Introduction
Estrogen deficiency commonly occurs in
post-menopausal women and results in systemic osteoporosis.
Osteoporosis is characterized by decreased bone mineral density
(BMD) and microarchitectural deterioration of bone, due to
increased bone resorption compared with formation. Thus,
osteoporosis has become one of the most prevalent and complex
skeletal disorders for post-menopausal women, the aged and those
with other associated medical conditions, or those who develop the
condition as a result of certain therapeutic interventions
(1,2).
A positive association has also been observed
between post-menopausal osteoporosis (PMO) and periodontitis. The
treatment of osteoporosis, particularly of osteoporosis with loss
of alveolar crestal height, remains key (3). Sphingosine-1-phosphate (S1P) is
formed by phosphorylation of sphingosine catalyzed by sphingosine
kinases (two isozymes designated sphingosine kinase 1 and
sphingosine kinase 2), and it has previously been recognized as an
important coupling molecule of osteoclast and osteoblast activity
that results in osteoanabolic effects (4–6). S1P
is pleiotropic, autocrine- and paracrine-signaling sphingolipid
released into the blood upon platelet activation. It binds to a
family of five high affinity G-coupled receptors [sphingosine 1
phosphate receptor (S1P); S1P1–S1P5] and
results in a wide range of biological processes, including the
stimulation of osteoblast migration and promotion of mature cell
survival during endogenous bone wound healing (7). S1P inhibits the differentiation of
osteoclast precursors to mature osteoclasts (5), and regulates osteoclast-osteoblast
coupling (7). S1P also enhances
the survival and migration of osteoblasts (7). Thus, S1P may be a potential strategy
to support bone formation in PMO and in fracture repair. FTY720
(fingolimod) is isolated from a Chinese herb and exerts
immunomodulating activity (8). It
has been demonstrated to be a S1P receptor agonist (for
S1P1 and S1P3–5 receptors). Compared with
native S1P (half life =2 h) (9)
and BMP-2 ligand (half life =16 min) (10), FTY720 has a longer systemic
half-life (20 h) (11) and
increased physiological stability for clinical applications. S1P
and FTY720 are important role in numerous tissue-repairing
processes, including bone regeneration, improvement of
microvascular remodeling, and osseous tissue growth in vivo
(12–14). However, whether S1P and FTY720
could enhance osteogenic differentiation of bone marrow mesenchymal
stem cells (BM-MSCs) remains to be elucidated.
The ovariectomized (OVX) rat exhibits the majority
of the characteristics of human PMO, and this animal model has been
ratified by the United States Food and Drug Administration as the
primary model to evaluate the prevention and treatment of PMO
(15,16). In the present study, BM-MSCs were
collected from femurs of the OVX rats. Following induction of
osteogenic differentiation with different concentrations of FTY720,
the stimulating effects of FTY720 on the BM-MSCs were evaluated.
The results of the present study demonstrated that FTY720 may
enhance the bone-forming ability of the BM-MSCs derived from OVX
and sham rats. In addition, the impaired bone-forming ability of
the BM-MSCs derived from OVX rats, due to estrogen deficiency, was
partly repaired following administration of FTY720.
Materials and methods
Animals and surgical procedures
Female Sprague-Dawley rats (n=40; age, 12 weeks;
weight, 240±20 g) were purchased from the Laboratory Animal
Research Center of the Fourth Military Medical University (Xi'an,
China) and all interventions were performed in full accordance with
the National Institutes of Health Guidelines for the Care and Use
of Laboratory Animals (17). The
rats were housed one per cage in a room exposed to artificial
12/12-h light-dark cycles at 24±2°C with 60±20% relative humidity.
All animals were fed 15 g of a rodent diet containing 0.3% calcium
and deionized water ad libitum per day to prevent weight
gain in OVX rats as recommended by Sims et al (18). The study was approved by the ethics
committee of the Fourth Military Medical University.
Following one week for acclimatizing to the new
laboratory surroundings, the rats were randomly divided into
experimental OVX and sham surgery control groups (n=20/group). All
the animals were anesthetized with an intraperitoneal injection of
8% chloral hydrate solution (6 ml/kg body weight). Bilateral
ovariectomy was performed in 20 rats in the OVX group under sterile
conditions as previously described (19). The remaining 20 rats had their
ovaries surgically exteriorized and replaced in sham surgery
control group.
Micro-computed tomography (CT)
measurement
Retrieved femoral samples of the animals in the OVX
and sham groups were scanned with a micro-CT scanner (SCANCO
Medical AG, Brüttisellen, Switzerland) 12 weeks after the surgery
to ensure that the PMO animal model was established successfully
(20). The scanner was operated at
70 kV, 145 μA, 300 msec integration time, 500 projections on
360°, 1024 CCD detector array and 8.6 μm/pixel for scan
resolution. Trabecular bone parameters were calculated using the
SCANCO Medical microCT systems for scanning, 3D analysis,
visualization, image management and data import/export (SCANCO
Medical AG). Morphometric indices of the trabecular bone region
were determined from microtomographic data sets using direct
three-dimensional morphometry. The microarchitecture parameters of
BMD, trabecular thickness (Tb.Th) and bone volume/total volume
ratio (BV/TV, bone volume fraction) were assessed.
Isolation and culture of rat BM-MSCs
(rBM-MSCs)
Following ovariectomy (after 12 weeks), the OVX and
control rats were sacrificed using sodium pentobarbital at a dose
of >60 mg/kg (Sigma-Aldrich St. Louis, MO, USA). Bilateral
femurs were harvested under aseptic conditions and all soft tissues
were removed. Metaphysis from the two ends of the femurs were
removed. The marrow was flushed out with 10 ml Dulbecco's modified
Eagle's medium (DMEM, Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and supplemented with 10% fetal bovine serum
(FBS, Gibco; Thermo Fisher Scientific, Inc.) and penicillin 100
U/ml and streptomycin 100 μg/ml (Invitrogen; Thermo Fisher
Scientific, Inc.). The cells were cultured at 37°C in a 5%
CO2 incubator for ~2 weeks, washed with phosphate
buffered saline (PBS) and passaged with 0.25%
trypsin/ethylenediaminetetraacetic acid (Gibco; Thermo Fisher
Scientific, Inc.). The rBM-MSCs from passage 2 were used for
treatment with different concentrations of FTY720
(Sigma-Aldrich).
