Introduction
Mesenchymal stem cells (MSCs) are an important
resource for tissue repair and regeneration. The multilineage
differentiation and immunomodulatory capabilities of MSCs make them
highly useful for cardiac repair (1), improving marrow engraftment (2), treating graft-versus-host disease
(3,4) and generating connective tissue
elements (5–9).
MSCs also secrete various cytokines and angiogenic
mediators that repair damaged tissues (10). A previous comparative study has
indicated that the umbilical cord (UC) is an excellent alternative
to bone marrow (BM) as a source of MSCs for cell therapy (11). UC-MSCs share numerous
characteristics with BM-MSCs, including morphology,
immunophenotype, cell cycle status, potential to differentiate and
hematopoiesis-supporting functions (11–13).
Experiments in animal models have previously
demonstrated that MSCs tend not to persist in the graft
environment, they either do not incorporate well into the host
tissue, or if incorporation occurs, the majority of the cells are
lost within a month of adoptive transfer (1,14,15).
The low incorporation rate of MSCs may be due to decreased cell
viability caused by ischemia and loss of a suitable
microenvironment, or local inflammation at the graft site (16). The extracellular matrix (ECM) may
aid MSCs to survive and expand. Furthermore, chemotaxis and homing
of MSCs are closely associated with ECM components. A previous
study demonstrated that three ECM proteins, fibronectin (FN),
vitronectin and collagen I (Col-I), induce significant mitogenic
activity in MSCs (17). The ECM
also stimulates neuronal differentiation of MSCs and serves as a
nerve-regenerative scaffold (18,19).
However, despite its importance, the regulation of the functional
activities of the ECM remains to be completely understood.
Transforming growth factor (TGF) -β1 stimulates ECM
synthesis in cultured cells, including renal, vascular smooth
muscle and type-II pulmonary epithelial cells (20–22).
TGF-β1 is also involved in MSC proliferation (23). In BM-derived adult human MSCs, SMAD
family member 3 (SMAD3) -dependent nuclear translocation of
β-catenin is required for TGF-β1-induced proliferation (24). Previous studies suggested that
TGF-β1 induces increased expression levels of vascular smooth
muscle cell (SMC) -like ion channels and the differentiation of
human adipose tissue-derived MSCs into contractile vascular SMCs
(25).
The present study investigated the effect of TGF-β1
on the proliferation of MSCs from human (h)UC-MSCs and the
expression levels of ECM genes, including Col-I, Col-IV, FN,
laminin (LN), integrin and tenascin-C. The effects of TGF-β1 on the
differentiation and cell phenotype of MSCs were also examined. An
effective approach to increase ECM expression by MSCs was
determined. A lipopolysaccharide (LPS) -induced rat model of acute
lung injury (ALI) was selected to assess the survival and
therapeutic benefit of TGF-β1-treated MSCs.
Materials and methods
Generation and culture of UC-MSCs
UCs (n=10, male newborns, clinically normal
pregnancies) were dissected and the blood vessels were removed. The
remaining tissues were cut into small pieces (1–2 mm3)
and placed in plates with α-minimum essential medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin, 100 µg/ml streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) 2 ng/ml vascular endothelial growth factor
(VEGF; R&D Systems, Inc., Minneapolis, MN, USA), 2 ng/ml
epidermal growth factor (EGF; R&D Systems, Inc.) and 2 ng/ml
fibroblast growth factor (FGF; R&D Systems, Inc.). Cultures
were maintained at 37°C in a humidified atmosphere of 5%
CO2 in air. The culture medium was changed every 3–4
days. Adherent cells proliferated from individual explanted tissues
7–12 days after incubation. These cells were subsequently
trypsinized using 0.25% trypsin (Gibco; Thermo Fisher Scientific,
Inc.) and cultured at a density of 1×104
cells/cm2 in the medium described. The cells were used
at ≤5 passages. The present study was approved by the Human
Research Ethics Committee of Qilu Hospital (Jinan, China). Informed
consent was obtained from all donors.
Proliferation of MSCs in response to
TGF-β1
The proliferation of UC-MSCs at passage 4 was
measured. The cells (6,000 cells/cm2) were seeded into
24-well culture plates and allowed to adhere. Different
concentrations (0.1, 0.5, 1, 5, 10 and 20 ng/ml) of TGF-β1 (R&D
Systems, Inc.) were then added to the plates. The plates were
maintained at 37°C in a humidified atmosphere of 5% CO2
and 95% air for 24, 48 or 72 h. Proliferation was measured after
these incubations using a Cell Counting kit-8 assay (Beyotime
Institute of Biotechnology, Haimen, China). The average optical
density values of triplicate measurements were calculated and used
for comparisons.
Measuring mRNA expression levels of ECM
and ECM-associated genes following TGF-β1 treatment
MSCs were cultured in 6-well plates at a density of
3×105 cells/well in 2 ml culture medium. Following cell
adherence, the cells were incubated for 24 h in different
concentrations of TGF-β1 (0.1, 1 or 10 ng/ml). The total RNA was
extracted from each sample using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) was performed to analyze the expression levels of ECM
genes, including Col-I, Col-IV, FN, LN, integrin and tenascin-C,
and ECM-associated genes, including matrix metalloproteinase (MMP)
-1, MMP-2 and MMP-9, and tissue inhibitor of metalloproteinase
(TIMP) -1 and TIMP-2. cDNA was generated from the total RNA via RT
using an Omniscript cDNA Synthesis kit (Qiagen GmbH, Hilden,
Germany), according to the manufacturer's instructions. Typically,
0.2 µg total RNA was reverse transcribed in a 20 µl
final reaction volume containing the following components: 1X RT
buffer, deoxynucleotide triphosphate mix (5 mM), RNase inhibitor
(RNaseOUT; 10 U/µl; Invitrogen; Thermo Fisher Scientific,
Inc.), oligo dT primers and 4 units Omniscript RT. The samples were
incubated at 37°C for 60 min, and the resulting cDNA was stored at
−80°C prior to RT-qPCR analysis using an ABI 500 PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and SYBR green
I dye (Toyobo Co., Ltd., Osaka, Japan). The primers used are listed
in Table I. All reagents and
primers were obtained from Bioasi Co., Ltd. (Shanghai, China).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
internal control. The expression of each gene was determined using
the 2−ΔΔCq method. The qPCR conditions were as follows:
1 cycle at 95°C for 4 min; 1 cycle at 94°C for 15 sec; and 1 cycle
at 60°C for 1 min; for a total of 40 cycles.
 | Table IPrimers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward sequence
(5′–3′) | Reverse sequence
(5′–3′) | Product (bp) |
|---|
| Human GAPDH |
GACAACTTTGGTATCGTGGA |
AGGCAGGGATGATGTTCTGG | 114 |
| Col-I |
TGACCGAGACGTGTGGAAAC |
GTCTCGTCACAGATCACGTCATC | 90 |
| Col-IV |
GCTGTGGATCGGCTACTCTT |
AAGCGTTTGCGTAGTAATTGC | 158 |
| Fibronectin |
AAATTCTGTAGGCCGTTGGAA |
CTTCTTGGTGGCCGTACTGC | 131 |
| Laminin |
CTTTCAAGACATTCCGTCCA |
AACGAGGCCTCACAGTCATAG | 106 |
| Integrin |
TGCCCTCCAGATGACATAGAAA |
CCTTTGCTACGGTTGGTTACATT | 80 |
| Tenascin-C |
CTGACATAACTCCCGAGAGC |
CTGAGATATGGGCAGTTCGTT | 150 |
| MMP-1 |
TGATGGACCTGGAGGAAATCTT |
AAAATGAGCATCCCCTCCAA | 70 |
| MMP-2 |
ACTGTGACGCCACGTGACA |
CGTATACCGCATCAATCTTTTCC | 88 |
| MMP-9 |
TGCGTGGAGAGTCGAAATCTC |
GTCTCGGGCAGGGACAGTT | 70 |
| TIMP-1 |
GCTGACATCCGGTTCGTCTAC |
GGTTGTGGGACCTGTGGAAGT | 70 |
| TIMP-2 |
TGGATGGACTGGGTCACAGA |
TTCTCTTGATGCAGGCGAAGA | 70 |
| Sry |
GCGAAGTGCAACTGGACAAC |
GCCTAGCTGGTGCTCCATTC | 80 |
| Rat GAPDH |
GTTACCAGGGCTGCCTTCTC |
GCCTAGCTGGTGCTCCATTC | 152 |
The data were analyzed using Sequence Detection
software (version 1.4; Applied Biosystems; Thermo Fisher
Scientific, Inc.). The data are presented as the mean ± standard
deviation of ≥3 independent experiments. mRNA expression is
presented as the fold difference with respect to the untreated
control groups and the control group values are set at a fold
change equal a value of 1.
Cell surface antigen phenotyping
Based on the above experimental results, 0.1 ng/ml
was the concentration of TGF-β1 most effective at promoting the
proliferation of MSCs and ECM gene transcription in MSCs. Thus, all
subsequent experiments assessing MSC gene expression used TGF-β1 at
a concentration of 0.1 ng/ml.
