Introduction
The immune response to microbial pathogens relies on
innate and adaptive components (1,2). The
innate and adaptive immune responses are lowered in diseases
associated with immunodeficiency (3,4).
Deficiency in minerals and vitamins induces the attenuation of
immune functions, including phagocytic activity, natural killer
cell activity, delayed type hypersensitivity, antigen-specific
antibody production and T cell proliferation (5). Immunomodulatory agents aid in
improving the immune response against pathogens by activating
immune cells (6). The innate
immune response is mediated predominantly by immune cells,
including neutrophils and macrophages. Macrophages are cells in the
host defense system, which inhibit the invasion of microorganisms
and foreign materials through phagocytic activities, and induce
additional adaptive immune responses by synthesizing various
inflammatory mediators and cytokines, including nitric oxide (NO)
and tumor necrosis factor (TNF) -α (1,6,7). NO
is synthesized by inducible NO synthase (iNOS) (8). The expression levels of TNF-α and
iNOS are increased by the translocation of nuclear factor-κB
(NF-κB) to the nucleus and the degradation of inhibitor of NF-κB
(IκB) (9).
T cells are also important in immune functions. In
particular, helper T cells (Th cells) have two subsets, Th1 and Th2
(10). Th1 cells produce Th1
cytokines, including interferon (IFN) -γ, interleukin (IL) -2 and
TNF-α, which increase cell-mediated immunity. Th2 cytokines
released from Th2 cells and promote the humoral antibody-mediated
immune response (11). T cell
deficiency causes acquired immune deficiency syndrome (12).
Bamboo salt (BS) is a processed salt, produced
according to a traditional recipe using sun-dried salt and bamboo
in Korea. BS is known to have therapeutic effects in the treatment
of diseases, including viral disease, dental plaque, diabetes,
circulatory organ disorders, cancer, inflammatory disorders,
allergic rhinitis and cisplatin-induced ototoxicity (13,14).
Compared with sun-dried salts, BS has a lower toxicity and a higher
content of iron, silicon, potassium and phosphate (15–17).
In addition, BS contains hydrogen sulfide (H2S), which
is not contained in sun-dried salts. H2S is an
endogenous gaseous signaling molecule involved in diverse
biological processes, including inflammatory responses, energy
metabolism, cell proliferation, apoptosis and oxidative stress
(18).
In the present study, the effects of BS and sodium
hydrosulfide (NaSH; a H2S donor) on the production of
TNF-α and activation of NF-κB were examined in RAW264.7 cells, a
macrophage-like cell line. Furthermore, the immune-enhancing
effects of BS and NaSH were investigated in a forced swimming test
(FST) animal model.
Materials and methods
Cell culture
The RAW264.7 cells were grown in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% heat inactivated fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.,) and 1%
penicillin-streptomycin at 37°C in 5% CO2 and 95% air.
The RAW264.7 cells (3×105; Korean Cell Line Bank, Seoul,
Korea) were treated with BS (1 mg/ml; Hongik Bio, Damyang, Korea),
NaSH (0.01, 0.1 and 1 µg/ml; Samchun Pure Chemical Co.,
Ltd., Pyeongtaek, Korea) or lipopolysaccharide (LPS; 10
µg/ml; Sigma-Aldrich, St. Louis, MO, USA) for 24 h at 37°C
in 5% CO2 and 95% air. The BS and NaSH were dissolved in
distilled water (D.W.). The concentrations of BS (1 mg/ml) and NaSH
(0.01, 0.1 and 1 µg/ml) were selected in accordance with
previous reports (13,19).
Enzyme-linked immunosorbent assay
(ELISA)
The levels of TNF-α IFN-γ, and IL-2 cytokines were
measured using ELISA, which was performed, as described previously
(20). The plates were read at 405
nm by a microplate reader.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Using an Easy-BLUETM RNA extraction kit (iNtRON
Biotech, Sungnam, Korea), total RNA was isolated from the RAW264.7
cells, according to the manufacturer's protocol. The concentration
of total RNA in the final elutes was determined by NanoDrop (Thermo
Scientific, Inc.). Total RNA (2.5 µg) was heated at 65°C for
10 min and then chilled on ice. Each sample was reverse-transcribed
to cDNA for 90 min at 37°C using a cDNA synthesis kit (Bioneer
Corporation, Daejeon, Korea). The polymerase chain reaction (PCR)
was performed in a C1000 Touch Thermal Cycler (Bio-Rad
Laboratories, Hercules, CA, USA) with the following primers: Mouse
TNF-α, forward 3′-TACAGGCTTGTCACTCGAAT-3′ and reverse
5′-ATGAGCACAGAAAGCATGAT-3′; actin, forward 5′-GTGGGCCGCTAGGCACCA-3′
and reverse 5′-CGGTTGGCCTTAGGGTTCAGGGGGG-3′. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used to verify whether equal
amounts of RNA were used for reverse transcription and
amplification from different experimental conditions. PCR was
conducted under the following conditions: 94°C for 5 min, 94°C for
45 sec, 60°C (TNF-α) or 62°C (GAPDH) for 45 sec and 72°C for 2 min,
for 39 cycles; the dinal cycle was followed by an extension for 5
min at 72°C. Products were electrophoresed on a 1.5% agarose gel
and visualized by staining with ethidium bromide.
Measurement of nitrite concentration
The RAW264.7 cells were stimulated with BS (1
mg/ml), NaSH (0.01, 0.1 and 1 µg/ml) or LPS for 48 h. The
concentrations of NO in the cell cultures were measured using a
Griess method, as previously described (9).
MTT assay
Cell viabilities were assessed using an MTT assay.
Briefly, 500 µl of the RAW 264.7 cell (3×105)
suspension was treated with BS or NaSH for 24 h, followed by
treatment with MTT solution (5 mg/ml) at 37°C for 4 h. The
insoluble formazan product was dissolved in dimethyl sulfoxide and,
the optical density was measured using an ELISA reader at 540
nm.
