Introduction
MS is a progressive autoimmune demyelinating
disorder of the central nervous system (CNS), which is mediated via
various inflammatory cells and cytokines. Experimental autoimmune
encephalomyelitis (EAE) has been universally acknowledged as an
animal model for MS, as it has similar clinical and
neuropathological features (1). In
MS/EAE, once activated, circulating T cells travel from the
periphery to the CNS and generate large amounts of proinflammatory
cytokines. Then microglia and invaded macrophages are consequently
activated, and stimulate autoimmune reactions, leading to myelin
damage. CD4+ T cells are the predominant cell type
involved in the pathology of MS/EAE. Mice without functional
CD4+ T cells do not develop the relevant clinical signs
of disease (2). CD8+ T
cells also accumulate and activate microglia to an extent, causing
tissue damage during CNS autoimmunity (3). In the CNS, Mac-1 is expressed
predominantly on the surface of resident microglia cells and
infiltrating inflammatory macrophages, and therefore used to
identify activated microglia/macrophages in EAE (4).
The interleukin (IL)-23/IL-17 axis performs
important functions in MS pathogenesis. IL-23 is predominantly
secreted from activated macrophages/microglia and dendritic cells
(5), inducing Th0 cell
differentiation into Th17 cells (6). This type of shift facilitates CNS
inflammation and the development of EAE. Th17 cells are
characterized by the secretion of IL-17. IL-23 and IL-17 in the
serum and CNS have been reported to serve an important role in the
pathology and immunotherapy of MS (7).
MS is a debilitating disease with high disability
and recurrence rates and there are over one million people
worldwide suffering from the disease (8). The treatment of MS is limited to
chemically synthesized immunomodulatory or immunosuppressive
reagents, which are not always effective and are often associated
with severe side-effects (9).
Thus, the identification of more effective and safe agents is
urgently required. Salvia miltiorrhiza, a Chinese herbal
medicine, has traditionally been used to treat cardiovascular and
cerebrovascular diseases (10,11).
Tanshinone IIA (TSIIA), its major bioactive constituent, has been
shown to exert immunomodulatory effects on various immune cells and
cytokines, with anti-inflammatory, anti-oxidative and
neuroprotective functions (12).
TSIIA has been shown to exert a therapeutic effect in
neurodegenerative diseases, such as Parkinson's (13) and Alzheimer's disease (14). It has also been shown to be
effective in inflammatory and autoimmunity diseases, including
acute lung inflammation (15),
sepsis (16) and systemic
sclerosis (17).
Considering these findings, the present study
examined the hypothesis that TSIIA can be effectively used for EAE
treatment. To the best of our knowledge, this is the first study to
demonstrate that TSIIA alleviates EAE by downregulating the
IL-23/IL-17 inflammatory pathway and reducing the infiltration of
immune cell populations supporting its potential as an effective
therapeutic agent for MS.
Materials and methods
Reagents and animals
Mycobacterium tuberculosis H37Ra was
purchased from Difco Laboratories, Inc. (Detroit, MI, USA). TSIIA
was obtained from Xi'an Guan Sheng Yuan Co. Ltd. (Xi'an, China).
Complete Freund's adjuvant (CFA), Luxol Fast Blue (LFB) and
protease inhibitors were purchased from Sigma-Aldrich (St. Louis,
MO, USA). Rat IL-17 (SEA063Ra) and rat IL-23 (SEA384Ra)
enzyme-linked immunosor-bent assay (ELISA) kits were obtained from
Cloud-Clone Corp. (Wuhan, China). Mouse anti-β-actin monoclonal
antibody (sc-130300), rabbit anti-CD4 polyclonal antibody
(sc-7219), rabbit anti-CD8 (sc-7188) polyclonal antibody,
peroxidase-conjugated goat anti-rabbit secondary antibody, luminol
reagent and radioimmunoprecipitation assay (RIPA) buffer were
purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA).
Rabbit anti-Mac-1 polyclonal antibody (DF6476) and rabbit
anti-IL-17 polyclonal antibody (DF6127) were obtained from Affinity
Biosciences (Cincinnati, OH, USA). Rabbit anti-IL-23 polyclonal
antibody (bs-18146R) was purchased from Beijing Bioss Biological
Technology Co., Ltd. (Beijing, China). Biotin-labeled anti-rabbit
IgG (SP KIT-C3) was purchased from Beijing Dingguo Changsheng
Biotechnology Co., Ltd. (Beijing, China). The goat anti-mouse
secondary antibody (SA00001-1) and peroxidase-conjugated goat
anti-rabbit secondary antibody (SA00001-2) were purchased from
Proteintech Group, Inc. (Chicago, IL, USA). The bicinchoninic acid
protein assay kit was purchased from Novagen Inc. (Madison, WI,
USA). polyvinylidene difluo-ride membranes were acquired from
Millipore (Billerica, MA, USA). Image-Pro Plus 6.0 was purchased
from Media Cybernetics (Rockville, MD, USA). Sodium dodecyl sulfate
(SDS) gels for electrophoresis were purchased from ZSGB-BIO Co.
Ltd. (Beijing, China). The Electrophoresis Gel Imaging Analysis
system was obtained from DNR Bio-Imaging Systems Ltd. (Jerusalem,
Israel). ImageJ 1.36 software was purchased from National
Institutes of Health (Bethesda, MD, USA). GraphPad PRISM 6.0
software was obtained from GraphPad Software, Inc. (La Jolla, CA,
USA). In total, 40 female Sprague-Dawley (SD) rats (6-8 weeks,
180–200 g) and 10 guinea pigs (4–5 weeks, 250–350 g) were obtained
from the Experimental Animal Center of China Medical University
(Shenyang, China). All the animals were kept under pathogen-free
conditions in the Experimental Animal Center of China Medical
University.
EAE induction
With the approval of the Bioethics Committee of
China Medical University, the EAE model was established by
following standard universally accepted procedures (18). In brief, guinea pig spinal cord
homogenate was mixed with same amount of CFA, which contained 5
mg/ml Mycobacterium tuberculosis H37Ra. Each rat received a
subcutaneous injection into the back and the hind footpads of 0.5
ml mixture to induce EAE. The day of immunization was regarded as
Day 0 postimmunization (p.i.).
TSIIA treatment and EAE assessment
Forty rats were separated at random into four
groups. Phosphate-buffered saline (PBS) (5 ml/kg) with dimethyl
sulfoxide (DMSO) (5%) and Tween-80 (5%) was used as a drug solvent.
Ten non-EAE rats that administered solvent intraperitoneally (i.p.)
every day beginning from day 0 p.i. served as the naive group. Ten
EAE rats administered i.p. injection of equal volume of solvent
every day served as the vehicle group. In the last two groups, EAE
was induced and rats were administered two different TSIIA
concentrations i.p. TSIIA was dissolved in solvent at low (25
mg/kg; TSIIA-L group) and high (50 mg/kg; TSIIA-H group)
concentrations, respectively. From day 0 p.i. the body weight of
all rats was measured daily and clinical signs were also evaluated
by two independent observers using the scale shown in Table I (19).
 | Table IClinical signs scales. |
Table I
Clinical signs scales.
| Clinical score | Clinical sign |
|---|
| 0 | No clinical
score |
| 1 | Loss of tail
tone |
| 2 | Hindlimb
weakness |
| 3 | Hindlimb
paralysis |
| 4 | Forelimb
paralysis |
| 5 | Moribund or
death |
Histopathological assessment
On day 18 p.i., all the rats were sacrificed by
transcardial perfusion of PBS (pH 7.4) through the left ventricle
under anesthesia with injection of 10% chloral hydrate (3 ml/kg;
Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) into the
abdominal cavity, and spinal cord and brain samples were obtained.