Flow cytometry analysis
The surface markers of mesenchymal and
non-mesenchymal stem cells are identifying characteristics of the
immunophenotype of ex vivo-expanded rBM-MSCs. The expression
levels of these markers were measured by flow cytometric analysis
at passage 2. Briefly, ~1×106 liberated adherent cells
were harvested and washed with PBS containing 3% FBS in different
Eppendorf tubes. The single-cell suspension was then resuspended
and incubated with phycoerythrin-conjugated anti-rat antibodies as
follows: Monoclonal armenian hamster cluster of differentiation
(CD)29 (1:100; #562154); and monoclonal mouse CD45 (1:100;
#554878); CD90 (1:100; #551401); and CD106 (1:100; #559229)
obtained from BD Biosciences (Franklin Lakes, NJ, USA); and mouse
monoclonal CD34 (1:50; #ab187284) and CD44 (1:200; #ab23396) from
Abcam (Cambridge, UK), at 4°C in the dark. Cell suspension without
antibodies served as a control to determine background
fluorescence. The cells were washed three times after 1 h with PBS
containing 3% FBS and 300 μl suspension was added to the
testing tubes. Finally, the samples were measured by flow
cytometric analysis using a Accuri C6 flow cytometer (BD
Biosciences).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay
The MTT assay was performed to evaluate the
proliferation of the OVX and sham passage 2 BM-MSCs separately
incubated in basic medium (DMEM supplemented with 10% FBS).
Briefly, cells were seeded in 96-well plates at 5×103
cells/well and cultured in a humidified atmosphere of 5%
CO2 at 37°C for 16 h. The medium was then discarded and
MTT (Amresco, LLC, Solon, OH, USA) solution was added. After 4 h
incubation at 37°C in a 5% CO2, 200 μl dimethyl
sulfoxide (Sigma-Aldrich) was added and agitated for 15 min to
dissolve the formazan crystals. The optical density (OD) of the
solution was measured at a wavelength 570 nm by ELx808 Absorbance
Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA).
The MTT assay, as described above, was performed once a day for 10
days.
Osteogenic differentiation induced by
different concentrations of FTY720
To determine the effects of FTY720 at different
concentrations, 0, 1, 10 and 100 nM were selected in preliminary
experiments and 0 nM served as a control. rBM-MSCs from the OVX and
sham groups were seeded in 24-well tissue culture plates at a
density of 1×106 cells/ml/well and divided into 8
groups: i) OVX rBM-MSCs without FTY720 (0 nM, blank control); ii)
sham rBM-MSCs without FTY720 (0 nM, control); iii) OVX rBM-MSCs
with 1 nM FTY720; iv) sham rBM-MSCs with 1 nM FTY720; v) OVX
rBM-MSCs with 10 nM FTY720; vi) sham rBM-MSCs with 10 nM FTY720;
vii) OVX rBM-MSCs with 100 nM FTY720; and viii) sham rBM-MSCs with
100 nM FTY720.
Following grouping, FTY720 was added to the
osteogenic differentiation medium [10% v/v FBS, DMEM, 10 nM
dexamethasone, 10 mM sodium β-glycerophosphate (Sigma-Aldrich), 50
μg/ml vitamin C (Gibco; Thermo Fisher Scientific, Inc.), 100
U/l penicillin and 100 U/l streptomycin]. The cells were allowed to
reach 80% confluency, and then osteogenesis was initiated with
osteogenic differentiation medium containing 0, 1, 10 or 100 nM
FTY720. The medium was changed every 3 days.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cells were incubated at 37°C in 5%
CO2 and harvested to assess mRNA expression levels at 1,
2 and 3 weeks. The relative gene expression levels of Runt-related
transcription factor 2 (Runx2), Sp7 transcription factor (Sp7, also
termed osterix), osteocalcin (OCN) and alkaline phosphatase (ALP)
in rBM-MSCs were compared among the 8 groups to detect the
extracellular matrix and genes of the osteogenesis marker. Total
RNA was purified from the cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. For reverse transcription of mRNA and the PCR reaction
system, conditions and analysis method were used as described
previously (20). The first-strand
cDNAs were synthesized from 5 μg total RNA using ReverTra
Ace qPCR RT Master Mix with gDNA Remover (Toyobo Co., Ltd., Osaka,
Japan) as follows: Genomic DNA removal (degradation), 37°C for 5
min; cDNA synthesis, 37°C for 15 min; reverse transcriptase
inactivation reaction, 98°C for 5 min; total time, ~30 min. A DNase
step was performed to dissociate the irrelevant DNA prior to qPCR.
DNase I from the RT kit was used in the present experiment. RT-qPCR
was performed using a SYBR Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and a GeneAmp 9700
thermal cycler (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following PCR conditions were used: 95°C for 10 sec; 40
cycles of 95°C for 5 sec and 60°C for 30 sec; and dissociation
program of 95°C for 15 sec, 60°C for 30 sec, and 95°C for 15 sec.
GAPDH served as an internal control. The expression levels of the
target genes were calculated from ∆∆Cq values (21). Primers for the RT-qPCR were
synthesized by Shanghai Shenggong Biology Engineering Technology
Service, Ltd. (Shanghai, China) based on the GenBank database
(www.ncbi.nlm.nih.gov/genbank). The
primer sequences are listed in Table
I.
 | Table IPrimers used in the present study. |
Table I
Primers used in the present study.
| Gene | Forward primer (5′ to
3′) | Reverse primer (5′ to
3′) | GenBank
accession | Size (bp) |
|---|
| Runx2 |
CCTCTGACTTCTGCCTCTGG |
GATGAAATGCCTGGGAACTG | NM_001278483 | 106 |
| Sp7 |
TTCACCTGTCTGCTCTGCTC |
GCTGATTGGCTTCTTCTTCC | NM_181374 | 154 |
| OCN |
ACAAGTCCCACACAGCAACTC |
CCAGGTCAGAGAGGCAGAAT | NM_013414 | 103 |
| ALP |
GGCTGGAGATGGACAAGTTC |
ACGCCACACAAGTAGGCAGT | J03572 | 106 |
| GAPDH |
ACAGCAACAGGGTGGTGGAC |
TTTGAGGGTGCAGCGAACTT | BC059110 | 105 |
Western blot analysis
Protein expression levels of Runx2, Sp7, OCN and ALP
were detected by western blot analysis 3 weeks after induction of
osteogenic differentiation. Cells were washed with cold PBS (pH
7.0) and the whole cell-aggregate lysates were extracted by
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing 50 mM Tris (pH 7.4), 150
mM NaCl, 1% Triton X-100, 1% sodium deoxycholate and 0.1% sodium
dodecyl sulfate (SDS) with a protease inhibitor cocktail
(Sigma-Aldrich). The concentration of total protein was determined
with a bicinchoninic acid assay (Beyotime Institute of
Biotechnology) following the manufacturer's protocol. Briefly, the
protein samples were loaded on 12% SDS-PAGE gels and, following
electrophoresis, transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA) at 100 V for 1.5 h.
