.
Protein profiles of rat kidney tissues
from the sham surgery group and those with PUUO
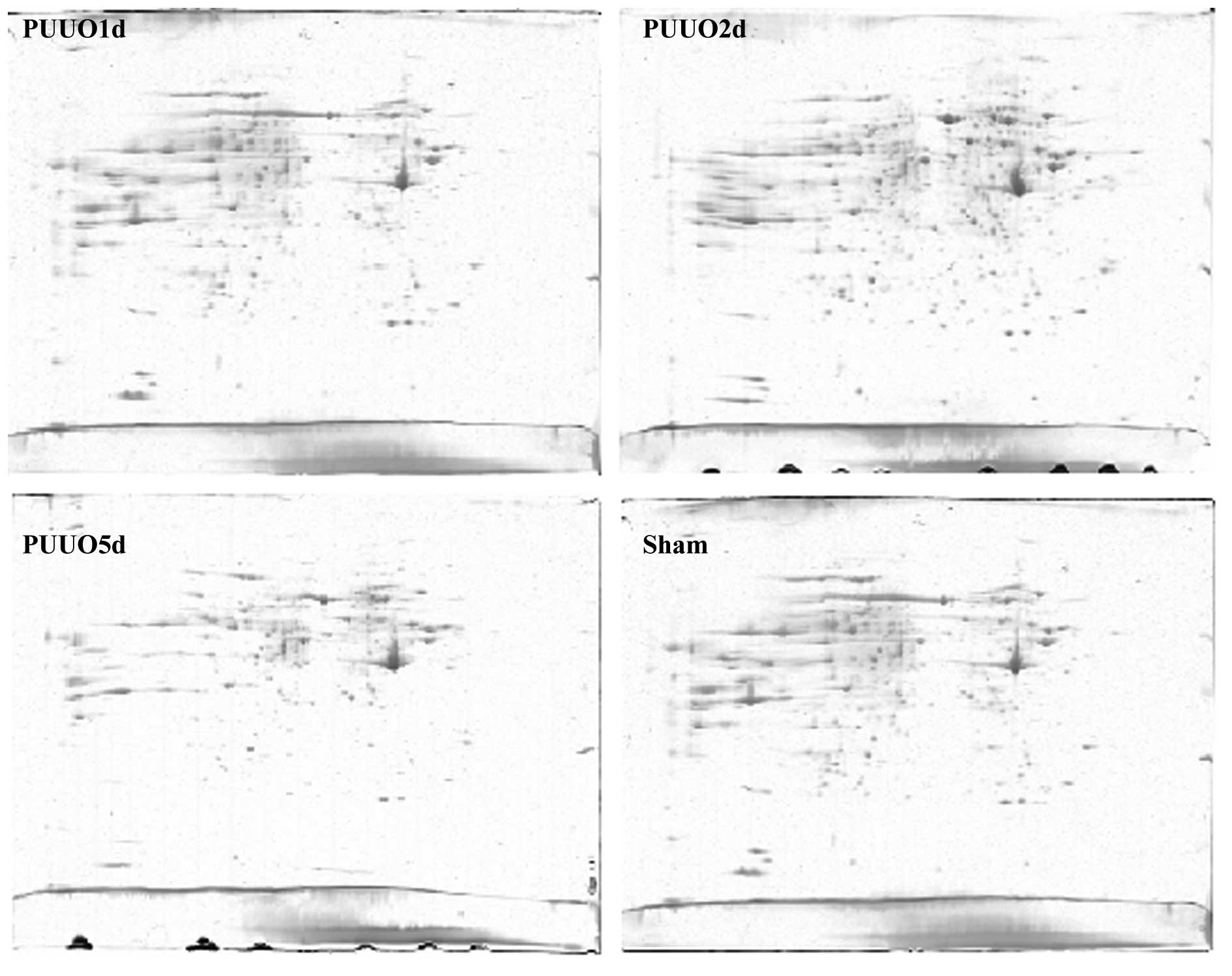
The rat kidney tissue proteome contained ~600
detectable proteins on a single mcCBB-stained 2-DE gel (Fig. 2). More than 80% of the protein
spots were matched on the 4 sets of CBB-stained gels. Overall, the
2-DE protein spot patterns across all the gels were similar in all
groups. However, 46 protein spots with at least 2-fold difference
in % volume (P<0.05) between kidney tissues from the sham
surgery group and the PUUO groups were detected and were dissected
for further analysis. Of the 46 dissected spots, 43 were also
identified to be differentially expressed by MS (Table I).