Western blot analysis
The RAW264.7 cells were stimulated with BS, NaSH or
LPS for 1 h. The cell extracts were heated at 95°C for 5 min and
briefly cooled on ice. Cell extracts were prepared by a detergent
lysis procedure. Cells were scraped, washed once with
phosphate-buffered saline (PBS) and resuspended in the
radioimmunoprecipitation assay lysis buffer containing 10 mM
Tris-HCL pH 7.4, 30 mM NaCl, 1 mM EDTA, 1% Nonidet P-40,
supplemented with 1 mM Na3VO4, 1 µg/ml
leupeptin, 1 µg/ml pepstatin A, 1 µg/ml aprotinin and
1 mM PMSF. Samples were vortexed for lysis for a few seconds every
15 min at 4°C for 1 h and centrifuged at 12,000 × g for 10 min at
4°C. The protein was determined using a bicinchoninic acid assay
(Pierce, Rockford, IL, USA) method. Following centrifugation at
12,000 × g at 4°C for 10 min, 50 µg aliquots were resolved
using 12% SDS-polyacrylamide gel electrophoresis. The resolved
proteins were electrotransferred overnight onto nitrocellulose
membranes in 25 mM Tris (pH 8.5), 200 mM glycerin and 20% methanol
at 25 V. The blots were blocked for at least 2 h with 1X PBS
containing 0.05% Tween 20 with 5% nonfat dry milk. The blots were
then incubated with rabbit polyclonal anti-NF-κB (sc-7151), mouse
monoclonal anti-phosphorylated IkB (pIκB) -a (sc-8404), mouse
monoclonal anti-tubulin (sc-8035), mouse monoclonal anti-actin
(sc-8432) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for
1 h at room temperature. The blots were developed with monoclonal
mouse anti-rabbit peroxidase conjugated-IgG (sc-2357) and
monoclonal goat anti-mouse peroxidase-IgG (sc-2005). for 1 h at
room temperature, and the proteins were visualized using enhanced
chemiluminescence procedures, (GE Healthcare, Piscataway, NJ, USA)
according to the manufacturer's protocol.
Immunocytochemistry and confocal
microscopy
The cells were washed with PBS, fixed with 3.7%
paraformaldehyde for 30 min and permeabilized with wash buffer
(0.5% Triton-X in PBS) for 20 min. The cells were then blocked with
wash buffer containing 10% FBS for 1 h, and incubated with
anti-NF-κB (p65) primary antibody for 1 h at room temperature at
1:500 dilution. Following washing with PBS, the cells were
incubated with anti-rabbit fluorescein isothiocyanate-conjugated
secondary antibody for 1 h at room temperature. Following extensive
washing with PBS, the slides were scanned under fluorescence with
an Olympus confocal microscope (Olympus Corporation, Tokyo,
Japan).
Animals
Male ICR mice (10–12 g; 3 weeks old) were obtained
from the Dae-Han Experimental Animal Center (Daejon, Korea).
Experiments were performed following 1 week of adaptation to the
laboratory environment. The animals were housed (five animals per
cage) in a laminar air-flow room maintained at a temperature of
22±1°C, a relative humidity of 55±10% and under a 12:12 light/dark
cycle on at 07:00 h throughout the experiment. Food and water were
available ad libitum. All experiments were performed between
09:00 and 16:00 h, and no animals was used in more than one
experiment. All protocols were approved by the Institutional Animal
Care and Use Committee of Kyung Hee University [Seoul, Korea;
KHUASP (SE)-10-032].
FST
Immobility time was defined as the amount of time
that the mouse remained floating in the water without struggling
and made only those movements necessary to keep its head above the
water. Following the first measurement of immobility times, the
mice were divided into a control group, Chlorella vulgaris
extract (CVE; 0.3 g/kg) group, BS (1 g/kg) group and NaSH (0.1 and
1 mg/kg) groups, based on the recorded swimming times (equivalent
average swim time/group). The CVE was supplied by Daesang
Corporation (Seoul, Korea), and was dissolved in D.W. as a positive
control. BS (1 g/kg), NaSH (0.1 and 1 mg/kg), CVE (0.3 g/kg) and
D.W. were orally administered to the mice in the respective groups
once a day for 4 weeks using an atraumatic feeding needle. The FST
was performed on 0 and 28 days after administration of BS or NaSH.
The BS, NaSH, CVE and D.W. were administered 1 h prior to the FST.
During the 6 min of the FST, the immobility time was analyzed, as
previously described by Porsolt et al (21). The FST was recorded using a Canon
camcorder (Canon, Inc., Tokyo, Japan). The immobility times were
measured using a stopwatch by a trained observer, who was blind to
the experimental treatments. There were five mice in each
group.