Following blocking with 5% bovine serum albumin (Gibco; Thermo
Fisher Scientific, Inc.) at room temperature for 2 h, the membranes
were incubated with primary antibodies purchased from Abcam
overnight at 4°C as follows: Rabbit polyclonal Runx2 (1:1,000;
#ab23981); rabbit monoclonal Sp7 (1:2,000; #ab187158); mouse
monoclonal OCN (1:500; #ab13418); rabbit polyclonal ALP (1:1,000;
#ab95462); and rabbit polyclonal GAPDH (1:2,500; ab9485), followed
by incubation with corresponding secondary antibodies from Abcam as
follows: Horseradish peroxidase-conjugated goat polyclonal
anti-rabbit (1:20,000; #ab97051) and anti-mouse (1:10,000; #ab6789)
IgGs. The bands were visualized using the eEcl Western Blot kit
(CWBIO, Ltd., Beijing, China) and were quantified using ImageQuant
TL 7.0 Image Analysis Software (GE Healthcare Life Sciences,
Pittsburgh, PA, USA).
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. Differences between values were analyzed statistically
using Student's t-test, one-way analysis of variance with
Student-Newman-Keuls post-hoc test (GraphPad Prism 5.0; GraphPad
Software, Inc., La Jolla, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
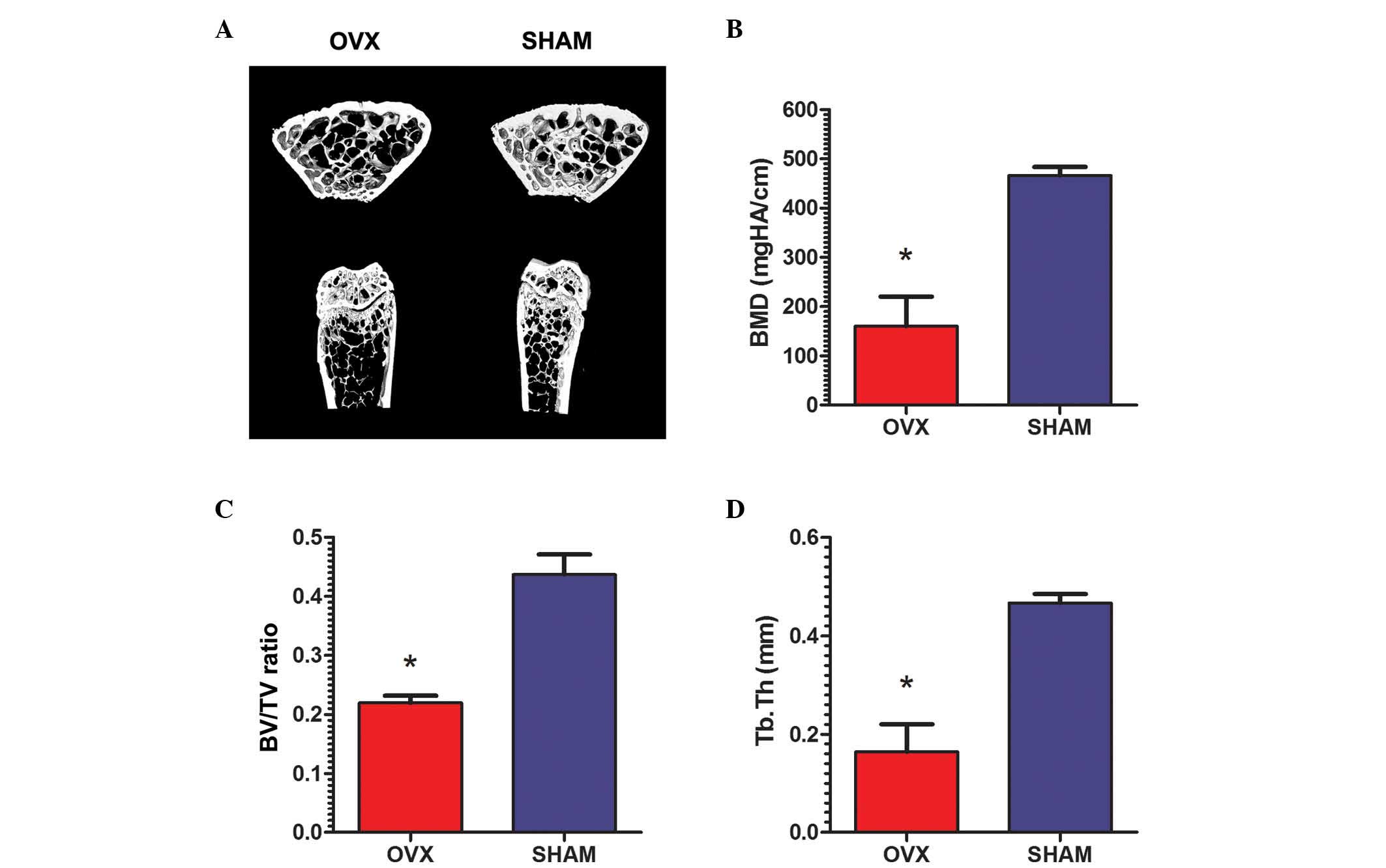
The animal model was successfully
generated
All rats remained healthy 3 months after surgery.
Micro-CT validation of all rats demonstrated essential structures
of the normal tibiae in the rats from the sham group, while the
proximal tibiae were observed to be thinner and sparser in OVX rats
(Fig. 1A). The micromorphology of
the tibiae the sham group rats was characterized by significantly
higher BMD (466.0±8.90 mgHA/cm) compared with that in OVX rats
(160.1±30.08 mgHA/cm; P<0.01; Fig.
