Introduction
Obesity is a serious issue worldwide, it is
associated with a marked risk for disease, including risk of
cardiovascular disorders, type 2 diabetes mellitus (T2DM) and
hypertension (1–3). The etiology of obesity is an
imbalance between energy intake and energy consumption (4). As a type of endocrine organ, adipose
tissue contributes to the release of metabolites, including free
fatty acids (FFAs), leptin, resistin, tumor necrosis factor-α
(TNF-α) and interleukin 6 (5,6). The
changes in adipose tissue secretion have been well-illustrated to
associate obesity with IR and inflammatory responses (7,8),
however, the detailed underlying mechanism requires further
elucidation.
MicroRNAs (miRNAs) are a class of non-coding RNAs
(length, 19–22 nt) that post-transcriptionally modulate gene
expression via specifically binding to the 3′-untranslated regions
(3′-UTRs) of target mRNAs (9).
Previous studies have suggested that miRNAs are important in
cellular differentiation and development (10,11),
tumorigenesis (12,13), and metabolic diseases (14). miRNAs, including miR-143 (15), miR-146b (16), and miR-378/378* (17) have also been demonstrated to
modulate adipocyte differentiation in mouse and human cell line
models. Furthermore, miRNA dysregulation has been reported in human
obesity and IR (18,19).
miRNA-199a-3p (miR-199a-3p) is a conserved miRNA in
mice and humans, it has been reported to potentially be important
in T2DM. A meta-analysis performed by Zhu and Leung (20) observed that miR-199a-3p expression
was dysregulated in T2DM patients, presenting a pancreas- and
liver- specific disturbance. In addition, upregulation of
miR-199a-3p expression occurred in islet cells from mouse models of
T2DM (21). Our previous study
identified miR-199a-3p was highly induced in differentiated human
adipose-derived mesenchymal stem cells (hMSCs-Ad) (22). However, the association between the
differential expression of miR-199a-3p in adipocytes and
obesity-associated IR and inflammatory responses remains to be
elucidated.
In the present study, increased expression of
miR-199a-3p in mature human adipocytes (visceral) was observed
compared with pre-adipocytes. In addition, miR-199a-3p expression
was higher in visceral adipose tissues from obese subjects. FFAs,
TNF-α, IL-6 and leptin were observed to significantly induce
miR-199a-3p expression in mature human adipocytes, while resistin
had the opposite effect. miR-199a-3p may exert an effect in
modulation of obesity-associated IR and inflammatory reactions.
Materials and methods
Human subjects
Visceral white adipose tissue samples were obtained
from 12 subjects undergoing surgery for various disorders at
Nanjing Maternity and Child Health Care Hospital Affiliated with
Nanjing Medical University. Patients did not have any notable
conditions, including malignant tumors, severe genetic diseases,
infectious disorders or autoimmune dysregulation. Based on body
mass index (BMI), patients were defined as normal subjects with a
BMI of 18-24 kg/m2 (n=6) and obese subjects with a BMI
of >28 kg/m2 (n=6). Biopsies were maintained in
RNAlater RNA Stabilization reagent (Qiagen, Inc., Valencia, CA,
USA) and maintained at −80°C for total RNA extraction. Prior to
participation in the present study, all patients provided written
informed consent. The current study was approved by the Ethics
Committee of Nanjing Maternity and Child Health Care Hospital
Affiliated to Nanjing Medical University (Nanjing, China).
Cell culture
Human pre-adipocytes from visceral (omental) adipose
were purchased from ScienCell Research Laboratories (Carlsbad, CA,
USA). Pre-adipocytes were maintained and induced to differentiate
as previously described (23).
Briefly, pre-adipocyte differentiation medium (cat. no. 7221;
ScienCell Research Laboratories) containing fetal bovine serum
(5%), growth supplement (1%) and penicillin/streptomycin solution
(1%) was used as basal medium for cell expansion. Upon reaching
confluence, cells were exposed to induction medium for the first 4
days and shifted to differentiation medium until the appearance of
mature lipid droplets was observed (15 days). The induction medium
contained pre-adipocyte medium without serum, supplemented with 500
µM 3-isobutyl-L-methylxanthine (Sigma-Aldrich, St. Louis,
MO, USA), 100 nM dexamethasone (Sigma-Aldrich), 50 nM insulin
(Sigma-Aldrich) and 100 µM rosiglitazone (Sigma-Aldrich).
The differentiation medium was serum-free pre-adipocyte medium
containing 50 nM insulin.
Oil red O staining
Following induction on day 4 and day 15, adipocytes
were washed with phosphate-buffered saline (PBS) three times. They
were fixed in 4% paraformaldehyde for 30 min prior to washing again
with PBS. Lipid accumulation was measured by staining with 0.2%
(m/v) oil red O (Sigma-Aldrich) dissolved in isopropanol for 30 min
at 37°C. The cells were examined by fluorescence microscopy
(ImagingA1; Carl Zeiss AG, Oberkochen, Germany).