Statistical analysis
The results are expressed as the mean ± standard
error of the mean. Statistical significance was compared among each
treated group and the control using an independent t-test
and one-way analysis of variance with Tukey's post-hoc test using
SPSS statistical software (SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
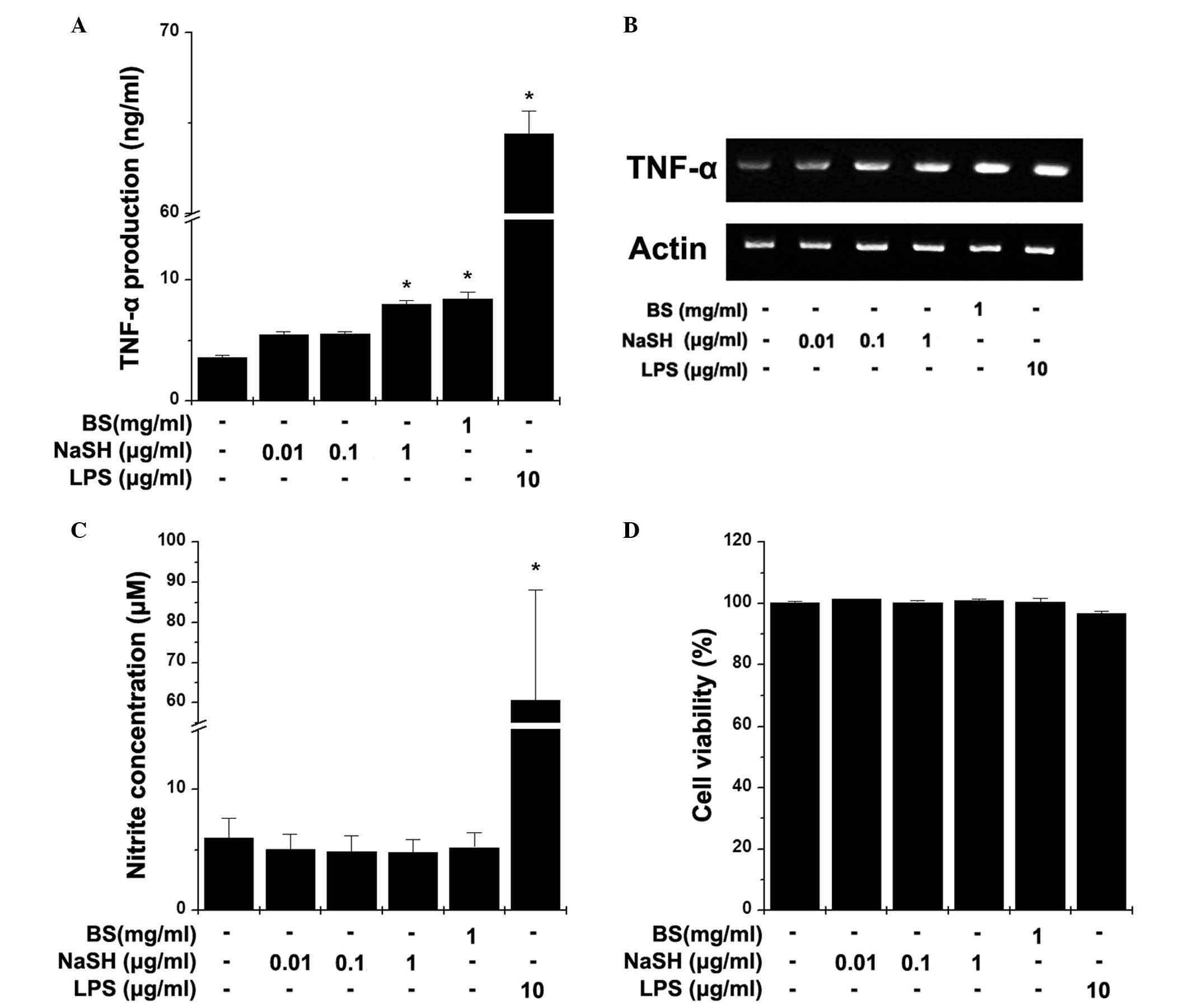
Effects of BS and NaSH on the production
of TNF-α and NO in RAW264.7 cells
Macrophages control the immune system directly
through their innate immune functions. Activated macrophages
secrete TNF-α and NO. To evaluate the effect of BS and NaSH on the
production of TNF-α, RAW264.7 cells were treated with BS and NaSH
for 24 h. As shown in Fig. 1A, BS
and NaSH (1 µg/ml) significantly increased the production of
TNF-α, compared with the unstimulated cells (P<0.05). The mRNA
levels of TNF-α were also increased by treatment with BS or NaSH
(Fig. 1B). To determine the
effects of BS and NaSH on the production of NO, the RAW264.7 cells
were treated with BS and NaSH for 48 h. BS and NaSH had no
significant effects on the production of NO (Fig. 1C). However, LPS significantly
increased the levels of TNF-α and NO (Fig. 1A–C). No cytotoxic effects of BS and
NaSH were observed (Fig. 1D).
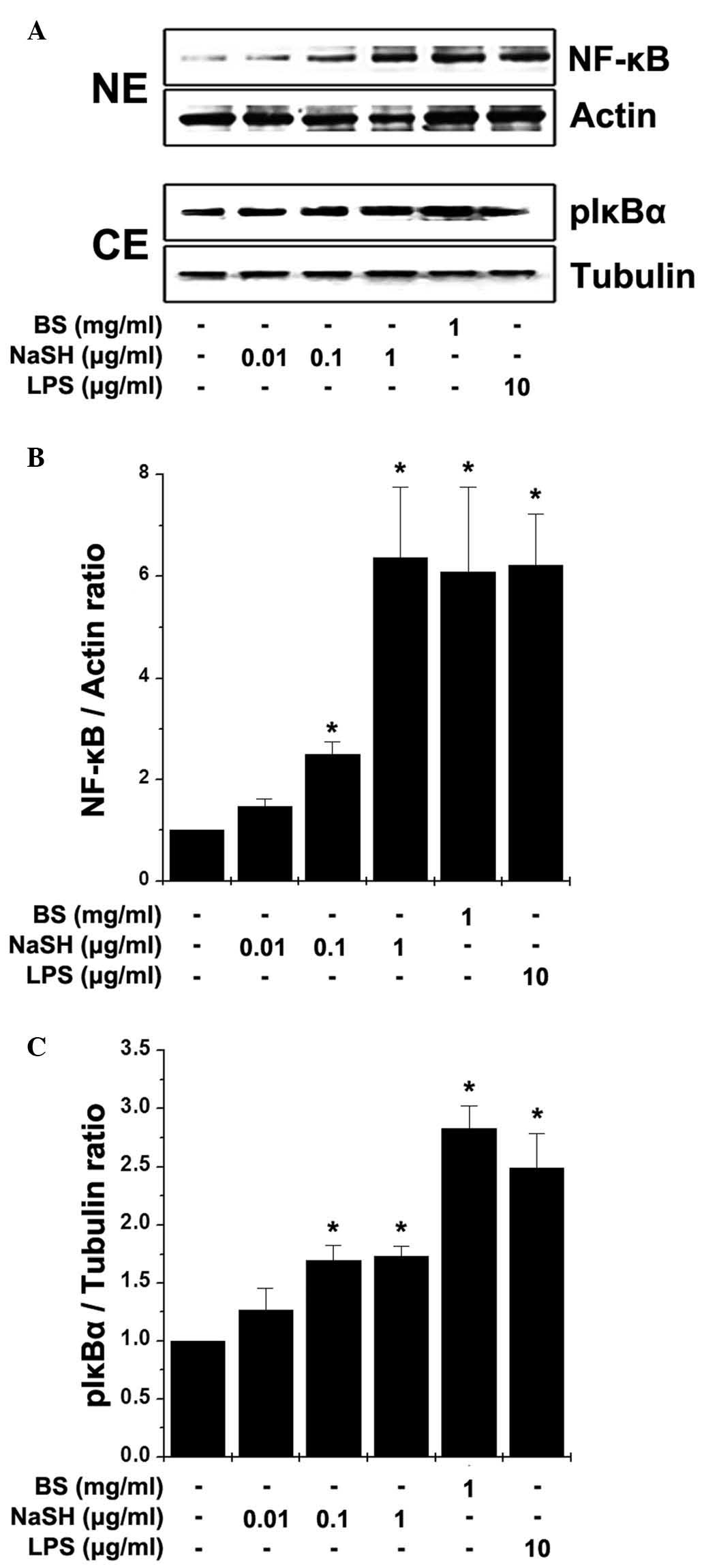
Effects of BS and NaSH on the activation
of NF-κB in RAW264.7 cells
NF-κB is a transcription factor, which regulates the
expression of TNF-α and is important in immunity (9). Thus, the present study examined the
effects of BS and NaSH on the activation of NF-κB in the RAW264.7
cells. Stimulation with BS and NaSH induced the translocation of
NF-κB (p65) to the nuclei following the phosphorylation of IκBα
(Fig. 2). Immunocytochemistry for
NF-κB (p65) was also performed; when the RAW 264.7 cells were
treated with BS or NaSH, immunoreactive NF-κB was localized to the
nuclei (Fig. 3).