1B). Increased BV/TV was also observed in the sham group
(43.69±1.7%) compared with the OVX group (21.98±0.6%; P<0.01;
Fig. 1C). Furthermore, the Tb.Th
in the rats from the sham group (0.467±0.009 mm) was significantly
higher than the OVX rats (0.164±0.028 mm; P<0.01; Fig. 1D).
rBM-MSCs from the OVX and sham groups
were determined to be similar
The rBM-MSCs derived from OVX and sham group rats
demonstrated similar expression of surface markers. The cells were
positive for CD29, CD44, CD90 and CD106, as demonstrated by the
detection of 79.2% CD29-, CD44-, CD90- and CD106-positive rBM-MSCs.
However, 4.62% CD34- and CD45-positive rBM-MSCs were observed,
suggesting the cells are negative for CD34 and CD45 (Fig. 2A). These results were compatible
with the characteristics of stem cells proposed in previous studies
(22,23).
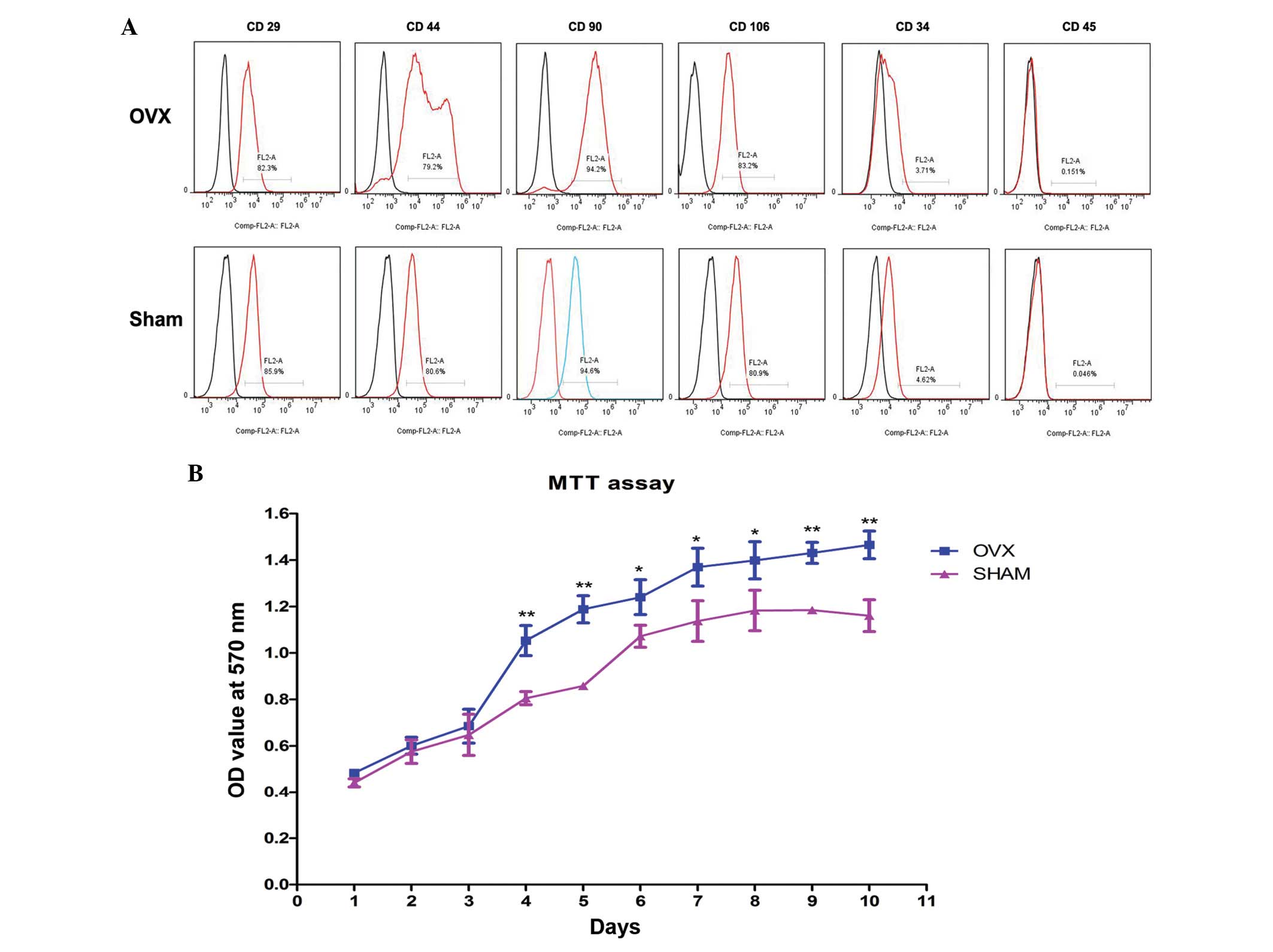 | Figure 2Characterization of rBM-MSCs and
analysis of proliferation. (A) rBM-MSCs harvested from the OVX and
sham groups were positive for mesenchymal stem cell-like markers,
including CD29 (OVX=82.3%; sham=85.9%), CD44 (OVX=79.2%;
sham=80.6%), CD90 (OVX=94.2%; sham=94.6%) and CD106 (OVX=83.2%;
sham=80.9%), while these cells were negative for hematopoietic
markers, including CD34 (OVX=3.71%; sham=4.62%) and CD45
(OVX=0.151%; sham=0.046%). (B) For the first 3 days, no significant
difference was detected in the OD values of rBM-MSCs between the
OVX and sham groups. From the 4th day, the OD values of rBM-MSCs
increased and increased cell proliferation was observed in the OVX
group. *P<0.05, **P<0.01 vs. the sham
group. rBM-MSCs, rat bone marrow mesenchymal stem cells; OVX,
ovariectomized; CD, cluster of differentiation; OD, optical
density. |
In vitro self-renewal potential of
rBM-MSCs increased in the OVX rBM-MSCs
The MTT assay indicated a higher proliferating
ability of rBM-MSCs in the OVX group compared with the sham group
(Fig. 2B). rBM-MSCs cultured in
basic medium were predominantly fusiform, asteroid, triangle or
polygonal with abundant cytoplasm and large, round or ovoid
nucleoli. For the first 3 days, there was no significant difference
in the OD values of rBM-MSCs in basic medium from the OVX group
compared with the sham group (P>0.05), however, after day 4, the
OD values of cultured rBM-MSCs significantly increased in OVX group
compared with those in sham group in a time-dependent manner
(P<0.05). Notably, rBM-MSCs from the OVX group are more
proliferative than rBM-MSCs from the sham group.
