Introduction
Osteoclasts are associated with bone homeostasis,
and their formation and function is based on the fusion of
macrophage precursor cells (1).
Receptor-activator of nuclear factor κB (NF-κB) (RANK) ligand
(RANKL) is a tumor-necrosis factor-associated cytokine, and a key
osteoclast differentiation factor. Through combination with RANK,
RANKL stimulates cytoplasmic tumor-necrosis factor
receptor-associated factor 6 (2)
and correspondingly activates the downstream signaling pathways,
including p38 mitogen-activated protein kinase (MAPK),
c-jun-N-terminal kinase (JNK), NF-κB and extra-cellular
signal-regulated kinase (ERK) (3–6). As
a result of a complex series of signal activation, osteoclast
progenitors fused into mature multi-nucleated osteoclasts,
expressing a specific group of various gene products, including
cathepsin K (CTSK), tartrate-resistant acid phosphatase (TRAP),
calcitonin receptor (CTR) and matrix metalloproteinase 9 (MMP-9)
(7).
Osteoporosis is regarded as a metabolic disease,
with characteristics of bone mass loss and increased fracture risk,
which is a public health problem in an aging society (8). Several anti-resorptive agents
including bisphosphonates, calcitonin and estrogen have been used
in the treatment of osteoporosis, however, each agent possesses
clinical limitations and side-effects include the induction of
breast cancer, osteonecrosis and vaginal bleeding (9,10).
Thus, a safer therapeutic strategy is required for the use in the
prevention and/or treatment of lytic bone diseases including
osteoporosis.
Aspirin is a common and safe compound used as an
effective antipyretic, analgesic and anti-inflammatory drug.
However, additional effects have been identified. Based on an
epidemiological survey and preliminary studies, aspirin is
suggested to possess anti-postmenopausal osteoporosis effects in
the ovariectomized rat model (11,12),
which indicate a possible clinical application for aspirin in the
prevention of bone loss. However, its detailed molecular mechanisms
remain to be fully elucidated.
The current study aimed to investigate the influence
of aspirin on osteoclastogenesis in RANKL-induced RAW264.7 cells
and identify the molecular mechanisms.
Materials and methods
Chemicals and reagents
Aspirin (over 99% purity) and the RANKL and TRAP
Staining kits were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), Dulbecco's modified Eagle's medium (DMEM) and
fluorescein isothiocyanate-conjugated secondary antibodies were
purchased from Invitrogen (Thermo Fisher Scientific, Inc.). NF-κB
(anti-p50, anti-p65 and anti-IκB) and MAPKs (anti-ERK, anti-JNK and
anti-p38) mouse antibodies and their phosphorylated antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell culture
RAW264.7 (TIB-71; American Type Culture Collection,
Manassas, VA, USA) murine-macrophage cells were cultured in DMEM
with 10% FBS, 100 U/ml penicillin, and 100 µg/ml
streptomycin under 5% CO2 at 37°C in a humidified
atmosphere. In each experiment, the cells were grown to 80%
confluence. They were induced by RANKL (100 ng/ml) in the presence
or absence of aspirin for the experiments that followed.
Cytotoxicity assay for aspirin
The cytotoxicity of aspirin was determined by the
conventional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. RAW264.7 cells were seeded into 96-well plates
at a density of 104 cells/well and cultured as described
above for 24 h in a 37°C, 5% CO2 incubater. Various
concentrations of aspirin were added to each well and the cells
were incubated for 2 h, then for 4 h in 0.5 mg/ml MTT solution. The
medium in the wells was carefully removed, then 15% sodium dodecyl
sulfate (SDS) was added into each well for solubilization of
formazan and measured at 540 nm with a microplate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA).
TRAP staining
The cells (2×105 cells/ml) were plated
into a 24-well microplate, cultured in DMEM with 10% FBS, and
incubated with different concentration of aspirin (0.25, 0.5, 1.0
and 1.5 mM) in the presence of RANKL (100 ng/ml) for 5 days. The
TRAP Staining kit was used to fix and stain the cells according to
the manufacturer's protocol. If TRAP-positive cells had greater
than three nuclei, they were regarded as multinucleated
osteoclasts. The multinucleated osteoclasts were assessed using a
light microscope by counting each field of total three fields. Each
group of cells were plated in triplicate, and the mean values were
calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The RAW264.7 cells were plated at a density of
2×105 cells/ml in a 6-well plate, were incubated with
aspirin (0.25, 0.5, 1.0 and 1.5 mM) and were cultured for 5 days in
DMEM with 10% FBS in the presence of RANKL (100 ng/ml). Total RNA
was separated from the cultured cells using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.). An RNA PCR kit (Thermo Fisher
Scientific, Inc.) was used to reverse transcribe the mRNA into cDNA
and five-fold sterile distilled water was added to dilute the
resultant cDNA mixture. Diluted cDNA (0.2 µg/2 µl)
was subjected to qPCR using SYBR Green I dye according to the
manufacturer's instructions. The reaction was conducted in 25
µl SYBR® premixed-Ex Taq™ solution (Takara Bio,
Inc., Otsu, Japan), which contained 20 µM anti-sense and
sense primers (Table I). Primer3
software (version 0.4.0; Whitehead Institute for Biomedical
Research, Cambridge, MA, USA) was used to design the primers. Each
RT-qPCR experiment was performed in triplicate, and data were
collected and normalized to relative expression using Rotor-Gene
6000 Series software, version 1.7.87. All values were normalized to
that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) based on
2−ΔΔCq formula (13).