Treatment of adipocytes with adipokines
and FFAs
After 15 days induction of differentiation, >85%
of the adipocytes exhibited lipid droplet accumulation. Following
serum starvation for 12 h, the human adipocytes were treated with a
1 mM FFA cocktail and adipokines, including 10 ng/ml TNF-α
(Sigma-Aldrich), 30 ng/ml IL-6 (Sigma-Aldrich), 100 ng/ml leptin
(Sigma-Aldrich) and 60 ng/ml resistin (Sigma-Aldrich) for different
durations (4, 8 and 24 h) (24–26),
then cells were treated with TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for the following
experiment.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from adipose tissues and
mature adipocytes using an RNeasy Mini kit (Qiagen). Concentration
and purity of extracted total RNA were quantified using a Nanodrop
spectrophotometer 2000 (Thermo Fisher Scientific, Inc.). For
analysis of mature miRNA quantification, 200 ng total RNA was
reverse transcribed using TaqMan miRNA Reverse Transcriptase kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and the
following temperature conditions: 16°C for 30 min, 42°C for 30 min,
85°C for 5 min, 4°C. qPCR was performed using TaqMan Universal
Master Mix II, no UNG (Thermo Fisher Scientific, Inc.) and
commercial primers for miR-199a-3p, miR-103 and U6 (cat. no.
A25576; Thermo Fisher Scientific, Inc.) on an ABI 7500 real-time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols as previously described
(16). Briefly, samples were
incubated at 95°C for 10 min for an initial denaturation, followed
by 40 cycles consisting of incubation at 95°C for 15 sec and 60°C
for 1 min. The expression of miR-199a-3p was normalized to U6 small
nucleolar RNA or miR-103 expression (27) and the fold change was calculated
using the 2−ΔΔCq method (28).
Statistical analysis
The data are presented as mean ± standard deviation.
Differences between groups were analyzed by Student's two-tailed
t-test when only two groups were present or by one way analysis of
variance and Bonferroni correction for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of miR-199a-3p increased
during human adipocyte differentiation
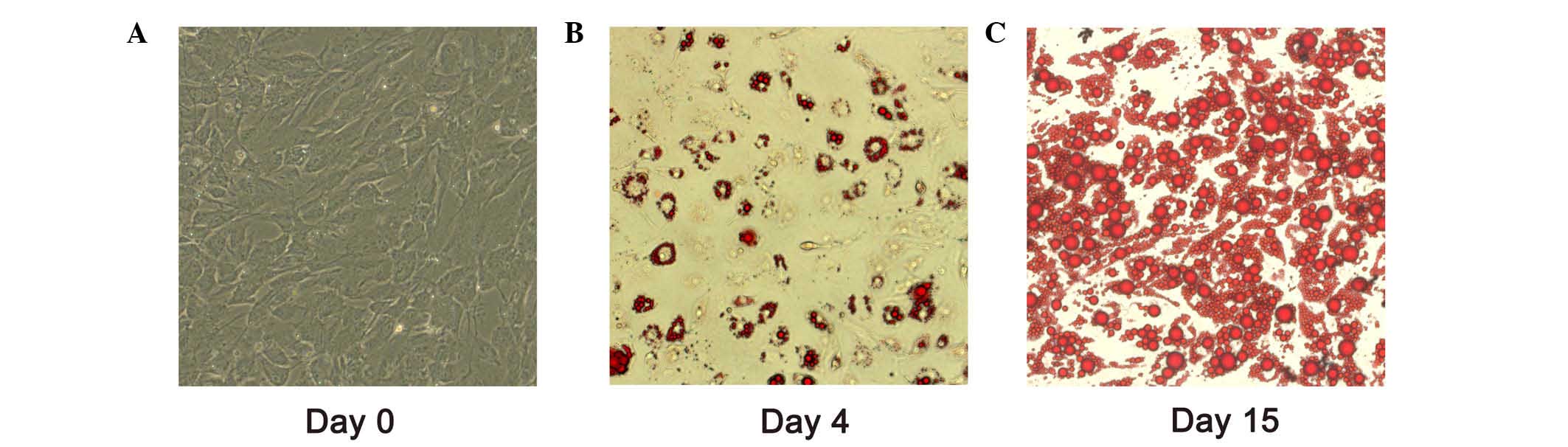
For adipocyte differentiation, cells were grown to
confluence as day 0 (Fig. 1A).
Adipocytes differentiated for 4 and 15 days exhibited oil red O
staining (Fig. 1B and C). After 15
days induction of differentiation, >80% of the adipocytes were
observed with lipid droplet accumulation. miR-199a-3p expression
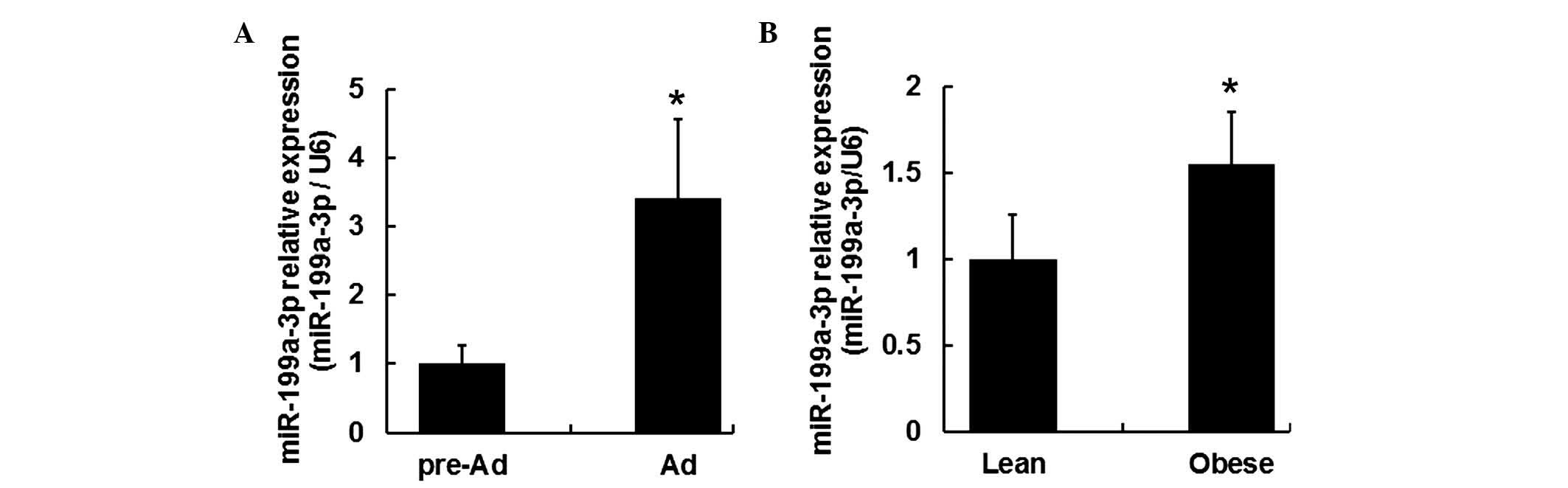
between pre-adipocytes (day 0) and mature adipocytes (day 15) was
evaluated by RT-qPCR. It was demonstrated the expression level of
miR-199a-3p in mature adipocytes increased ~3.4 fold change
compared with that in human pre-adipocytes (Fig. 2A; P<0.05).