FTY720 alters osteogenic gene expression
in rBM-MSCs
The primer sequences were based on data from GenBank
(Table I). The mRNA expression
levels of osteogenesis-associated genes, including Runx2, Sp7, OCN
and ALP were examined at 1, 2 and 3 weeks (Fig. 3). Compared with the control groups
cultured in media containing 0 nM FTY720, the cells cultured in 1,
10 or 100 nM FTY720 demonstrated significantly increased expression
levels (P<0.01) of Runx2 and Sp7 at 2 weeks, and ALP at 3 weeks.
The expression levels of OCN cultured in 1, 10 or 100 nM was
significantly increased (P<0.01) at 2 and 3 weeks compared with
the results observed at 1 week. Furthermore, among the three
concentrations, experimental groups treated with 10 nM FTY720
exhibited significantly higher expression levels of Runx2 and Sp7
at 2 weeks, while ALP in the sham group was highest at 3 weeks.
There was no significant difference (P>0.05) in expression of
OCN at 2 and 3 weeks. The increased gene expression suggested an
improved promotion of osteogenesis (24).
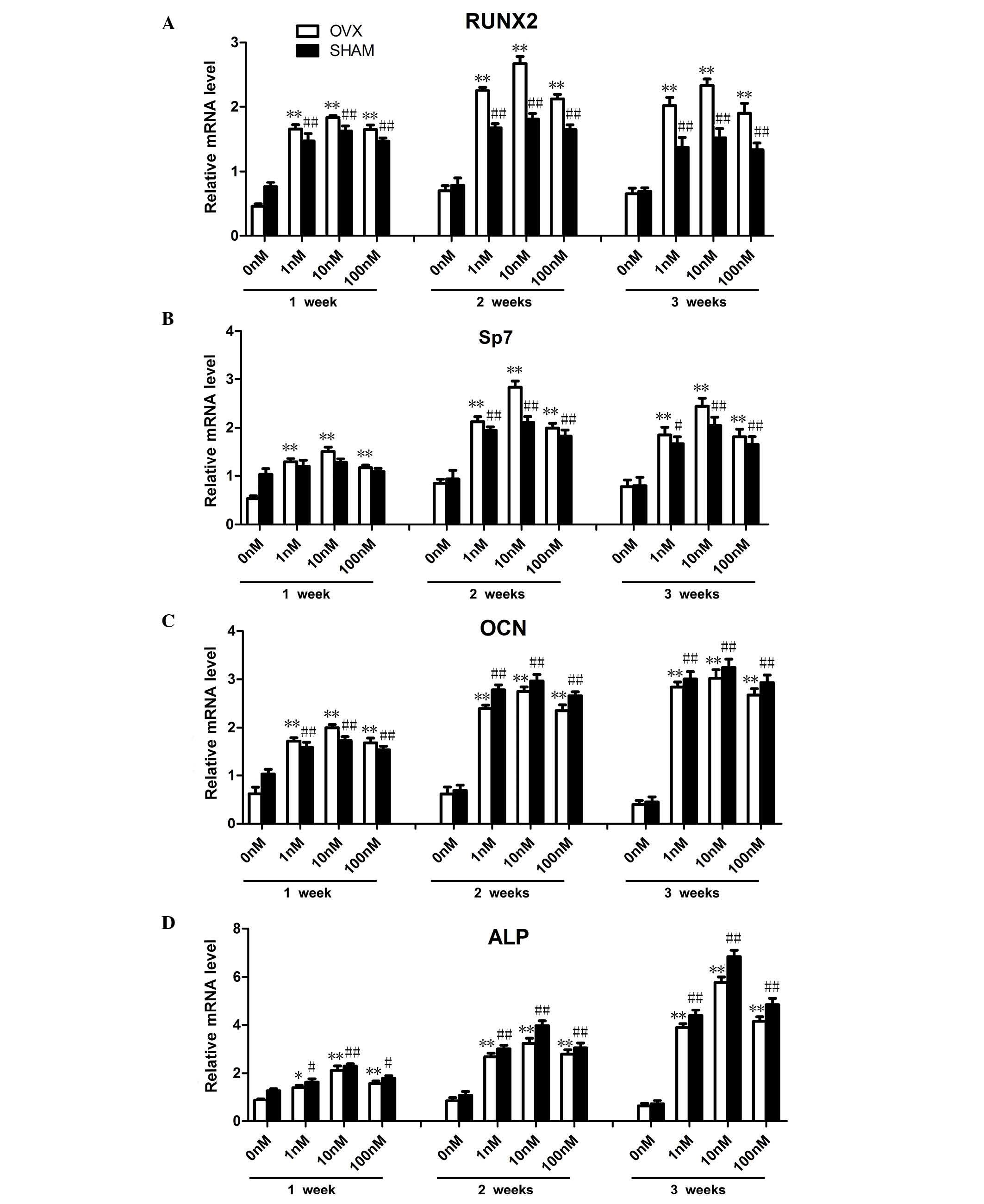 | Figure 3Osteogenic differentiation associated
gene expression levels in rBM-MSCs treated with three different
concentrations of FTY720 at 1, 2 and 3 weeks in OVX and sham
Groups. (A and B) Runx2 and Sp7 mRNA expression levels increased
with administration of 1, 10 or 100 nM FTY720 at 2 weeks, the
highest expression levels were observed in the 10 nM group at 2
weeks. The expression levels in the OVX groups were higher
comapared with the sham groups at 2 weeks. (C) OCN mRNA expression
levels significantly increased with 1, 10 and 100 nM FTY720 at 2
and 3 weeks. (D) ALP mRNA expression levels significantly increased
with 1, 10 or 100 nM FTY720 at 3 weeks. The highest expression was
observed in the 10 nM group at 3 weeks. The mRNA expression levels
of ALP in the OVX group were lower than those in the sham groups at
3 weeks. The mRNA expression levels were calculated as the fold
expression relative to the control levels at the indicated time
points. *P<0.05, **P<0.01 vs. the
control in the OVX group.#P<0.05,
##P<0.01 vs. the control in the sham group. rBM-MSCs,
rat bone marrow-mesenchymal stem cells; OVX, ovariectomized; RunX2,
Runt-related transcription factor 2; Sp7, Sp7 transcription factor;
OCN, osteocalcin; ALP, alkaline phosphatase. |
Notably, in contrast to OCN and ALP, the expression
levels of Runx2 and Sp7 in the OVX groups were significantly
increased (P<0.05) compared with the sham group at 2 weeks,
while ALP expression in sham groups increased to a higher level
(P<0.05) than that in OVX group at 3 weeks. For the 10 nM OVX
sub-group, the highest expression levels of Runx2 and Sp7 were
observed at 2 weeks (P<0.01) while the highest expression level
of ALP was detected at 3 week (P<0.01). The expression levels of
OCN were similar (P>0.05) at 2 and 3 weeks.
