Introduction
Cases of cancer arising in the larynx (voice box)
are predominantly squamous cell carcinoma, which is a rare form of
cancer that develops inside the tissue of the larynx. It has been
reported that laryngeal cancer accounts for ~200,000 mortalities
worldwide each year, which represents 2–5% of all malignant tumors
(1). However, laryngeal cancer is
particularly detrimental to the life quality of patients due to the
effects on the voice and swallowing abilities of patients. It is
estimated that >12,000 cases are diagnosed each year in the
United States, and the incidence is increasing whilst it is
decreasing in other types of cancer, thus, the research and
treatment of laryngeal cancer is crucial (2).
Tumor suppressor genes, which protect cells from
cancer, are critical for regulating tumor formation and
development. When these genes mutate leading to reduction or loss
of their function, cells may progress to a cancerous state, usually
in combination with other genetic changes. The loss of tumor
suppressor genes may be of greater importance than
proto-oncogene/oncogene activation for the formation of numerous
types of human cancer (3). Tumor
suppressor genes can be divided into a variety of categories,
including caretaker genes, gatekeeper genes and landscaper genes.
Among them, the tumor suppressor gene, P16, has previously been
reported to be altered in a variety of human tumors (4). It was reported that P16 is involved
in the progression of multiple types of human cancer, including
skin, lung, brain and laryngeal cancer, and the P16 protein was
demonstrated to be deleted or mutated in these tumors (5).
It has been previously reported that P16 expression
was commonly decreased in laryngeal tumors. Yuen et al
(6) reported that P16 expression
was decreased in 48% of breast cancer tumors, and that there was a
higher frequency of reduced P16 expression in tumors of the larynx
compared with those from the pharynx and oral cavity. Kalfert et
al (7) demonstrated that P16
overexpression in glottic laryngeal squamous cell carcinoma may be
associated with a low risk of disease recurrence.
The current study aimed to assess the importance of
P16 expression on cell apoptosis and the antitumor effect on Hep2
laryngeal carcinoma cells. A recombinant adenovirus carrying the
P16 gene was used to infect cells and result in expression of high
levels of P16 protein in P16-null Hep2 laryngeal carcinoma cells.
Cell proliferation, invasion assays and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) were
used to evaluate the effect of the P16 gene on cell apoptosis and
the antitumor effect on Hep2 cells.
Materials and methods
Cell culture
Hep2 (P16 null) cells were purchased from the
Chinese Academy of Medical Sciences (Beijing, China) in March 2014.
This cell line is known to be contaminated, but it was tested in
the present study and no bacterial or cell line contamination was
identified, thus the correct identity of the cells were confirmed.
The cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), penicillin (100 U/ml) and streptomycin (100
µg/ml; Gibco; Thermo Fisher Scientific, Inc.) and incubated at 37°C
in 5% CO2.
Adenovirus infection
Adenoviral vectors containing wild-type P16 cDNA and
a cytomegalovirus promoter (Agilent Technologies, Inc., Santa
Clara, CA, USA) were inserted into the E1-deleted region of
modified adenovirus (Ad-P16) as described previously (8). Monolayer cells were cultured in DMEM
with 10% FBS and were infected with P16 cDNA-adenoviral vectors as
the experimental group. Cells receiving empty vector adenovirus and
untreated cells were used as control groups.
Western blot analysis
After 1 week of infection with Ad-P16 and control
adenovirus (Ad-control), the expression levels of P16 in
Ad-P16-treated cells, Ad-control-treated cells and untreated cells
were examined by western blotting as previously described (9).
Briefly, protein was extracted from ~107
cells by lysis in extraction buffer [50 mM Tris-HC1, pH=8; 150 mM
NaCl; 1% NP-40; 0.1% sodium dodecyl sulfate (SDS), 1 mM
phenylmethylsulfonyl fluoride; Thermo Fisher Scientific, Inc.].
Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used to determine the concentration of protein. Equivalent
amounts of protein (100 µg) were fractionated by electrophoresis in
10% SDS-polyacrylamide gels. The proteins were subsequently
transferred to Immobilon-P nitrocellulose membrane (Bio-Rad
Laboratories, Inc.) under semi-dry conditions and blocked with 5%
nonfat dried milk for 1 h. Primary antibodies were incubated for 1
h at room temperature and were as follows: Monoclonal rabbit
anti-P16 (Biodesign International, Inc., Saco, MA, USA; cat. no.
K59470R) and mouse monoclonal anti-β-actin (dilution, 1:2,000;
Sigma-Aldrich, St. Louis, MO, USA; cat. no. A1978) were used. The
blots were incubated in horse anti-mouse (cat. no. 7076) or goat
anti-rabbit (cat. no. 7074) IgG horseradish peroxidase-conjugated
secondary antibody (dilution, 1:5,000; Cell Signaling Technology,
Inc., Danvers, MA, USA) for 1 hour at room temperature. The
expression of P16 and β-actin proteins were detected by an enhanced
chemiluminescence system (Amersham ECL Western Blotting Detection
reagent; GE Healthcare Life Sciences, Chalfont, UK) according to
the manufacturer's instruction and visualized using Quantity One
1-D software version 4.2.1 (Bio-Rad Laboratories, Inc.).
Cell proliferation
The cells were seeded in a 96-well flat-bottomed
microplate (1,000 cells/well) and cultured in 100 µl DMEM at 37°C
and 5% CO2. At days 1, 3, 5 and 7, the cell culture
medium in each well was then replaced with 100 µl of cell growth
medium containing 10 µl cell counting kit-8 (CCK-8) solution
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan). After
incubation for 4 h at 37°C, the absorbance at 450 nm were measured
on a SpectraMax Plus 384 microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA) and presented as the mean ± standard deviation
from triplicate wells (10).
Animal models and in vivo
experiments
Hep2 cells (0.1 ml, 1×108 cells/ml) were
subcutaneously injected into 18 male BALB/c (nu/nu) mice (age, 6
weeks; weight, 20 g) obtained from the Experimental Animal Center
of Shandong (Jinan, China). The mice were maintained at 18–23°C,
35–65% humidity, under a 12-h light/dark cycle with access to food
and water ad libitum. The present study was approved by the
ethics committee of Shangong University (Jinan, China). When the
tumor xenografts reached 50 mm3 in size, mice were
divided randomly into 3 groups (n=6 per group) as follows:
Adeno-virus-P16 (Ad-P16); Ad-control; and phosphate-buffered saline
(PBS). The mice received 3 intratumoral injections every other day
for 3 weeks at a total dosage of 109 pfu virus per mouse
in the virus-treated groups and 100 µl PBS per mouse in the control
group. Tumor size was measured at weeks 1, 2 and 3, and tumor
volume was estimated with the following formula: a × b2
× 0.5 (a, maximal diameter; b, minimal diameter) (11). The mice were sacrificed by
CO2 inhalation.
Cancer cell invasion assay
Cancer cell invasion in vitro was measured
using an invasion assay according to a previously described
protocol (12). Briefly, Transwell
inserts with 8 mm pore size were coated with a final concentration
of 0.78 mg/ml of Matrigel (Corning Incorporated, Corning, NY, USA)
in cold serum-free DMEM. Cells were trypsinized (Thermo Fisher
Scientific, Inc.) and 200 ml cell suspension (1×106
cells/ml) from each treatment was added in triplicate wells. After
48 h incubation, the cells that passed through the filter into the
lower wells were quantified by CCK-8 assay, and expressed as a
percentage of the sum of the cells in the upper and lower
wells.