 | Table IProteins with differential expression
between the PUUO groups and corresponding sham rat kidney tissues
identified be matrix-assisted laser desorption/ionization
time-of-flight mass spectrometry. |
Table I
Proteins with differential expression
between the PUUO groups and corresponding sham rat kidney tissues
identified be matrix-assisted laser desorption/ionization
time-of-flight mass spectrometry.
| Protein name | Accession no. | Mr (kDa)/Pi | Expression | Mascot score | Function |
|---|
| Hsp90b1 | IPI00365985 | 93/4.72 | ↑ | 385 | Endoplasmic reticulum
associated degradation |
| Sucla2 | IPI00951645 | 48/6.08 | ↑ | 125 | Tricarboxylic acid
cycle |
| Vcp | IPI00212014 | 90/5.14 | ↑ | 478 | Export of misfolded
proteins |
| Acy1a | IPI00464791 | 46/6.03 | ↑ | 437 | Amino acid
metabolism |
| Gsn | IPI00923716 | 81/5.46 | ↑ | 152 | Actin-modulating
protein |
| Hnrnpf | IPI00210357 | 46/5.31 | ↑ | 121 | Processing
pre-mRNAs |
| Cox5a | IPI00192246 | 16/6.08 | ↑ | 116 | Oxidoreductase
family |
| RGD1565368 | IPI00769218 | 36/8.44 | ↑ | 116 | Energy
metabolism |
| Ezr | IPI00470254 | 69/5.83 | ↑ | 214 | Connection
cytoskeletal structures to the plasma membrane |
| Hnrnpk | IPI00780608 | 51/5.39 | ↑ | 257 | Pre-mRNA-binding
protein |
| Tpm4 | IPI00214905 | 29/4.66 | ↑ | 184 | Stabilize
cytoskeleton actin filaments |
| Aldh9a1 | IPI00203690 | 57/6.94 | ↑ | 121 | Aldehyde
dehydrogenase family |
| Eef1b2 | IPI00476899 | 25/4.55 | ↑ | 141 | EF-1-β/EF-1-α
family |
| Pepd | IPI00364304 | 56/5.61 | ↑ | 100 | Collagen
metabolism |
| Prdx6 | IPI00231260 | 25/5.64 | ↑ | 500 | Oxidoreductase
family |
| Dlst | IPI00551702 | 49/8.89 | ↑ | 64 | Tricarboxylic acid
cycle |
| Park7 | IPI00212523 | 20/6.32 | ↑ | 125 | Oxidoreductase
family |
| Actr3 | IPI00949517 | 48/5.61 | ↑ | 229 | Actin
polymerization and actin networks |
| Rbm3 | IPI00367437 | 17/7.98 | ↑ | 62 | Cold-inducible mRNA
binding protein |
| Psmc2 | IPI00421600 | 49/5.59 | ↑ | 385 | Degradation of
ubiquitinated proteins |
| Erp44 | IPI00949066 | 47/5.14 | ↑ | 244 | Protein
folding |
| Gdi2 | IPI00197568 | 47/6.5 | ↑ | 140 | Regulate the
GDP/GTP exchange |
| Gsn | IPI00923716 | 81/5.46 | ↑ | 86 | Actin-modulating
protein |
| Ahcy | IPI00476295 | 48/6.07 | ↑ | 99 | Amino acid
metabolism |
| Ezr | IPI00948980 | 69/5.83 | ↑ | 194 | Connection
cytoskeletal structures to the plasma membrane |