Upregulation of miR-199a-3p was observed
in visceral fat tissues from obese human subjects
To determine the role of miR-199a-3p within obese
subjects, miR-199a-3p expression levels were measured in the
visceral fat tissues of lean and obese human subjects. Visceral fat
tissues samples were acquired from 6 lean and 6 obese human
subjects (Table I). RT-qPCR
results indicated the miR-199a-3p expression levels were
upregulated in the visceral fat tissue samples from obese subjects
(Fig. 2B; P<0.05).
 | Table IVariables between obese and lean
subjects. |
Table I
Variables between obese and lean
subjects.
| Variable (mean ±
SD) | Obese group
(n=6) | Lean group
(n=6) |
|---|
| Age (year) | 50.33±18.27 | 46.23±11.41a |
| Weight (kg) | 85.50±20.95 | 56.50±10.28b |
| Height (m) | 1.64±0.04 | 1.69±0.06a |
| BMI
(kg/m2) | 31.38±6.30 | 20.42±3.52b |
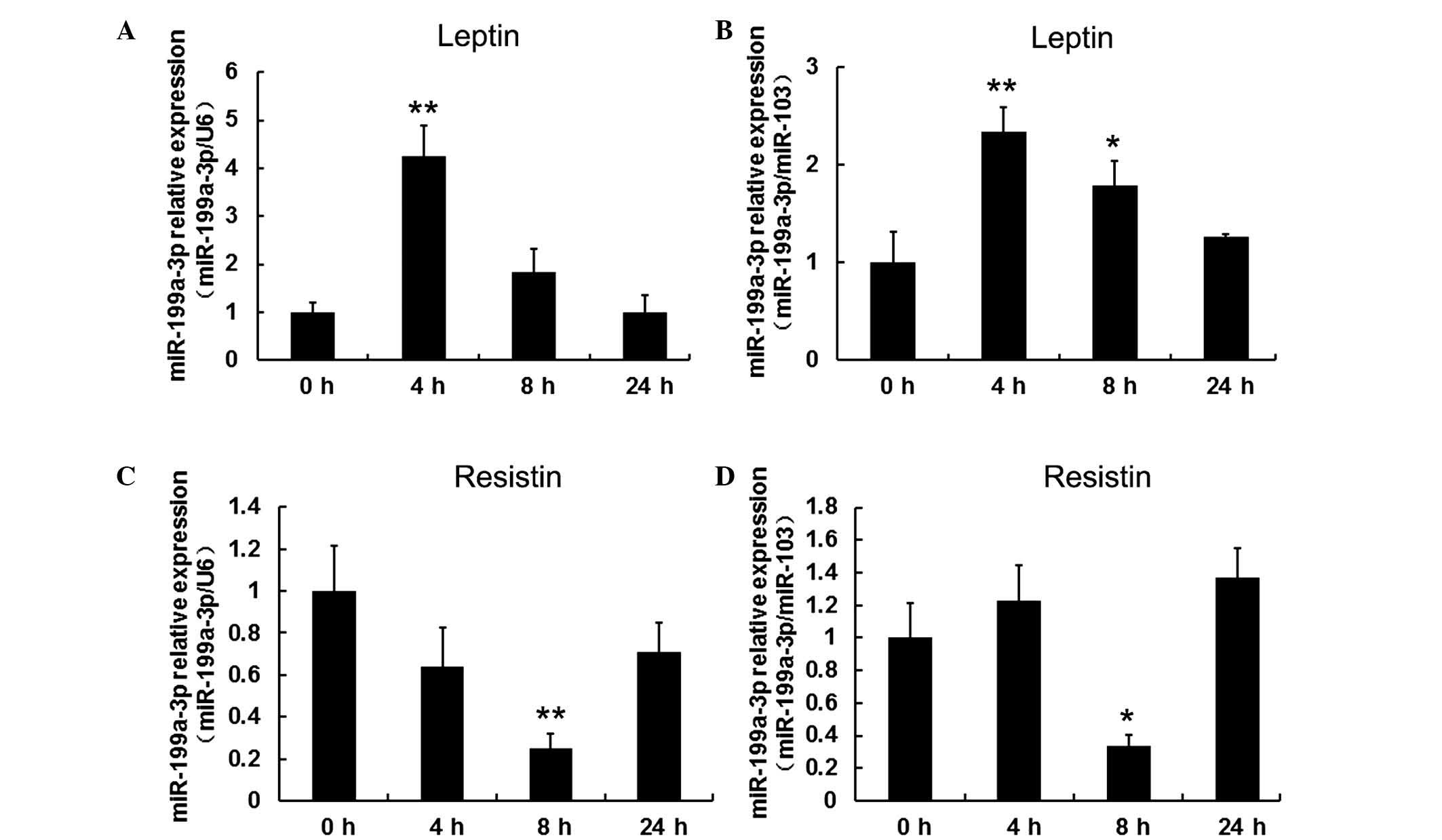
Leptin initially increased and resistin
decreased miR-199a-3p expression levels in human differentiated
adipocytes
To investigate the effect of leptin and resistin on
miR-199a-3p, mature adipocytes, following 15 days induced
differentiation, were treated with 100 ng/ml leptin and collected
at 4, 8 and 24 h. Cells without treatment served as the control
group (defined as 0 h). miR-103 and U6 served as reference genes
for normalization in order to obtain consistent results. It was