The cells were collected and treated with 0.25%
trypsin. The cells were blocked with phosphate-buffered saline
(PBS) containing 1% FBS for 10 min at room temperature in the dark.
Subsequently, the cells were incubated with either fluorescein
isothiocyanate- or phycoerythrin-conjugated monoclonal antibodies
(single labeling experiments) in 100 µl phosphate buffer,
for either 15 min at room temperature or 30 min at 4°C, as
recommended by the manufacturers. Antibodies against human cluster
of differentiation (CD)45 (cat. no. 10894), CD31 (cat. no. 16284),
CD34 (cat. no. 28906), CD29 (cat. no. 05556), CD44 (cat. no.
16070), CD73 (cat. no. 21589), CD90 (cat. no. 15421) and CD105
(cat. no. 17813) were purchased from AbD SeroTec (Raleigh, NC, USA)
and used at a 1:10 dilution. Immunofluorescence was detected using
flow cytometry (Guava easyCyte 6HT; EMD Millipore, Billerica, MA,
USA) and the data were analyzed using Guava Incyte (version 2.8;
EMD Millipore). Positive cells were counted and compared with the
signal of corresponding immunoglobulin isotypes.
Differentiation of MSCs in response to
TGF-β1
To investigate the differentiation potential of MSCs
in response to administration of TGF-β1, MSCs at passage 4 were
treated with 0.1 ng/ml TGF-β1 for 24 h prior to inducing the
differentiation of each lineage. The cell density was adjusted to
2×104 cells/cm2, and the inducing medium was
changed every 3–4 days for differentiation assays. The osteogenic
differentiation medium consisted of low-glucose Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS, 0.1 µM dexamethasone, 50 mM
β-glycerol phosphate and 0.2 mM ascorbic acid (Sigma-Aldrich, St.
Louis, MO, USA). Adipogenic differentiation medium consisted of
high-glucose DMEM supplemented with 0.25 mM
3-isobutyl-1-methyl-xanthine, 0.1 µM dexamethasone, 0.1 mM
indomethacin (Sigma-Aldrich), 6.25 µg/ml insulin (PeproTech
EC Ltd., London, UK) and 10% FBS. Cells were maintained in regular
culture medium served as a control.
Mineralized matrix was evaluated by fixing the cells
with 4% formaldehyde and staining with 1% alizarin-red S
(Sigma-Aldrich) solution in water for 10 min. Alkaline phosphatase
(ALP) staining was performed using an ALP staining kit (Tuo Yang
Biotechnology Co., Ltd., Shanghai, China), according to the
manufacturer's recommendations. Briefly, the cells were fixed in 4%
paraformaldehyde and incubated with a substrate buffer at room
temperature for 30 min. For oil red O staining, the cells were
fixed with 4% formaldehyde, stained with oil red O (Sigma-Aldrich)
for 10 min, and then counterstained with Mayer's hematoxylin
(Sigma-Aldrich) for 1 min. Then, images were obtained with a light
microscope (Olympus BX53; Olympus Corporation, Tokyo, Japan) fitted
with a digital camera (Olympus cellSens Standard; Olympus
Corporation).
Immunocytochemical analysis of ECM
expression levels following treatment of MSCs with TGF-β1
For immunocytochemical analyses, the cells were
cultured in 6-well plates at a density of 3×105
cells/well in 2 ml culture medium. Following cell adherence, the
cells were incubated for 24 h with 0.1 ng/ml TGF-β1. Subsequently,
the cells were fixed in 4% paraformaldehyde and washed in 0.01 M
PBS (pH 7.2–7.4). Endogenous peroxidase was blocked with 0.3%
hydrogen peroxide in absolute methanol for 30 min. Prior to the
immunocytochemical procedure, proteolytic treatment was applied
using 0.4% pepsin in 0.01 M HCl for 30 min at 37°C. Non-spicific
binding was blocked using 5% goat serum (Beijing Kang Century
Biotechnology Co., Ltd., Beijing, China). Primary anti-Col-I (cat.
no. ab34710), -Col-IV (cat. no. ab6586), -FN (cat. no. ab45688) and
-integrin (cat. no. ab15459) antibodies (all obtained from Abcam,
Cambridge, UK) were added to two wells of each group at 1:300
dilution, and allowed to bind for 1 h at 37°C.
Peroxidase-conjugated secondary antibody (cat. no. CW0220S; Beijing
Kang Century Biotechnology Co., Ltd.) were added for 30 min at room
temperature. The cells were washed three times in PBS, incubated
with diaminobenzidine chromogen (Biocare Medical, LLC., Concord,
CA, USA) for 10 min at room temperature, and then counterstained
with hematoxylin.
Western blot analysis
Following stimulation with 0.1 ng/ml TGF-β1 for 24
h, the cells were washed twice with ice-cold PBS and lysed in
radioimmunoprecipitation assay buffer [50 mM Tris-HCl (pH 8.0); 150
mM NaCl; 1% Nonidet P-40; 0.5% deoxycholate and 0.1% sodium dodecyl
sulphate (SDS)], containing protease and phosphatase inhibitors
(Roche Diagnostics GmbH, Mannheim, Germany). Protein concentrations
in cleared cell lysates were determined using a Bradford assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein extracts
(3 µg/µl) were subjected to 7% SDS-polyacrylamide gel
electrophoresis and transferred onto Immobilon-P membranes (EMD
Millipore). The membranes were blocked with 5% milk in
Tris-buffered saline Tween-20 [20 mM Tris-HCl (pH 7.6); 137 mM NaCl
and 0.1% Tween-20] at room temperature for 1 h. Subsequently, the
membranes were incubated with the following primary antibodies in
blocking buffer (1:1,000) at 4°C for 16 h: Monoclonal rabbit
anti-RhoA (clone 67B9; cat. no. 2117), anti-SMAD3 (clone C69H7;
cat. no. 9523), monclonal rabbit anti-phospho-SMAD3 (Ser423/425;
clone C25A9; cat. no. 9520) and anti-GAPDH (clone D16H11; cat. no.
5174), all from Cell Signaling Technology, Inc. Danvers, MA, USA.
Following incubation with horseradish peroxidase-conjugated
secondary antibody (1:2,000; cat. no. CW0103S; Beijing Kang Century
Biotechnology Co., Ltd.), immunoreactive bands were detected by
electrochemiluminescence (Beyotime Institute of Biotechnology) and
quantified using a C-DiGit blot scanner (model 3600; Li-cor,
Lincoln, NE, USA) and Image Studio software (version 4.0;
Li-cor).
Creation of the rat model of ALI and MSC
transplantation
Female Sprague-Dawley rats (weighing 240–280 g)
obtained from Shandong University Experimental Animal Centre
(Jinan, China) were randomly divided into four groups (n=16/group)
as follows: i) Control; ii) ALI/PBS; iii) ALI/MSC; iv) ALI/MSC +
TGF-β1. The animals were maintained on a standard 12-h light/dark
cycle, at a constant temperature (22±2°C) and a relative humidity
of 55±10% with free access to water ad libitum and standard
laboratory rodent food. ALI was induced by intraperitoneal
injection of LPS (10 mg/kg) from Escherichia coli O111:B4
(Sigma-Aldrich). After 1 h, the rats were administered with either
MSCs (MSCs or MSC + TGF-β1; 5×105 cells in 300 µl
PBS) or 300 µl PBS (as the control) via injection into the
tail vein. For the MSC + TGF-β1 group, MSCs were stimulated with
0.1 ng/ml TGF-β1 for 24 h prior to injection. At each time point
(6, 24 and 48 h post-injection of LPS), three rats from each group
were anesthetized by intraperitoneal injection of sodium
pentobarbital (50 mg/kg) and sacrificed to assess lung injury. All
procedures were approved by the Animal Care and Use Committee of
Shandong University (Jinan, China).
Collection of bronchoalveolar lavage
fluid (BALF) and tissue samples
The rats were sacrificed, the trachea was isolated
and the right bronchial tube was ligated. BALF was obtained by
placing a 20-gauge catheter into the trachea, through which 3 ml
cold PBS was flushed back and forth three times. BALF was
centrifuged at 2,400 × g for 20 min at 4°C. A counter (Beckman
Coulter, Inc., Brea, CA, USA) was used to determine the total cell
count in the resulting cell pellet. The cells were smeared with
Wright-Giemsa stain to confirm the neutrophil percentage. The
protein concentration of cell-free BALF from all groups was
measured using a Bio-Rad protein assay kit (Bio-Rad Laboratories),
and was used to indicate endothelial and epithelial
permeabilities.
The right upper lung lobes were used for wet-dry
analysis to quantify the magnitude of pulmonary edema. The lungs
were placed into previously weighed microcentrifuge tubes and were
subsequently weighed. Subsequently, tissues were desiccated under a
vacuum overnight at 80°C and weighed again. The wet lung mass was
divided by the dry lung mass to obtain the wet-dry ratio. The right
middle lung lobes were stored in TRIzol at −20°C to detect male
UC-MSC survival in vivo. The right lower lobes were used for
histological evaluation. Paraffin-embedded lungs were cut into 5
µm thick sections and were subsequently stained with
hematoxylin and eosin for histological analysis.