FTY720 alters osteogenic
differentiation-associated protein expression in rBM-MSCs
The osteogenesis-associated protein expression
levels of Runx2, Sp7, OCN and ALP with 3 different concentrations
of FTY720 in the osteogenic differentiation medium were
significantly higher (P<0.01) than those with no FTY720
treatment (0 nM control groups) at 3 weeks in the OVX and sham
groups (Fig. 4). The experimental
groups treated with 10 nM FTY720 exhibited the highest protein
expression levels of Runx2, Sp7, OCN and ALP (P<0.01),
suggesting that 10 nM FTY720 exerts the largest effect to promote
osteogenesis (Fig. 4).
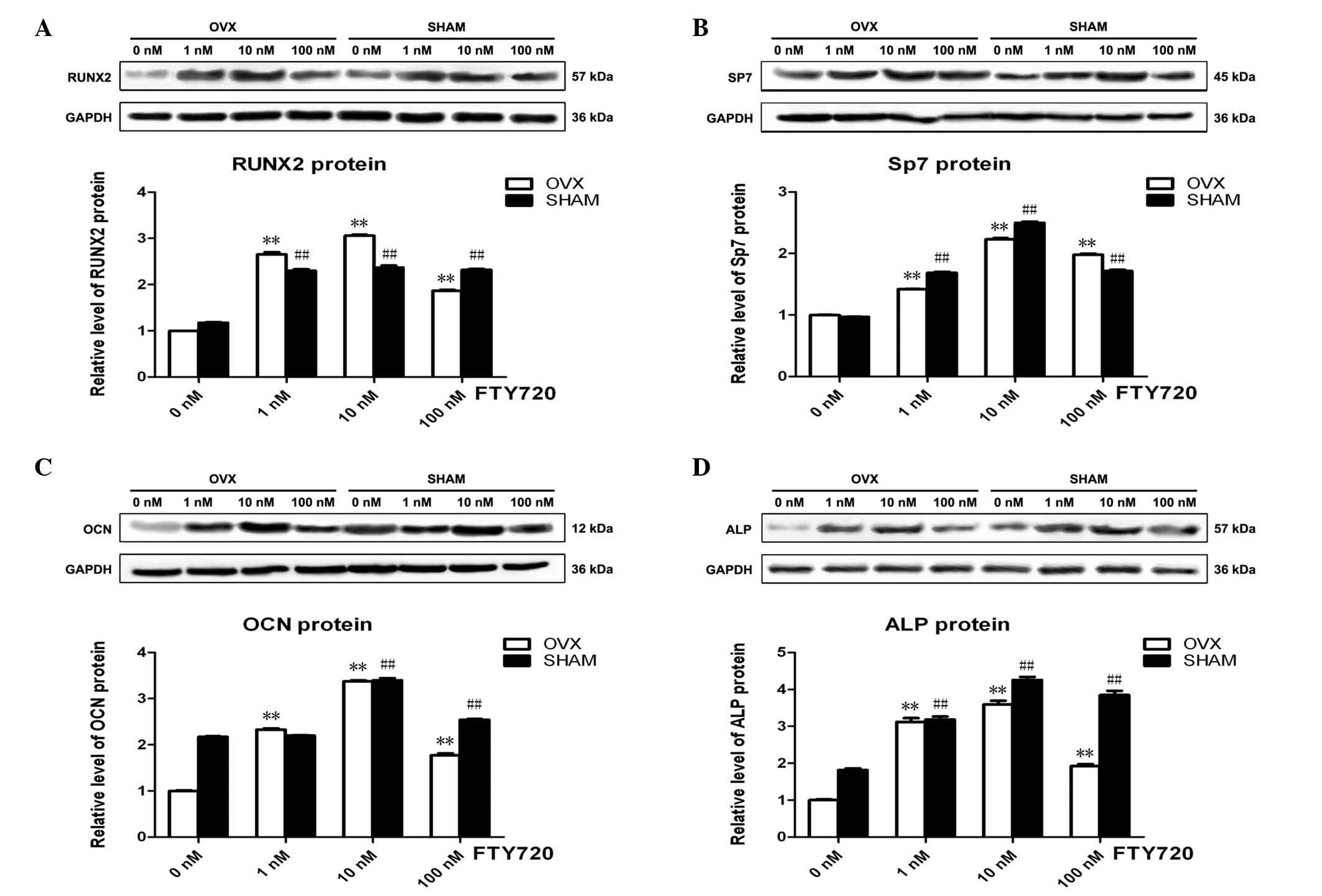 | Figure 4The expression profile of key proteins
obtained from rBM-MSCs in the OVX and sham groups. The protein
expression levels of (A) Runx2, (B) Sp7, (C) OCN and (D) ALP were
significantly upregulated following treatments with 1, 10 or 100 nM
FTY720, with the highest expression levels observed following 10 nM
FTY720 in the OVX and sham groups. Band intensity was quantified
from at least three independent experiments and calculated as the
fold change in expression relative to the control levels.
**P<0.01 vs. the control in the OVX group.
##P<0.01 vs. the control in the sham group. rBM-MSCs,
rat bone marrow-mesenchymal stem cells; OVX, ovariectomized; RunX2,
Runt-related transcription factor 2; Sp7, Sp7 transcription factor;
OCN, osteocalcin; ALP, alkaline phosphatase. |
Discussion
Previous studies have demonstrated that S1P may
result in osteoanabolic effects (4–6), and
improve bone defect healing (12).
The present study demonstrated that the S1P analog FTY720 promoted
the expression of osteogenesis-associated genes and proteins, thus,
inducing the osteogenic differentiation of rBM-MSCs from either OVX
or sham rats. It was observed that FTY720 increased expression of
markers for osteoblast differentiation, including ALP, OCN, Runx2
and Sp7 in rBM-MSCs from the OVX group. However, osteogenesis of
rBM-MSCs from OVX rats was not induced without FTY720. Thus, to the
best of our knowledge, the current study is the first to
demonstrate FTY720 induces osteoblast differentiation and
mineralization by altering expression levels of
osteogenesis-associated genes or proteins and osteoblast
differentiation in rBM-MSCs from the OVX group.