observed that leptin significantly stimulated miR-199a-3p
expression at 4 h (P<0.01) but this effect decreased over time
(Fig. 3A and B). By contrast,
exposure of mature adipocytes to 60 ng/ml resistin resulted in a
significant decrease in miR-199a-3p expression at 8 h (P<0.05
when normalized to U6, P<0.01 when normalized to miR-103;
Fig. 3C and D).
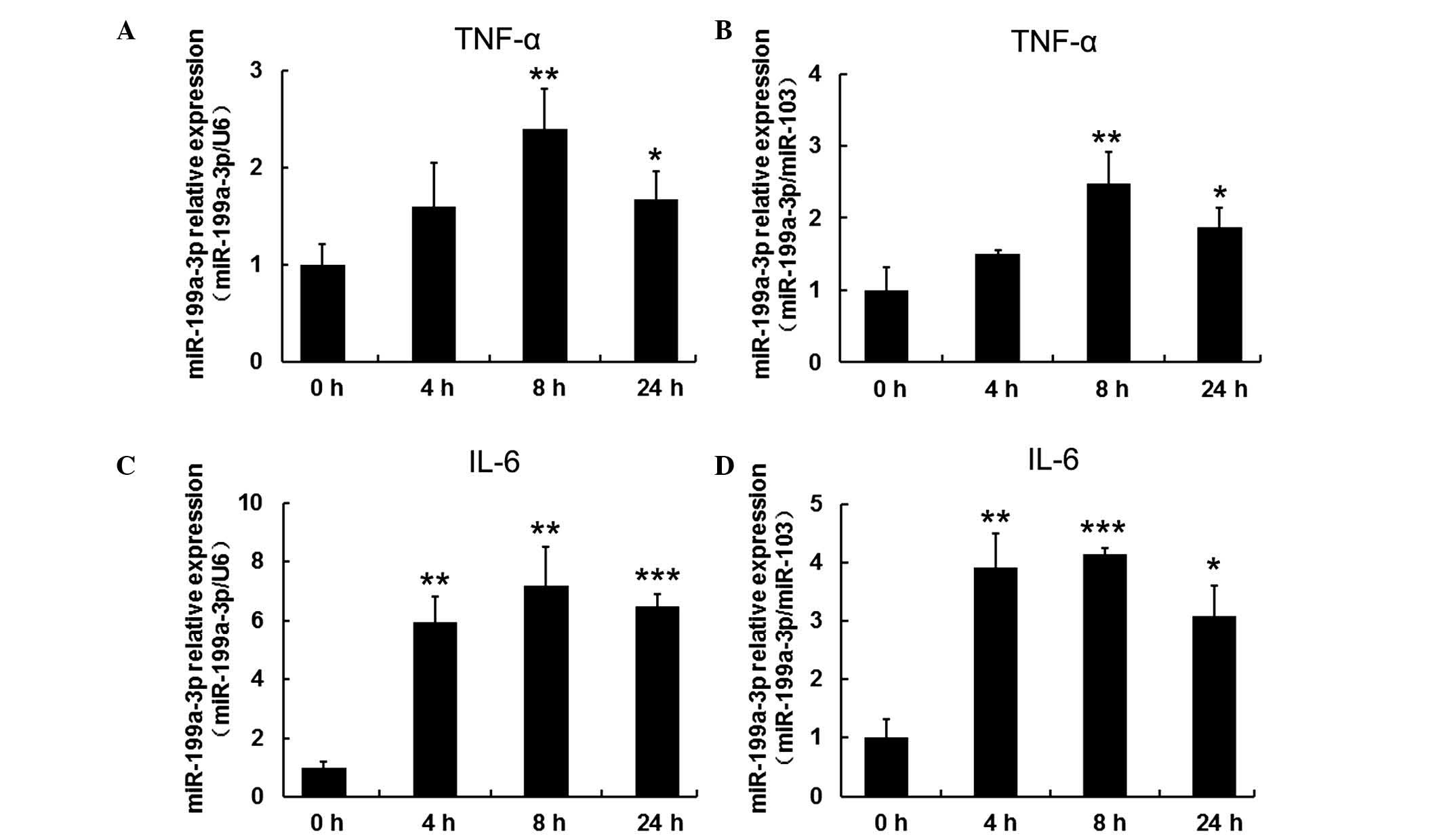
TNF-α and IL-β upregulated expression of
miR-199a-3p in human mature adipocytes
To investigate the response of miR-199-3p to
inflammatory cytokines, including TNF-α and IL-β, mature human
adipocytes that had been differentiated for 15 days were treated
with 10 ng/ml TNF-α and 30 ng/ml IL-β and harvested at different
time points (4, 8 and 24 h). Cells without treatment served as the
control group (defined as 0 h). miR-103 and U6 expression were used
as reference genes for normalization in order to obtain consistent
results. RT-qPCR results indicated that miR-199a-3p expression was
gradually increased when mature human adipocytes were exposed to 10
ng/ml TNF-α, reaching a maximum at 8 h (P<0.01). The
upregulation was sustained to 24 h (P<0.05; Fig. 4A and B). IL-β treatment exhibited a
similar trend in mir-199a-3p expression (P<0.01 at 4 h,
P<0.01 when normalized to U6 and P<0.001 when normalized to
miR-103 at 8 h, P<0.001 when normalized to U6 and P<0.05 when
normalized to miR-103; Fig. 4C and
D).