Measurement of MSC survival in vivo
To measure male MSC survival in vivo, 27
female Sprague-Dawley rats were randomly divided into three groups
(n=9/group) as follows: i) ALI/PBS, ii) ALI/MS; iii) ALI/MSC +
TGF-β1. ALI was induced as described, and the rats were
administered with either male MSCs (MSC or MSC + TGF-β1;
5×105 cells in 300 µl PBS) or 300 µl PBS
via injection into the tail vein. At each time point (days 1, 7 and
14 post-injection of LPS), three rats from each group were
anesthetized and sacrificed, as previously, to assess MSC survival.
The survival of male donor human MSCs, which expressed the
sex-determining region Y (Sry) gene, in the female recipient rat
lung was calculated using RT-qPCR. Different numbers of male MSCs
(100, 50, 25, 10, 5, 1 and 0×103 MSCs/10 mg lung tissue)
were added to female DNA (from rat lung tissue) as standards, to
allow the construction of a calibration curve. Sry expression
levels were quantified by normalizing the values relative to the
rat housekeeping gene, GAPDH. The number of stem cells was
calculated according to the calibration curve, which plotted the
cycle threshold (Cq) of Sry expression against the number of
serially diluted MSCs (26).
Statistical analysis
The data were analyzed using SPSS software (version
14.0; SPSS Inc., Chicago, IL, USA). Quantitative data are presented
as the mean ± standard deviation. Analysis of variance with
Fisher's protected least significant difference as a post hoc
analysis was used for multigroup comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Biological characteristics of
UC-MSCs
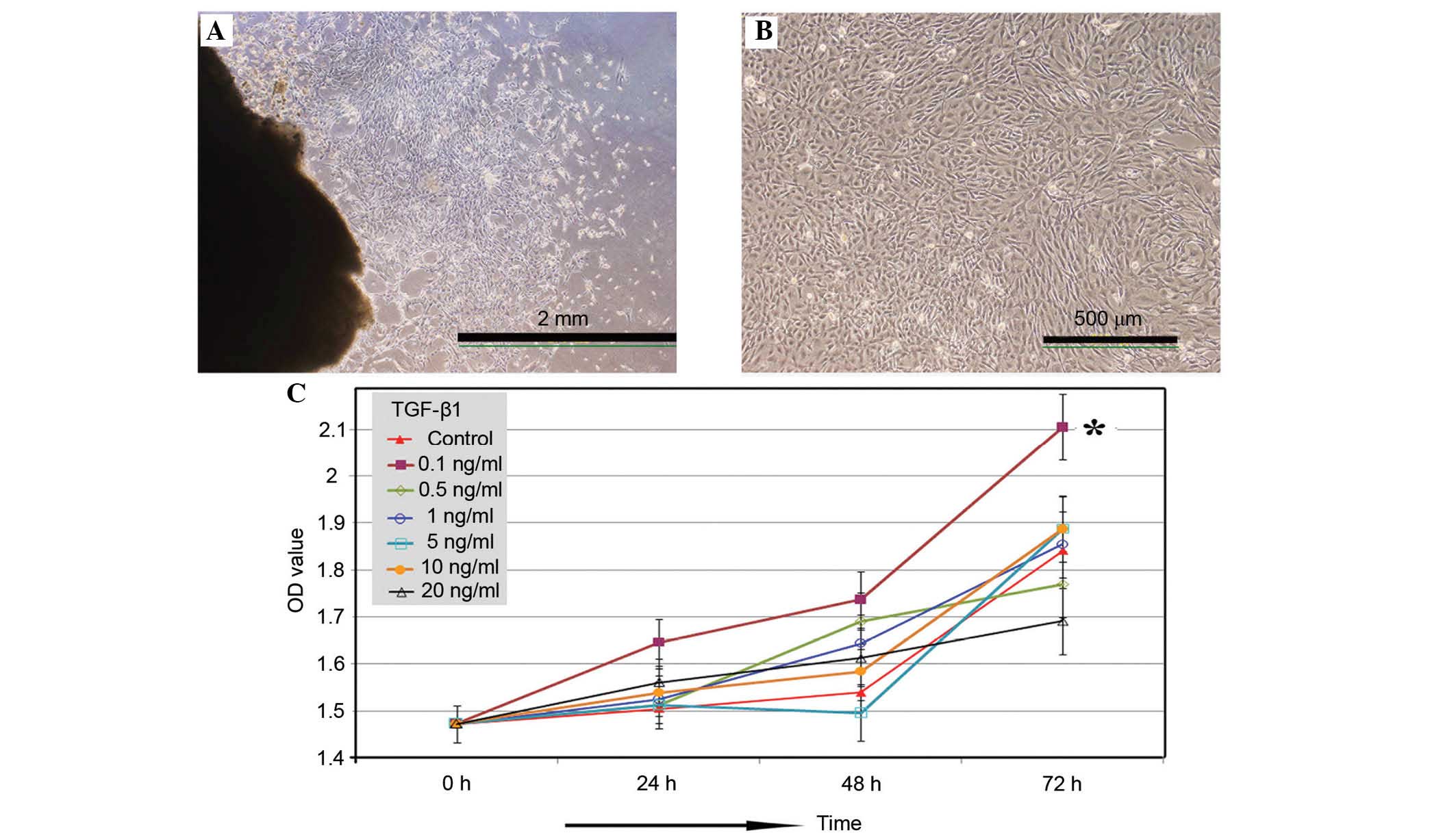
Adherent cells with fibroblastic morphology were
observed as early as 48 h using the tissue-explant adherent method
(Fig. 1A). The cells formed a
monolayer of homogeneous bipolar spindle-like cells with a
whirlpool-like array within 2 weeks. Following several passages,
adherent cells from the UC formed a monolayer of typical
fibroblastic cells (Fig. 1B).
Effect of TGF-β1 on UC-MSC
proliferation
The concentration-dependent effects of TGF-β1 on
UC-MSC proliferation were further investigated (Fig. 1C). The results suggested that
exposure to 0.1 ng/ml TGF-β1 was optimal for enhancing UC-MSC
proliferation from 24 h after treatment, with a significant effect
on proliferation after treatment for 72 h compared with control
(P=0.0241). Higher concentrations of TGF-β1 demonstrated minimal
effects on UC-MSC proliferation. Thus, all subsequent experiments
investigating the effects of TGF-β1 on MSCs utilized a TGF-β1
concentration of 0.1 ng/ml, and the TGF-β1 treatment group was
denoted as MSC + TGF-β1.
TGF-β1 causes no effect on the UC-MSC
phenotype
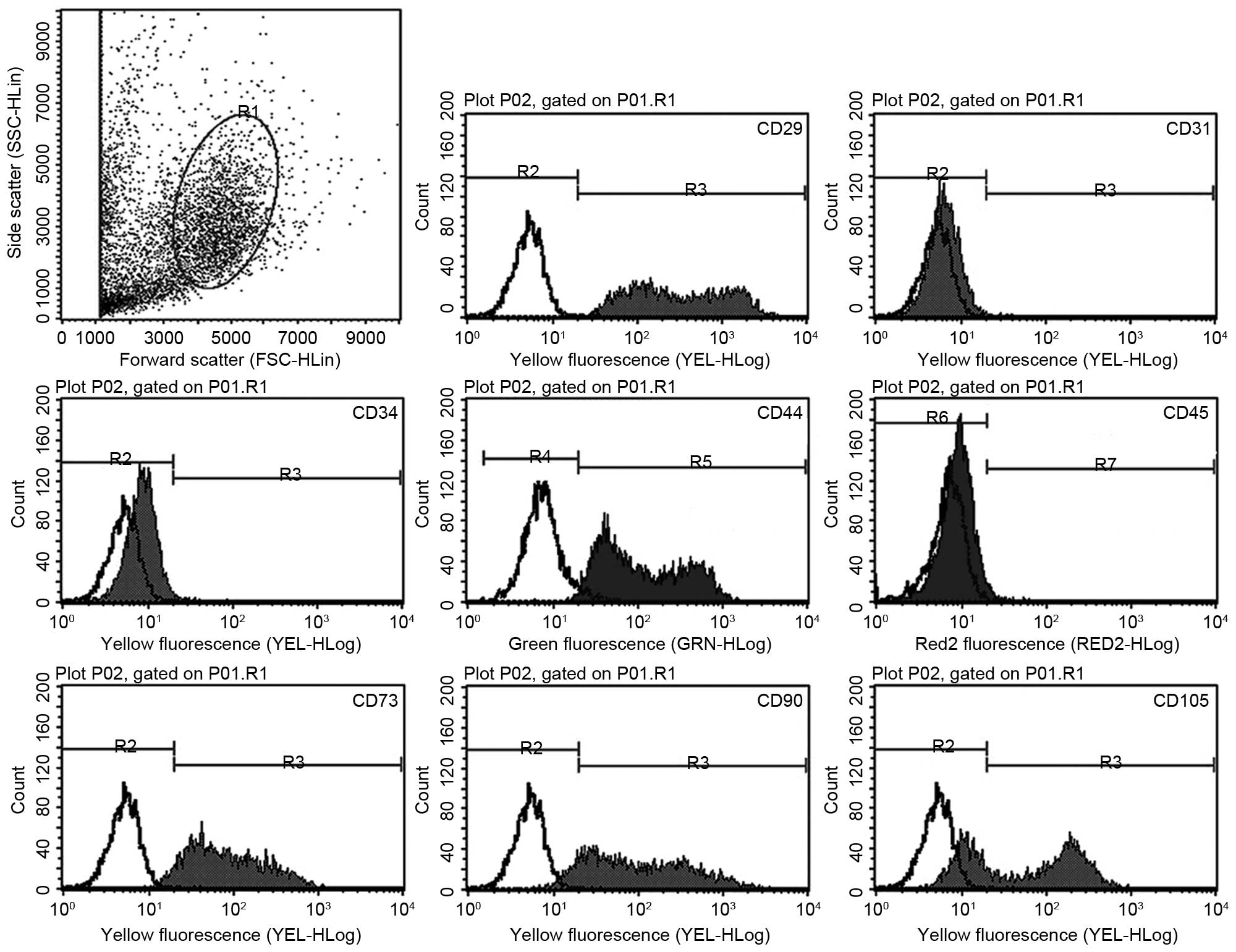
Flow cytometry results (Fig. 2) demonstrated that the UC-derived
cells exhibited a similar immunophenotype to MSCs, including
positive expression of stromal markers (CD29, CD44, CD73, CD90 and
CD105), and negative expression of endothelial (CD31) and
hematopoietic (CD34 and CD45) markers. Treatment with 0.1 ng/ml
TGF-β1 demonstrated no significant effect on the percentage of
cells expressing each marker (Table
II).
