Introduction
Cervical cancer occurs frequently in females. In
recent years, the incidence of cervical cancer has increased,
particularly among young women below the age of 50 years (1). Currently, cervical cancer treatments
include surgery, chemotherapy and radiation therapy. These are
usually effective treatments, however, the structure and function
of the reproductive system may become damaged as a result. The
pregnancy rate of postoperative patients is low, and the risk of
miscarriage and premature birth is increased (2). Loss of fertility is an undesirable
side effect in the majority of young patients, therefore, further
research is required to establish an effective treatment for
cervical cancer that allows patients to retain full reproductive
and sexual function.
The application of pulsed electric fields is a novel
method used to treat tumors. Varied biological and cellular effects
are observed following application of the different pulse widths of
electric field, including reversible electroporation, irreversible
electroporation (IRE) and intracellular electromanipulation
(3,4). IRE has been widely studied and the
main mechanism of its action on tumor cells is the irreversible
breakdown of the cell membrane (5,6).
Previous studies have confirmed the validity of the IRE treatment
on tumor cells (7–9). IRE presents the following benefits:
i) The treatment time is short; ii) the curative effect does not
depend on the thermal effect (10); and iii) the tissue scaffolding,
large blood vessels and other tissue structures surrounding target
areas are not damaged (11,12).
Therefore, IRE has broad application prospects in the field of
tumor treatment.
Tumor tissue often does not share boundaries with
the surrounding normal tissue due to its invasion; therefore,
performing a treatment that targets only the tumor tissue is
difficult. Residual tumor cells at the edge of the therapeutic
range of the pulsed electric fields is inevitable. The behavior of
the residual tumor cells is important in order to determine the
progress of the disease and for the safe use of IRE as a clinical
treatment. The behavior of residual tumor cells following IRE
treatment has not been fully elucidated. Therefore, in the present
study, HeLa and SiHa cells were treated with a sublethal dose of
pulsed electric fields. The changes in cellular behaviors of the
residual tumor cells following treatment was observed, including
proliferation, migration, invasion and adhesive ability.
Materials and methods
Chemicals and reagents
RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from GE Healthcare Life Sciences (Logan, UT, USA). A Cell
Counting Kit-8 (CCK-8) was obtained from Guangzhou Yiyuan
Biological Technology Co., Ltd. (Guangzhou, China). Propidium
iodide (PI) and Carboxyfluorescein diacetate-succinimidyl ester
(CFDA-SE) were obtained from Beyotime Institute of Biotechnology
(Shanghai, China). Transwell inserts were purchased from Corning
Incorporated (Corning, NY, USA). Matrigel was purchased from BD
Biosciences (Franklin Lakes, NJ, USA). The primary antibodies
against p53, p21, cyclin-dependent kinase 2 (CDK2), proliferating
cell nuclear antigen (PCNA) and β-actin were obtained from BIOSS
(Beijing, China).
Cell culture and exposure to electric
pulses
HeLa and SiHa human cervical cancer cell lines (The
Institute of Biological Engineering in Chongqing Medical
University, Chongqing, China) were cultured in RPMI-1640 enriched
with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin
at 37°C and 5% CO2. The cell suspensions were exposed to
the electric pulse therapeutic system in the State Key Laboratory
of Power Transmission Equipment and System Security and New
Technology at Chongqing University (Chongqing, China) as presented
in Fig. 1. The pulse parameters
were selected as 16 electric pulses, 1 Hz frequency for 100
µsec with 1,000 V/cm strength, based on a previous study
(13). These parameters were used
in order to ensure partial survival of the tumor cells, for their
behavior to be examined.
CCK-8 assay
The following three groups were established: i) The
treatment group, cells were treated with electric pulses; ii) the
control group, cells without any treatment; and iii) the blank
group, which did not contain cells. Cells were adjusted to a
density of 5.0×104 cells/ml and seeded in 96-well
plates, in 100 µl 10% FBS and cultured for 2, 4, 6, 8, 10,
12 and 24 h at 37°C in a humidified 5% CO2 atmosphere.
Next, 10 µl CCK-8 reagent was added to each well for 2 h.
The optical density (OD) value of each well was determined using a
microculture plate reader at a wavelength of 450 nm. Each
experimental group comprised of 6 wells and all experiments were
repeated in triplicate. The survival rate of cells was calculated
as follows: Cell survival rate = ODexperimental
group/ODcontrol group × 100.