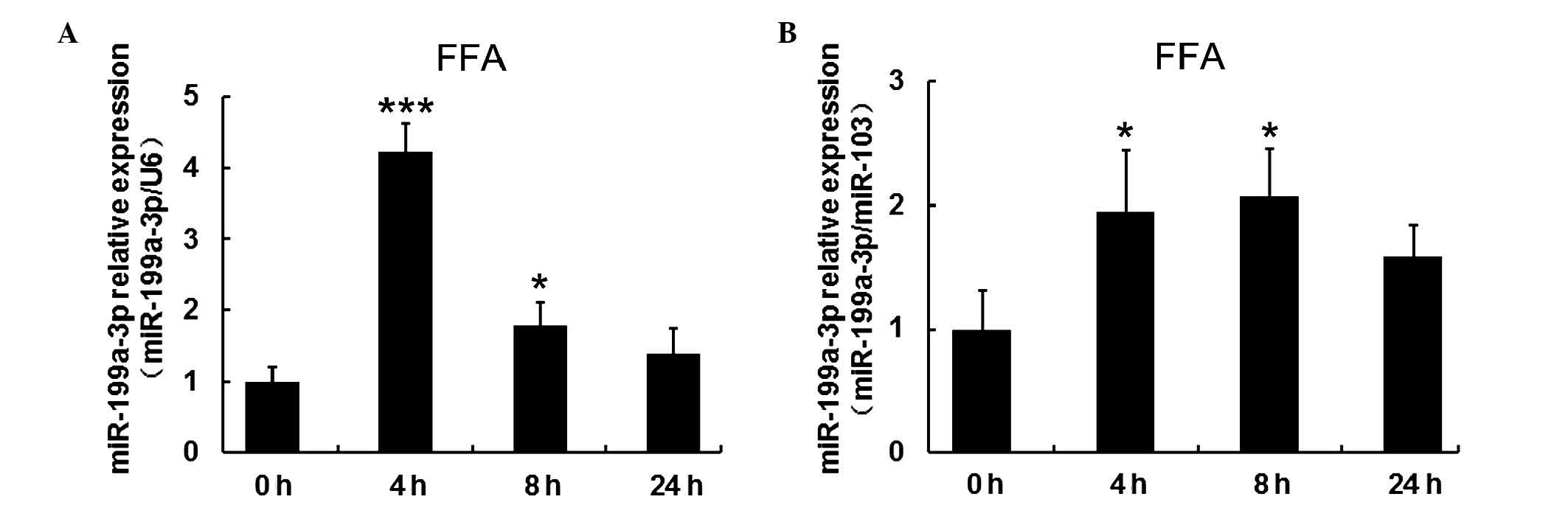
FFAs increased expression of miR-199a-3p
in human mature adipocytes
To investigate the effect of FFAs on miR-199a-3p
expression, mature human adipocytes following 15 days induced
differentiation, were treated with 1 mM FFA at different time
points (4, 8 and 24 h). Cells without treatment served as the
control group (defined as 0 h). To confirm the regulation by FFAs,
miR-103 and U6 expression were reference genes for normalization.
RT-qPCR results indicated miR-199a-3p expression was significantly
increased (P<0.001 when normalized to U6 and P<0.05 when
normalized to miR-103 at 4 h) within 8 h, but decreased again over
time (Fig. 5A and B).
Discussion
Altered adipose tissue function is observed between
normal and obese individuals. The dysregulation of adipose tissue
in obese subjects is commonly characterized by the following
features (6,29): i) Adipocyte hypertrophy; ii)
ectopic fat accumulation; and iii) adipose tissue inflammation. The
alteration of fat tissue function exerts a key effect in the
development of IR and T2DM (8).