| Atp5b | IPI00551812 | 56/5.19 | ↑ | 148 | Mitochondrial
membrane ATP synthase |
| RGD1309537 | IPI00564409 | 20/4.67 | ↑ | 192 | Myosin regulatory
subunit |
| Fbp1 | IPI00231745 | 40/5.54 | ↑ | 198 | Rate-limiting
enzyme in gluconeogenesis |
| Aldh1a2 | IPI00211419 | 57/5.58 | ↑ | 186 | Aldehyde
dehydrogenase family |
| Clta | IPI00230870 | 24/4.43 | ↑ | 160 | Clathrin light
chain family |
| Calr | IPI00191728 | 48/4.33 | ↑ | 126 | Chaperone in
protein folding |
| Anxa5 | IPI00471889 | 36/4.93 | ↑ | 440 | Anticoagulant
protein |
| Cct2 | IPI00366218 | 58/6.01 | ↑ | 202 | Folding of
actin |
| Prdx1 | IPI00211779 | 22/8.27 | ↑ | 75 | Oxidoreductase
family |
| Atp5b | IPI00551812 | 56/5.19 | ↑ | 98 | Mitochondrial
membrane ATP synthase |
| Etfb | IPI00364321 | 28/7.6 | ↓ | 150 | Specific electron
acceptor |
| Dld | IPI00365545 | 55/7.96 | ↓ | 153 | Oxidoreductase
family |
| Tufm | IPI00371236 | 50/7.23 | ↓ | 144 | Protein
biosynthesis |
| Ruvbl2 | IPI00364340 | 51/5.49 | ↓ | 222 | Nucleotide-binding,
ATP-binding, DNA repair |
| LOC100360986 | IPI00559028 | 35/9.4 | ↓ | 189 | Nucleic acid
binding, nucleotide binding |
| Tf | IPI00679202 | 79/7.14 | ↓ | 461 | Stimulate cell
proliferation |
| Apoa4 | IPI00324272 | 44/5.12 | ↓ | 62 | Chylomicrons and
VLDL secretion and catabolism |
| Aldh4a1 | IPI00921682 | 62/8.26 | ↓ | 236 | Aldehyde
dehydrogenase family |
Immunoblot analysis of selected
proteins
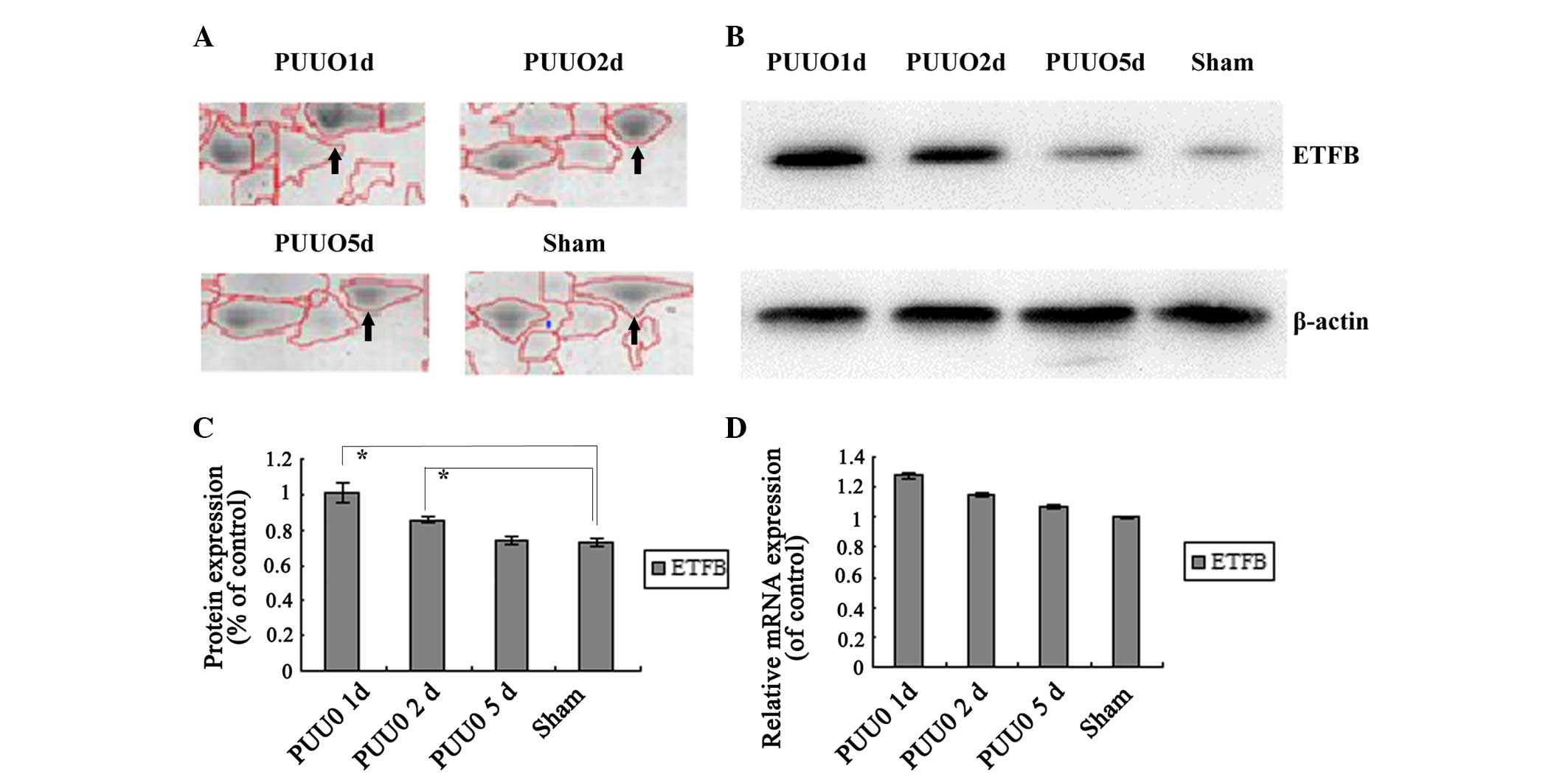
ETFB was selected for further immunoblot analysis.
ETFB was enhanced initially following PUUO compared with the sham
group, and was then continuously downregulated with sustained
obstruction. In the PUUO5d group, no significant difference was
identified in ETFB levels compared with the sham group. The result
is consistent with the results of 2D-E (P<0.05) (Fig. 3A–C).
Transcription levels
According to the relative mRNA levels (Fig. 3D), the mRNA expression level of
ETFB was not identified to be significantly different in the PUUO
groups compared with the sham group (P>0.05).
Discussion
Despite numerous clinical and experimental studies
over the past several decades, the pathogenesis of obstructive
nephropathy remains to be fully understood. Following urinary tract
obstruction and tubular dilatation, a cascade of events results in
cellular proliferation and tubular apoptosis, which leads to