It was recently reported that S1P modulates the
differentiation of MSCs into cardiomyocytes and smooth muscle cells
(25,26). However, the effects of S1P or its
analog FTY720 on osteoblast differentiation of BM-MSCs had not been
elucidated. Furthermore, whether the osteogenic capability of
BM-MSCs in osteoporosis could be recovered remained unknown. To
increase understanding of the recovery of impaired bone healing
ability in OVX rats, the evaluation of expression levels of genes
and proteins associated with osteoblast differentiation, including
ALP, OCN, Runx2 and Sp7, was performed. Runx2 is essential to
osteoblast differentiation and osteogenesis, and regulates the
expression of numerous extracellular matrix protein genes during
differentiation (27). Similarly,
ALP is an important marker of early osteoblast differentiation and
the bone mineralization process (27). Furthermore, Sp7 is an
osteoblast-specific transcription factor that activates a variety
of genes during differentiation of preosteoblasts into mature
osteoblasts and osteocytes (28).
OCN, a 49 amino acid non-collagenous matrix protein of calcified
tissue, is synthesized by osteoblasts. It has been hypothesized to
be involved in bone resorption and mineralization, and is currently
described as the most specific marker of mature osteoblasts
(29). Thus, the present study
aimed to elucidate the role of FTY720 on osteoblast differentiation
in normal BM-MSCs (sham group) and BM-MSCs with osteoporosis (OVX
group).
Previous studies have demonstrated that S1P or
FTY720 induce differentiation of osteoblast-like cells, including
the SaOS-2 (30), MC3T3-E1
(30), C2C12 (4), and C3H10T1/2 (31) cell lines into osteoblasts. Although
these results are encouraging, clinical application requires
further research. The findings of the present study regarding the
effects of FTY720 for BM-MSC osteoblast differentiation suggest
further research into clinical application should be conducted.
Notably, an abnormal result was detected in the
present study. The gene expression levels of Runx2 and Sp7 in the
OVX group were higher than those in sham group, however, the
expression levels of ALP and OCN in the OVX group were lower than
those in the sham group. Although the mechanism underlying this
result remains unknown, it may be predicted that FTY720 is key in
the process. It is well known that Runx2 and Sp7 are essential
upstream transcription factors during the early processes of
osteoblast differentiation (32).
Kido et al (33) recently
comparatively evaluated the process of bone repair in healthy and
ovariectomized rats 14 days after tibial bone defect. It was
hypothesized that ovariectomy-induced bone loss would suppress
tissue healing, predominantly due to the decreased expression of
genes and proteins associated with bone cell differentiation, and
result in reduced formation of new bone. The RT-qPCR findings in
the present study suggested that FTY720 may repair
osteogenesis-associated gene expression, particularly the upstream
transcription factors, such as Runx2 and Sp7, and thus promote bone
regeneration.
It was also demonstrated that FTY720 increased gene
and protein expression levels of ALP and OCN, the
osteoblastic-specific phenotype markers secreted by mature
osteoblasts. It has been reported that S1P is important in a number
of tissue repairing processes, including bone regeneration. S1P or
FTY720 improves microvascular remodeling and osseous tissue growth
in vivo (12,13). Thus, the present study hypothesized
that FTY720 may be a potential therapeutic target for the promotion
of bone regeneration.
In conclusion, all different concentrations of
FTY720 administered to rBM-MSCs enhances osteogenic differentiation
from either OVX or sham rats, and 10 nM FTY720 induced the highest
level of bone-associated gene expression in the present study.
These findings suggested a beneficial effect of FTY720 on the
impaired osteogenesis in BM-MSCs derived from the OVX rats.
Furthermore, the results of the present study may also provide a
potential therapeutic method for the improvement of bone loss in
osteoporosis and alveolar bone resorption in patients with PMO.
Acknowledgments
The present study was supported by the Chinese
National Key Project for Basic Research (grant nos. 2014CB542202)
and the National Natural Science Foundations of China (grant nos.
81470631 and 81170982).
References
|
1
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang W, Li X, Li Y, Li C, Gao B, Gan H,
Li S, Shen J, Kang J, Ding S, et al: Tongue coating microbiome
regulates the changes in tongue texture and coating in patients
with post-menopausal osteoporosis of Ganshen deficiency syndrome
type. Int J Mol Med. 32:1069–1076. 2013.PubMed/NCBI
|
|
3
|
Khan AA, Morrison A, Hanley DA, Felsenberg
D, McCauley LK, O'Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis
S, et al: Diagnosis and management of osteonecrosis of the Jaw: A
systematic review and international consensus. J Bone Miner Res.
30:3–23. 2015. View Article : Google Scholar
|
|
4
|
Sato C, Iwasaki T, Kitano S, Tsunemi S and
Sano H: Sphingosine 1-phosphate receptor activation enhances
BMP-2-induced osteoblast differentiation. Biochem Biophys Res
Commun. 423:200–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishii M, Egen JG, Klauschen F,
Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL and Germain RN:
Sphingosine-1-phosphate mobilizes osteoclast precursors and
regulates bone homeostasis. Nature. 458:524–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lotinun S, Kiviranta R, Matsubara T,
Alzate JA, Neff L, Lüth A, Koskivirta I, Kleuser B, Vacher J,
Vuorio E, et al: Osteoclast-specific cathepsin K deletion
stimulates S1P-dependent bone formation. J Clin Invest.
123:666–681. 2013.PubMed/NCBI
|
|
7
|
Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y
and Kim HH: Sphingosine 1-phosphate as a regulator of osteoclast
differentiation and osteoclast-osteoblast coupling. EMBO J.