 | Table IIEffect of TGF-β1 on the
immunophenotype of umbilical cord-derived cells. |
Table II
Effect of TGF-β1 on the
immunophenotype of umbilical cord-derived cells.
| Phenotype | MSC | MSC + TGF-β1 |
|---|
| CD29 | 97.31±3.04 | 97.55±2.43 |
| CD31 | 2.79±0.75 | 2.12±1.50 |
| CD34 | 0.69±0.47 | 0.72±0.52 |
| CD44 | 79.10±4.70 | 89.96±7.80 |
| CD45 | 3.19±2.01 | 3.62±0.87 |
| CD73 | 94.96±6.11 | 95.09±2.42 |
| CD90 | 90.20±2.83 | 94.24±2.32 |
| CD105 | 66.45±5.68 | 62.70±7.39 |
Osteogenic and adipogenic differentiation
capacities of TGF-β1-treated UC-MSCs
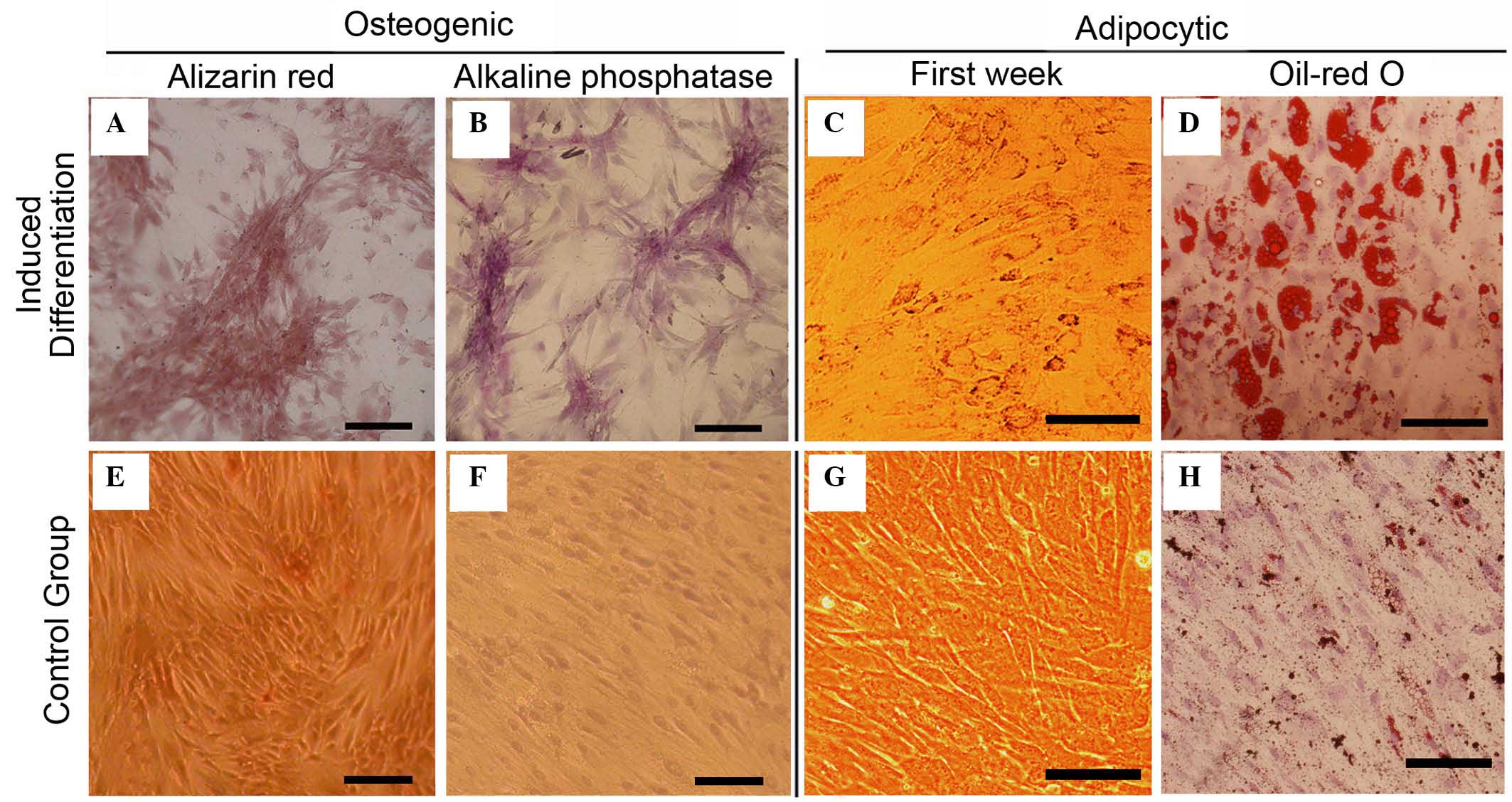
MSCs treated with TGF-β1 retained the ability to
differentiate into osteoblasts and adipocytes. When induced to
differentiate under osteogenic conditions, the TGF-β1-treated MSCs
began to grow in clusters with increasing induction time, and the
cells formed a mineralized matrix, confirmed by alizarin red
staining (Fig. 3A). The majority
of the cells became alkaline phosphatase-positive by day 14
(Fig. 3B). No mineralized matrix
was observed in cells maintained in regular growth medium (Fig. 3E). The spindle shape of
TGF-β1-treated MSCs flattened and broadened after 1 week of
adipogenic induction (Fig. 3C).
Small oil droplets gradually appeared in the cytoplasm, and after 2
weeks, almost all cells contained numerous oil red O-positive lipid
droplets (Fig. 3D). The observed
morphological changes and staining characteristics indicated that
treatment with TGF-β1 caused no affect on the ability of UC-MSCs to
undergo osteogenic and adipogenic differentiation.
Influence of TGF-β1 on the mRNA and
protein expression levels of ECM components and ECM-related
molecules
The effect of TGF-β1 on the mRNA expression levels
of ECM components and ECM-associated molecules in cultured UC-MSCs
was also investigated. RT-qPCR assays were performed following
treatment of UC-MSCs with various concentrations of TGF-β1 for 24
h. A low concentration of TGF-β1 (0.1 ng/ml) significantly
increased the expression level of five ECM genes (Col-I, Col-IV,
FN, integrin and tenascin-C; P=0.0387, 0.0219, 0.0274, 0.0402 and
0.0336, respectively), whereas TGF-β1 at 1 ng/ml significantly
increased only Col-I expression (P= 0.0057; Fig. 4A). Furthermore, higher
concentrations of TGF-β1 (1 and 10 ng/ml) significantly inhibited
LN (P=0.0004 and 0.0023, respectively) and integrin (P=0.0011 and
0.0048, respectively) expression levels (Fig. 4A). Regarding the ECM-associated
genes, including MMP-1, MMP-2 and MMP-9, the mRNA expression levels
were maximally increased by TGF-β1 at a concentration of 1 ng/ml
(Fig. 4A). Treatment with 0.1
ng/ml TGF-β1 significantly inhibited MMP-1 expression in UC-MSCs
(P=0.0012). The mRNA expression levels of TIMP-1 and -2 were not
significantly altered by TGF-β1 at any concentration (Fig. 4A). Notably, high concentrations of
TGF-β1 (10 ng/ml) did not increase the mRNA expression levels of
various ECM components and ECM-associated molecules, except
MMPs.