tubular atrophy and inevitably progressive fibrosis (6). EMT, which is often preceded by and
closely associated with chronic interstitial inflammation, could be
an adaptive response of epithelial cells to a hostile or changing
microenvironment, and it is increasingly recognized as an integral
part of tissue fibrogenesis following injury (5). In addition to tubular epithelial
cells, recent studies indicate that glomerular podocytes may also
undergo transition following injury (9). Phenotypic alteration of podocytes
results in functional impairment, resulting in proteinuria and
glomerulosclerosis (9). Thus, it
was hypothesized that EMT of epithelial cells and podocytes is
involved in the initial phases of renal fibrosis.
Double labeling revealed that ED-1 and α-SMA were
undetectable in the majority of glomeruli in healthy neonatal
kidneys, whereas ED-1 was detected in glomeruli from the PUUO1d
group and levels progressively increased after day 2 of PUUO. In
addition, α-SMA was observed in renal tubular epithelial cells and
was detectable in the majority of renal tubular epithelial cells
after day 5 of PUUO. Thus, podocytes took on mesenchymal
characteristics 2 days after obstruction and phenotypic changes in
tubular epithelial cells occurred 5 days after PUUO.
Therefore, identification of the protein expression
profile of renal tissues at the time point of podocyte and tubular
cell EMT aids in the determination of the pathogenesis of renal
injury and progressive interstitial fibrosis, and also increased
the understanding of the onset of podocyte dysfunction, proteinuria
and glomerulosclerosis.
Numerous studies using radiology have demonstrated
that tubular atrophy and interstitial fibrosis develop prior to
significant renal pelvic dilatation (11–13).
However, the present study investigated EMT pathological changes of
podocytes and tubular cells; determination of the differentially
expressed proteins associated with EMT of podocytes and tubular
cells could rule out the bias caused by different degrees of
partial obstruction, and establish the diagnosis on a pathological,
rather than radiological, basis. Thus, the present study used a
comparative proteomics 2-DE system to observe the protein
expression profile during obstructive nephropathy and a total of 43
differentially expressed proteins were successfully identified.