However, understanding of the underlying mechanisms linking obesity
and adipose tissue dysfunction to IR requires further research.
miR-199a-3p, which is commonly identified as a
suppressor gene, has been observed to be involved in the
progression of various types of cancer, including hepatocellular
carcinoma (30), osteosarcoma
(31), ovarian carcinoma (32) and thyroid carcinoma (33). In addition, previous studies have
demonstrated the potential implication of miR-199a-3p in T2DM
(20,21). However, the differential
miR-199a-3p expression during adipocyte differentiation and the
progression obesity has not been fully elucidated from experimental
studies in animal models or in humans.
hMSCs-Ad are a well-characterized type of adult stem
cell that may differentiate into adipocytes, osteoblasts and
chondrocytes under various conditions (34). Use of monoclonal antibodies and
cell sorting on the basis of specific markers (35), resulted in obtaining a common early
precursor of MSC and adipocytes that was termed human
pre-adipocyte, which differentiates into mature adipocytes under
suitable stimulatory condition (36). Using pre-adipocytes for in
vitro experiments is a effective strategy as they most closely
resemble the physiological situation in vivo. The present
study used human pre-adipocytes as a model to examine miR-199a-3p
expression during differentiation and the results of the current
study demonstrated miR-199a-3p expression was higher in mature
adipocytes compared with pre-adipocytes. This trend was consistent
with our previous study in hMSCs-Ad (22). It was also observed that the
expression level of miR-199a-3p increased in the visceral fat
tissue samples from obese subjects relative to the lean subjects
(Fig. 2B). Further experiments are
required to validate the function of miR-199a-3p in human adipocyte
differentiation and the progression of obesity.
Among adipokines secreted by adipose tissue, leptin
is the best characterized (37).
The predominant function of leptin is to control adipose tissue
growth, reduce appetite and increase energy consumption (38,39).
By contrast, leptin plasma concentration and mRNA expression levels
in adipose tissue were elevated in obese individuals (40). The high dose of leptin levels
directly results in IR in the liver and adipose tissue (41). Resistin, another adipocyte derived
factor, was induced in adipogenesis and in genetic and diet-induced
obesity (42). Previous studies
have demonstrated the role of resistin in the modulation of the
insulin signaling pathway and that resistin contributes to IR and
T2DM (43,44). In the present study, it was
observed that exposure of mature adipocytes to 100 ng/ml leptin
resulted in a significant upregulation of miR-199a-3p at 4 h,
however, this effect decreased over time. By contrast, resistin has
a negative effect on miR-199a-3p expression. These observations
demonstrated that miR-199a-3p was involved in obesity-associated
IR.
Pro-inflammatory adipokines, including TNF-α and
IL-6 commonly participate in different inflammatory and autoimmune
diseases (45,46). The level of TNF-α is increased in
chronic obesity and it has been demonstrated to impair insulin
secretion and induce IR (47).
Moderate elevation of IL-6 levels has also been observed in obese
individuals (48), and it may be a
contributing factors in the development of obesity-associated
diseases, including IR and T2DM (49). In the current study, miR-199a-3p
expression was increased following TNF-α or IL-6 exposure and
reached the maximum at 8 h, further indicating an association
between miR-199a-3p and pro-inflammatory adipokines in the
development of obesity-associated IR.
It is well-known that an increase in basal lipolysis
is observed and results in the increased free fat acid release
during obesity (50). The excess
free fatty acid in circulation induces ectopic accumulation in
non-adipose tissues and, in turn, alters the secretory pattern of
adipose tissue (51). Increased
free fatty acid level may lower insulin sensitivity, resulting in
IR and T2DM development (52). In
the present study, miR-199a-3p increased significantly at 8 h,
though this decreased over time. This observation may indicate the
negative role of miR-199a-3p in the regulation of insulin
sensitivity of adipocytes.
miRNAs modulate genes expression by specifically
binding to the 3′-UTRs of target mRNAs. By usage of online tools
including TargetScan (www.targetscan.org), miRanda (www.microrna.org) and miRDB (www.mirdb.org), three candidate genes were selected
for further research. These were NLK (nemo-like kinase), VAMP3
(vesicle associated membrane protein 3) and Cdk7 (cyclin-dependent
kinase 7), which are reported to be associated with obesity,
adipocyte differentiation and IR (53–55).
However, the association between the potential target mRNAs and
miR-199a-3p requires further validation by luciferase reporter
assays.
In conclusion, the present study identified
miR-199a-3p as an obesity-associated miRNA induced during adipocyte
adipogenesis. The effective response of miR-199a-3p expression to
different adipokines and FFAs in mature adipocytes indicated it was
associated with obesity-associated IR. However, additional studies
are required to elucidate the role of miR-199a-3p in adipogenesis
and its contribution to the development of obesity.
Acknowledgments
The present study was supported by grants from the
National Key Basic Research Program of China (grant no.
2013CB530604), the Key project of the National Natural Science
Foundation of China (grant no. 81330067), the National Natural
Science Foundation of China (grant nos. 81300683, 81300706 and
81500649), the Program for Innovative Research Teams of Jiangsu
Province (grant no. LJ201108), Key Project supported by Medical
Science and Technology Development Foundation Nanjing Department of
Health (grant nos. YKK13141 and JQX13012).
References
|
1
|
Van Gaal LF, Mertens IL and De Block CE:
Mechanisms linking obesity with cardiovascular disease. Nature.
444:875–880. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dorresteijn JA, Visseren FL and Spiering
W: Mechanisms linking obesity to hypertension. Obes Rev. 13:17–26.
2012. View Article : Google Scholar
|
|
3
|
Bell JA, Kivimaki M and Hamer M:
Metabolically healthy obesity and risk of incident type 2 diabetes:
A meta-analysis of prospective cohort studies. Obes Rev.
15:504–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cummings DE and Schwartz MW: Genetics and
pathophysiology of human obesity. Annu Rev Med. 54:453–471. 2003.