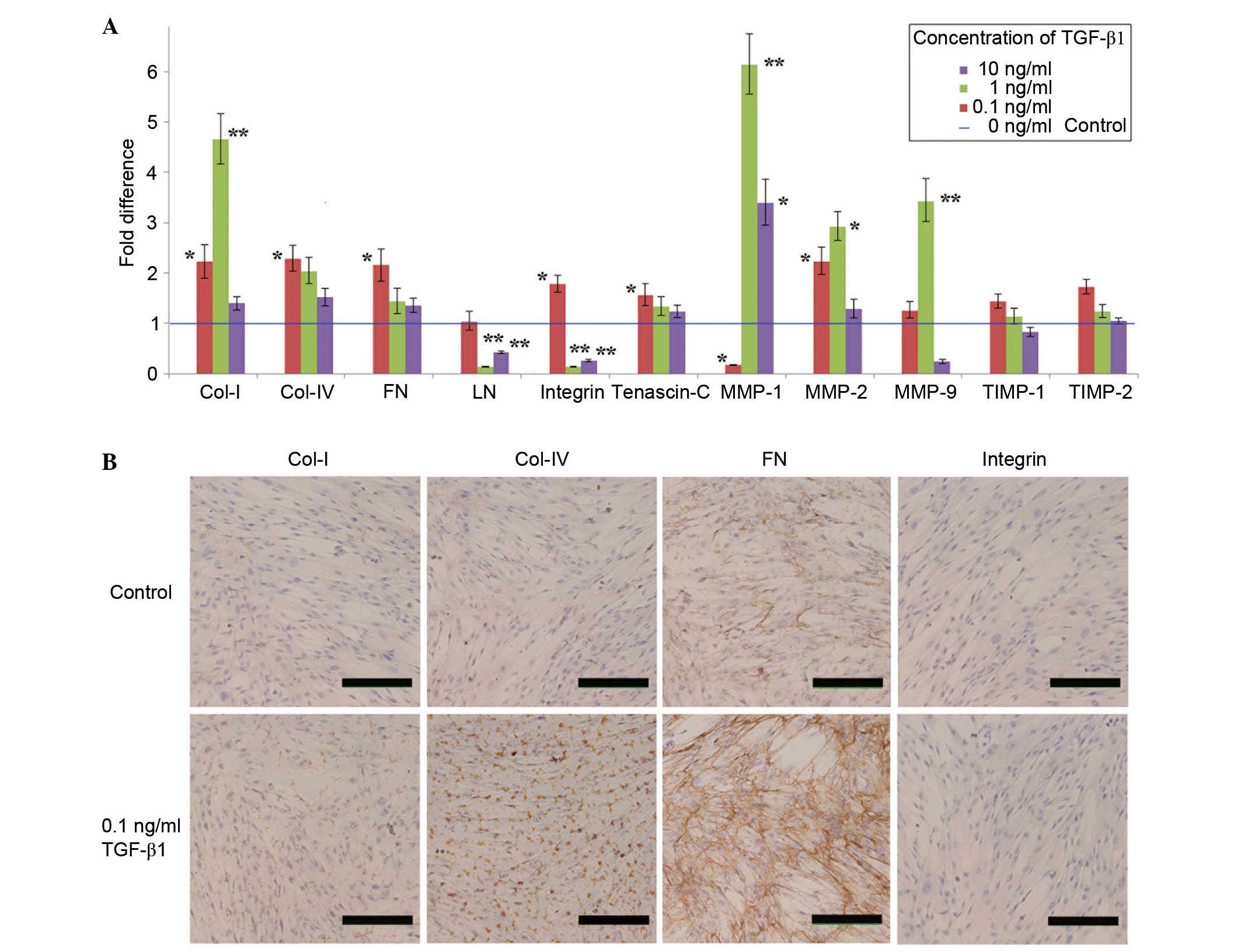 | Figure 4Effect of TGF-β1 (0.1 ng/ml) on the
expression levels of ECM components and ECM-associated molecules.
(A) The mRNA expression is presented as the fold difference with
respect to the control group, with the control values set at fold
change=1 (blue line). The data are presented as the mean ± standard
deviation of ≥3 independent experiments (*P<0.05,
**P<0.01 vs. control). (B) The effect of TGF-β1 (0.1
ng/ml) on the protein expression levels of Col-I, Col-IV, FN and
integrin. As demonstrated by the increased brown-coloured staining,
0.1 ng/ml TGF-β1 promoted the expression of Col-I, Col-IV, and
particularly FN (scale bar, 200 µm). TGF, transforming
growth factor; Col, collagen I; FN, fibronectin; MMP, matrix
metalloproteinase; TIMP, tissue inhibitor of metal-loproteinase;
ECM, extracellular matrix. |
Based on these data, a TGF-β1 concentration of 0.1
ng/ml was selected to examine the effects of TGF-β1 on ECM protein
expression. The expression levels of four ECM components (Col-I,
Col-IV, FN and integrin) were examined by immunocytochemistry prior
to and following treatment with TGF-β1 (Fig. 4B). UC-MSCs in the TGF-β1 group
maintained their morphology compared with the control group. MSCs
in the untreated control group demonstrate no immunoreactivity
toward Col-I, Col-IV or integrin. Furthermore, 0.1 ng/ml TGF-β1
increased the protein expression levels of Col-I, Col-IV and, in
particular, FN compared with the untreated control. However, TGF-β1
failed to observably increase the protein expression of
integrin.
TGF-β1 upregulates RhoA expression and
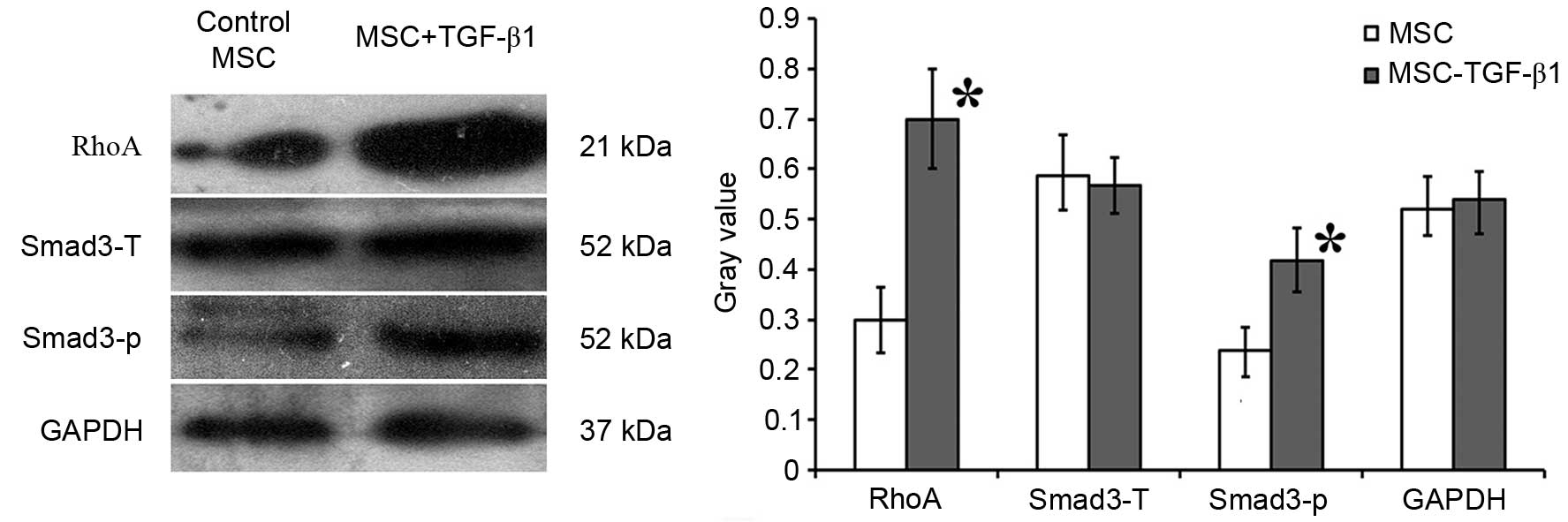
activates phosphorylation of SMAD3 in UC-MSCs
To further investigate the mechanisms underlying the
effects of TGF-β1 described above, the expression levels of RhoA
and SMAD3, which are important genes in FN synthesis, were
analyzed. Western blot analysis demonstrated that 0.1 ng/ml TGF-β1
significantly increased the expression level of RhoA compared with
control MSCs (P=0.0215), whereas the total expression level of
SMAD3 was not significantly altered. However, the levels of
phosphorylated SMAD3 were significantly higher in TGF-β1-treated
MSCs compared with the control MSCs (P=0.0240; Fig. 5).
Treatment with UC-MSCs attenuates
LPS-induced systemic injury in rats
Animals receiving LPS exhibited physical signs of
systemic illness, including lethargy, piloerection and diarrhea.