These proteins participate in the regulation of cytoskeleton and
actin, glucose metabolism, cell apoptosis, mitochondrial energy
metabolism, oxidative stress and endoplasmic reticulum stress.
Results of previous studies have shown that actin is
one of two monomer subunits of microfilaments, which are the major
cytoskeleton component. In addition, actin is critical in a number
of cellular functions, such as cell motility, cell division,
transfer of information between cells, the maintenance of cell
shape, cell connections, cell polarity and the regulation of
transcription (14).
Actin-regulating proteins including Gsn, Tpm4, Actr3 and Cct2 were
significantly upregulated in the early stages following PUUO. GSN
is a calcium-regulated actin-modulating protein, Tpm4 is implicated
in stabilizing cytoskeleton actin filaments, Actr3 is involved in
the regulation of actin polymerization and formation of branched
actin networks, and Cct2 is known to be involved in the folding of
actin in vitro (Gene Ontology). It was hypothesized that rat
kidneys subjected to PUUO produce more actin filaments to control
and maintain kidney shape and internal structure. However, with
severe and persistent obstruction, this leads to progressive smooth
muscle hyperplasia, enhanced peristalsis and finally smooth muscle
fibrosis.
A total of six differentially expressed proteins
were successfully identified, which participate in the regulation
of mitochondrial energy metabolism. Sucla2 and D1st are involved in
the tricarboxylic acid cycle, and Acy1a and Ahcy are associated
with amino acid metabolism, ATP5b is involved in mitochondrial
membrane ATP synthase and Tufm promotes protein biosynthesis (Gene
Ontology). The expression of the majority of proteins in the kidney
following obstruction were upregulated (with the exception of
Tufm), suggesting that mitochondrial energy metabolism
abnormalities may be the basis for early renal pathological changes
in obstructive nephropathy. Due to mitochondrial dysfunction,
caused by the imbalance between biogenesis and degradation, the
induction of oxidative stress is induced (15), which contributes significantly to
renal energy loss, redox environment damage and transforming growth
factor-β (6) pathway activation.
This ultimately results in cell apoptosis and renal fibrosis.
In eukaryotic cells, newly synthesized secretory
proteins enter the secretory pathway via the endoplasmic reticulum
(ER). After proper folding, proteins are then transported out of
the ER and folding-defective products are retained in the ER, thus
initiating the ER-associated degradation process (ERAD) (16,17).
ER stress was demonstrated to induce EMT in human proximal tubule
cells, a finding that directly connects ER stress to renal fibrosis
(18). Among the differentially
expressed proteins, several were clustered functionally with
protein folding. Calr acts as a chaperone in promoting protein
folding, oligomeric assembly and quality control in the ER via the
calreticulin/calnexin cycle. Heat shock protein 90b1 is a member of
the heat shock protein family, which can perform specific chaperone
functions in the processing and transport of secreted proteins to
ERAD. In addition, Erp44 was found to be closely associated with
protein folding, response to ER stress and unfolded proteins. Psmc2
is involved in the ATP-dependent degradation of ubiquitinated
proteins and vcp is required for the export of misfolded proteins
from the ER to the cytoplasm, where they are degraded by the
proteasome (Gene Ontology). Thus, the elevation of these five
proteins in neonatal rats with PUUO may aid in understanding the
association between ER stress and obstructive nephropathy.
Among the differently expressed proteins, Prdx1,
Prdx6, Park7, Dld and Cox5a, which are members of different
oxidoreductase families have functions in the regulation of
antioxidation related to oxidative stress. Following obstruction,
increased production of intracellular reactive oxygen species
(19) induces oxidative stress,
leading to monocyte/macrophage (ED-1) infiltration (20) followed by apoptosis and eventually
renal fibrosis. This process is regulated by pro-apoptotic and
anti-apoptotic factors simultaneously (21) and involves the TGF-β1 signaling
pathway (22). Therefore,
oxidative stress is vital in the regulation of inflammation,
apoptosis and fibrosis.