View Article : Google Scholar
|
|
5
|
Morigny P, Houssier M, Mouisel E and
Langin D: Adipocyte lipolysis and insulin resistance. Biochimie.
2015.Epub ahead of print. PubMed/NCBI
|
|
6
|
Fasshauer M and Blüher M: Adipokines in
health and disease. Trends Pharmacol Sci. 36:461–470. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bluher M: Adipose tissue dysfunction
contributes to obesity related metabolic diseases. Best Pract Res
Clin Endocrinol Metab. 27:163–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guilherme A, Virbasius JV, Puri V and
Czech MP: Adipocyte dysfunctions linking obesity to insulin
resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 9:367–377.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kusakabe R and Inoue K: Developmental
regulation and evolution of muscle-specific microRNAs. Semin Cell
Dev Biol. 47–48:9–16. 2015. View Article : Google Scholar
|
|
11
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun M, Liu XH, Li JH, Yang JS, Zhang EB,
Yin DD, Liu ZL, Zhou J, Ding Y, Li SQ, et al: MiR-196a is
upregulated in gastric cancer and promotes cell proliferation by
downregulating p27(kip1). Mol Cancer Ther. 11:842–852. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung KH, Zhang J, Zhou C, Shen H, Gagea M,
Rodriguez-Aguayo C, Lopez-Berestein G, Sood AK and Beretta L:
Differentiation therapy for hepatocellular carcinoma: Multifaceted
effects of miR-148a on tumor growth and phenotype and liver
fibrosis. Hepatology. 63:864–879. 2016. View Article : Google Scholar :
|
|
14
|
Thomas H: Diabetes: Enterovirus
dysregulates islet miRNAs. Nat Rev Endocrinol. 12:22016.
|
|
15
|
Esau C, Kang X, Peralta E, Hanson E,
Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, et
al: MicroRNA-143 regulates adipocyte differentiation. J Biol Chem.
279:52361–52365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Dai YM, Ji CB, Yang L, Shi CM, Xu
GF, Pang LX, Huang FY, Zhang CM and Guo XR: MiR-146b is a regulator
of human visceral preadipocyte proliferation and differentiation
and its expression is altered in human obesity. Mol Cell
Endocrinol. 393:65–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gerin I, Bommer GT, McCoin CS, Sousa KM,
Krishnan V and MacDougald OA: Roles for miRNA-378/378* in adipocyte
gene expression and lipogenesis. Am J Physiol Endocrinol Metab.
299:E198–E206. 2010.PubMed/NCBI
|
|
18
|
Martinelli R, Nardelli C, Pilone V,
Buonomo T, Liguori R, Castanò I, Buono P, Masone S, Persico G,
Forestieri P, et al: miR-519d overexpression is associated with
human obesity. Obesity (Silver Spring). 18:2170–2176. 2010.
View Article : Google Scholar
|
|
19
|
Williams MD and Mitchell GM: MicroRNAs in
insulin resistance and obesity. Exp Diabetes Res. 2012:4846962012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu H and Leung SW: Identification of
microRNA biomarkers in type 2 diabetes: A meta-analysis of
controlled profiling studies. Diabetologia. 58:900–911. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nesca V, Guay C, Jacovetti C, Menoud V,
Peyot ML, Laybutt DR, Prentki M and Regazzi R: Identification of
particular groups of microRNAs that positively or negatively impact
on beta cell function in obese models of type 2 diabetes.
Diabetologia. 56:2203–2012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi C, Zhang M, Tong M, Yang L, Pang L,
Chen L, Xu, Chi X, Hong Q, Ni Y, et al: miR-148a is associated with
obesity and modulates adipocyte differentiation of mesenchymal stem
cells through Wnt signaling. Sci Rep. 5:99302015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang L, Shi CM, Chen L, Pang LX, Xu GF, Gu
N, Zhu LJ, Guo XR, Ni YH and Ji CB: The biological effects of
hsa-miR-1908 in human adipocytes. Mol Biol Rep. 42:927–935. 2015.
View Article : Google Scholar
|
|
24
|
Wellen KE, Fucho R, Gregor MF, Furuhashi
M, Morgan C, Lindstad T, Vaillancourt E, Gorgun CZ, Saatcioglu F
and Hotamisligil GS: Coordinated regulation of nutrient and
inflammatory responses by STAMP2 is essential for metabolic
homeostasis. Cel. 129:537–548. 2007. View Article : Google Scholar
|
|
25
|
Kralisch S, Klein J, Lossner U, Bluher M,
Paschke R, Stumvoll M and Fasshauer M: Interleukin-6 is a negative
regulator of visfatin gene expression in 3T3-L1 adipocytes. Am J
Physiol Endocrinol Metab. 289:E586–E590. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi C, Zhu L, Chen X, Gu N, Chen L, Zhu L,
Yang L, Pang L, Guo X, Ji C and Zhang C: IL-6 and TNF-α induced
obesity-related inflammatory response through transcriptional
regulation of miR-146b. J Interferon Cytokine Res. 34:342–348.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Neville MJ, Collins JM, Gloyn AL, McCarthy
MI and Karpe F: Comprehensive human adipose tissue mRNA and
microRNA endogenous control selection for quantitative
real-time-PCR normalization. Obesity (Silver Spring). 19:888–892.
2011. View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Leal VO and Mafra D: Adipokines in
obesity. Clin Chim Acta. 419:87–94. 2013. View Article : Google Scholar
|
|
30
|
Fornari F, Milazzo M, Chieco P, Negrini M,
Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L and Gramantieri
L: MiR-199a-3p regulates mTOR and c-Met to influence the
doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res.