Lumbar enlargement of the spinal cord and right-hand side of the
brain were paraformalde-hyde-fixed and paraffin-embedded.
Subsequently, 3-µm slices of brain were sectioned and
stained with hematoxylin-eosin (H&E) to evaluate inflammatory
infiltration. In addition, 3-µm slices of spinal cord were
sectioned and stained with LFB to evaluate demyelination.
Histopathological assessment was performed in a blinded according
to Table II (19).
 | Table IIHistopathological assessment. |
Table II
Histopathological assessment.
| Score | Inflammation | Demyelination |
|---|
| 0 | No inflammatory
cells | None |
| 1 | A few scattered
inflammatory cells | Rare foci |
| 2 | Organization of
inflammatory infiltrates around blood vessels | A few areas of
demyelination |
| 3 | Extensive
perivascular cuffing with extension into adjacent parenchyma or
parenchyma | Large (confluent)
areas of demyelination |
Immunohistochemical analysis of CD4, CD8,
Mac-1, IL-17 and IL-23
Slices (3 µm) of brain and spinal cord were
used for immunohistochemical staining. After deparaffinization with
xylene and washing with PBS, sections of spinal cords were
incubated with rabbit antibodies specific for CD4 (diluted 1:200),
CD8 (diluted 1:200), Mac-1 (diluted 1:200), IL-17 (diluted 1:200)
and IL-23 (diluted 1:200). Biotin-labeled anti-rabbit IgG was used
as a secondary antibody for the detection of primary antibodies.
Color was developed with DAB. CD4-, CD8- and Mac-1-positive cells
were counted under 400-fold magnification in the ventricornu of
spinal cord sections. Expression of IL-23 and IL-17 was assessed
based on the integral optical density (IOD) of positive cells under
200-fold magnification in a restricted area of brain sections. Five
fields in the restricted area were randomly selected for
calculations. All measurements and data analysis were performed
independently by two pathologists in a blinded manner. Morphometric
analysis was conducted using Image-Pro Plus 6.0.
Western blot analysis
Brain and spinal cord from each group was
respectively homogenized in lysis buffer with protease inhibitors
and RIPA for protein extraction. Tissue homogenate was centrifuged
at 12,000 × g for 10 min at 4°C to obtain the supernatants. The BCA
protein assay kit was used to measure protein concentrations.
Protein samples (30 µg/well) were subjected to
electrophoresis on 10% SDS-PAGE gel and elec-trotransferred onto a
PVDF membrane. After blocking with 5% non-fat dry milk in
Tris-buffered saline with 0.05% Tween-20 (TBST) for 2 h at normal
temperature, the membranes were separately incubated with rabbit
anti-CD4 (diluted 1:500), rabbit anti-CD8 (diluted 1:500), rabbit
anti-Mac-1 (diluted 1:300), rabbit anti-IL-17 (diluted 1:500),
rabbit anti-IL-23 (diluted 1:500) and mouse anti-β-actin (diluted
1:1,000) overnight at 4°C. The following day, membranes were washed
three times with TBST (5 min/wash), then were incubated with
peroxidase-conjugated goat anti-rabbit secondary or goat anti-mouse
secondary (diluted 1:2,000) antibody for 2 h. Following incubation,
the membranes were washed three times with TBST (5 min/wash). Bands
were treated with Luminol reagent for 1 min and visualized using
the Electrophoresis Gel Imaging Analysis system. Band density was
calculated via Image J 1.36 software. Protein bands were compared
with that of β-actin to determine the relative expression level of
target protein.
ELISA analysis of IL-17 and IL-23
On day 18 p.i., all the rats were sacrificed by
transcardial perfusion of PBS (pH 7.4) through the left ventricle
under anesthesia with injection of 10% chloral hydrate (3 ml/kg)
into the abdominal cavity, and blood was collected via
retro-orbital bleeding to collect the serum. IL-17 and IL-23
concentrations were detected using ELISA according to the
specifications strictly.
Statistical analysis
The results are presented as the mean ± standard
deviation. GraphPad PRISM 6.0 software (GraphPad Software, Inc., La
Jolla, CA, USA) was utilized to conduct all statistical analyses.
Multiple comparisons were performed by Kruskal-Wallis test or
one-way analysis of variance, followed by the least significant
differences test, as appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
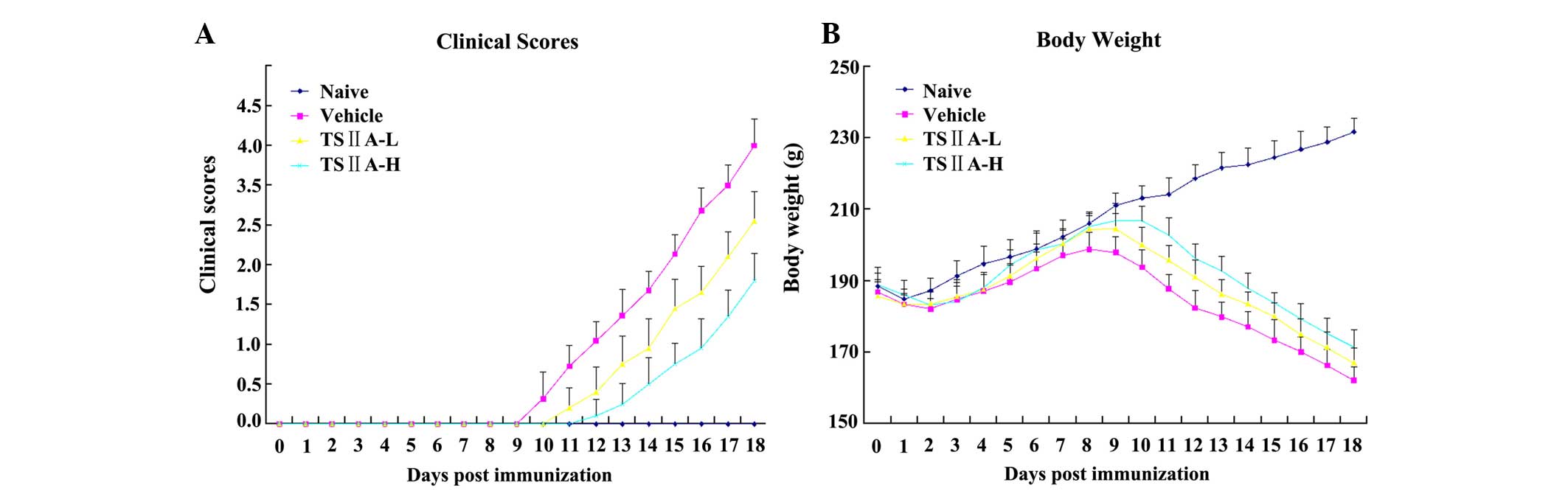
TSIIA treatment relieves clinical
signs
Clinical signs of EAE development in rats from the
vehicle group began to appear on day 9 p.i., including loss of
appetite, reduced physical activity, and tail and limb paralysis.
However, EAE onset in TSIIA-treated rats occurred on day 11 p.i.