 | Table IPrimer sequences and product lengths
of the genes in reverse transcription-quantitative polymerase chain
reaction analysis. |
Table I
Primer sequences and product lengths
of the genes in reverse transcription-quantitative polymerase chain
reaction analysis.
| Gene | Primer sequences | Fragment size
(bp) |
|---|
| TRAP | F
5′ATCCCTCTGTGCGACATCAACG3′ | 214 |
| R
5′TTAGCGGACAAGCAGGACTCTC3′ | |
| CTSK | F
5′TGACTTCCGCAATCCTTAC3′ | 133 |
| R
5′GCAGCAGAAACTTGAACAC3′ | |
| MMP-9 | F
5′AGGGAGATGCCCATTTCG3′ | 203 |
| R
5′GCCGTCCTTATCGTAGTCAG3′ | |
| CTR | F
5′AGGGCTACTACACAGAGG3′ | 174 |
| R
5′CGGAGTCAGTGAGATTGG3′ | |
| GAPDH | F
5′ATCACTGCCACCCAGAAG3′ | 191 |
| R
5′TCCACGACGGACACATTG3′ | |
Western blot analysis
RAW264.7 cells were plated at a density of
2×105 cells/ml into 6-well plates for 18 h, then were
pretreated with aspirin (0.25, 0.5, 1.0 or 1.5 mM) for 2 h prior to
treatment with RANKL (100 ng/ml) for 30 min. Ice-cold cell lysis
buffer was used to prepare the whole-cell lysates (Cell Signaling
Technology, Danvers, MA, USA). The whole cell lysate samples were
separated using 10% SDS-polyacrylimide gel electrophoresis, and
then these samples were transferred onto nitrocellulose membranes
using the wet-transfer method and blocked in 5% milk for 1 h prior
to immunoblotting with the following primary antibodies from Cell
Signaling Technology, Inc. at 1:2,000 dilution: GAPDH (cat. no.
2118), p65 (cat. no. 8242), phosphorylated (p)-p65 (cat. no.3039),
p50 (cat. no.12540), p-p50 (cat. no. 4806), IκB-α (cat. no. 4814)
and p-IκB-α (cat. no. 5209) and p-p38 (cat. no. 4511), p38 (cat.
no. 8609), p-ERK (cat. no. 4370), ERK (cat. no. 4695), p-JNK (cat.
no. 9252) and JNK (cat. no. 9255). The membranes were washed with
Tris-buffered saline with Tween-20 (TBST), and then incubated with
horseradish peroxidase-conjugated secondary antibody (cat. no.
7074; Cell Signaling Technology, Inc.; 1:2,000 in TBST). GAPDH
protein was used as the internal control for the normalization of
protein loading. A chemiluminescence detection system (GE
Healthcare Life Sciences, Chalfont, UK) was used to detect each
protein according to the manufacturer's instructions.
p65 subunit translocations by
immunofluorescence
RAW264.7 cells (2×105 cells/ml) were put
in 6-well plates on glass cover slips and cultured for 18 h,
pre-treated with 1.0 mM aspirin for 2 h prior to treatment with
RANKL (100 ng/ml) for 30 min. The cells were washed with
phosphate-buffered saline (PBS), then fixed in 4% formaldehyde for
30 min. 1% Triton X-100 was used for the permeabilization of the
cells for 10 min, and then PBS including 5% bovine serum albumin
was also used to block these cells for 30 min. The anti-NF-κB p65
polyclonal antibodies were added and incubated overnight at 4°C,
followed by 45 min incubation at room temperature with the
fluorescein-conjugated IgG secondary antibody (1:2,000 dilution;
cat. no. 4414; Cell Signaling Technology, Inc.). The cover slips
were then mounted onto the slides, and fluorescence microscopy
(Olympus Corporation, Tokyo, Japan) was used to analyze the
fluorescence signal.
Statistical analysis
The results are presented as the mean ± standard
deviation of more than three experiments. One-way analysis of
variance with post hoc Dunnett's test was used, and Student's
t-test was additionally used to measure the differences among the
mean values of normally-distributed data. P<0.05 was considered
to indicate a statistically significant difference.