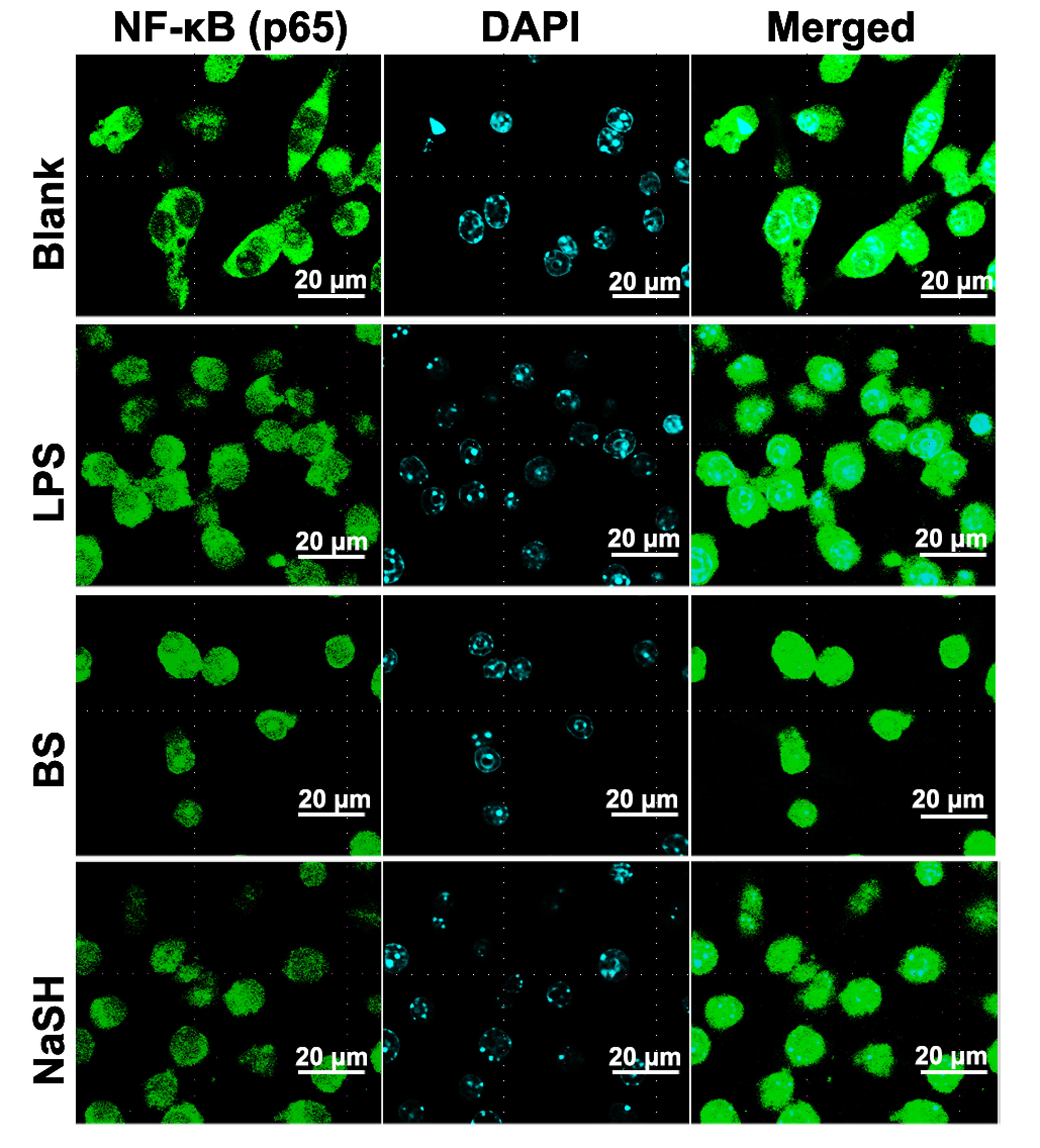 | Figure 3Effect of BS and NaSH on the
translocation of NF-kB into the nucleus of RAW264.7 cells. RAW264.7
cells were treated with BS (1 mg/ml), NaSH (1 µg/ml) or LPS
(10 µg/ml) for 1 h. NF-κB was stained using primary
antibody, anti-p65, for 1 h and then incubated with secondary
fluorescein isothiocyanate-conjugated IgG for 30 min. Results are
representative of three independent experiments. (Original
magnification, ×138; scale bar=20 µm). Blank, unstimulated
cells, BS, bamboo salt; LPS, lipopolysaccharide; NaSH, sodium
hydrosulfide; NF-κB, nuclear factor-κB. |
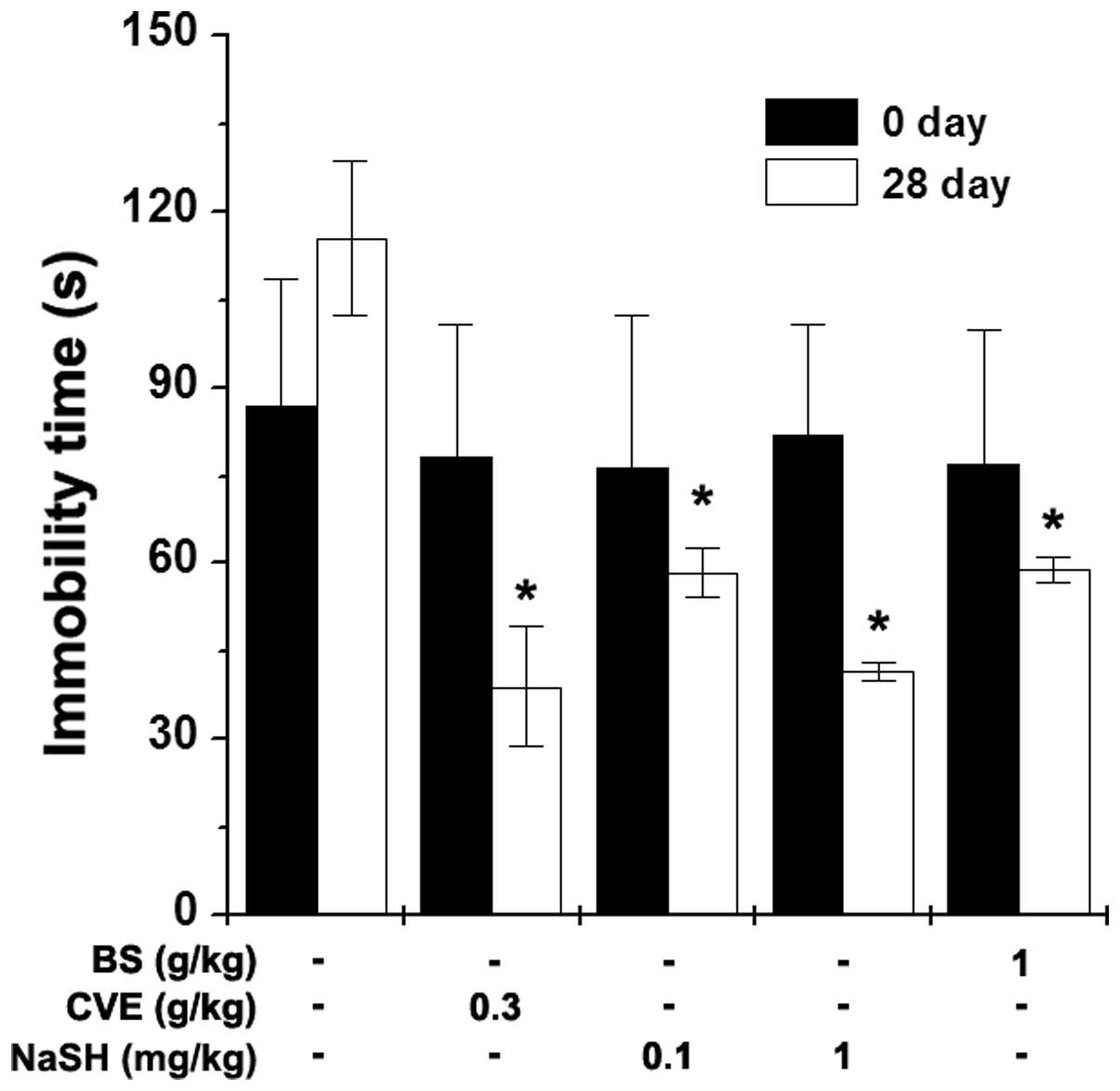
Effects of BS and NaSH on immobility time
during the FST
The FST is a behavioral animal model for the
evaluation of immune-enhancing drugs (22). The present study investigated the
effects of BS and NaSH on the immobility time during the FST. BS (1
g/kg), NaSH (0.1 and 1 mg/kg) and CVE (0.3 g/kg) were orally
administered to the mice once a day for 28 days. Measurements of
immobility times were performed 1 h following BS, NaSH and CVE
administration. The immobility times were significantly decreased
in the BS- and NaSH-administered groups, compared with the control
group (Fig. 4; P<0.05). CVE
also significantly reduced the immobility time (Fig. 4; P<0.05).
Effects of BS and NaSH on the levels of
Th1 cytokines in the spleen and serum
Th cells are important in the cellular immune
response, and are key in host defense systems against bacterial
products and viruses (23). The
Th1 cytokines secreted by Th1 cells increase cell-mediated immune
responses (24), therefore, the
present study analyzed the levels of Th1 cytokines (IFN-γ, IL-2 and
TNF-α) in the spleen and serum following the FST. BS (1 g/kg) and
NaSH (1 mg/kg) significantly increased the levels of IFN-γ, IL-2
and TNF-α in the spleen (Fig.
5A–C; P<0.05). The serum levels of IFN-γ were also
significantly increased by BS, NaSH and CVE (Fig. 5D; P<0.05).