(TSIIA-H) and 10 p.i. (TSIIA-L). Compared with the vehicle group,
the two TSIIA-treated groups received significantly lower clinical
scores (P<0.01). Significant differences were also observed
between the treated groups, which were dose-dependent (P<0.01;
Fig. 1A). Furthermore, the body
weights of untreated EAE rats were significantly decreased,
compared with the naive and TSIIA-treated rats (all P<0.01)
while those of TSIIA-treated groups were only marginally reduced,
with the smallest recorded weight loss in the TSIIA-H group.
Significant differences in body weight were observed between the
two treatment groups (P<0.01; Fig.
1B).
TSIIA treatment improves CNS
histopathology
Since inflammatory cell invasion and CNS
demyelination are the key characteristics of EAE, the impact of
TSIIA on these parameters was verified. Consistent with clinical
scores, rats in the vehicle group exhibited typical inflammatory
cell infiltration in the brain, as determined via H&E staining.
This cell infiltration was dose dependently attenuated following
TSIIA treatment (Fig. 2A).
Similarly, LFB staining revealed large areas of demyelination in
the spinal cord of rats from the vehicle group, which were
significantly decreased in the treated groups (both P<0.01;
Fig. 2B). These results clearly
indicate a beneficial effect of TSIIA in reducing inflammatory cell
infiltration and demyelination, which provide the basement of
mitigated clinical signs following TSIIA treatment.
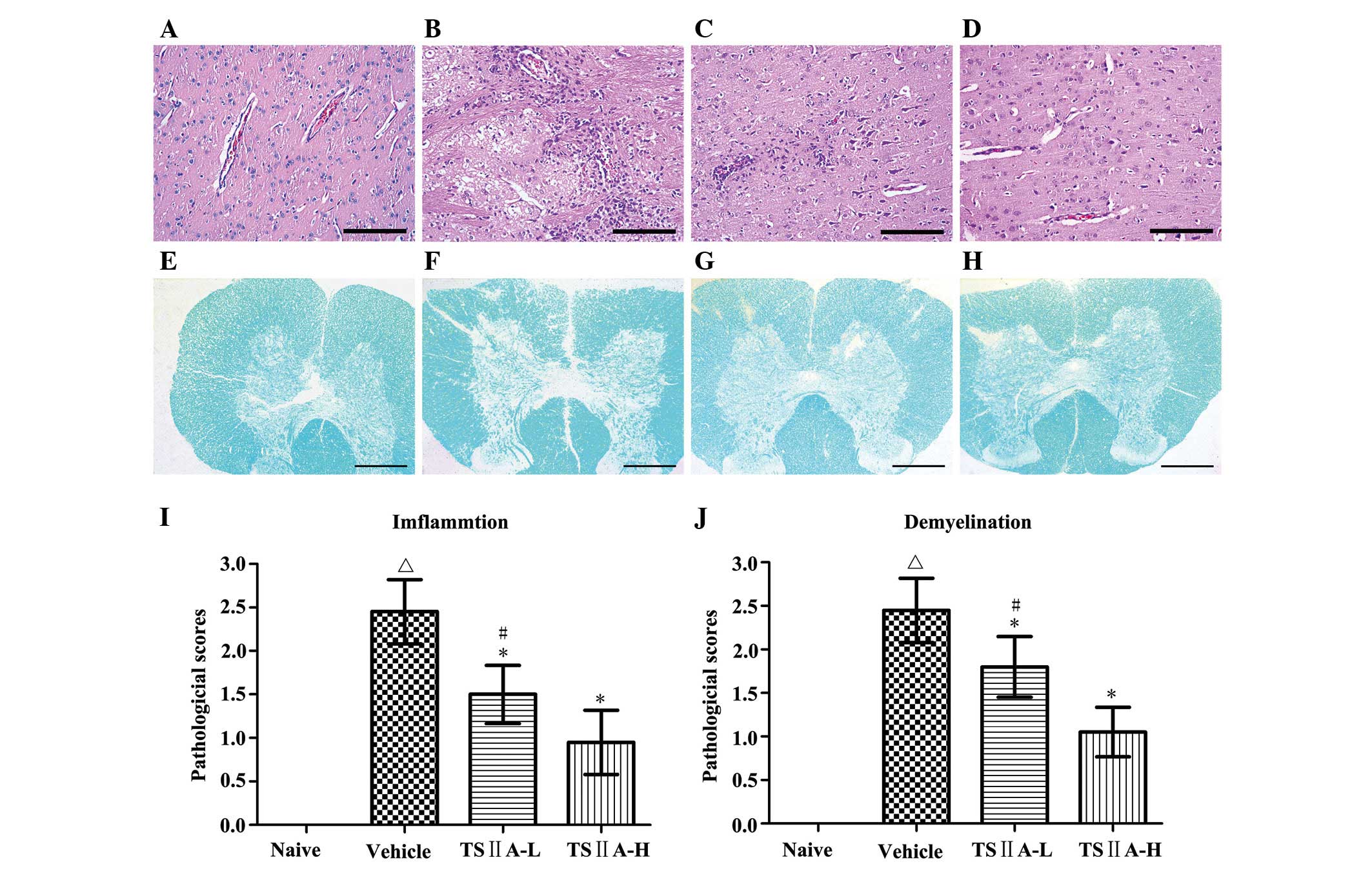 | Figure 2Histological analysis of inflammation
and demyelination. (A–D) H&E staining. Magnification, ×200;
(E–H) LFB staining. Magnification, ×5; (A and E) naive group; (B
and F) vehicle group; (C and G) TSIIA-L group; (D and H) TSIIA-H
group. (I) Quantification of inflammatory cell infiltration
(H&E staining, A–D), (J) demyelination quantification (LFB
staining, E–H). Values are presented as the mean ± standard
deviation (n=10 per group). ΔP<0.01, compared with
the naive group; *P<0.01, compared with the vehicle
group; #P<0.01, compared with the TSIIA-H group.
TSIIA, Tanshinone IIA; LFB, Luxol Fast Blue; H&E, hematoxylin
and eosin. |
TSIIA treatment suppresses the expression
of CD4, CD8 and Mac-1
To identify the types of infiltrating cells in the
CNS of EAE rats, immunohistochemistry was performed. Inflammatory
exudates included a mixture of cell types, including
CD4+ T cells, CD8+ T cells, microglia and
macrophages (Fig. 3A–L). Compared
with the vehicle group, TSIIA administration at the two doses
induced a significant decrease in the quantity of CD4+ T
cells (P<0.01), CD8+ T cells (P<0.01), macrophages
and microglia (P<0.01) (Fig.
3M–O). The results were confirmed by quantification of the
western blots and were consistent with the results of
immunostaining (P<0.05; Fig. 4A, B
and C).