Results
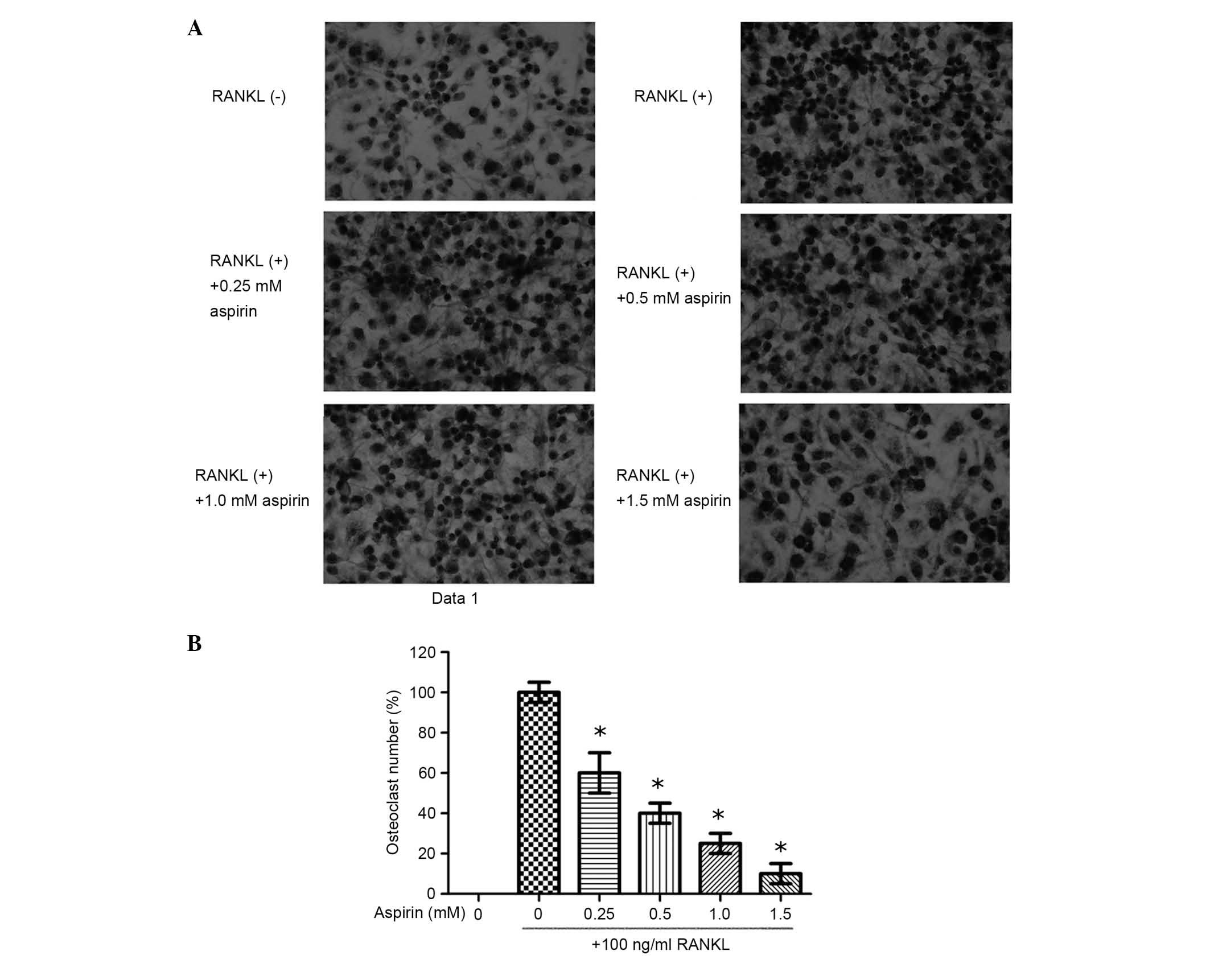
Effects of aspirin on osteoclast
differentiation in the RANKL-stimulated RAW264.7 cells
RAW264.7 cells were cultured with aspirin (0.25,
0.5, 1.0 or 1.5 mM) in the presence of 100 ng/ml RANKL. These cells
were stained following 5-day culture (Fig. 1). The results demonstrate that
aspirin significantly suppressed the formation of RANKL-induced
osteoclast-like cells of RAW264.7 cells in a dose-dependent manner
(Fig. 1). In order to exclude the
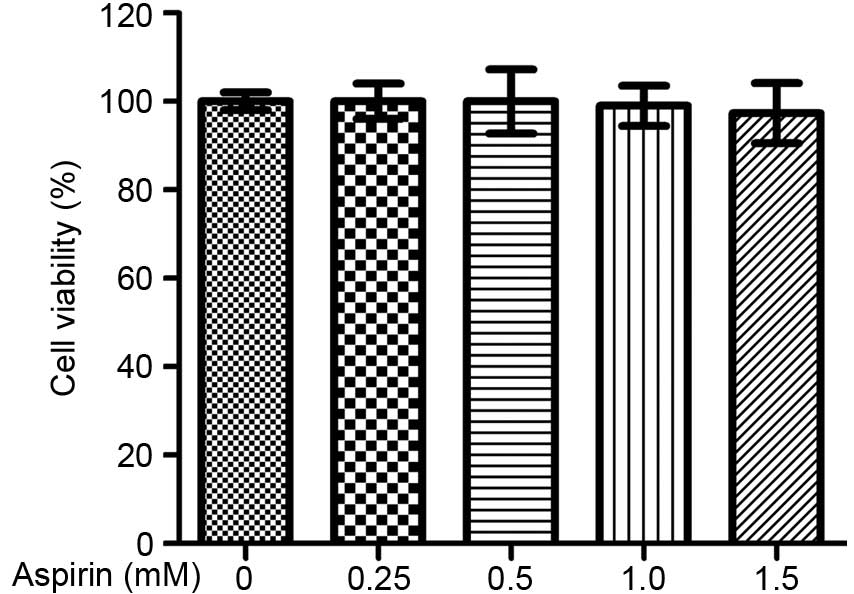
possible cytotoxicity of aspirin, its effects on survival of
RAW264.7 cells were further assessed. As presented in Fig. 2, aspirin does not exhibit
significant cytotoxicity at the concentrations tested.