RT-qPCR
The expression levels of epidermal growth factor
receptor (EGFR), survivin and cyclin D1 after 48 h were determined
by RT-qPCR as previous described (13). Total RNA was extracted using the
phenol-chloroform method (Ambion; Thermo Fisher Scientific, Inc.)
and treated with DNase I. The RNA was reverse transcribed using the
High-Capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. The cDNA was diluted to ~1 ng/µl and qPCR was conducted
with SYBR® Green PCR Master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) in a ABI 7300 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Specific
primers with the following sequences were used: EGFR, forward
5′TATTGATCGGGAGAGCCGGA and reverse 5′GCGCACGTGATCTCAAGGAA;
survivin, forward 5′GCC CAG TGT TTC TTC TGCTT and reverse
5′CCGGACGAATGCTTTTTATG; cyclin D1, forward 5′AAGGCGGAGGAGACCTGCGCG
and reverse 5′ATCGTGCGGCATTGCGGC; and GAPDH, forward
5′AGCCACATCGCTCAGACAC and reverse 5′GCCCAATACGACCAAATCC. The qPCR
cycling conditions were as follows: Initial denaturation at 95°C
for 10 min; 45 cycles of amplification at 95°C for 10 sec, 60°C for
30 sec and 72°C for 1 sec; and a single fluorescence acquisition.
GAPDH was used as an internal control. Relative gene expression was
calculated using the 2−ΔΔCq method (14,15).
Statistical analysis
All analysis was performed with SPSS software
(version 17.0; SPSS, Inc., Chicago, IL, USA). Differences between
groups were tested using two-way analysis of variance and Fisher's
least significant difference test. P<0.05 was considered to
indicate a statistically significant difference.
Results
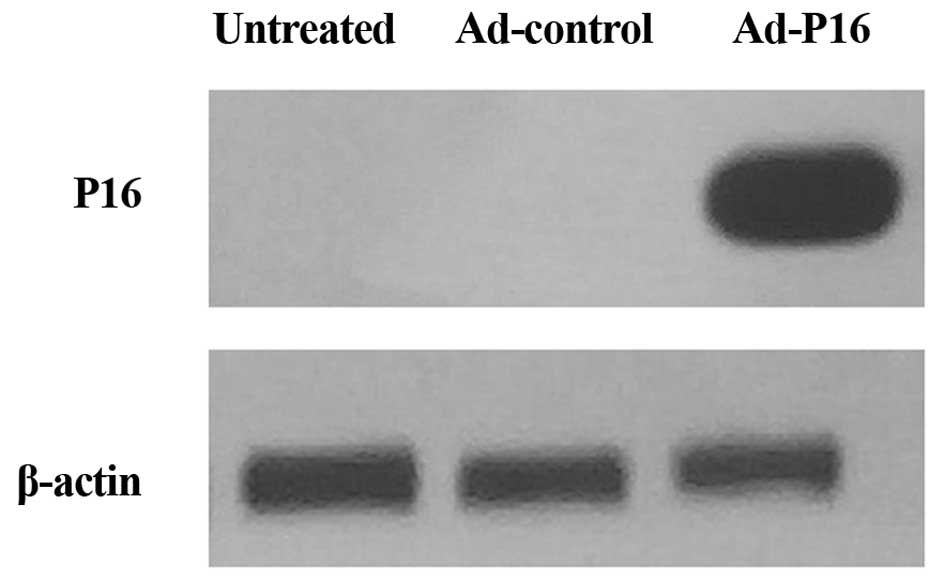
Western blot analysis of P16 protein
expression
Western blotting was performed to detect the P16
protein expression level in Hep2 cells. The results demonstrated
that the level of expression of P16 was barely detectable in
untreated cells and Ad-control cells, whereas P16 was markedly
increased following infection with Ad-P16 (Fig. 1).
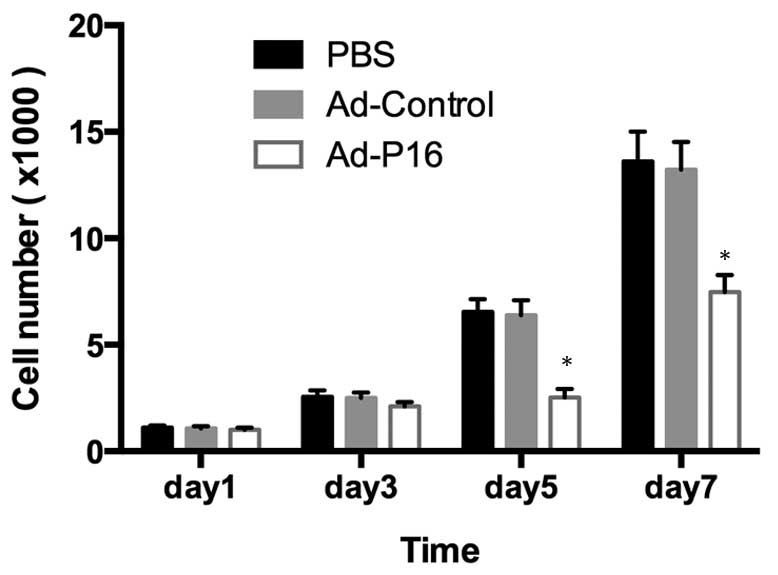
Cell proliferation and tumor growth
After 3 days of infection, Ad-P16 cells exhibited
reduced levels of proliferation compared with the untreated cells
and Ad-control cells (P<0.05), indicating that P16 exerts a
significant inhibitory effect on Hep2 cell growth (Fig. 2).