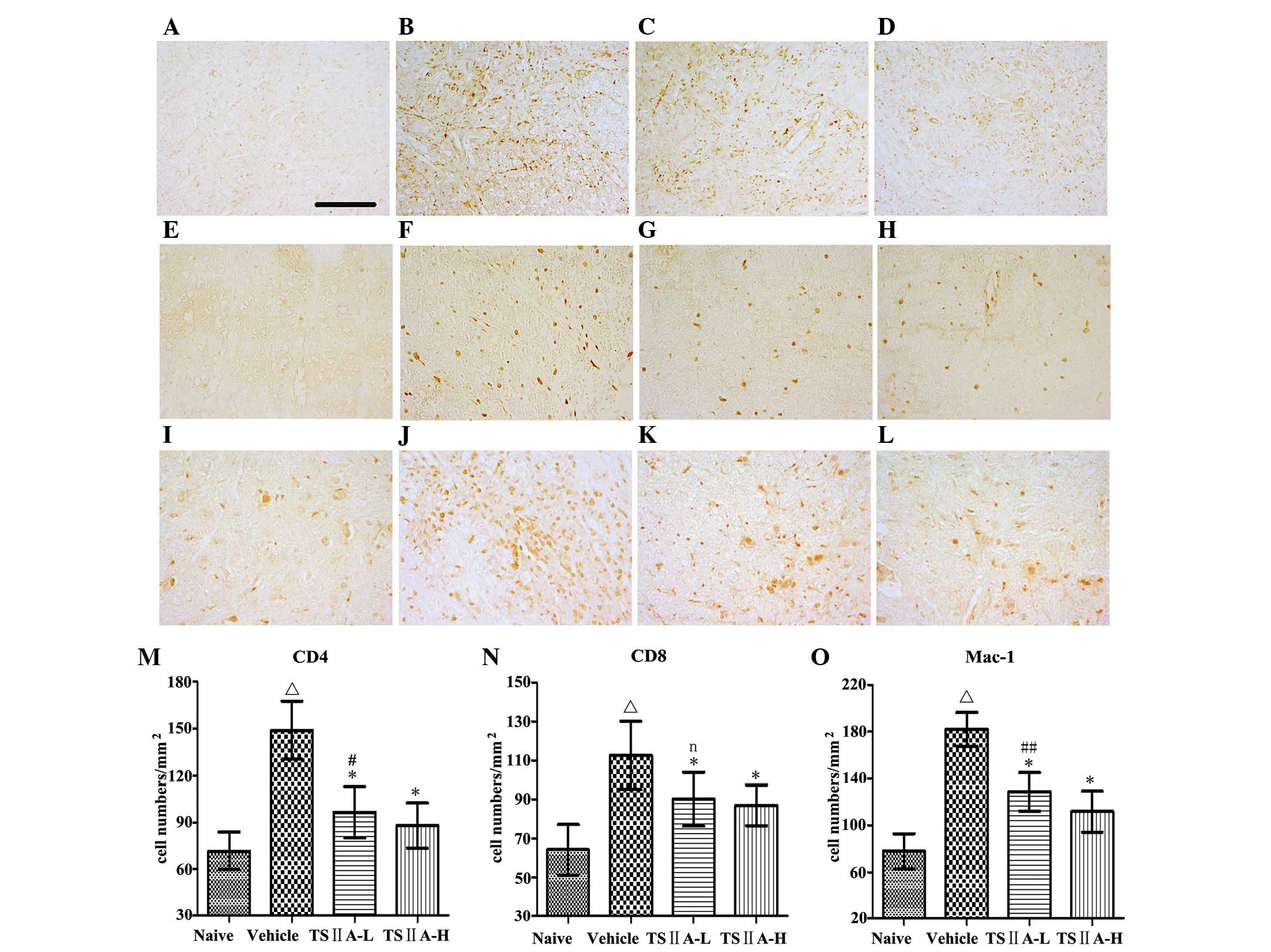 | Figure 3Immunohistochemistry of CD4, CD8 and
Mac-1. (A–D) CD4; (E–H) CD8; (I–L) Mac-1; Magnification, ×400. (A,
E and I) naive group; (B, F and J) vehicle group; (C, G and K)
TSIIA-L group; and (D, H and L) TSIIA-H group. (M–O) Quantitative
analysis of the above immune cells. Values are presented as the
mean ± standard deviation (n=10 per group). ΔP<0.01,
compared with the naive group; *P<0.01, compared with
the vehicle group; #P<0.01, ##P<0.05 or
n (not significant) compared with the TSIIA-H group. TSIIA,
Tanshinone IIA; TSIIA-L, TSIIA low dose; TSIIA-H, TSIIA high
dose. |
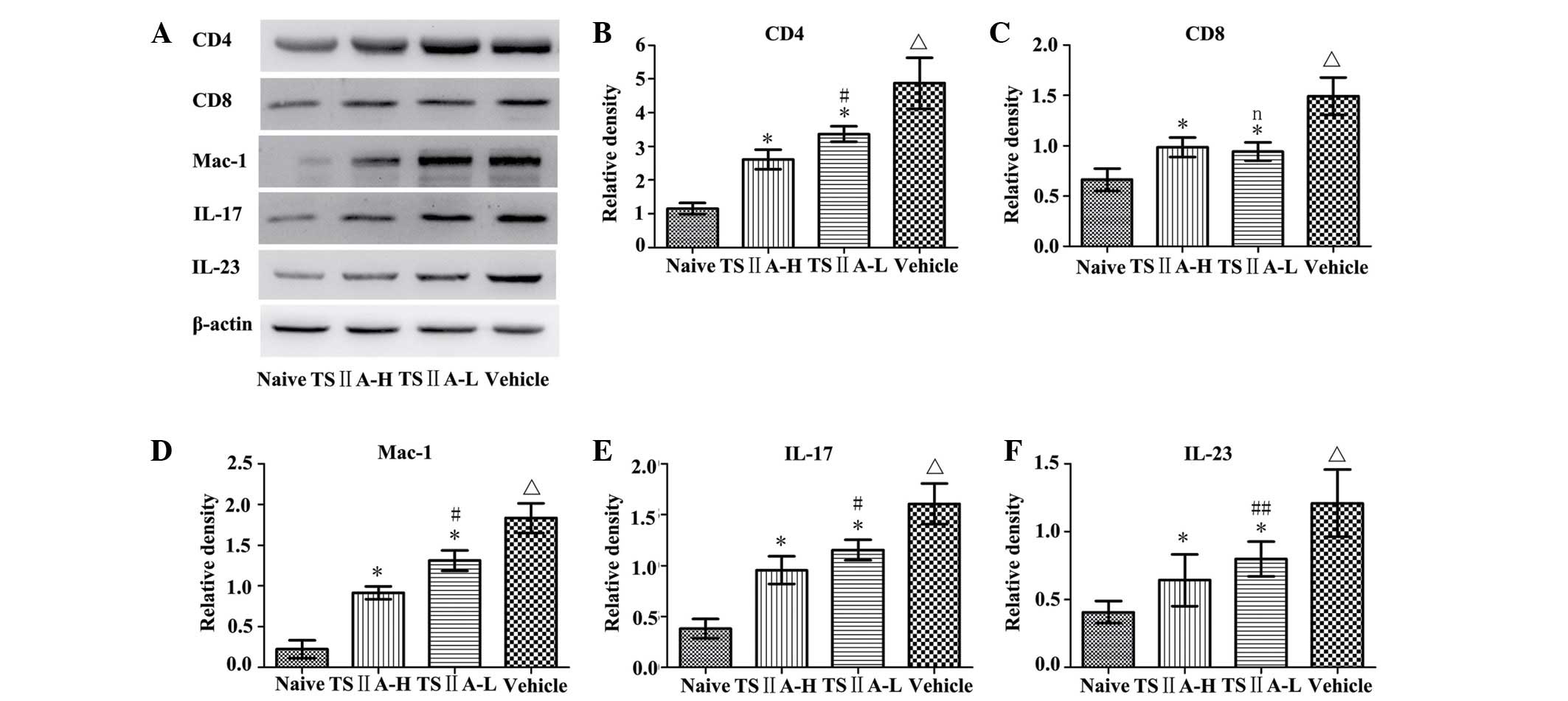 | Figure 4(A) Determination of CD4, CD8, Mac-1,
IL-17 and IL-23 protein expression via western blot analysis.