The RNA expression of osteoclastic
markers in RANKL-stimulated RAW264.7 cells
In order to analyze the function of aspirin in the
differentiation of osteoclasts, its effects on the RNA expression
levels of the osteoclastic marker gene were assessed by RT-qPCR
analysis. Osteoclastic markers, including MMP-9, TRAP, CTR and
CTSK, were significantly upregulated following RANKL treatment.
This RANKL-mediated upregulation of osteoclastic marker gene
expression was reduced by the addition of aspirin (Fig. 3).
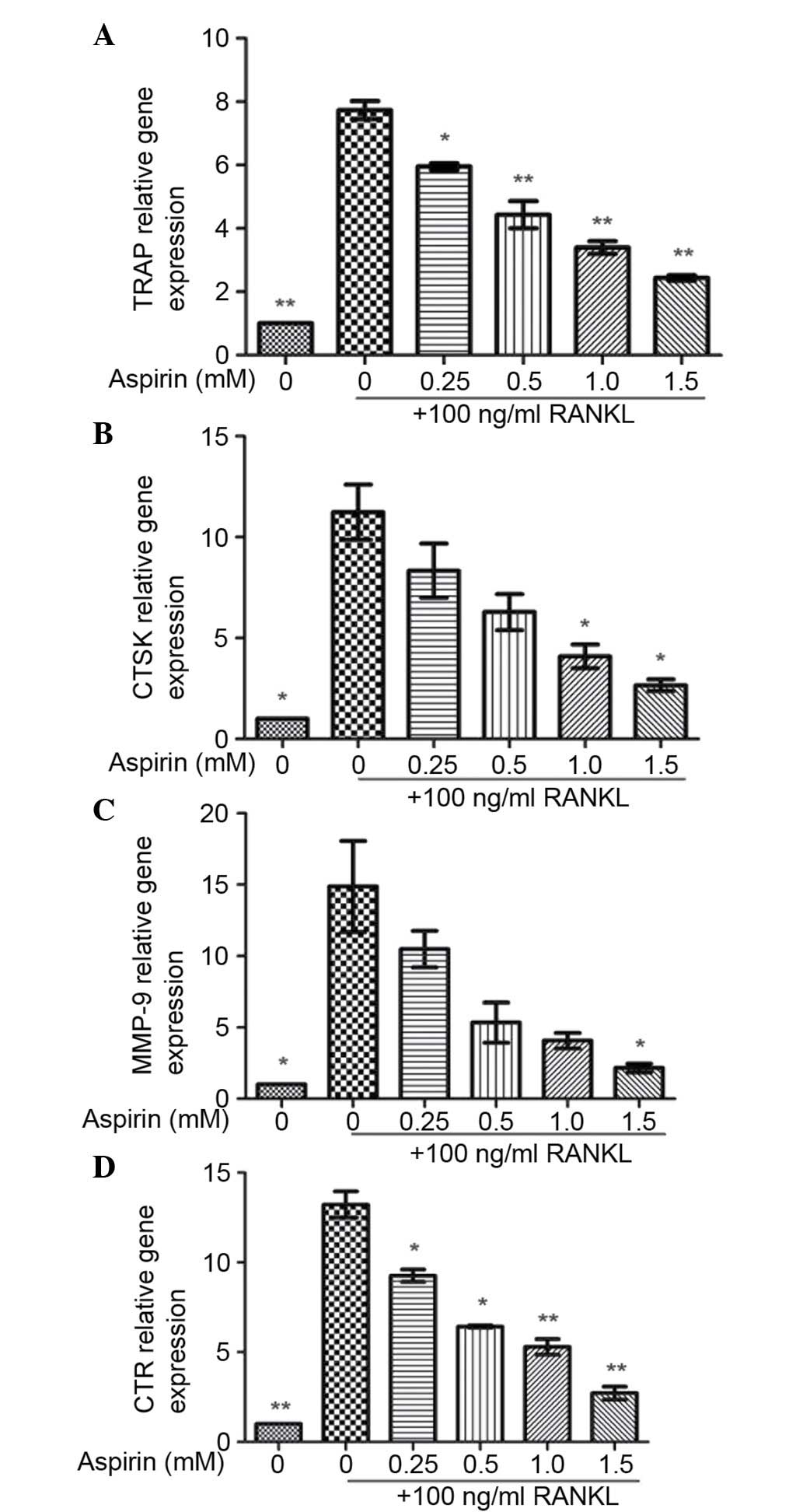 | Figure 3Effects of aspirin on RNA expression
of osteoclastic marker genes. Total RNA was extracted from RAW264.7
cells cultured for 24 h in the presence of RANKL (100 ng/ml) and
aspirin (0.25, 0.5, 1.0 or 1.5 mM) for 30 min. Relative gene
expression of (A) TRAP, (B) CTSK, (C) MMP-9 and (D) CTR was
analyzed by reverse transcription-quantitative polymerase chain
reaction. Values presented as the mean ± standard deviation of
three independent experiments.\ *P<0.05,
**P<0.01 vs. RANKL-treated control. RANKL,
receptor-activator of nuclear factor κB ligand; TRAP,
tartrate-resistant acid phosphatase; CTSK, cathepsin K; MMP-9,