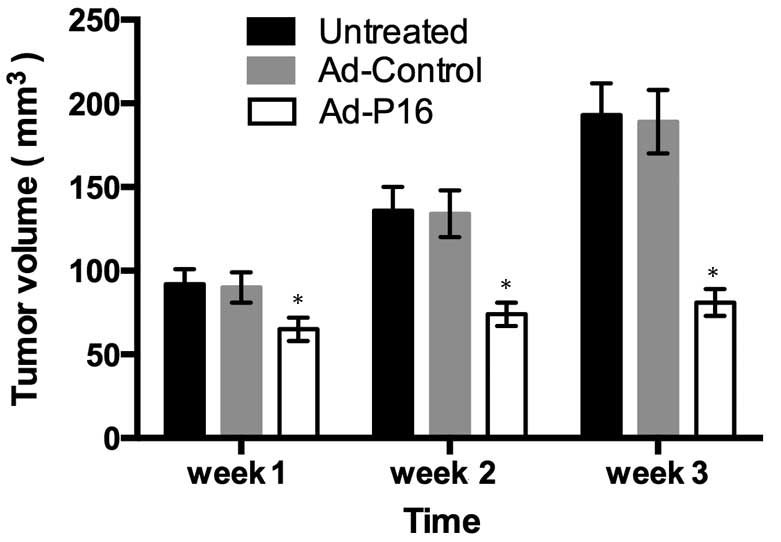
In order to determine the effect of elicited by P16
overexpression on tumor growth, the tumor formation was measured at
different times following viral infection to evaluate the antitumor
effects. Tumors injected with Ad-P16 exhibited a reduced growth
rate compared with those injected with PBS or Ad-control (Fig. 3; P<0.05).
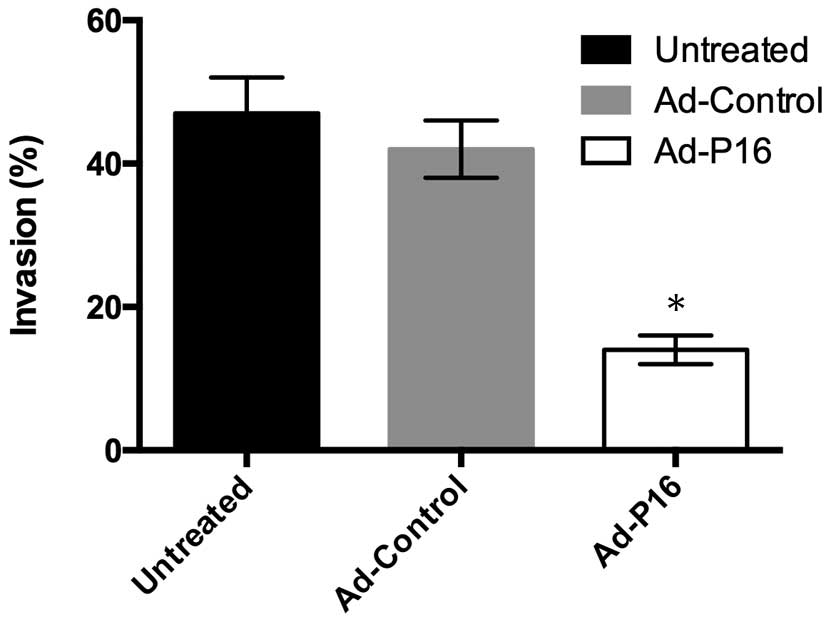
P16 inhibits laryngeal cancer cell
invasion
An important hallmarks of malignant laryngeal cancer
cells is invasion. Cells (1×106 cells/ml density) were
seeded in the upper chamber of a Transwell insert and cells that
invaded through the Matrigel were detected by CCK-8 assay. Compared
with untreated cells (47%) and Ad-control cells (42%), the number
of invasive Ad-P16 Hep2 cells (14%) was decreased significantly
(Fig. 4; P<0.05).