Quantitative analysis of (B) CD4, (C) CD8, (D) Mac-1, (E) IL-17 and
(F) IL-23. Values are presented as the mean ± standard deviation
(n=10 per group). ΔP<0.01, compared with the naive
group; *P<0.01, compared with the vehicle group;
#P<0.01, ##P<0.05 or n (not
significant) compared with the TSIIA-H group. TSIIA, Tanshinone
IIA; IL, interleukin; TSIIA-L, TSIIA low dose; TSIIA-H, TSIIA high
dose. |
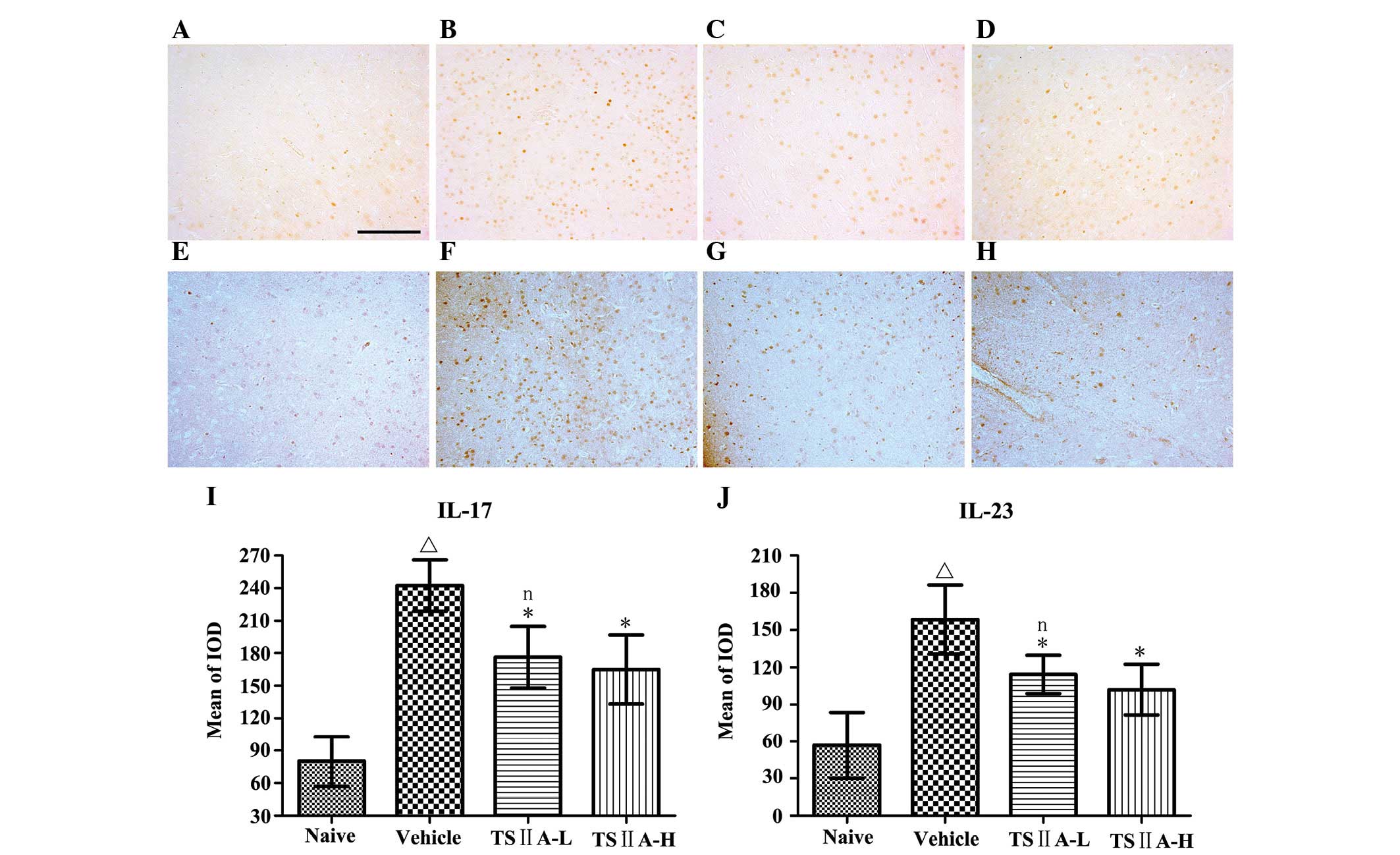
TSIIA treatment suppresses the levels of
IL-17 and IL-23 in the brain
Expression of the inflammatory cytokines, IL-17 and
IL-23, is usually increased in the CNS in EAE. To assess whether
TSIIA exerts anti-inflammatory activity in EAE in vivo, its
effects on IL-23 and IL-17 levels in the brain were determined
using immunohistochemistry. Compared with the naive group,
expression of IL-17 and IL-23 in the vehicle groups was
significantly increased (P<0.01). Compared with the vehicle
group, TSIIA treatment significantly induced a reduction in IL-17
and IL-23 expression (P<0.01). However, there were no
significant differences between the two treatment groups
(P>0.05; Fig. 5). The effects
of TSIIA on these indicators were further confirmed by quantitative
western blot analysis (P<0.01), and significant differences were
identified between the TSIIA-L and TSIIA-H groups in IL-17
(P<0.01) and IL-23 (P<0.05) expression (Fig. 4 A, E and F).
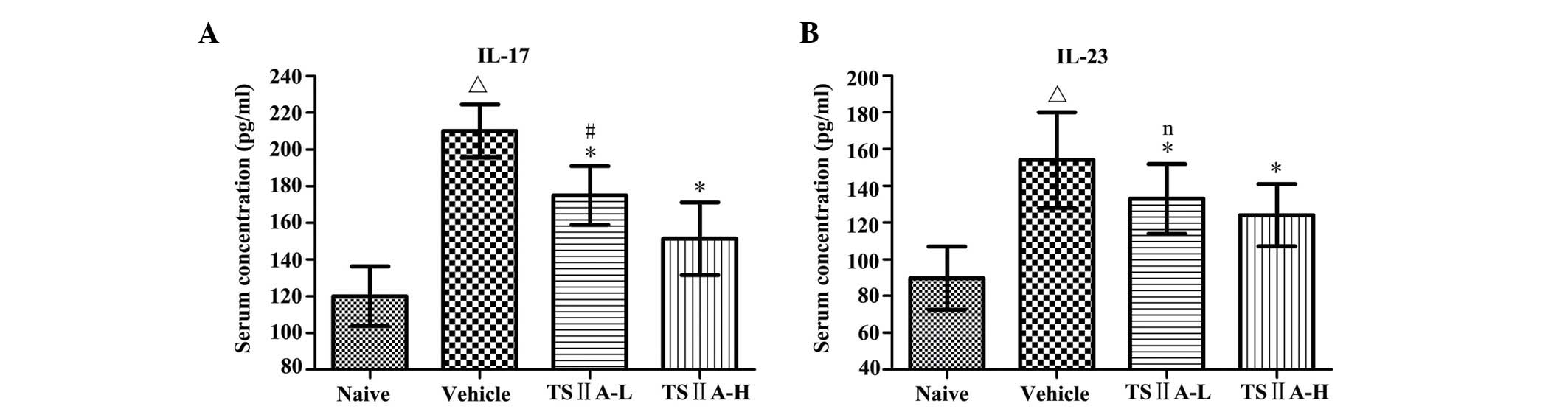
TSIIA treatment reduces the expression of
IL-17 and IL-23 in the serum
The serum contents of IL-17 and IL-23 are usually
upregulated and closely associated with the development of EAE. The
highest concentrations of serum IL-17 and IL-23 among all groups
were found in vehicle-treated rats, which decreased significantly
in the two TSIIA-treated groups (P<0.01). The serum level of
IL-17 in the TSIIA-H group was lower than that in the TSIIA-L group
(P<0.01). However, no significant difference was identified in
the serum concentrations of IL-23 between the two TSIIA-treated
groups (Fig. 6).