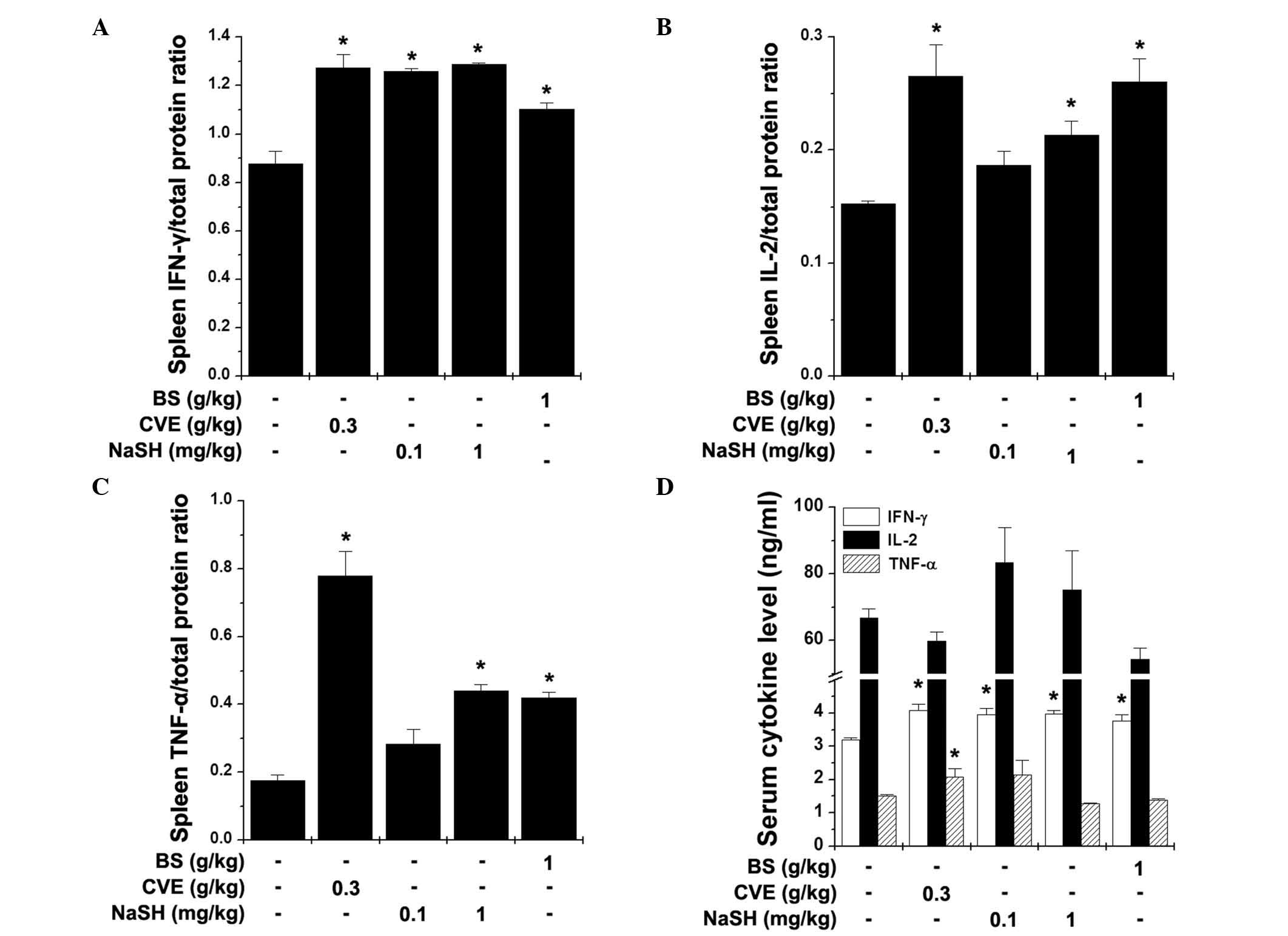 | Figure 5Effects of BS and NaSH on the levels
of Th1 cytokines in the spleen and serum. The levels of (A) IFN-γ,
(B) IL-2 and (C) TNF-α in the spleen, and the (D) serum levels of
these three cytokines were measured following the FST using an
enzyme-linked immunosorbent assay. Values are presented as the mean
± standard error of the mean. *P<0.05, vs. distilled
water-treated control group. BS, bamboo salt; CVE, Chlorella
vulgaris extract; NaSH, sodium hydrosulfide; FST, forced
swimming test; IFN-γ, interferon-γ; IL-2, interleukin-2; TNF-α,
tumor necrosis factor-α. |
Discussion
In the present study, it was shown that BS and NaSH
increased the production of TNF-α and activation of NF-κB in RAW
264.7 cells. BS and NaSH significantly reduced the immobility time
in the FST on day 28. In addition, BS and NaSH significantly
increased the levels of IFN-γ, IL-2 and TNF-α.
Macrophages are involved in homeostasis, wound
repair, tissue remodeling during embryogenesis and the removal of
damaged or senescent cells subsequent to injury or infection
(7). The activation of macrophages
by LPS stimulation increases the production of NO and TNF-α
(9). NO is a key molecule for
inducing pathogen and tumor cell death, and is synthesized from
L-arginine by iNOS (25). The
expression of TNF-α is dependent on the activation of the
transcription factor, NF-κB (9).
NF-κB is a major transcription factor for the expression of innate
and adaptive immunity-associated genes (26). In Korea, red ginseng has been known
to improve immunity and increase the levels of TNF-α and NO in
RAW264.7 cells (27). In the
present study, BS and NaSH increased the levels of TNF-α and
activation of NF-kB, suggesting that BS and NaSH increased the
levels of TNF-α via the activation of NF-κB.
Several psychotropic drugs have been developed using
the FST (21,28). Although a number of antidepressants
reduce immobility time during FSTs (29), the attenuation of lymphocyte
proliferation and IL-2 production, damage to natural killer cell
cytotoxic responses and reduced neutrophil phagocytosis have been
reported following exposure to the FST (30,31).
An et al (22) reported
that the observed reduction in immobility time by CVE in the FST
indicated enhanced immune function and improved physical stamina.
Panax ginseng has been used as a traditional Korean medicine
for improving physical stamina and enhancing the immune response.
The immobility time in the FST has been found to be reduced in mice
administered with Panax ginseng (32). In the present study, BS and NaSH
reduced the immobility time during the FST, which indicated that BS
and NaSH had an immune-enhancing effect.
The spleen is organized as a tree of branching
arterial vessels, and is composed of T cells, B cells, fibroblasts,
marginal-zone macrophages and dendritic cells. In particular, Th1
cells are a key factor in the cellular immune response and have a
central role in host defense systems against various pathogens
(12). Th1 cytokines, including
IFN-γ, IL-2 and TNF-α are produced from Th1 cells and are vital in
regulation of the immune response, activating lymphocytes,
macrophages and polymorphonuclear cells to destroy bacterial
pathogens (24). IFN-γ is crucial
in immunity against intracellular pathogens and in the control of
tumors (33). IL-2 is a cytokine
messenger protein, which activates components of the immune system.