Following intraperitoneal injection of LPS, the capillaries in the
lung tissue expanded and became congested by a significant increase
in neutrophils compared with untreated rats (Fig. 6A and B). Histological assessment of
the lungs of animals injected with LPS demonstrated various
pathological changes associated with ALI, including lung tissue
hyperemia, hemorrhage, infiltration of inflammatory cells and
neutrophil accumulation. These results indicated that the rat model
of ALI was successfully developed. Additionally, the lung septa
were observably thickened, and exhibited no improvement at the 48 h
time-point (Fig. 6A and B). The
MSC + LPS and MSC + TGF-β1 + LPS groups also exhibited signs of
moderate injury, however, the severity of the injury was observably
reduced compared with the LPS alone group at the same time points
(Fig. 6C and D).
 | Figure 6Injection of untreated MSC or MSC
pre-treated with 0.1 ng/ml TGF-β1 attenuated LPS-induced lung
injury in rats. The severity of lung injury was determined from
assessments of lung oedema, lung wet/dry weight ratio, BALF
neutrophil count and BALF protein concentration. Histological
analysis of (A) normal, (B) ALI/PBS, (C) ALI/MSC and (D) ALI/MSC +
TGF-β indicated that injection of LPS caused pulmonary capillary
expansion and congestion, as well as neutrophil infiltration into
the lung tissue (ALI/PBS group) at 48 h. In addition, the lung
septa were noticeably thickened. Administration of MSC or MSC ±
TGF-β reduced the severity of the lung injury at 48 h. (E)
Injection of MSC or MSC ± TGF-β reduced pulmonary oedema induced by
LPS. Pulmonary oedema was measured as the wet-dry weight ratio. (F)
Neutrophil counts were higher in the LPS group compared with the
control group. Treatment with MSC significantly reduced the
LPS-induced increase in BALF neutrophil count at 24 h. The effects
of MSC + TGF-β were not statistically significant, although the
neutrophil count at 24 h was lower compared with that in the
ALI/PBS group. (G) BALF protein concentration increased
significantly after LPS injection, peaking at 24 h. Administration
of MSC or MSC ± TGF-β significantly attenuated the LPS-induced
increase in BALF protein concentration at 24 h.
(*P<0.05 vs. normal healthy control group;
#P<0.05 vs. ALI/PBS group). ALI, acute lung injury;
PBS, phosphate-buffered saline; LPS, lipopolysaccharide; MSC,
mesenchymal stem cells; TGF, transforming growth factor; BALF,
bronchoalveolar lavage fluid. |
The lung wet-dry weight ratio was significantly
elevated at 24 and 48 h after LPS administration compared with the
normal group (P<0.05), with the ratio marginally lower at 48 h
compared with 24 h. However, the administration of MSC or MSC +
TGF-β1 significantly attenuated these increases in lung wet-dry
weight ratio at 24 h compared with the ALI group (P=0.0148 and
0.0102, respectively), while at 48 h only MSC + TGF-β1
administration significantly attenuated the wet-dry weight ratio
compared with the ALI group (P=0.0144; Fig. 6E). LPS injection also resulted in
significant increases in the BALF neutrophil count (P=0.0207,
0.0153 and 0.0119 for 6h, 24h and 48h, respectively; Fig. 6F) and protein concentration (an
indicator of endothelial and epithelial permeability; P=0.0298,
0.0131 and 0.0243 for 6h, 24h and 48h, respectively; Fig. 6G) compared with the normal group,
both of which peaked at 24 h. The increase in BALF neutrophil count
at 24 h was significantly reduced in the MSC-treated group compared
with the ALI group (P<0.05), and although the count was also
numerically lower in the MSC + TGF-β1-treated group, this did not
reach statistical significance (Fig.
6F). Furthermore, BALF protein concentration at 24 h was
significantly lower in the MSC-treated and MSC + TGF-β1-treated
groups compared with the ALI group (P=0.0155 and 0.0130,
respectively; Fig. 6G).
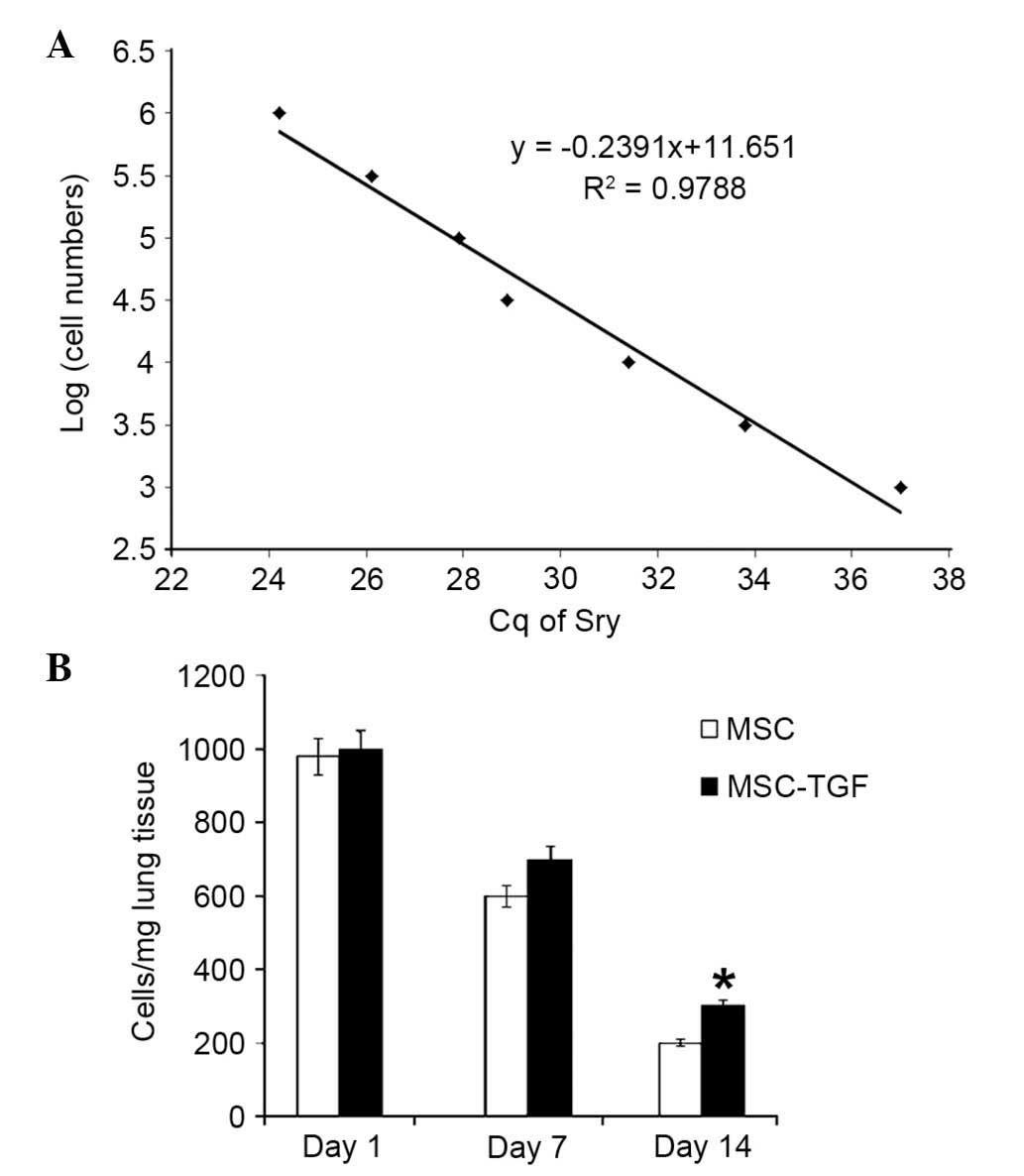
MSC survival in damaged lungs
To characterize the survival of transplanted MSCs in
damaged lungs, a calibration curve was established to calculate the
number of MSCs in the recipient lung compared with Sry gene
expression (Fig. 7A). Cq values of
the Sry gene were plotted against a semi-logarithmic scale of MSC
number to obtain a balanced contribution of all reference
dilutions. Subsequently, lungs (3/group) were harvested from rats
at 1, 7 and 14 days after their respective treatment. The number of
MSCs was calculated from the above calibration curve. The
expression of the Sry gene in the lungs at each time-point was
significantly higher in the MSC + TGF-β1 group compared with the
MSC group. TGF-β1 pre-treatment (0.1 ng/ml) significantly increased
MSC survival in damaged lungs at day 14 compared with untreated
MSCs (P=0.0326; Fig. 7B). Sry
expression was not detected in the control animals that were
administered with medium without MSCs.
Discussion
Numerous pre-clinical studies have demonstrated the
potential of using MSCs for tissue repair in type-1 diabetes
(27), ALI (28), radiation-induced injury (29) and nephropathy (30). However, the use of MSCs is limited
by their rare occurrence in BM, with MSCs constituting only
0.001-0.01% of the BM population (31). Thus, MSCs require expansion prior
to their use in tissue regeneration. A variety of growth factors,
including hepatocyte growth factor, VEGF, platelet-derived growth
factor, EGF and FGF, have previous been used to amplify MSCs
(32). The present study
successfully isolated, cultured and expanded MSCs from hUC using
three cytokines (EGF, VEGF and FGF). It was additionally
demonstrated that 0.1 ng/ml TGF-β1 promoted UC-MSC proliferation.