Discussion
TSIIA has been shown to alleviate several CNS
disorders in animal models, possibly through its anti-inflammatory
and neuroprotective properties. However, the effects of TSIIA on
EAE have not been determined to date. In the current study, an
experimental model was established to determine the effects of
TSIIA on EAE and its mechanisms of action.
In MS and EAE, CD4+ T cells,
CD8+ T cells, macrophages and resident microglia
orchestrate a series of inflammatory reactions in the CNS of humans
and animals, resulting in persistent disability. CD4+
cells are undoubtedly the pivotal pathogenic cells in MS and EAE
that facilitate inflammatory reactions and subsequent
neurodegeneration. These cells induce neuronal death under
pathological conditions through a variety of mechanisms, such as
triggering antigen-independent calcium oscillations and
TRAIL-mediated injury in neurons (20,21).
A number of therapeutic options that reduce the T cell- and
particularly, CD4+ cell-mediated damage of neurons are
extensively applied in the clinic. For example, natalizumab, an
effective drug for MS, decreases the number of CD4+
cells in the brain and inhibits peripheral lymphocyte migration
into the CNS (22).
CD8+ T cells are also crucial in CNS autoimmunity. These
cells are abundant in active demyelinating lesions in MS, and
induce inflammation and demyelination in EAE (23). Furthermore, there is a positive
correlation between the degree of axonal injury and the quantity of
CD8+ T cells as well as macrophages/microglia in the
brain tissue from patients with MS, suggesting their important
effects on axon loss (24).
Cytotoxic CD8+ T cells secrete various pro-inflammatory
cytokines, including perforin (25) and granzymes (26), which trigger autoimmune injury
mediated by cells in the CNS. Sodium tanshinone IIA sulfonate, a
water-soluble derivative of TSIIA, has been shown to markedly
suppress the proliferation of spleen T lymphocytes and reduce the
CD4+ and CD8+ T cell percentage in peripheral
blood in a rat skin transplantation model (27). Additionally, TSIIA inhibits the
maturation of dendritic cells and suppresses the expression of
pro-inflammatory cytokines, weakening their capacity to stimulate
T-cell proliferation (28). Data
from the current study showed that TSIIA downregulates the increase
in CD4+ and CD8+ T cells in the CNS and
relieves clinical symptoms.
Macrophages in the CNS are derived from peripheral
monocytes, and microglia are resident macrophages. Once activated,
microglia and macrophages cause mitochondrial dysfunction through
induction of reactive oxygen and nitrogen species, which contribute
to axon injury and subsequent neuronal cell death (29). Activated microglia and macrophages
produce pro-inflammatory cytokines and enhance sensitization of
axons to glutamate, with subsequent initiation of an indirect
immunological attack on oligodendrocytes and neurons (30,31).
TSIIA reduces the release of LPS-induced pro-inflammatory
cytokines, such as IL-1, IL-6 and tumor necrosis factor (TNF)-α,
from macrophages during inflammation (32–34).
Microglial activation, a hallmark of CNS pathology in MS and other
neurode-generative diseases, triggers cytotoxic effects and drives
neuronal damage, which can be suppressed by TSIIA in experimental
models of SNL-induced neuropathic pain (35) and Parkinson's disease (13). Macrophage depletion (36) and microglial paralysis (37) markedly alleviate disease
progression. Consistently, compared with wild-type mice,
Mac-1-deficient mice displayed attenuated EAE with lower levels of
gliosis, axonal degeneration and demyelination (38). To the best of our knowledge, no
studies regarding the impact of TSIIA on microglia/macrophages in
CNS of EAE are have been conducted. The results from this study
showed that following TSIIA treatment, microglia/macrophage numbers
are decreased following decreased demyelination and inflammatory
cell infiltration in the CNS of EAE rats.
The IL-17/IL-23 pathway is associated with the
pathogenesis of autoimmune disorders, including MS, psoriasis and
inflammatory bowel disease (39–41).
IL-23-deficient mice are unable to induce EAE (42). IL-23 is reported to promote
polarization, development and expansion of pathogenic T cells;
thus, it is essential for EAE induction. (43–45).
As a lineage of major pathogenic T cells, Th17 cells not only
autosynthesize but also promote other types of cells to generate
pro-inflammatory cytokines (46).
Furthermore, Th17 cells transmigrate efficiently across the blood
brain barrier, damage neurons and contribute to CNS inflammation
through CD4+ lymphocyte accumulation (47). Targeting IL-23-p19 with
neutralizing antibodies has been shown to reduce the IL-17 level in
the CNS and serum, which was also shown to prevent EAE relapse
during disease remission (48).
Serum IL-17 levels are positively correlated with disease severity
(49) and EAE is inhibited in
IL-17−/− mice whose CD4+ T cells are
incapable of inducing EAE efficiently, compared with wild-type T
cells (50). IL-17 also stimulates
microglia (51) and astrocytes
(52) to secrete inflammatory
cytokines and chemokines, resulting in recruitment of neutro-phils
(53). In collaboration with
TNF-α, IL-17 promotes oxidative stress-induced apoptosis of
oligodendrocytes, causing axonal loss and subsequent neurological
deficits (54,55). In an earlier phase II clinical
trial, following treatment with secukinumab, an antibody that
neutralizes IL-17, MS patients displayed fewer new CNS lesions
observed using magnetic resonance imaging, and lower annualized
recurrence rates, compared with placebo-treated patients (56). Therefore, blockage of the
IL-23/IL-17 pathway in the clinical treatment of MS has recently
received considerable research attention, in view of accumulating
data highlighting its vital role in MS/EAE. TSIIA has been shown to
inhibit IL-17-induced vascular remodeling in systemic sclerosis
patients (17). However, no
research to date has investigated the impact of TSIIA on IL-17 and
IL-23 levels in EAE/MS. To the best of our knowledge, this is the
first study to demonstrate a significant decrease in serum and
brain expression of IL-17 and IL-23 in EAE following TSIIA
treatment.
In conclusion, this study provides preliminary
evidence supporting the use of TSIIA as a potential novel
therapeutic option for MS. However, rats in this study were only
treated acutely, and the feasibility and safety of TSIIA require
validation in the clinic.
Acknowledgments
This study was supported by the Liaoning Province
Science and Technology Project-Animal Scientific Research and
Clinical Application for Major Disease of Liaoning Province (grant
no. 2012225021), Program of Basic and Clinical Research Platform of
China Medical University (grant no. CMU-201406) and from Technology
Projects of Liaoning Province (grant no. 2009225010-2) to Dr Juan
Feng.