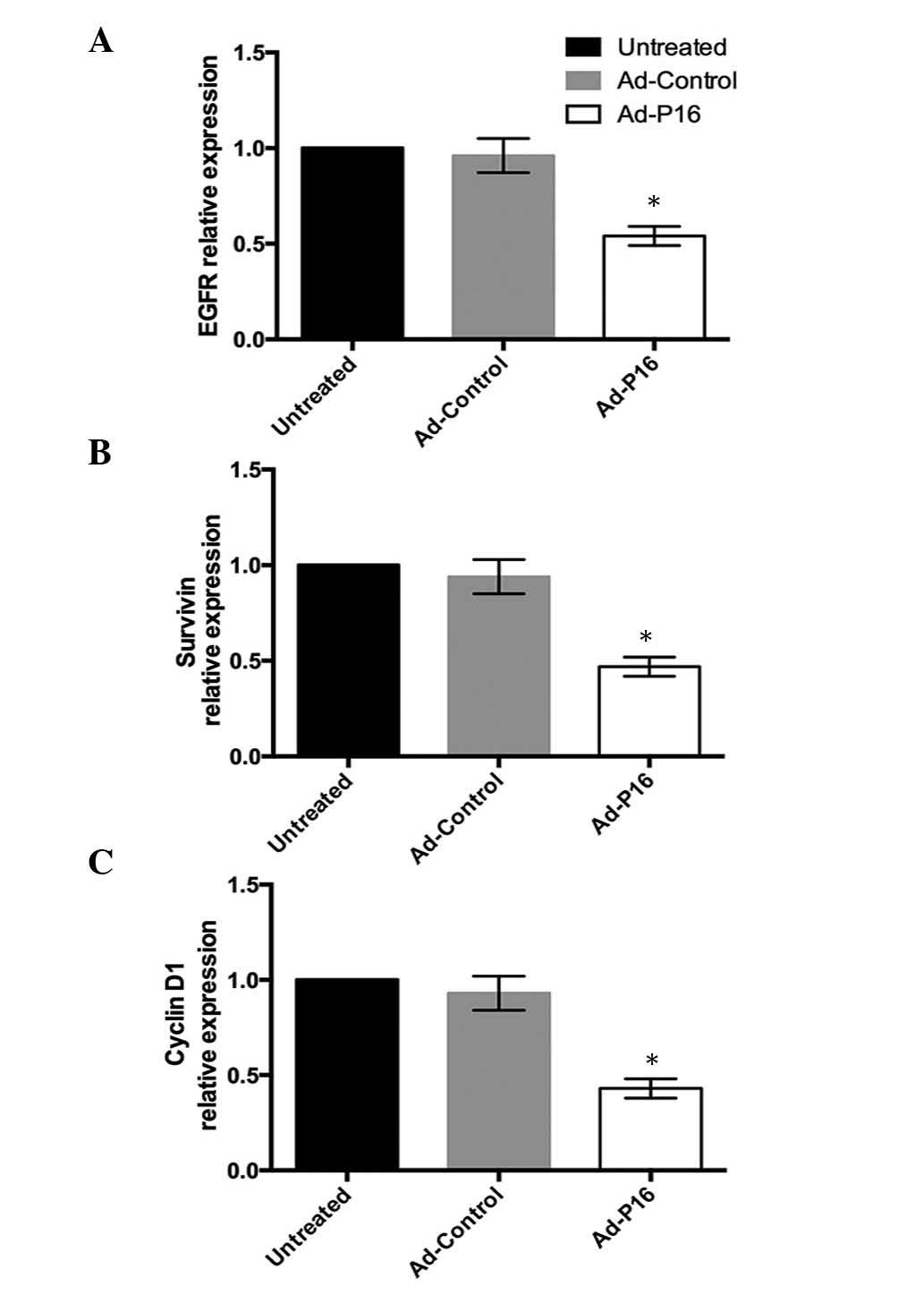
Expression of laryngeal cancer-associated
genes
High expression of EGFR, survivin and cyclin D1 are
commonly observed in the development of laryngeal cancer, thus, the
expression patterns of the 3 genes were analyzed to evaluate the
effect of Ad-P16 on Hep2 cells. Compared with untreated cells and
Ad-control cells, the mRNA expression levels of EGFR, survivin and
cyclin D1 were significantly decreased in Ad-P16 cells (P<0.05;
Fig. 5).