matrix metalloproteinase 9; CTR, calcitonin receptor. |
Effects of aspirin on NF-κB activation in
RANKL-stimulated RAW 264.7 cells
To ascertain whether aspirin suppresses the
phosphorylation and degradation of IκB in RANKL-induced RAW264.7
cells, RAW264.7 cells were pre-treated for 2 h in the presence of
aspirin, and the IκB-α protein level was confirmed following 30 min
additional exposure with RANKL (100 ng/ml). It was identified that
aspirin markedly suppressed the RANKL-induced degradation in
addition to the phosphorylation of IκB-α (Fig. 4). In addition, the phophorylation
of p50/p65 was examined and aspirin was identified to markedly
reduce RANKL-induced p50/p65 phosphorylation (Fig. 4).
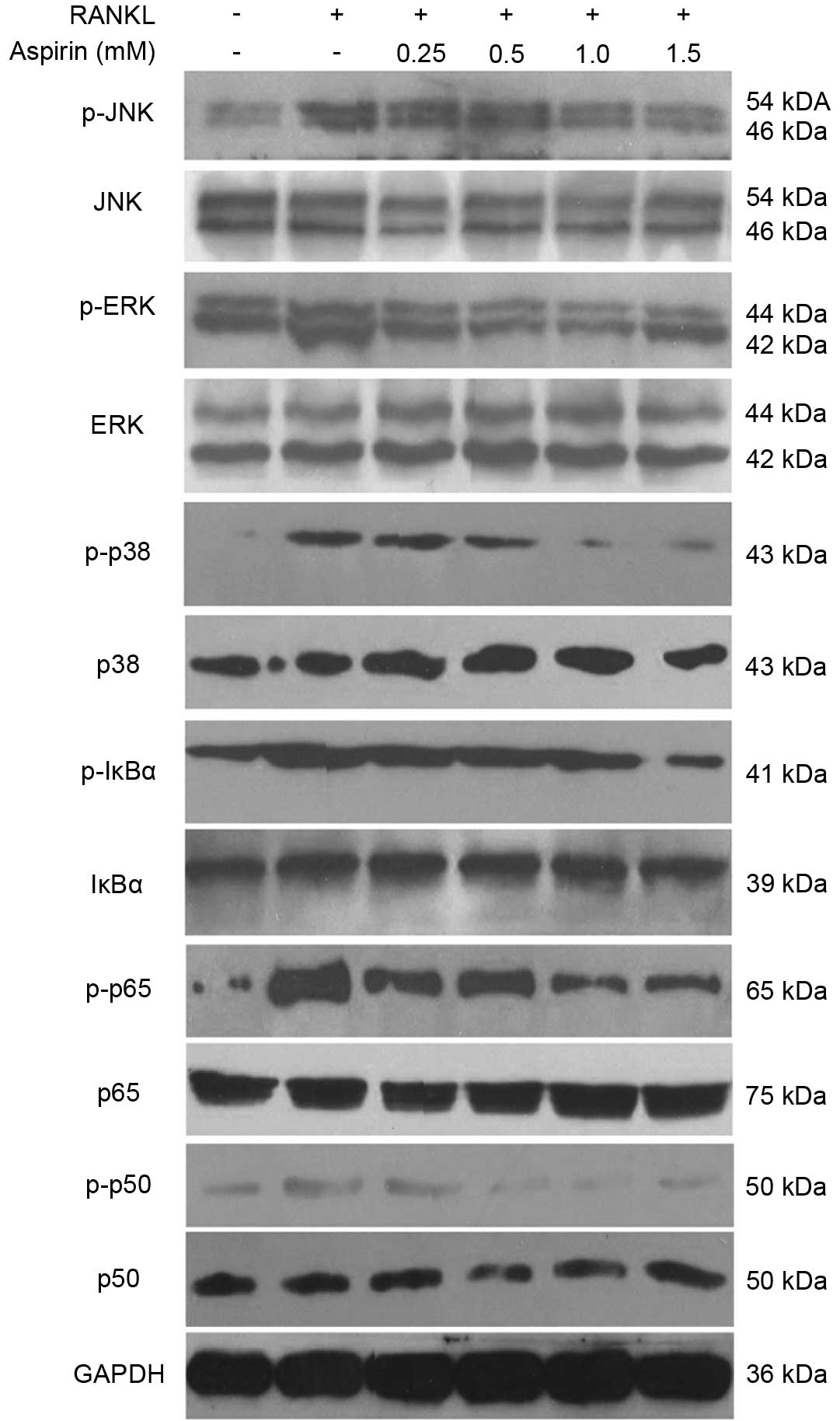 | Figure 4Effects of aspirin on nuclear factor
κB and mitogen-activated protein kinase protein levels in
RANKL-stimulated RAW264.7 cells. RAW264.7 cells (2.0×105
cells/ml) were cultured for 18 h, preincubated with aspirin (0.25,
0.5, 1.0 or 1.5 mM) for 2 h, and then stimulated with RANKL (100
ng/ml) for 30 min. Protein levels were determined using
immunoblotting. RANKL, receptor-activator of nuclear factor κB
ligand; p-, phosphorylated; JNK, c-Jun N-terminal kinases; ERK,