References
|
1
|
Rangachari M and Kuchroo VK: Using EAE to
better understand principles of immune function and autoimmune
pathology. J Autoimmun. 45:31–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leuenberger T, Paterka M, Reuter E, Herz
J, Niesner RA, Radbruch H, Bopp T, Zipp F and Siffrin V: The role
of CD8 (+) T cells and their local interaction with CD4 (+) T cells
in myelin oligodendrocyte glycoprotein 35–55-induced experimental
autoimmune encephalomyelitis. J Immunol. 191:4960–4968. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cabarrocas J, Bauer J, Piaggio E, Liblau R
and Lassmann H: Effective and selective immune surveillance of the
brain by MHC class I-restricted cytotoxic T lymphocytes. Eur J
Immunol. 33:1174–1182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fischer HG and Reichmann G: Brain
dendritic cells and macrophages/microglia in central nervous system
inflammation. J Immunol. 166:2717–2726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Chu N, Hu A, Gran B, Rostami A and
Zhang GX: Increased IL-23p19 expression in multiple sclerosis
lesions and its induction in microglia. Brain. 130:490–501. 2007.
View Article : Google Scholar
|
|
6
|
Langrish CL, Chen Y, Blumenschein WM,
Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA and
Cua DJ: IL-23 drives a pathogenic T cell population that induces
autoimmune inflammation. J Exp Med. 201:233–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luchtman DW, Ellwardt E, Larochelle C and
Zipp F: IL-17 and related cytokines involved in the pathology and
immunotherapy of multiple sclerosis: Current and future
developments. Cytokine Growth Factor Rev. 25:403–413. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McFarland HF and Martin R: Multiple
sclerosis: A complicated picture of autoimmunity. Nat Immunol.
8:913–919. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castro-Borrero W, Graves D, Frohman TC,
Flores AB, Hardeman P, Logan D, Orchard M, Greenberg B and Frohman
EM: Current and emerging therapies in multiple sclerosis: A
systematic review. Ther Adv Neurol Disord. 5:205–220. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu B, Liu M and Zhang S: Cochrane
systematic review: danshen agents for acute ischaemic stroke.
Chinese Journal of Evidence-Based Medicine. 2:101–105. 2005.
|
|
11
|
Cheng TO: Cardiovascular effects of
Danshen. Int J Cardiol. 121:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu S and Liu P: Tanshinone II-A: New
perspectives for old remedies. Expert Opin Ther Pat. 23:149–153.
2013. View Article : Google Scholar
|
|
13
|
Ren B, Zhang YX, Zhou HX, Sun FW, Zhang
ZF, Wei Z, Zhang CY and Si DW: Tanshinone IIA prevents the loss of
nigrostriatal dopaminergic neurons by inhibiting NADPH oxidase and
iNOS in the MPTP model of Parkinson's disease. J Neurol Sci.
348:142–152. 2015. View Article : Google Scholar
|
|
14
|
Jiang P, Li C, Xiang Z and Jiao B:
Tanshinone IIA reduces the risk of Alzheimer's disease by
inhibiting iNOS, MMP-2 and NF-κBp65 transcription and translation
in the temporal lobes of rat models of Alzheimer's disease. Mol Med
Rep. 10:689–694. 2014.PubMed/NCBI
|
|
15
|
Zhang K, Wang J, Jiang H, Xu X, Wang S,
Zhang C, Li Z, Gong X and Lu W: Tanshinone IIA inhibits
lipopolysaccharide-induced MUC1 overexpression in alveolar
epithelial cells. Am J Physiol Cell Physiol. 306:C59–C65. 2014.
View Article : Google Scholar
|
|
16
|
Zhu W, Lu Q, Chen HW, Feng J, Wan L and
Zhou DX: Protective effect of sodium tanshinone II A sulfonate on
injury of small intestine in rats with sepsis and its mechanism.
Chin J Integr Med. 18:496–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu M, Yang J and Li M: Tanshinone IIA
attenuates interleukin-17A-induced systemic sclerosis
patient-derived dermal vascular smooth muscle cell activation via
inhibition of the extracellular signal-regulated kinase signaling
pathway. Clinics (Sao Paulo). 70:250–256. 2015. View Article : Google Scholar
|
|
18
|
Mao YS, Lu CZ, Wang X and Xiao BG:
Induction of experimental autoimmune encephalomyelitis in Lewis
rats by a viral peptide with limited homology to myelin basic
protein. Exp Neurol. 206:231–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jokubaitis VG, Gresle MM, Kemper DA,
Doherty W, Perreau VM, Cipriani TL, Jonas A, Shaw G, Kuhlmann T,
Kilpatrick TJ and Butzkueven H: Endogenously regulated Dab2 worsens
inflammatory injury in experimental autoimmune encephalomyelitis.
Acta Neuropathol Commun. 1:322013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nitsch R, Pohl EE, Smorodchenko A,
Infante-Duarte C, Aktas O and Zipp F: Direct impact of T cells on
neurons revealed by two-photon microscopy in living brain tissue. J
Neurosci. 24:2458–2464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aktas O, Smorodchenko A, Brocke S,
Infante-Duarte C, Schulze Topphoff U, Vogt J, Prozorovski T, Meier
S, Osmanova V, Pohl E, et al: Neuronal damage in autoimmune
neuroinflammation mediated by the death ligand TRAIL. Neuron.
46:421–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
del Pilar Martin M, Cravens PD, Winger R,
Frohman EM, Racke MK, Eagar TN, Zamvil SS, Weber MS, Hemmer B,
Karandikar NJ, et al: Decrease in the numbers of dendritic cells
and CD4+ T cells in cerebral perivascular spaces due to
natalizumab. Arch Neurol. 65:1596–1603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Babbe H, Roers A, Waisman A, Lassmann H,
Goebels N, Hohlfeld R, Friese M, Schröder R, Deckert M, Schmidt S,
et al: Clonal expansions of CD8 (+) T cells dominate the T cell
infiltrate in active multiple sclerosis lesions as shown by
micro-manipulation and single cell polymerase chain reaction. J Exp
Med. 192:393–404. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuhlmann T, Lingfeld G, Bitsch A,
Schuchardt J and Brück W: Acute axonal damage in multiple sclerosis
is most extensive in early disease stages and decreases over time.
Brain. 125:2202–2212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meuth SG, Herrmann AM, Simon OJ, Siffrin
V, Melzer N, Bittner S, Meuth P, Langer HF, Hallermann S, Boldakowa
N, et al: Cytotoxic CD8+ T cell-neuron interactions:
Perforin-dependent electrical silencing precedes but is not
causally linked to neuronal cell death. J Neurosci. 29:15397–15409.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zang YC, Li S, Rivera VM, Hong J, Robinson
RR, Breitbach WT, Killian J and Zhang JZ: Increased CD8+ cytotoxic
T cell responses to myelin basic protein in multiple sclerosis. J
Immunol. 172:5120–5127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Q, Chen H, Sheng L, Liang Y and Li Q:
Sodium tanshinone IIA sulfonate prolongs the survival of skin
allografts by inhibiting inflammatory cell infiltration and T cell
proliferation. Int Immunopharmacol. 22:277–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li HZ, Lu YH, Huang GS, Chen Q, Fu Q and
Li ZL: Tanshinone II A inhibits dendritic cell-mediated adaptive
immunity: Potential role in anti-atherosclerotic activity. Chin J
Integr Med. 20:764–769. 2014. View Article : Google Scholar
|
|
29
|
Nikić I, Merkler D, Sorbara C, Brinkoetter
M, Kreutzfeldt M, Bareyre FM, Brück W, Bishop D, Misgeld T and
Kerschensteiner M: A reversible form of axon damage in experimental
autoimmune encephalomyelitis and multiple sclerosis. Nat Med.