Discussion
Laryngeal cancer is a disease in which malignant
cells form in the tissues of the larynx. P16 has been previously
demonstrated to be important in the development of laryngeal
cancer. P16 is a cyclin-dependent kinase-4 inhibitor expressed in
certain normal tissues and tumors (16). P16 is frequently inactivated in
multiple types of human cancer and the major biochemical effect of
P16 is to halt cell-cycle progression at the G1/S boundary. Loss of
P16 function may lead to cancer progression by allowing unregulated
cellular proliferation (4).
Cyclin-dependent kinases 4/6/2 (Cdk4/6/2) are proteins that lead
progression through the G1-S transition and are regulated in the
process of cell proliferation. P16 binds to Cdk4/6 and inhibits
phosphorylation of the retino-blastoma protein, forcing cells to
remain in the G1 phase and therefore, arresting cell division
(17). Thus, the overexpression
and restoration of P16 in cancer cells may be able to inhibit
cancer development. The current study transfected P16 cDNA into
Hep2 cells to restore the expression of P16. The results of western
blot analysis demonstrated that P16 cDNA was successfully
transfected into the cell line.
Cell proliferation is an important process to
evaluate the effect of P16 on cancer cells. The current study
demonstrated that after 3 days of infection with Ad-P16, the cell
proliferation in the experimental group was significantly reduced
compared with the Ad-control-treated cells and untreated cells,
indicating that P16 inhibits cell growth. Nalabothula et al
(18) demonstrated that
restoration of P16 reduces glioma growth in nude mice and
downregulates αvβ3 integrin receptor expression. Furthermore, they
demonstrated that a sense P16 and anti-sense plasminogen activator
urokinase receptor bicistronic construct significantly inhibited
angiogenesis, induced apoptosis by deregulation of the
phosphatidylinositol-4,5-bisphosphate 3-kinase/v-akt murine
thymoma viral oncogene homolog 1 pathway and down-regulated αvβ5
integrin receptor expression. The result was further corroborated
by the tumor formation assay performed in the current study. The
tumor injected with Ad-P16 exhibited reduced growth compared with
those injected with PBS or Ad-control virus. By contrast, the loss
of nuclear P16 protein expression has been demonstrated to be
correlated with increased tumor cell proliferation. Straume et
al (19) demonstrated that the
loss of nuclear P16 protein expression in vertical growth phase
melanomas is associated with increased tumor cell proliferation and
independently predicts decreased patient survival.
Cell invasion assays, based on the invasive and
metastatic abilities of cancer cells, have been widely used to
study the interactions between tumor cells and the extracellular
matrix. In the current study, Matrigel was used to provide
structural support for the cells to grow and move. Cells secrete
enzymes that degrade certain components of the Matrigel to migrate
towards chemoattractants. Due to increased motility and/or
enzymatic activity degrading Matrigel components, metastatic tumor
cells often exhibit increased invasiveness through the Matrigel.
The results of the current study demonstrated that the Ad-P16
transfection inhibited Hep2 cell invasion. The results are
consistent with those from a study by Chintala et al
(12), which demonstrated that
restoring wild-type P16 activity into P16-null SNB19 glioma cells
significantly inhibited tumor cell invasion, thus, suggesting the
P16 gene may inhibit the invasion of multiple tumor cell types.