Several studies have established IL-2 as the lymph cytotropic
cytokine, responsible for signaling helper T lymphocyte
(CD4+ T cell) proliferation (34). TNF-α performs important functions
in the protection of cells from viral infection or in promoting the
selective elimination of virally-infected cells via an
IFN-independent mechanism (35).
Anticancer drugs induce potent cellular immune responses leading to
the production of IFN-γ, IL-2 and TNF-α (36). CVE, which functions in immune
enhancing, increases the levels of IL-2 and IFN-γ in T cells
(37). In the present study, it
was shown that BS and NaSH induced a significant increase in Th1
cytokines in the spleen and serum. These results suggested that BS
and NaSH may have useful effects in the treatment of cancer and
infections via immune enhancement. However, further investigation
is required to clarify the anticancer and antiviral effects of BS
and NaSH.
The administration of minerals enhances the host
immune response (38). Zinc has
been shown to be necessary for physiological functioning of the
innate and adaptive immune systems, and it is particularly
important for the development of T cells and their peripheral
functions following maturation (39). Copper and magnesium are known to be
important in the development and maintenance of the immune system
(40,41). Iron contributes to the regulation
of body temperature following physical exercise and controlling
immune defenses (42).
H2S involves the cell signaling pathways, which may be
involved in cytoprotective, anti-inflammatory and anti-apoptotic
actions, and in the modulation of ion channels and metabolism,
including the production of mitochondrial ATP (43,44).
In the present study, it was shown that bamboo salt and NaSH had
immune-enhancing effects. Bamboo salt contains H2S, in
addition to 70 essential minerals and micronutrients (17). Therefore, the present study
hypothesized that H2S is an active component of BS in
immune functions.
In conclusion, the present study showed for the
first time, to the best of our knowledge, that BS and
H2S significantly increased the production of TNF-α via
the activation of NF-κB in the RAW264.7 cells. BS and
H2S significantly reduced the immobility times in the
FST, and significantly increased the levels of IFN-γ, IL-2 and
TNF-α. Taken together, these results suggested that BS and
H2S may offer potential as essential agents for the
enhancement of immune function.
Acknowledgments
This study was supported by a grant (grant no.
20130290) to the Solar Salt Research Center from the Ministry of
Oceans and Fisheries of Korea.
References
|
1
|
Aderem A and Ulevitch RJ: Toll-like
receptors in the induction of the innate immune response. Nature.
406:782–787. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoffmann JA, Kafatos FC, Janeway CA and
Ezekowitz RA: Phylogenetic perspectives in innate immunity.
Science. 284:1313–1318. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonilla FA, Bernstein IL, Khan DA, Ballas
ZK, Chinen J, Frank MM, Kobrynski LJ, Levinson AI, Mazer B, Nelson
RP Jr, et al: Practice parameter for the diagnosis and management
of primary immunodeficiency. Ann Allergy Asthma Immunol. 94(5 Suppl
1): S1–S63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Younger EM, Epland K, Zampelli A and
Hintermeyer MK: Primary immunodeficiency diseases: A primer for
PCPs. Nurse Pract. 40:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaminogawa S and Nanno M: Modulation of
immune functions by foods. Evid Based Complement Alternat Med.
1:241–250. 2004. View Article : Google Scholar
|
|
6
|
Park HJ, Yang HJ, Kim KH and Kim SH:
Aqueous extract of Orostachys japonicus A. Berger exerts
immunostimulatory activity in RAW 264.7 macrophages. J
Ethnopharmacol. 170:210–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams OD and Hamilton TA: Molecular basis
of macrophage activation and its origins. Oxford University Press;
New York: pp. 75–114. 1992
|
|
8
|
Nathan C: Nitric oxide as a secretory
product of mammalian cells. FASEB J. 6:3051–3064. 1992.PubMed/NCBI
|
|
9
|
Jeong HJ, Han NR, Kim KY, Choi IS and Kim
HM: Gomisin A decreases the LPS-induced expression of iNOS and
COX-2 and activation of RIP2/NF-κB in mouse peritoneal macrophages.
Immunopharmacol Immunotoxicol. 36:195–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Del Prete GF, De Carli M, Mastromauro C,
Biagiotti R, Macchia D, Falagiani P, Ricci M and Romagnani S:
Purified protein derivative of Mycobacterium tuberculosis and
excretory-secretory antigen(s) of Toxocara canis expand in vitro
human T cells with stable and opposite (type 1 T helper or type 2 T
helper) profile of cytokine production. J Clin Invest. 88:346–350.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carter LL and Dutton RW: Type 1 and type
2: A fundamental dichotomy for all T-cell subsets. Curr Opin
Immunol. 8:336–342. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Linder J: The thymus gland in secondary
immunodeficiency. Arch Pathol Lab Med. 111:1118–1122.
1987.PubMed/NCBI
|
|
13
|
Kim KY, Nam SY, Shin TY, Park KY, Jeong HJ
and Kim HM: Bamboo salt reduces allergic responses by modulating
the caspase-1 activation in an OVA-induced allergic rhinitis mouse
model. Food Chem Toxicol. 50:3480–3488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YS, Lee EH and Kim HM: Surprisingly,
traditional purple bamboo salt, unlike other salts does not induce
hypertension in rats. TANG. 3:e162013.
|
|
15
|
Nam SY, Oh HA, Choi Y, Park KY, Kim HM and
Jeong HJ: Inhibition of IL-32 signaling by bamboo salt decreases
pro-inflammatory responses in cellular models of allergic rhinitis.