This finding is in accordance with a previous study that reported
that low levels of TGF-β1 promotes MSC proliferation in the
presence of fetal calf serum (33). TGF-β1 inhibits the proliferation of
the majority of cell types, including epithelial cells, endothelial
cells, embryonic fibroblasts and hematopoietic cells (34). By contrast, TGF-β1 stimulates the
proliferation of mesenchymal cells (35). However, the mechanisms responsible
for the effects of low TGF-β1 concentrations on MSC proliferation
remain to be fully elucidated. The results of the present study
demonstrate that higher TGF-β1 concentrations do not promote MSC
proliferation. Thus, it is speculated that TGF-β1 may stimulate
different signaling pathways in MSCs, in a manner that is tightly
regulated and concentration-dependent.
Subsequent experiments of the present study
demonstrated that low doses of TGF-β1 cause no affect on the
phenotype of the UC-MSCs, with no significant changes demonstrated
in any of the markers assessed. Similarly, the in vitro
differentiation capacity of UC-MSCs was not affected by a low
concentration of TGF-β1, with TGF-β1-treated UC-MSCs retaining
adipogenic and osteogenic differentiation potential. Additionally,
low-concentration TGF-β1-treated UC-MSCs were demonstrated to
perform a function in cell therapy.
Further analyses in the present study demonstrated
that a low concentration of TGF-β1 increases the mRNA expression
level of several ECM components and their regulatory elements,
including MMPs and TIMPs, in UC-MSCs. The ECM is an intricate
network of proteins surrounding cells and performs a central
function in establishing the stem cell phenotype. The composition
and mechanical properties of the ECM can affect cell shape and stem
cell differentiation (36). The
predominant fibrillar components of the ECM can be divided into two
groups: Collagens and cell-adhesive glycoproteins (e.g. FN)
(34). The PCR and
immunocytochemical analyses of the present study demonstrated that
the expression levels of Col-I, Col-IV and FN were enhanced by 0.1
ng/ml TGF-β1. Notably, FN exhibited the greatest upregulation in
response to the low concentration of TGF-β1. FN is a multidomain
protein that contains binding sites for integrins, col and other
ECM proteins (37,38). The multidomain structure of these
proteins provides a mechanism for connecting cells to the ECM
network. FN primarily supports mesenchymal cell migration during
embryonic development (39).
Additionally, FN signals support cell survival and growth (40), and may contribute to cell viability
during differentiation. For example, hematopoietic stem cells
express α4β1 integrin, which binds to FN and receptors on
neighboring cells (41). Thus,
this receptor can tether stem cells to the ECM and support cells
within the BM microenvironment. Tenascin-C, a multimeric ECM
protein, is also a stem cell marker present in the BM (42). Tenascin-C binds to FN and modulates
cell adhesion (43). Notably, the
PCR results of the present study also demonstrated increased
expression levels of tenascin-C following treatment with 0.1 ng/ml
TGF-β1.
SMAD signaling is a major pathway stimulated by
TGF-β1 in the regulation of the ECM. In the signaling cascade,
activated TGF-β receptor 1 induces the phosphorylation and
activation of SMAD2 and 3. The activated SMAD2/3 complex forms
oligomers with SMAD4, which translocate into the nucleus to
regulate the expression levels of target ECM genes (44). Additionally, the RhoA/Rho-kinase
pathway is also involved in TGF-β1-induced synthesis of FN and
Col-I (45). The western blot
results of the present study demonstrated that the expression
levels of RhoA and phosphorylated SMAD3 were increased in the
TGF-β1-treated UC-MSCs. This data suggested that these signaling
pathways may be involved in the regulation of MSCs by the ECM.
To investigate whether pre-treatment of MSCs with
0.1 ng/ml TGF-β1 demonstrated any benefit when the cells were used
as a therapeutic intervention in vivo, a rat model of
endotoxemia was induced. This model was generated using
intraperitoneal injection of LPS to simulate sepsis-associated lung
injury, and the effects of TGF-β1-treated and untreated UC-MSCs on
ALI were observed. As previously demonstrated in several animal
studies, MSCs can alleviate LPS-induced ALI and improve survival by
restoring lung function through anti-inflammatory, antiapoptotic
and immune regulatory actions (46–48).
Histological analysis from the present study demonstrated that
intravenous injection of control UC-MSCs or TGF-β1-pre-treated
UC-MSCs 1 h after endotoxin injury ameliorates LPS-induced lung
injury. Furthermore, rats administered with TGF-β1 or untreated
UC-MSCs demonstrated less prominent elevations in BALF neutrophil
count and protein content (indicative of epithelial permeability)
in response to LPS. Additionally, measurements of lung wet-dry
weight ratio indicated that therapy with TGF-β1 or untreated
UC-MSCs resulted in reduced pulmonary edema. However, no clear
differences were demonstrated in the therapeutic benefits of
TGF-β1-treated and untreated UC-MSCs, indicating that pre-treatment
of UC-MSCs with a low concentration of TGF-β1 provided no
additional benefits in this model of ALI over the short-term (48
h). However, it cannot be excluded that TGF-β1-treated UC-MSCs
provided longer-term advantages due to improved survival.
Transplanted MSCs can be identified in the damaged
tissue area through the use of an eGFP marker or
4′,6-diamidino-2-phenylindole staining. However, given the
autofluorescence of pulmonary vascular endothelial cells, comparing
differences in survival between various MSCs is challenging. A
previous study has evaluated stem cell survival by observing Sry
gene expression in the heart (26). Since the Sry gene is located on the
Y-chromosome, gene-positive stem cells can be inferred to be of
male origin. In the present study, qPCR results demonstrated that
pre-treatment with TGF-β1 significantly increased the number of
UC-MSCs in the lung 2 weeks after transplantation. This novel
finding indicated that pre-treatment with 0.1 ng/ml TGF-β1 enhances
MSC survival in vivo. Taken together with the other
observations of the present study, it is speculated that enhanced
expression levels of ECM components in UC-MSCs by 0.1 ng/ml TGF-β1,
particularly the upregulation of FN, is favorable for their
survival in vivo, and thus may augment their longer term
therapeutic benefit when used for tissue repair.
In conclusion, pre-treatment with 0.1 ng/ml TGF-β1
resulted in the upregulation of FN and other ECM components in
MSCs, and improved the survival of engrafted MSCs in a rat model of
LPS-induced ALI. Furthermore, as TGF-β1-treated MSCs retain
multilineage differentiation and tissue repair functions, these
cells may demonstrate promise in regenerative medicine and adoptive
stem cell therapy.
Acknowledgments
The present study was supported by the Major State
Basic Research Development Program of China (no. 2012CB966504), the
Shandong Province Natural Science Foundation (no. 2013GSF11812),
the Jinan Science and Technology Development Plan (no. 201403010)
and the Innovation Fund Project of Shandong University (no.
2014QLKY02).
Abbreviations:
|
ALI
|
acute lung injury
|
|
ALP
|
alkaline phosphatase
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
BM
|
bone marrow
|
|
Col-1
|
collagen I
|
|
Col-IV
|
collagen IV
|
|
ECM
|
extracellular matrix
|
|
EGF
|
epidermal growth factor
|
|
FGF
|
fibroblast growth factor
|
|
FN
|
fibronectin
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
hUC-MSC
|
human umbilical cord mesenchymal stem
cell
|
|
LN
|
laminin
|
|
LPS
|
lipopolysaccharide
|
|
MMP
|
matrix metalloproteinase
|
|
MSC
|
mesenchymal stem cell
|
|
SMC
|
smooth muscle cell
|
|
Sry
|
sex-determining region Y
|
|
TGF-β1
|
transforming growth factor beta-1
|
|
TIMP
|
tissue inhibitor of
metalloproteinase
|
|
UC
|
umbilical cord
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Cir Res.
95:9–20. 2004. View Article : Google Scholar
|
|
2
|
Wu KH, Tsai C, Wu HP, Sieber M, Peng CT
and Chao YH: Human application of ex vivo expanded umbilical
cord-derived mesenchymal stem cells: Enhance hematopoiesis after
cord blood transplantation. Cell Transplant. 22:2041–2051. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Blanc K, Rasmusson I, Sundberg B,
Götherström C, Hassan M, Uzunel M and Ringdén O: Treatment of
severe acute graft-versus-host disease with third party
haploidentical mesenchymal stem cells. Lancet. 363:1439–1441. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sudres M, Norol F, Trenado A, Grégoire S,
Charlotte F, Levacher B, Lataillade JJ, Bourin P, Holy X, Vernant
JP, et al: Bone marrow mesenchymal stem cells suppress lymphocyte
proliferation in vitro but fail to prevent graft-versus-host
disease in mice. J Immunol. 176:7761–7767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marion NW and Mao JJ: Mesenchymal stem
cells and tissue engineering. Methods Enzymol. 420:339–361. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi S, Bartold PM, Miura M, Seo BM, Robey
PG and Gronthos S: The efficacy of mesenchymal stem cells to
regenerate and repair dental structures. Orthod Craniofac Res.
8:191–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fiedler J, Etzel N and Brenner RE: To go
or not to go: Migration of human mesenchymal progenitor cells
stimulated by isoforms of PDGF. J Cell Biochem. 93:990–998. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao J and Caplan AI: Mesenchymal stem
cells and tissue engineering for orthopaedic surgery. Chir Organi
Mov. 88:305–316. 2003.In English, Italian.
|
|
9
|
Tuan RS, Boland G and Tuli R: Adult
mesenchymal stem cells and cell-based tissue engineering. Arthritis
Res Ther. 5:32–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsiao ST, Asgari A, Lokmic Z, Sinclair R,
Dusting GJ, Lim SY and Dilley RJ: Comparative analysis of paracrine
factor expression in human adult mesenchymal stem cells derived
from bone marrow, adipose, and dermal tissue. Stem Cells Dev.