extracellular signal-related kinase; IκBα, inhibitor of κB; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Effects of aspirin on the phosphorylation
of MAPKs in RANKL-stimulated RAW264.7 cells
In order to confirm whether the MAPK signaling
pathway serves an important role in the inhibition of
osteoclastogenesis by aspirin, the phosphorylation of three MAPK
signaling molecules, including ERK, p38 and JNK, were evaluated. It
is was observed that following RANKL activation, the proteins were
phosphorylated (Fig. 4). Aspirin
markedly suppressed p-ERK, p-p38 and p-JNK stimulation in a
dose-dependent manner (Fig. 4).
However, the quantity of unphosphorylated JNK, ERK and p38 did not
appear to be affected by aspirin treatment with RANKL.
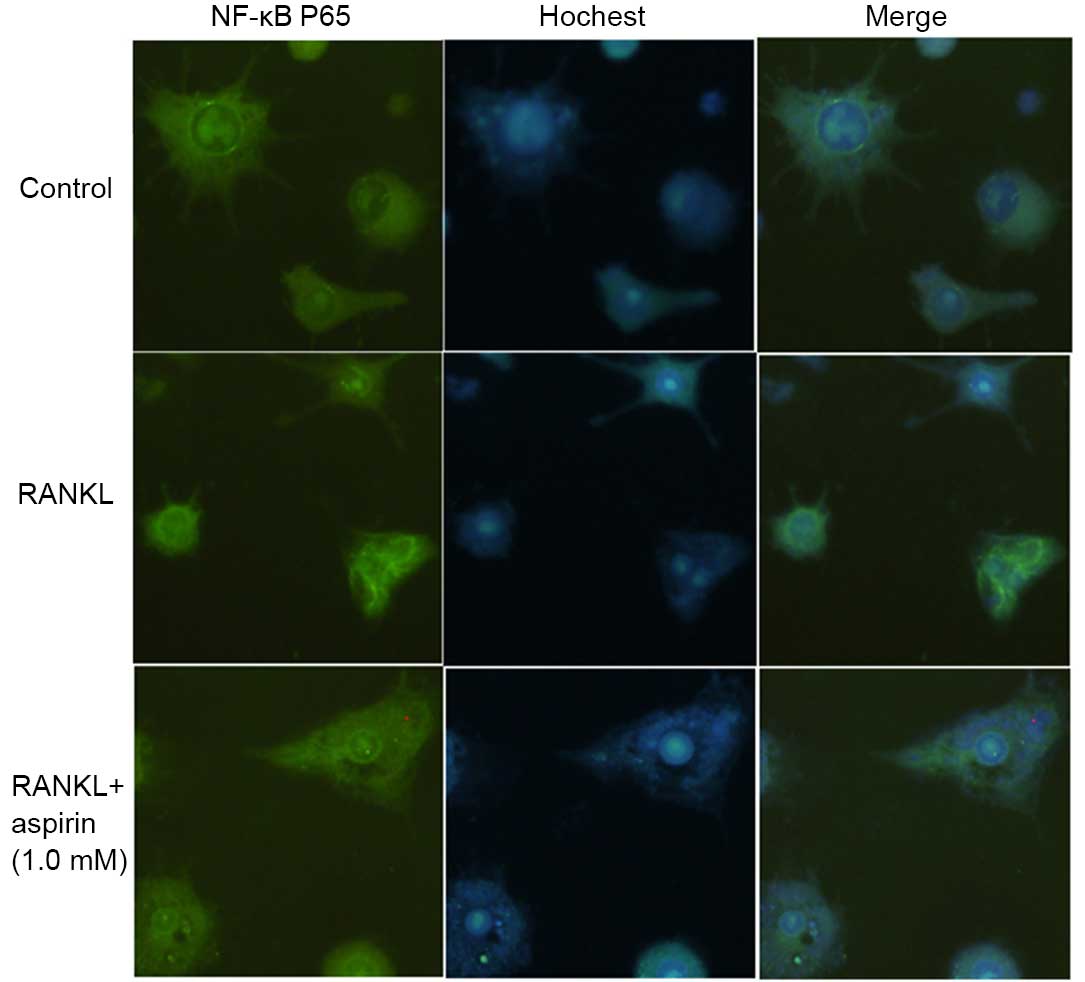
Effects of aspirin on p65 translocation
to the nucleus in RANKL-stimulated RAW 264.7 cells
The effects of aspirin on RANKL-induced NF-κB p65
nuclear translocation were assessed, due to the fact that p65
translocation to the nucleus is essential for NF-κB-dependent
transcription following RANKL stimulation. Thus, an indirect
immunofluorescence assay was used to analyze the NF-κB p65
translocation. As presented in Fig.