Liang et al (20) also
demonstrated that reduced glioma cell invasion due to decreased
BMI-1 proto-oncogene was rescued by P16 downregulation, and that
BMI-1 enhances to the motility of glioma cells by regulating the
expression of P16, indicating that P16 is important during tumor
cell invasion (20).
Numerous genes contribute to the development of
cancer. EGFR is a transmembrane tyrosine kinase involved in cell
transformation and tumor progression. High EGFR levels are
associated with poor prognosis in patients with laryngeal cancer
(21,22). Survivin, an anti-apoptotic protein,
is highly expressed in numerous types of cancer and is associated
with chemotherapy resistance, increased tumor recurrence and
shorter patient survival, making anti-survivin therapy an
attractive cancer treatment strategy (23). Cyclin D1 is an important regulator
of cell cycle progression and functions as a transcriptional
co-regulator. The overexpression of cyclin D1 has previously been
associated with the development and progression of cancer (24). Cyclin D1 genotype and protein
expression are important risk markers for laryngeal cancer, and
future trials targeting upstream regulators of cyclin D1
transcription may be useful (25).
The present study evaluated the effect of P16 on 3 genes in tumor
cells. After 72 h infection, Ad-P16-infected Hep2 cells
demonstrated reduced expression of EGFR, survivin and cyclin D1
compared with Ad-control and untreated cells.
In conclusion, a recombinant adenovirus carrying the
P16/gene was used to infect and express high levels of P16 protein
in P16-null Hep2 cells. Cell proliferation, invasion assays and
RT-qPCR were used to evaluate the antitumor effect of P16. The
results demonstrate that restoring wild-type P16 activity into
P16-null carcinoma cells exerts an antitumor effect. This may be
useful in the development of novel anti-tumor therapeutic agents or
gene therapy.
Acknowledgments
The present study is supported by Shandong
Provincial Natural Science Foundation, China (nos. ZR2013HM107 and
ZR2013HQ055).
References
|
1
|
Jemal A, Clegg LX, Ward E, Ries LA, Wu X,
Jamison PM, Wingo PA, Howe HL, Anderson RN and Edwards BK: Annual
report to the nation on the status of cancer, 1975–2001, with a
special feature regarding survival. Cancer. 101:3–27. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. Ca: Cancer J Clin. 65:5–29. 2015.
|
|
3
|
Weinberg RA: pRb and Control of the Cell
Cycle Clock In: The Biology of Cancer. 2nd edition. Garland
Science; New York, NY: pp. 275–329. 2014
|
|
4
|
Liggett WH Jr and Sidransky D: Role of the
p16 tumor suppressor gene in cancer. J Clin Oncol. 16:1197–1206.
1998.PubMed/NCBI
|
|
5
|
Attri J, Srinivasan R, Majumdar S, Radotra
BD and Wig J: Alterations of tumor suppressor gene p16INK4a in
pancreatic ductal carcinoma. BMC Gastroenterol. 5:222005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuen PW, Man M, Lam KY and Kwong YL:
Clinicopathological significance of p16 gene expression in the
surgical treatment of head and neck squamous cell carcinomas. J
Clin Pathol. 55:58–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalfert D, Celakovsky P, Laco J and
Ludvikova M: The role of protein p16 (INK4a) in glottic laryngeal
squamous cell carcinoma. Pathol Oncol Res. 20:909–915. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Craig C, Kim M, Ohri E, Wersto R, Katayose
D, Li Z, Choi YH, Mudahar B, Srivastava S, Seth P and Cowan K:
Effects of adenovirus-mediated p16INK4A expression on cell cycle
arrest are determined by endogenous p16 and Rb status in human
cancer cells. Oncogene. 16:265–272. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piao CQ, Zhao YL and Hei TK: Analysis of
p16 and p21 (Cip1) expression in tumorigenic human bronchial
epithelial cells induced by asbestos. Oncogene. 20:7301–7306. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Zhu J, Man Z, Ao Y and Chen H:
Investigation on the structure and upconversion fluorescence of
Yb3+/Ho3+ co-doped fluorapatite crystals for
potential biomedical applications. Sci Rep. 4:44462014.
|
|
11
|
Ma J, He X, Wang W, Huang Y, Chen L, Cong
W, Gu J, Hu H, Shi J, Li L and Su C: E2F promoter-regulated
oncolytic adenovirus with p16 gene induces cell apoptosis and
exerts antitumor effect on gastric cancer. Dig Dis Sci.