17:495–499. 2011. View
Article : Google Scholar
|
|
30
|
Pitt D, Werner P and Raine CS: Glutamate
excitotoxicity in a model of multiple sclerosis. Nat Med. 6:67–70.
2000. View Article : Google Scholar
|
|
31
|
Jack C, Ruffini F, Bar-Or A and Antel JP:
Microglia and multiple sclerosis. J Neurosci Res. 81:363–373. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen TH, Hsu YT, Chen CH, Kao SH and Lee
HM: Tanshinone IIA from Salvia miltiorrhiza induces heme
oxygenase-1 expression and inhibits lipopolysaccharide-induced
nitric oxide expression in RAW 264.7 cells. Mitochondrion.
7:101–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi HS, Cho DI, Choi HK, Im SY, Ryu SY
and Kim KM: Molecular mechanisms of inhibitory activities of
tanshinones on lipopolysaccharide-induced nitric oxide generation
in RAW 264.7 cells. Arch Pharm Res. 27:1233–1237. 2004. View Article : Google Scholar
|
|
34
|
Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu
LM, Su YF, Kang LY and Zhang BL: The anti-inflammatory activities
of Tanshinone IIA, an active component of TCM, are mediated by
estrogen receptor activation and inhibition of iNOS. J Steroid
Biochem Mol Biol. 113:275–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao FL, Xu M, Wang Y, Gong KR and Zhang
JT: Tanshinone IIA attenuates neuropathic pain via inhibiting glial
activation and immune response. Pharmacol Biochem Behav. 128:1–7.
2015. View Article : Google Scholar
|
|
36
|
Huitinga I, van Rooijen N, de Groot CJ,
Uitdehaag BM and Dijkstra CD: Suppression of experimental allergic
encephalo-myelitis in Lewis rats after elimination of macrophages.
J Exp Med. 172:1025–1033. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heppner FL, Greter M, Marino D, Falsig J,
Raivich G, Hövelmeyer N, Waisman A, Rülicke T, Prinz M, Priller J,
et al: Experimental autoimmune encephalomyelitis repressed by
microglial paralysis. Nat Med. 11:146–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bullard DC, Hu X, Schoeb TR, Axtell RC,
Raman C and Barnum SR: Critical requirement of CD11b (Mac-1) on T
cells and accessory cells for development of experimental
autoimmune encephalomyelitis. J Immunol. 175:6327–6333. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qin S, Wen J, Bai XC, Chen TY, Zheng RC,
Zhou GB, Ma J, Feng JY, Zhong BL and Li YM: Endogenous n-3
polyunsaturated fatty acids protect against imiquimod-induced
psoriasis-like inflammation via the IL-17/IL-23 axis. Mol Med Rep.
9:2097–2104. 2014.PubMed/NCBI
|
|
40
|
Ghadimi D, Helwig U, Schrezenmeir J,
Heller KJ and de Vrese M: Epigenetic imprinting by commensal
probiotics inhibits the IL-23/IL-17 axis in an in vitro model of
the intestinal mucosal immune system. J Leukoc Biol. 92:895–911.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gaffen SL, Jain R, Garg AV and Cua DJ: The
IL-23-IL-17 immune axis: From mechanisms to therapeutic testing.
Nat Rev Immunol. 14:585–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cua DJ, Sherlock J, Chen Y, Murphy CA,
Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al:
Interleukin-23 rather than interleukin-12 is the critical cytokine
for autoimmune inflammation of the brain. Nature. 421:744–748.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McGeachy MJ, Chen Y, Tato CM, Laurence A,
Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ and Cua
DJ: The interleukin 23 receptor is essential for the terminal
differentiation of interleukin 17-producing effector T helper cells
in vivo. Nat Immunol. 10:314–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gyuelveszi G, Haak S and Becher B:
IL-23-driven encephalo-tropism and Th17 pola rization during
CNS-inflammation in vivo. Eur J Immunol. 39:1864–1869. 2009.
View Article : Google Scholar
|
|
45
|
Ghoreschi K, Laurence A, Yang XP, Tato CM,
McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N,
et al: Generation of pathogenic T (H) 17 cells in the absence of
TGF-β signalling. Nature. 467:967–971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Goverman J: Autoimmune T cell responses in
the central nervous system. Nat Rev Immunol. 9:393–407. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kebir H, Kreymborg K, Ifergan I,
Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N,
Becher B and Prat A: Human TH17 lymphocytes promote blood-brain
barrier disruption and central nervous system inflammation. Nat
Med. 13:1173–1175. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen Y, Langrish CL, McKenzie B,
Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W,
Churakovsa T, Low J, Presta L, et al: Anti-IL-23 therapy inhibits
multiple inflammatory pathways and ameliorates autoimmune
encepha-lomyelitis. J Clin Invest. 116:1317–1326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tzartos JS, Friese MA, Craner MJ, Palace
J, Newcombe J, Esiri MM and Fugger L: Interleukin-17 production in
central nervous system-infiltrating T cells and glial cells is
associated with active disease in multiple sclerosis. Am J Pathol.
172:146–155. 2008. View Article : Google Scholar :
|
|
50
|
Komiyama Y, Nakae S, Matsuki T, Nambu A,
Ishigame H, Kakuta S, Sudo K and Iwakura Y: IL-17 plays an
important role in the development of experimental autoimmune
encephalomy-elitis. J Immunol. 177:566–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kawanokuchi J, Shimizu K, Nitta A, Yamada
K, Mizuno T, Takeuchi H and Suzumura A: Production and functions of
IL-17 in microglia. J Neuroimmunol. 194:54–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Trajkovic V, Stosic-Grujicic S, Samardzic
T, Markovic M, Miljkovic D, Ramic Z and Mostarica Stojkovic M:
Interleukin-17 stimulates inducible nitric oxide synthase
activation in rodent astrocytes. J Neuroimmunol. 119:183–191. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wojkowska DW, Szpakowski P,
Ksiazek-Winiarek D, Leszczynski M and Glabinski A: Interactions
between neutrophils, Th17 cells and chemokines during the
initiation of experimental model of multiple sclerosis. Mediators
Inflamm. 2014:5904092014. View Article : Google Scholar
|
|
54
|
Paintlia MK, Paintlia AS, Singh AK and
Singh I: Synergistic activity of interleukin-17 and tumor necrosis
factor-α enhances oxidative stress-mediated oligodendrocyte
apoptosis. J Neurochem. 116:508–521. 2011. View Article : Google Scholar :
|
|
55
|
Prineas JW, Barnard RO, Kwon EE, Sharer LR
and Cho ES: Multiple sclerosis: Remyelination of nascent lesions.
Ann Neurol. 33:137–151. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Miossec P and Kolls JK: Targeting IL-17and
TH17 cells in chronic inflammation. Nat Rev Drug Disco. 11:763–776.
2012. View Article : Google Scholar
|