5, compared with untreated cells, marked intracellular-p65
translocation to the nucleus from the cytoplasm was induced by
RANKL in RAW264.7 cells. The aspirin pre-treated cell were observed
to exhibit inhibition of this type of translocation.
Discussion
Osteoclasts are present only in the bone, and serve
an effective function in bone-resorption. The intervention of
functions and differentiation of osteoclasts are regarded as the
treatment for bone-metabolic diseases like osteoporosis (14). RANKL signaling triggers osteoclast
differentiation and has been significant for treating pathological
bone-loss. The combination of RANKL and its receptor, RANK, rapidly
stimulates MAPKs, including p38, ERK and JNK, which are all
essential for the differentiation, survival and activation of the
osteoclasts (15–17). These activated MAPKs lead to the
stimulation of transcription factors such as nuclear factor of
activated T-cells, cytoplasmic 1. In the current study, the rapid
phosphorylation of p38, ERK and JNK following treatment with RANKL
in RAW264.7 cell were suppressed by aspirin through a
dose-dependent manner, indicating that aspirin may suppress the
MAPK signaling cascade.
It is has been previously demonstrated that
hematopoietic cells of monocyte-macrophage lineage fuse to form
osteoclasts in the early stage of differentiation (18). The final differentiation
manifestations include alterations in gene expression of
characteristic markers including TRAP, CTSK, MMP-9 and CTR,
morphological conversions into multinucleated cell and the ability
of development of bone resorption lacunae (19–22).
Aspirin reduced the RANKL-induced TRAP, CTSK, MMP-9 and CTR gene
products in a dose-dependent manner, indicating an osteoclast
differentiation inhibiting effect.
NF-κB has been reported to be a crucial
transcription factor in RANKL-induced osteoclastogenesis (23). NF-κB is not active in the cytosol
due to its connections with IκB, however it becomes active once the
phosphorylation and corresponding degradation of IκB occurs
(24). In unstimulated cells,
NF-κB is composed of the p65/p50 heterodimer and is combined with
the inhibitor protein IκB. RANKL activation results in the
activation of the kinase of IκB and IκB-α phosphorylation. The
latter is effected by the proteasomes, and the dissociative p65
subunit enters the nucleus and binds with at a specific position on
the DNA, then target gene transcription is initiated (25). The current study observed that
aspirin was able to suppress the degradation of IκB-α/p65
translocation to the nucleus in the RANKL-induced RAW264.7 cells,
which were detected by western blotting and immunofluorescence,
implying that the NF-κB pathway may serve a role in the inhibition
of osteoclastogenesis of the RANKL-induced RAW264.7 cells.
In conclusion, the current study demonstrated that
aspirin suppressed the osteoclastogenesis of RANKL-induced RAW264.7
cells. Aspirin additionally reduced RANKL-induced expression of the
osteoclastic marker genes. Additionally, aspirin was observed to
suppress RANKL-induced activation of p38, JNK and NF-κB. Further
study is required to clarify the efficacy of aspirin in the
treatment of disease in vivo, however the results of the
current study indicate that it may have potential for the
development of a therapeutic drug for osteoporosis.
References
|
1
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Armstrong AP, Tometsko ME, Glaccum M,
Sutherland CL, Cosman D and Dougall WC: A RANK/TRAF6-dependent
signal transduction pathway is essential for osteoclast
cytoskeletal organization and resorptive function. J Biol Chem.
277:44347–44356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei S, Teitelbaum SL, Wang MW and Ross FP:
Receptor activator of nuclear factor-kappa b ligand activates
nuclear factor-kappa b in osteoclast precursors. Endocrinology.
142:1290–1295. 2001.PubMed/NCBI
|
|
4
|
Matsumoto M, Sudo T, Saito T, Osada H and
Tsujimoto M: Involvement of p38 mitogen-activated protein kinase
signaling pathway in osteoclastogenesis mediated by receptor
activator of NF-kappa B ligand (RANKL). J Biol Chem.