54:1425–1431. 2009. View Article : Google Scholar
|
|
12
|
Chintala SK, Fueyo J, Gomez-Manzano C,
Venkaiah B, Bjerkvig R, Yung WK, Sawaya R, Kyritsis AP and Rao JS:
Adenovirus-mediated p16/CDKN2 gene transfer suppresses glioma
invasion in vitro. Oncogene. 15:2049–2057. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knight BB, Oprea-Ilies GM, Nagalingam A,
Yang L, Cohen C, Saxena NK and Sharma D: Survivin upregulation,
dependent on leptin-EGFR-Notch1 axis, is essential for
leptin-induced migration of breast carcinoma cells. Endocr Relat
Cancer. 18:413–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408
|
|
15
|
Huang YP, Lin IJ, Chen CC, Hsu YC, Chang
CC and Lee MJ: Delivery of small interfering RNAs in human cervical
cancer cells by polyethylenimine-functionalized carbon nanotubes.
Nanoscale Res Lett. 8:2672013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Neill CJ and McCluggage WG: p16
expression in the female genital tract and its value in diagnosis.
Adv Anat Pathol. 13:8–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Villacañas O, Pérez JJ and Rubio-Martínez
J: Structural analysis of the inhibition of Cdk4 and Cdk6 by p16
(INK4a) through molecular dynamics simulations. J Biomol Struct
Dyn. 20:347–358. 2002. View Article : Google Scholar
|
|
18
|
Nalabothula N, Lakka SS, Dinh DH, Gujrati
M, Olivero WC and Rao JS: Sense p16 and antisense uPAR bicistronic
construct inhibits angiogenesis and induces glioma cell death. Int
J Oncol. 30:669–678. 2007.PubMed/NCBI
|
|
19
|
Straume O, Sviland L and Akslen LA: Loss
of nuclear p16 protein expression correlates with increased tumor
cell proliferation (Ki-67) and poor prognosis in patients with
vertical growth phase melanoma. Clin Cancer Res. 6:1845–1853.
2000.PubMed/NCBI
|
|
20
|
Liang J, Wang P, Xie S, Wang W, Zhou X, Hu
J, Shi Q, Zhang X and Yu R: Bmi-1 regulates the migration and
invasion of glioma cells through p16. Cell Biol Int. 39:283–290.
2015. View Article : Google Scholar
|
|
21
|
Maurizi M, Almadori G, Ferrandina G,
Distefano M, Romanini ME, Cadoni G, Benedetti-Panici P, Paludetti
G, Scambia G and Mancuso S: Prognostic significance of epidermal
growth factor receptor in laryngeal squamous cell carcinoma. Br J
Cancer. 74:1253–1257. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lionello M, Lovato A, Staffieri A,
Blandamura S, Turato C, Giacomelli L, Staffieri C and Marioni G:
The EGFR-mTOR pathway and laryngeal cancer angiogenesis. Eur Arch
Otorhinolaryngol. 271:757–764. 2014. View Article : Google Scholar
|
|
23
|
Fukuda S and Pelus LM: Survivin, a cancer
target with an emerging role in normal adult tissues. Mol Cancer
Ther. 5:1087–1098. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alao JP: The regulation of cyclin D1
degradation: Roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Papadimitrakopoulou V, Izzo JG, Liu DD,
Myers J, Ceron TL, Lewin J, William WN Jr, Atwell A, Lee JJ,
Gillenwater A, et al: Cyclin D1 and cancer development in laryngeal
premalignancy patients. Cancer Prev Res (Phila). 2:14–21. 2009.
View Article : Google Scholar
|