Cell proliferation assay
CFDA-SE was used to examine the proliferative
abilities of cells. It diffuses into the cytoplasm where it links
to the target protein by covalent bonding and releases green
fluorescence following hydrolysis. In the process of cell
proliferation, the fluorescence intensity reduces as the cells
divide. Fluorescence is evenly distributed between the two daughter
cells, therefore, cell proliferation can be measured from the cell
fluorescence intensity.
HeLa and SiHa cells were divided into the control
and treatment groups and labeled with 10 ml CFDA-SE at 37°C for 10
min in the dark, then washed twice with phosphate-buffered saline
(PBS) containing 10% FBS to remove excess CFDA-SE. Cells were then
plated in 6-well plates and incubated at 37°C with 5%
CO2. Cells were collected and analyzed 24 h after
seeding using a FACScan flow cytometer (BD Biosciences, San Jose,
CA, USA). Proliferation index and precursor frequency were analyzed
using ModFit LT, version 3.2 (Verity Software House, Inc., Topsham,
MA, USA).
Cell cycle analysis
The control and treatment groups of HeLa and SiHa
cells were collected 24 h after treatment and were cultivated at
37°C in a humidified 5% CO2 atmosphere, then fixed in
75% cold ethanol overnight. Following a wash with PBS, cells were
incubated with 100 ml RNase A (100 mg/ml) and 400 ml propidium
iodide for 30 min at 37°C. The cell cycle phase was determined by
flow cytometry at 488 nm, and the relative ratios of the G1, S and
G2 phases were analyzed using FlowJo, version 2.8 (FlowJo LLC,
Ashland, OR, USA). The experiment was performed in triplicate.
Wound healing assay
The control and treatment groups of HeLa and SiHa
cells were seeded in a 6-well plate. When the cells reached 80-90%
confluence, the middle of the culture was scraped with a sterile
pipette tip (10 µl). The floating cells were removed by
washing with PBS. Five fields of view were randomly selected and
viewed with a microscope and photographed. Cells were then cultured
in serum-free medium for 48 h and images were captured. The scratch
width was determined using Image-Pro Plus, version 6 (Media
Cybernetics, Inc., Rockville, MD, USA), and scrape repair rate =
[(width0 h − width24 h)/width0 h]
× 100. The experiment was repeated three times.
Cell invasion analysis
The cell invasion ability of cells was determined by
Transwell invasion assay in vitro. The upper chamber of
24-well Transwell plates with polycarbonate membrane (8-mm pore
size) were covered with 40 µl Matrigel (BD Biosciences; 1:4
dilution) and incubated for 24 h at 37°C. The lower chamber was
filled with 500 µl RPMI-1640 with 10% FBS. The non-invading
cells were removed from the membrane using a cotton swab and the
Transwell plate was fixed with 4% paraformaldehyde for 30 min. The
cells were then stained with crystal violet for 10 min at room
temperature. The cells that passed through the polycarbonate
membrane were counted using a Leica microscope. The experiment was
repeated three times for each group.
Matrigel adhesion analysis
The 96-well plate was covered with Matrigel for 1 h
and other protein binding sites were blocked using 100 µl
bovine serum albumin (5 mg/ml) in DMEM for 1 h. A total of
4.0×105 cells/well from each group were suspended in 100
µl RPMI-1640 and cultured for 2 h. Non-adherent cells were
washed away with PBS and adherent cells were treated with 20
µl MTT in 200 µl serum-free medium. The cells were
cultured for 4 h, then the solution was removed carefully and
replaced with 150 µl dimethyl sulfoxide. The cells were
oscillated in the dark for 15 min at a speed of 300 r/min. The OD
value of each well was determined using a microculture plate reader
at a wavelength of 490 nm. Each experimental group comprised 6
wells, and all experiments were repeated in triplicate.
Western blot analysis
Following IRE treatment, the cells were collected
and lysed in cold radioimmunoprecipitation assay buffer (Cell
Signaling Technology, Inc., Danvers, MA, USA). Proteins were
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis, and transferred onto a poly-vinylidene difluoride
membrane. The membrane was blocked for 2 h at room temperature in
PBS containing 5% non-fat milk, and then incubated overnight at 4°C
with the following primary antibodies: Rabbit polyclonal anti-P53
(cat no. bs-8687R), anti-P21 (cat no. bs-10129R), anti-CDK2 (cat.
no. bs-0757R), anti-PCNA (cat. no. bs-2007R) and anti-β-actin (cat.
no. bs-0061R) were obtained from BIOSS. Mouse anti-rabbit secondary
antibody conjugated with horseradish peroxidase (bs-0295M; BIOSS)
was used to visualize the stained bands with a Beyo Enhanced
chemiluminescence Plus kit (Beyotime Institute of Biotechnology).