21:2189–2203. 2012. View Article : Google Scholar :
|
|
11
|
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X,
Gong W, Han ZB, Xu ZS, Lu YX, Liu D, et al: Isolation and
characterization of human umbilical cord mesenchymal stem cells
with hematopoiesis-supportive function and other potentials.
Haematologica. 91:1017–1026. 2006.PubMed/NCBI
|
|
12
|
Liu S, Yuan M, Hou K, Zhang L, Zheng X,
Zhao B, Sui X, Xu W, Lu S and Guo Q: Immune characterization of
mesenchymal stem cells in human umbilical cord Wharton's jelly and
derived cartilage cells. Cell Immunol. 278:35–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng J, Wang Y, Zhang L, Zhao B, Zhao Z,
Chen J, Guo Q, Liu S, Sui X, Xu W and Lu S: Human umbilical cord
Wharton's jelly-derived mesenchymal stem cells differentiate into a
Schwann-cell phenotype and promote neurite outgrowth in vitro.
Brain Res Bull. 84:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamama K, Kawasaki H and Wells A:
Epidermal growth factor (EGF) treatment on multipotential stromal
cells (MSCs). Possible enhancement of therapeutic potential of MSC.
J Biomed Biotechnol. 2010:7953852010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Freyman T, Polin G, Osman H, Crary J, Lu
M, Cheng L, Palasis M and Wilensky RL: A quantitative, randomized
study evaluating three methods of mesenchymal stem cell delivery
following myocardial infarction. Eur Heart J. 27:1114–1122. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song H, Song BW, Cha MJ, Choi IG and Hwang
KC: Modification of mesenchymal stem cells for cardiac
regeneration. Expert Opin Biol Ther. 10:309–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thibault MM, Hoemann CD and Buschmann MD:
Fibronectin, vitronectin, and collagen I induce chemotaxis and
haptotaxis of human and rabbit mesenchymal stem cells in a
standardized transmembrane assay. Stem Cells Dev. 16:489–502. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Basile DP, Martin DR and Hammerman MR:
Extracellular matrix-related genes in kidney after ischemic injury:
Potential role for TGF-beta in repair. Am J Physiol. 275:F894–F903.
1998.PubMed/NCBI
|
|
19
|
Lee JH, Yu HS, Lee GS, Ji A, Hyun JK and
Kim HW: Collagen gel three-dimensional matrices combined with
adhesive proteins stimulate neuronal differentiation of mesenchymal
stem cells. J R Soc Interface. 8:998–1010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Callaghan CJ and Williams B: Mechanical
strain-induced extracellular matrix production by human vascular
smooth muscle cells: Role of TGF-beta(1). Hypertension. 36:319–324.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee YC and Rannels DE: Regulation of
extracellular matrix synthesis by TNF-alpha and TGF-beta1 in type
II cells exposed to coal dust. Am J Physiol. 275:L637–L644.
1998.PubMed/NCBI
|
|
22
|
Baarsma HA, Menzen MH, Halayko AJ, Meurs
H, Kerstjens HA and Gosens R: β-Catenin signaling is required for
TGF-β1-induced extracellular matrix production by airway smooth
muscle cells. Am J Physiol Lung Cell Mol Physiol. 301:L956–L965.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ng F, Boucher S, Koh S, Sastry KS, Chase
L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS and Tanavde
V: PDGF, TGF-beta, and FGF signaling is important for
differentiation and growth of mesenchymal stem cells (MSCs):
Transcriptional profiling can identify markers and signaling
pathways important in differentiation of MSCs into adipogenic,
chondrogenic, and osteogenic lineages. Blood. 112:295–307. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jian H, Shen X, Liu I, Semenov M, He X and
Wang XF: Smad3-dependent nuclear translocation of beta-catenin is
required for TGF-beta1-induced proliferation of bone marrow-derived
adult human mesenchymal stem cells. Genes Dev. 20:666–674. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park WS, Heo SC, Jeon ES, Hong da H, Son
YK, Ko JH, Kim HK, Lee SY, Kim JH and Han J: Functional expression
of smooth muscle-specific ion channels in TGF-β(1) -treated human
adipose-derived mesenchymal stem cells. Am J Physiol Cell Physiol.
305:C377–C391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang F, Zhu X, Hu XQ, Fang ZF, Tang L, Lu
XL and Zhou SH: Mesenchymal stem cells modified with miR-126
release angiogenic factors and activate Notch ligand Delta-like-4,
enhancing ischemic angiogenesis and cell survival. Int J Mol Med.
31:484–492. 2013.
|
|
27
|
Jurewicz M, Yang S, Augello A, Godwin JG,
Moore RF, Azzi J, Fiorina P, Atkinson M, Sayegh MH and Abdi R:
Congenic mesenchymal stem cell therapy reverses hyperglycemia in
experimental type 1 diabetes. Diabetes. 59:3139–3147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Curley GF, Hayes M, Ansari B, Shaw G, Ryan
A, Barry F, O'Brien T, O'Toole D and Laffey JG: Mesenchymal stem
cells enhance recovery and repair following ventilator-induced lung
injury in the rat. Thorax. 67:496–501. 2012. View Article : Google Scholar
|
|
29
|
Hu KX, Sun QY, Guo M and Ai HS: The
radiation protection and therapy effects of mesenchymal stem cells
in mice with acute radiation injury. Br J Radiol. 83:52–58. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zoja C, Garcia PB, Rota C, Conti S,
Gagliardini E, Corna D, Zanchi C, Bigini P, Benigni A, Remuzzi G
and Morigi M: Mesenchymal stem cell therapy promotes renal repair
by limiting glomerular podocyte and progenitor cell dysfunction in
adriamycin-induced nephropathy. Am J Physiol Renal Physiol.
303:F1370–F1381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rodrigues M, Griffith LG and Wells A:
Growth factor regulation of proliferation and survival of
multipotential stromal cells. Stem Cell Res Ther. 1:322010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Locklin RM, Oreffo RO and Triffitt JT:
Effects of TGFbeta and bFGF on the differentiation of human bone
marrow stromal fibroblasts. Cell Biol Int. 23:185–194. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang SS and Huang JS: TGF-beta control of
cell proliferation. J Cell Biochem. 96:447–462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roberts AB: Molecular and cell biology of
TGF-beta. Miner Electrolyte Metab. 24:111–119. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singh P and Schwarzbauer JE: Fibronectin
and stem cell differentiation-lessons from chondrogenesis. J Cell
Sci. 125:3703–3712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Singh P, Carraher C and Schwarzbauer JE:
Assembly of fibronectin extracellular matrix. Ann Rev Cell Dev
Biol. 26:397–419. 2010. View Article : Google Scholar
|
|
38
|
Yurchenco PD and Patton BL: Developmental
and pathogenic mechanisms of basement membrane assembly. Curr Pharm
Des. 15:1277–1294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
George EL, Georges-Labouesse EN,
Patel-King RS, Rayburn H and Hynes RO: Defects in mesoderm, neural
tube and vascular development in mouse embryos lacking fibronectin.
Development. 119:1079–1091. 1993.PubMed/NCBI
|
|
40
|
Schwartz MA and Assoian RK: Integrins and
cell proliferation: Regulation of cyclin-dependent kinases via
cytoplasmic signaling pathways. J Cell Sci. 114:2553–2560.
2001.PubMed/NCBI
|
|
41
|
Whetton AD and Graham GJ: Homing and
mobilization in the stem cell niche. Trends Cell Biol. 9:233–238.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Klein G, Beck S and Müller CA: Tenascin is
a cytoadhesive extracellular matrix component of the human
hematopoietic microenvironment. J Cell Biol. 123:1027–1035. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hsia HC and Schwarzbauer JE: Meet the
tenascins: Multifunctional and mysterious. J Biol Chem.
280:26641–26644. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signaling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Itoh Y, Kimoto K, Imaizumi M and Nakatsuka
K: Inhibition of RhoA/Rho-kinase pathway suppresses the expression
of type I collagen induced by TGF-beta2 in human retinal pigment
epithelial cells. Exp Eye Res. 84:464–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gupta N, Su X, Popov B, Lee JW, Serikov V
and Matthay MA: Intrapulmonary delivery of bone marrow-derived
mesenchymal stem cells improves survival and attenuates
endotoxin-induced acute lung injury in mice. J Immunol.
179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu J, Woods CR, Mora AL, Joodi R, Brigham
KL, Iyer S and Rojas M: Prevention of endotoxin-induced systemic
response by bone marrow-derived mesenchymal stem cells in mice. Am
J Physiol Lung Cell Mol Physiol. 293:L131–L141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rojas M, Xu J, Woods CR, Mora AL, Spears
W, Roman J and Brigham KL: Bone marrow-derived mesenchymal stem
cells in repair of the injured lung. Am J Respir Cell Mol Biol.
33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|