J Med Food. 17:939–948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shin HY, Na HJ, Moon PD, Shin T, Shin TY,
Kim SH, Hong SH and Kim HM: Inhibition of mast cell dependent
immediate-type hypersensitivity reactions by purple bamboo salt. J
Ethnopharmacol. 91:153–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
View Article : Google Scholar
|
|
18
|
Han J, Xuan JL, Hu HR and Chen ZW: Effects
and mechanisms of hyperoside on vascular endothelium function in
middle cerebral arteries of rats ex vivo. Zhongguo Zhong Yao Za
Zhi. 39:4849–4855. 2014.In Chinese.
|
|
19
|
Zhang Y, Li H, Zhao G, Sun A, Zong NC, Li
Z, Zhu H, Zou Y, Yang X and Ge J: Hydrogen sulfide attenuates the
recruitment of CD11b+Gr−1+myeloid cells and regulates Bax/Bcl−2
signaling in myocardial ischemia injury. Sci Rep. 4:47742014.
|
|
20
|
Jeong HJ, Koo HN, Na HJ, Kim MS, Hong SH,
Eom JW, Kim KS, Shin TY and Kim HM: Inhibition of TNF-alpha and
IL-6 production by Aucubin through blockade of NF-kappaB activation
RBL-2H3 mast cells. Cytokine. 18:252–259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Porsolt RD, Bertin A and Jalfre M:
Behavioral despair in mice: A primary screening test for
antidepressants. Arch Int Pharmacodyn Ther. 229:327–336.
1977.PubMed/NCBI
|
|
22
|
An HJ, Choi HM, Park HS, Han JG, Lee EH,
Park YS, Um JY, Hong SH and Kim HM: Oral administration of hot
water extracts of Chlorella vulgaris increases physical stamina in
mice. Ann Nutr Metab. 50:380–386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paul WE and Seder RA: Lymphocyte responses
and cytokines. Cell. 76:241–251. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abbas AK, Murphy KM and Sher A: Functional
diversity of helper T lymphocytes. Nature. 383:787–793. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho CW, Han CJ, Rhee YK, Lee YC, Shin KS,
Shin JS, Lee KT and Hong HD: Cheonggukjang polysaccharides enhance
immune activities and prevent cyclophosphamide-induced
immunosuppression. Int J Biol Macromol. 72:519–525. 2015.
View Article : Google Scholar
|
|
26
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park SY, Kim HB, Kim JH, Lee JM, Kim SR,
Shin HS and Yi TH: Immunostimulatory effect of fermented red
ginseng in the mouse model. Prev Nutr Food Sci. 19:10–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Connor TJ, Kelliher P, Shen Y, Harkin A,
Kelly JP and Leonard BE: Effect of subchronic antidepressant
treatments on behavioral, neurochemical, and endocrine changes in
the forced-swim test. Pharmacol Biochem Behav. 65:591–597. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeong HJ, Kim JH, Kim NR, Yoou MS, Nam SY,
Kim KY, Choi Y, Jang JB, Kang IC, Baek NI and Kim HM:
Antidepressant effect of Stillen. Arch Pharm Res. 38:1223–1231.
2015. View Article : Google Scholar
|
|
30
|
Irwin M, Smith TL and Gillin JC: Low
natural killer cytotoxicity in major depression. Life Sci.
41:2127–2133. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shu J, Stevenson JR and Zhou X: Modulation
of cellular immune responses by cold water swim stress in the rat.
Dev Comp Immunol. 17:357–371. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shin HY, Jeong HJ, Hyo-Jin-An, Hong SH, Um
JY, Shin TY, Kwon SJ, Jee SY, Seo BI, Shin SS, et al: The effect of
Panax ginseng on forced immobility time & immune function in
mice. Indian J Med Res. 124:199–206. 2006.PubMed/NCBI
|
|
33
|
Yamaguchi R, Kawata J, Yamamoto T,
Ishimaru Y, Sakamoto A, Ono T, Narahara S, Sugiuchi H, Hirose E and
Yamaguchi Y: Mechanism of interferon-gamma production by monocytes
stimulated with myeloperoxidase and neutrophil extracellular traps.
Blood Cells Mol Dis. 55:127–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shaker MA and Younes HM: Interleukin-2:
Evaluation of routes of administration and current delivery systems
in cancer therapy. J Pharm Sci. 98:2268–2298. 2009. View Article : Google Scholar
|
|
35
|
Lee JA, Kim YM, Hyun PM, Jeon JW, Park JK,
Suh GH, Jung BG and Lee BJ: Honeybee (Apis mellifera) venom
reinforces viral clearance during the early stage of infection with
porcine reproductive and respiratory syndrome virus through the
up-regulation of Th1-specific immune responses. Toxins (Basel).
7:1837–1853. 2015. View Article : Google Scholar
|
|
36
|
Dalgleish AG: Cancer vaccines. Br J
Cancer. 82:1619–1624. 2000.PubMed/NCBI
|
|
37
|
An HJ, Rim HK, Jeong HJ, Hong SH, Um JY
and Kim HM: Hot water extracts of Chlorella vulgaris improve immune
function in protein-deficient weanling mice and immune cells.
Immunopharmacol Immunotoxicol. 32:585–592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schafer AS, Leal MLR, Molento MB, et al:
Immune response of lambs experimentally infected with Haemonchus
contortus and parenterally treated with a combination of zinc and
copper. Small Ruminant Res. 123:183–188. 2015. View Article : Google Scholar
|
|
39
|
Jansen J, Karges W and Rink L: Zinc and
diabetes-clinical links and molecular mechanisms. J Nutr Biochem.
20:399–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Percival SS: Copper and immunity. Am J
Clin Nutr. 67(5 Suppl): 1064S–1068S. 1998.PubMed/NCBI
|
|
41
|
Tam M, Gómez S, González-Gross M and
Marcos A: Possible roles of magnesium on the immune system. Eur J
Clin Nutr. 57:1193–1197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Speich M, Pineau A and Ballereau F:
Minerals, trace elements and related biological variables in
athletes and during physical activity. Clin Chim Acta. 312:1–11.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Azizi F, Seifi B, Kadkhodaee M and Ahghari
P: Administration of hydrogen sulfide protects ischemia
reperfusion-induced acute kidney injury by reducing the oxidative
stress. Ir J Med Sci. 2015.Epub ahead of print. PubMed/NCBI
|
|
44
|
Hunter JP, Hosgood SA, Patel M, Furness P,
Sayers RD and Nicholson ML: Hydrogen sulfide reduces inflammation
following abdominal aortic occlusion in rats. Ann Vasc Surg.
29:353–360. 2015. View Article : Google Scholar
|