Equal loading of protein was confirmed by stripping the blots and
reprobing with β-actin antibody.
Statistical analysis
All data was processed with the statistical software
SPSS, version 19.0 (IBM SPSS, Armonk, NY, USA). Student's t-test,
one-way analysis of variance and χ2 test were used to
analyze differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Growth inhibition of HeLa and SiHa
cells
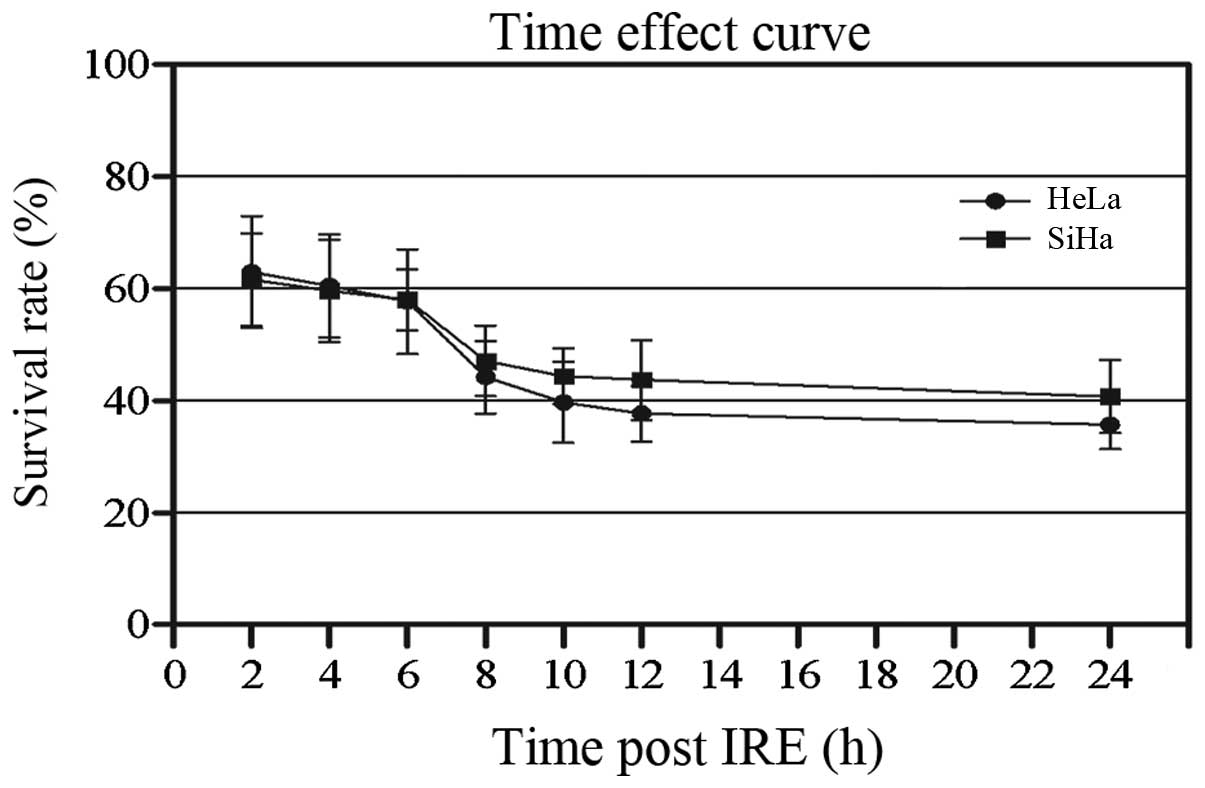
CCK-8 assay was used to detect the effect of IRE on
cellular growth. It was determined that IRE may inhibit the growth
of the two cell lines (Fig. 2).
The viability of HeLa and SiHa cells was decreased gradually. The
survival rate of HeLa cells decreased from 62.95±10.01% at 2 h to
39.69±4.34% at 24 h after IRE. The survival rate of SiHa cells
decreased from 61.58±8.28 to 40.71±6.48% over the same time
period.