70:5184–5193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao Y, Milazzo M, Chieco P, Negrini M,
Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L and Gramantieri
L: CD44 is a direct target of miR-199a-3p and contributes to
aggressive progression in osteosarcoma. Sci Rep. 5:113652015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kinose Y, Sawada K, Nakamura K, Sawada I,
Toda A, Nakatsuka E, Hashimoto K, Mabuchi S, Takahashi K, Kurachi
H, et al: The hypoxia-related microRNA miR-199a-3p displays tumor
suppressor functions in ovarian carcinoma. Oncotarget.
6:11342–11856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Minna E, Sawada K, Nakamura K, Sawada I,
Toda A, Nakatsuka E, Hashimoto K, Mabuchi S, Takahashi K, Kurachi
H, et al: miR-199a-3p displays tumor suppressor functions in
papillary thyroid carcinoma. Oncotarget. 5:2513–2528. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gesta S, Tseng YH and Kahn CR:
Developmental origin of fat: Tracking obesity to its source. Cell.
131:242–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boeuf S, Klingenspor M, Van Hal NL,
Schneider T, Keijer J and Klaus S: Differential gene expression in
white and brown preadipocytes. Physiol Genomics. 7:15–25. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bluher M and Mantzoros CS: From leptin to
other adipokines in health and disease: Facts and expectations at
the beginning of the 21st century. Metabolism. 64:131–145. 2015.
View Article : Google Scholar
|
|
38
|
Jéquier E: Leptin signaling, adiposity,
and energy balance. Ann N Y Acad Sci. 967:379–388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahima RS, Prabakaran D, Mantzoros C, Qu D,
Lowell B, Maratos-Flier E and Flier JS: Role of leptin in the
neuroendocrine response to fasting. Nature. 382:250–252. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Considine RV, Sinha MK, Heiman ML,
Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee
LJ, Bauer TL, et al: Serum immunoreactive-leptin concentrations in
normal-weight and obese humans. N Engl J Med. 334:292–295. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Müller G, Ertl J, Gerl M and Preibisch G:
Leptin impairs metabolic actions of insulin in isolated rat
adipocytes. J Biol Chem. 272:10585–10593. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Steppan CM, Bailey ST, Bhat S, Brown EJ,
Banerjee RR, Wright CM, Patel HR, Ahima RS and Lazar MA: The
hormone resistin links obesity to diabetes. Nature. 409:307–312.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen BH, Song Y, Ding EL, Roberts CK,
Manson JE, Rifai N, Buring JE, Gaziano JM and Liu S: Circulating
levels of resistin and risk of type 2 diabetes in men and women:
Results from two prospective cohorts. Diabetes Care. 32:329–334.
2009. View Article : Google Scholar :
|
|
44
|
Sheng CH, Di J, Jin Y, Zhang YC, Wu M, Sun
Y and Zhang GZ: Resistin is expressed in human hepatocytes and
induces insulin resistance. Endocrine. 33:135–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Akira S, Taga T and Kishimoto T:
Interleukin-6 in biology and medicine. Adv Immunol. 54:1–78. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lai Y and Dong C: Therapeutic antibodies
that target inflammatory cytokines in autoimmune diseases. Int
Immunol. 28:181–188. 2016. View Article : Google Scholar
|
|
47
|
Hotamisligil GS, Shargill NS and
Spiegelman BM: Adipose expression of tumor necrosis factor-alpha:
Direct role in obesity-linked insulin resistance. Science.
259:87–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roytblat L, Rachinsky M, Fisher A,
Greemberg L, Shapira Y, Douvdevani A and Gelman S: Raised
interleukin-6 levels in obese patients. Obes Res. 8:673–675. 2000.
View Article : Google Scholar
|
|
49
|
Eder K, Baffy N, Falus A and Fulop AK: The
major inflammatory mediator interleukin-6 and obesity. Inflamm Res.
58:727–736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Boden G: Role of fatty acids in the
pathogenesis of insulin resistance and NIDDM. Diabetes. 46:3–10.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gustafson B, Gogg S, Hedjazifar S,
Jenndahl L, Hammarstedt A and Smith U: Inflammation and impaired
adipogenesis in hypertrophic obesity in man. Am J Physiol
Endocrinol Metab. 297:E999–E1003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Boden G: Obesity, insulin resistance and
free fatty acids. Curr Opin Endocrinol Diabetes Obes. 18:139–143.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pei YF, Zhang L, Liu Y, Li J, Shen H, Liu
YZ, Tian Q, He H, Wu S, Ran S, et al: Meta-analysis of genome-wide
association data identifies novel susceptibility loci for obesity.
Hum Mol Genet. 23:820–830. 2014. View Article : Google Scholar :
|
|
54
|
Maier VH, Melvin DR, Lister CA, Chapman H,
Gould GW and Murphy GJ: v- and t-SNARE protein expression in models
of insulin resistance: Normalization of glycemia by rosiglitazone
treatment corrects overexpression of cellubrevin,
vesicle-associated membrane protein-2 and syntaxin 4 in skeletal
muscle of Zucker diabetic fatty rats. Diabetes. 49:618–625. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Helenius K, Yang Y, Alasaari J and Mäkelä
TP: Mat1 inhibits peroxisome proliferator-activated receptor
gamma-mediated adipocyte differentiation. Mol Cell Biol.
29:315–323. 2009. View Article : Google Scholar
|