Proteomics screening results reflected the
complexity of protein expression changes in the process of renal
injury. In order to confirm the proteomics results, in depth
investigation was necessary. The results of 2-DE demonstrated that
ETFB was enhanced initially following PUUO, and was then
continuously downregulated with sustained obstruction. Gene
ontology analyses mention that ETFB is a specific electron acceptor
(23,24) that is associated with tissue
remodeling and/or fibrotic processes (25,26).
Thus, the present study focused on ETFB out of all the
differentially expressed proteins. Immunoblot analysis and RT-qPCR
were performed to investigate the differential expression of
protein and mRNA between the obstructed and normal kidney. In the
neonatal kidney with PUUO, immunoblot analysis confirmed that ETFB
was upregulated compared with that in the sham group, which was
consistent with the results of 2-DE. ETFB was continuously
downregulated with persistence of the obstruction and no
significant difference was identified in the expression in the
PUUO5d group compared with that in the sham group. However, RT-qPCR
analysis demonstrated that ETFB was not altered at the mRNA level.
The inconsistency between mRNA and protein expression may be caused
by posttranscriptional regulation. ETFB is located in the inner
membrane of the mitochondrial matrix in a complex with ETFA, FAD
and AMP, which together act as an electron acceptor of 5 isoforms
of acyl-CoA dehydrogenase, glutaryl-CoA and sarcosine dehydrogenase
in the fatty acid β-oxidation cascade (23,24).
ETFB is normally active in the mitochondria, the energy-producing
centers of cells, and is involved in the process by which fats and
proteins are broken down to produce energy (Gene Ontology). Based
on data from Hirokawa et al (25,26),
ETFB participates in the mechanoregulation of fibroblast cell
number and ETFB knockdown can reduce TGF-β-induced α-SMA mRNA
expression and affect tissue remodeling and/or fibrotic processes.
In general, ETFB protein expression is relatively high in early
obstruction and relatively low in late obstruction. Thus, its
upregulation may be an attempt to promote renal fibrosis in the
early stages. With continued obstruction results in mitochondrial
dysfunction and kidney energy loss, ETFB may gradually decrease as
it is involved in energy metabolism. Its expression was not
significantly different compared with that in the sham surgery
group in advanced stages.
In conclusion, an overview of proteomic changes in
the kidney of neonatal rats with PUUO was demonstrated.
Differentially expressed proteins caused by the primary obstruction
or secondary injuries in the kidney tissues exposed to the
obstructive environment were determined. These proteins possess
diverse functions, such as involvement in cell cytoskeleton and
energy metabolism. However, their precise role in the pathology
obstructive nephropathy requires further investigation. This study
may provide insights into obstructive nephropathy and contribute to
the development of novel therapeutic strategies.
Acknowledgments
This study was supported by the National Nature
Science Foundation of China (grant no. 81370772) and the Nature
Science Foundation of Liaoning Province, China (grant no.
2013021036).
References
|
1
|
Chevalier RL and Peters CA: Congenital
urinary tract obstruction: Proceedings of the State-Of-The-Art
Strategic Planning Workshop-National Institutes of Health Bethesda,
Maryland, USA 11–12 March 2002. Pediatr Nephrol. 18:576–606.
2003.PubMed/NCBI
|
|
2
|
Eddy AA: Molecular basis of renal
fibrosis. Pediatr Nephrol. 15:290–301. 2000. View Article : Google Scholar
|
|
3
|
Madsen MG, Nørregaard R, Frøkiær J and
Jørgensen TM: Urinary biomarkers in prenatally diagnosed unilateral
hydronephrosis. J Pediatr Urol. 7:105–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen JG, Ringgaard S, Jorgensen TM,
Stodkilde-Jorgensen H, Djurhuus JC and Frokiaer J: Long-term
effects of partial unilateral ureteral obstruction on renal
hemodynamics and morphology in newborn rats: a magnetic resonance
imaging study. Urol Res. 30:205–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar
|
|
6
|
Chevalier RL: Obstructive nephropathy:
Towards biomarker discovery and gene therapy. Nat Clin Pract
Nephrol. 2:157–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ulm AH and Miller F: An operation to
produce experimental reversible hydronephrosis in dogs. J Urol.