25:5840–5851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mandala S, Hajdu R, Bergstrom J,
Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D,
Keohane C, et al: Alteration of lymphocyte trafficking by
sphingosine-1-phosphate receptor agonists. Science. 296:346–349.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Venkataraman K, Lee YM, Michaud J,
Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C and Hla T:
Vascular endothelium as a contributor of plasma sphingosine
1-phosphate. Circ Res. 102:669–676. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poynton AR and Lane JM: Safety profile for
the clinical use of bone morphogenetic proteins in the spine. Spine
(Phila Pa 1976). 27(16 Suppl 1): S40–S48. 2002. View Article : Google Scholar
|
|
11
|
Meno-Tetang GM, Li H, Mis S, Pyszczynski
N, Heining P, Lowe P and Jusko WJ: Physiologically based
pharmacokinetic modeling of FTY720 (2-amino-2
[2-(-4-octylphenyl)ethyl] propane-1,3-diol hydrochloride) in rats
after oral and intravenous doses. Drug Metab Dispos. 34:1480–1487.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sefcik LS, Aronin CE, Awojoodu AO, Shin
SJ, Mac Gabhann F, MacDonald TL, Wamhoff BR, Lynch KR, Peirce SM
and Botchwey EA: Selective activation of sphingosine 1-phosphate
receptors 1 and 3 promotes local microvascular network growth.
Tissue Eng Part A. 17:617–629. 2011. View Article : Google Scholar :
|
|
13
|
Petrie Aronin CE, Shin SJ, Naden KB, Rios
PD Jr, Sefcik LS, Zawodny SR, Bagayoko ND, Cui Q, Khan Y and
Botchwey EA: The enhancement of bone allograft incorporation by the
local delivery of the sphingosine 1-phosphate receptor targeted
drug FTY720. Biomaterials. 31:6417–6424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petrie Aronin CE, Sefcik LS, Tholpady SS,
Tholpady A, Sadik KW, Macdonald TL, Peirce SM, Wamhoff BR, Lynch
KR, Ogle RC and Botchwey EA: FTY720 promotes local microvascular
network formation and regeneration of cranial bone defects. Tissue
Eng Part A. 16:1801–1809. 2010. View Article : Google Scholar
|
|
15
|
Turner AS: Animal models of
osteoporosis-necessity and limitations. Eur Cell Mater. 1:66–81.
2001.
|
|
16
|
Thompson DD, Simmons HA, Pirie CM and Ke
HZ: FDA Guidelines and animal models for osteoporosis. Bone.
17(Suppl 4): S125–S133. 1995. View Article : Google Scholar
|
|
17
|
National Research Council of the National
Academies: Guide for the Care and Use of Laboratory Animals. 8th
edition. The National Academies Press; Washington D.C.: 2011
|
|
18
|
Sims NA, Morris HA, Moore RJ and Durbridge
TC: Increased bone resorption precedes increased bone formation in
the ovariectomized rat. Calcif Tissue Int. 59:121–127. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HL, Tung YT, Chuang CH, Tu MY, Tsai
TC, Chang SY and Chen CM: Kefir improves bone mass and
microarchitecture in an ovariectomized rat model of postmenopausal
osteoporosis. Osteoporosis Int. 26:589–599. 2015. View Article : Google Scholar
|
|
20
|
Liu Y, Ming L, Luo H, Liu W, Zhang Y, Liu
H and Jin Y: Integration of a calcined bovine bone and BMSC-sheet
3D scaffold and the promotion of bone regeneration in large
defects. Biomaterials. 34:9998–10006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Miyazaki M, Hardjo M, Masaka T, Tomiyama
K, Mahmut N, Medina RJ, Niida A, Sonegawa H, Du G, Yong R, et al:
Isolation of a bone marrow-derived stem cell line with high
proliferation potential and its application for preventing acute
fatal liver failure. Stem Cells. 25:2855–2863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y, Zhao RC and Tredget EE: Concise
review: Bone marrow-derived stem/progenitor cells in cutaneous
repair and regeneration. Stem Cells. 28:905–915. 2010.PubMed/NCBI
|
|
24
|
Wu C, Miron R, Sculean A, Kaskel S, Doert
T, Schulze R and Zhang Y: Proliferation, differentiation and gene
expression of osteoblasts in boron-containing associated with
dexamethasone deliver from mesoporous bioactive glass scaffolds.
Biomaterials. 32:7068–7078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Z, Chen Z, Zhao X, Pan F, Cai M, Wang
T, Zhang H, Lu JR and Lei M: Sphingosine-1-phosphate promotes the
differentiation of human umbilical cord mesenchymal stem cells into
cardiomyocytes under the designated culturing conditions. J Biomed
Sci. 18:372011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nincheri P, Luciani P, Squecco R, Donati
C, Bernacchioni C, Borgognoni L, Luciani G, Benvenuti S, Francini F
and Bruni P: Sphingosine 1-phosphate induces differentiation of
adipose tissue-derived mesenchymal stem cells towards smooth muscle
cells. Cell Mol Life Sci. 66:1741–1754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma A, Meyer F, Hyvonen M, Best SM,
Cameron RE and Rushton N: Osteoinduction by combining bone
morphogenetic protein (BMP)-2 with a bioactive novel nanocomposite.
Bone Joint Res. 1:145–151. 2012. View Article : Google Scholar
|
|
28
|
Sinha KM and Zhou X: Genetic and molecular
control of osterix in skeletal formation. J Cell Biochem.
114:975–984. 2013. View Article : Google Scholar :
|
|
29
|
Jang H, Kim EJ, Park JK, Kim DE, Kim HJ,
Sun WS, Hwang S, Oh KB, Koh JT, Jang WG and Lee JW: SMILE inhibits
BMP-2-induced expression of osteocalcin by suppressing the activity
of the RUNX2 transcription factor in MC3T3E1 cells. Bone. 61:10–18.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsuzaki E, Hiratsuka S, Hamachi T,
Takahashi-Yanaga F, Hashimoto Y, Higashi K, Kobayashi M, Hirofuji
T, Hirata M and Maeda K: Sphingosine-1-phosphate promotes the
nuclear translocation of β-catenin and thereby induces
osteoprotegerin gene expression in osteoblast-like cell lines.
Bone. 55:315–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hashimoto Y, Matsuzaki E, Higashi K,
Takahashi-Yanaga F, Takano A, Hirata M and Nishimura F:
Sphingosine-1-phosphate inhibits differentiation of C3H10T1/2 cells
into adipocyte. Mol Cell Biochem. 401:39–47. 2015. View Article : Google Scholar
|
|
32
|
Komori T: Regulation of osteoblast
differentiation by transcription factors. J Cell Biochem.
99:1233–1239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kido HW, Bossini PS, Tim CR, Parizotto NA,
da Cunha AF, Malavazi I and Renno AC: Evaluation of the bone
healing process in an experimental tibial bone defect model in
ovariectomized rats. Aging Clin Exp Res. 26:473–481. 2014.
View Article : Google Scholar : PubMed/NCBI
|