275:31155–31161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
David JP, Sabapathy K, Hoffmann O,
Idarraga MH and Wagner EF: JNK1 modulates osteoclastogenesis
through both c-Jun phosphorylation-dependent and -independent
mechanisms. J Cell Sci. 115:4317–4325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SE, Woo KM, Kim SY, Kim HM, Kwack K,
Lee ZH and Kim HH: The phosphatidylinositol 3-kinase, p38, and
extracellular signal-regulated kinase pathways are involved
inosteoclast differentiation. Bone. 30:71–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koga T, Inui M, Inoue K, Kim S, Suematsu
A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al:
Costimulatory signals mediated by the ITAM motif cooperate with
RANKL for bone homeostasis. Nature. 428:758–763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baltas CS, Balanika AP, Raptou PD, Tournis
S and Lyritis GP; Hellenic guidelines on bone densitometry working
group: Clinical practice guidelines proposed by the Hellenic
Foundation of Osteoporosis for the management of osteoporosis based
on DXA results. J Musculoskelet Neuronal Interact. 5:388–392.
2005.PubMed/NCBI
|
|
9
|
Valverde P: Pharmacotherapies to manage
bone loss-associated diseases: A quest for the perfect
benefit-to-risk ratio. Curr Med Chem. 15:284–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L and Seeram NP: Further investigation
into maple syrup yields 3 new lignans, a new phenylpropanoid, and
26 other phytochemicals. J Agric Food Chem. 59:7708–7716. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauer DC, Orwoll ES, Fox KM, Vogt TM, Lane
NE, Hochberg MC, Stone K and Nevitt MC: Aspirin and NSAID use in
older women: Effect on bone mineral density and fracture risk.
Study of Osteoporotic Fractures Research Group. J Bone Miner Res.
11:29–35. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen ZW, Wu ZX, Sang HX, Qin GL, Wang LS,
Feng J, Wang J, Li XJ, Wang JC and Zhang D: Effect of aspirin
administration for the treatment of osteoporosis in ovariectomized
rat model. Zhonghua Yi Xue Za Zhi. 91:925–929. 2011.In Chinese.
PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Raisz LG: Pathogenesis of osteoporosis:
Concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grigoriadis AE, Wang ZQ, Cecchini MG,
Hofstetter W, Felix R, Fleisch HA and Wagner EF: c-Fos: A key
regulator of osteoclast-macrophage lineage determination and bone
remodeling. Science. 266:443–448. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mansky KC, Sankar U, Han J and Ostrowski
MC: Microphthalmia transcription factor is a target of the p38 MAPK
pathway in response to receptor activator of NF-kappa B ligand
signaling. J Biol Chem. 277:11077–11083. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gingery A, Bradley E, Shaw A and Oursler
MJ: Phosphatidylinositol 3-kinase coordinately activates the
MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival.
J Cell Biochem. 89:165–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohamed SG, Sugiyama E, Shinoda K, Taki H,
Hounoki H, Abdel-Aziz HO, Maruyama M, Kobayashi M, Ogawa H and
Miyahara T: Interleukin-10 inhibits RANKL-mediated expression of
NFATc1 in part via suppression of c-Fos and c-Jun in RAW264.7 cells
and mouse bone marrow cells. Bone. 41:592–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anusaksathien O, Laplace C, Li X, Ren Y,
Peng L, Goldring SR and Galson DL: Tissue-specific and ubiquitous
promoters direct the expression of alternatively spliced
transcripts from the calcitonin receptor gene. J Biol Chem.
276:22663–22674. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Motyckova G, Weilbaecher KN, Horstmann M,
Rieman DJ, Fisher DZ and Fisher DE: Linking osteopetrosis and
pycnodysostosis: Regulation of cathepsin K expression by the
microphthalmia transcription factor family. Proc Natl Acad Sci USA.
98:5798–5803. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reddy SV, Hundley JE, Windle JJ, Alcantara
O, Linn R, Leach RJ, Boldt DH and Roodman GD: Characterization of
the mouse tartrate-resistant acid phosphatase (TRAP) gene promoter.
J Bone Miner Res. 10:601–606. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi HJ, Park YR, Nepal M, Choi BY, Cho
NP, Choi SH, Heo SR, Kim HS, Yang MS and Soh Y: Inhibition of
osteoclastogenic differentiation by Ikarisoside A in RAW 264.7
cells via JNK and NF-kappaB signaling pathways. Eur J Pharmacol.
636:28–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bharti AC, Takada Y and Aggarwal BB:
Curcumin (diferuloylmethane) inhibits receptor activator of
NF-kappa B ligand-induced NF-kappa B activation in osteoclast
precursors and suppresses osteoclastogenesis. J Immunol.
172:5940–5947. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karin M, Yamamoto Y and Wang QM: The IKK
NF-kappa B system: A treasure trove for drug development. Nat Rev
Drug Discov. 3:17–26. 2004. View
Article : Google Scholar : PubMed/NCBI
|