88:337–341. 1962.PubMed/NCBI
|
|
8
|
Zhao Q, Yang Y, Wang CL, Hou Y and Chen H:
Screening and identification of the differential proteins in kidney
with complete unilateral ureteral obstruction. Int J Clin Exp
Pathol. 8:2615–2626. 2015.PubMed/NCBI
|
|
9
|
Pappin DJ, Hojrup P and Bleasby AJ: Rapid
identification of proteins by peptide-mass fingerprinting. Curr
Biol. 3:327–332. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
Li Y, Kang YS, Dai C, Kiss LP, Wen X and
Liu Y: Epithelial-to-mesenchymal transition is a potential pathway
leading to podocyte dysfunction and proteinuria. Am J Pathol.
172:299–308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thornhill BA, Burt LE, Chen C, Forbes MS
and Chevalier RL: Variable chronic partial ureteral obstruction in
the neonatal rat: A new model of ureteropelvic junction
obstruction. Kidney Int. 67:42–52. 2005. View Article : Google Scholar
|
|
13
|
Seseke F, Thelen P, Heuser M, Zöller G and
Ringert RH: Impaired nephrogenesis in rats with congenital
obstructive uropathy. J Urol. 165:2289–2292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu XX, Wang CL, Sun RG, et al: Renal
pathological appearance and podocyte phenotype after the ureteral
obstruction. Journal of China Medical University. 39:1–3. 2010.
|
|
15
|
Dominguez R and Holmes KC: Actin structure
and function. Annu Rev Biophys. 40:169–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Small DM, Coombes JS, Bennett N, Johnson
DW and Gobe GC: Oxidative stress, anti-oxidant therapies and
chronic kidney disease. Nephrology (Carlton). 17:311–321. 2012.
View Article : Google Scholar
|
|
17
|
Hebert DN and Molinari M: In and out of
the ER: Protein folding, quality control, degradation, and related
human diseases. Physiol Rev. 87:1377–1408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ellgaard L, Molinari M and Helenius A:
Setting the standards: Quality control in the secretory pathway.
Science. 286:1882–1888. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dickhout JG, Carlisle RE and Austin RC:
Interrelationship between cardiac hypertrophy, heart failure, and
chronic kidney disease: Endoplasmic reticulum stress as a mediator
of pathogenesis. Circ Res. 108:629–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salahudeen AK, Huang H, Joshi M, Moore NA
and Jenkins JK: Involvement of the mitochondrial pathway in cold
storage and rewarming-associated apoptosis of human renal proximal
tubular cells. Am J Transplant. 3:273–280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manucha W, Kurbán F, Mazzei L, Benardón
ME, Bocanegra V, Tosi MR and Vallés P: eNOS/Hsp70 interaction on
rosuvastatin cytoprotective effect in neonatal obstructive
nephropathy. Eur J Pharmacol. 650:487–495. 2011. View Article : Google Scholar
|
|
22
|
Lodha S, Dani D, Mehta R, Bhaskaran M,
Reddy K, Ding G and Singhal PC: Angiotensin II-induced mesangial
cell apoptosis: Role of oxidative stress. Mol Med. 8:830–840.
2002.
|
|
23
|
Frerman FE: Acyl-CoA dehydrogenases,
electron transfer flavoprotein and electron transfer flavoprotein
dehydrogenase. Biochem Soc Trans. 16:416–418. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beckmann JD and Frerman FE:
Electron-transfer flavoprotein-ubiquinone oxidoreductase from pig
liver: Purification and molecular, redox, and catalytic properties.
Biochemistry. 24:3913–3921. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirokawa S, Shimanuki T, Kitajima H,
Nishimori Y and Shimosaka M: Identification of ETFB as a candidate
protein that participates in the mechanoregulation of fibroblast
cell number in collagen gel culture. J Dermatol Sci. 64:119–126.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirokawa S, Shimanuki T, Kitajima H,
Nishimori Y and Shimosaka M: Knockdown of electron transfer
flavoprotein β subunit reduced TGF-β-induced α-SMA mRNA expression
but not COL1A1 in fibroblast-populated three-dimensional collagen
gel cultures. J Dermatol Sci. 68:179–186. 2012. View Article : Google Scholar : PubMed/NCBI
|