Proliferation suppression of HeLa and
SiHa cells
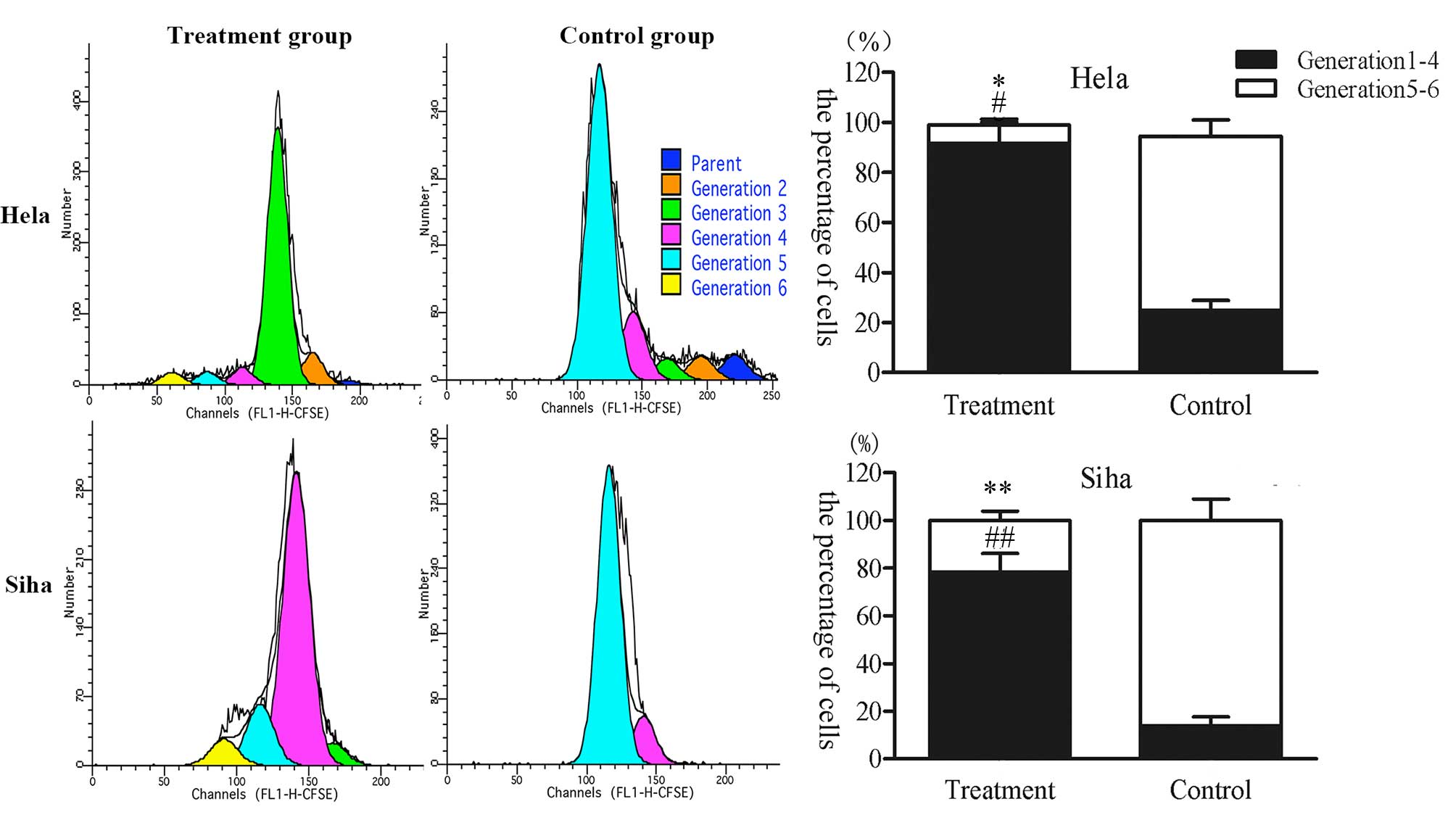
CFDA-SE fluorescence intensity was determined 24 h
after IRE treatment to evaluate effects on cell proliferation
(Fig. 3). Cell proliferation was
inhibited in HeLa and SiHa cells treated with IRE for 24 h compared
with the control group. The HeLa cells at generations 5 and 6
(7.38±2.21%) were decreased significantly compared with the control
group (69.77±6.56%) (P=0.03); however, there was a greater number
of cells in the treatment group at generations 1–4 compared with
the control group (P=0.009). The SiHa cells at generations 5 and 6
(21.72±3.99%) decreased significantly compared with the control
group (86.08±8.96%). Conversely, there was a greater number of
cells at generations 1–4 compared with the control group (P=0.008).
The results showed that IRE can inhibit cell proliferation.
Cell cycle arrest in HeLa and SiHa
cells
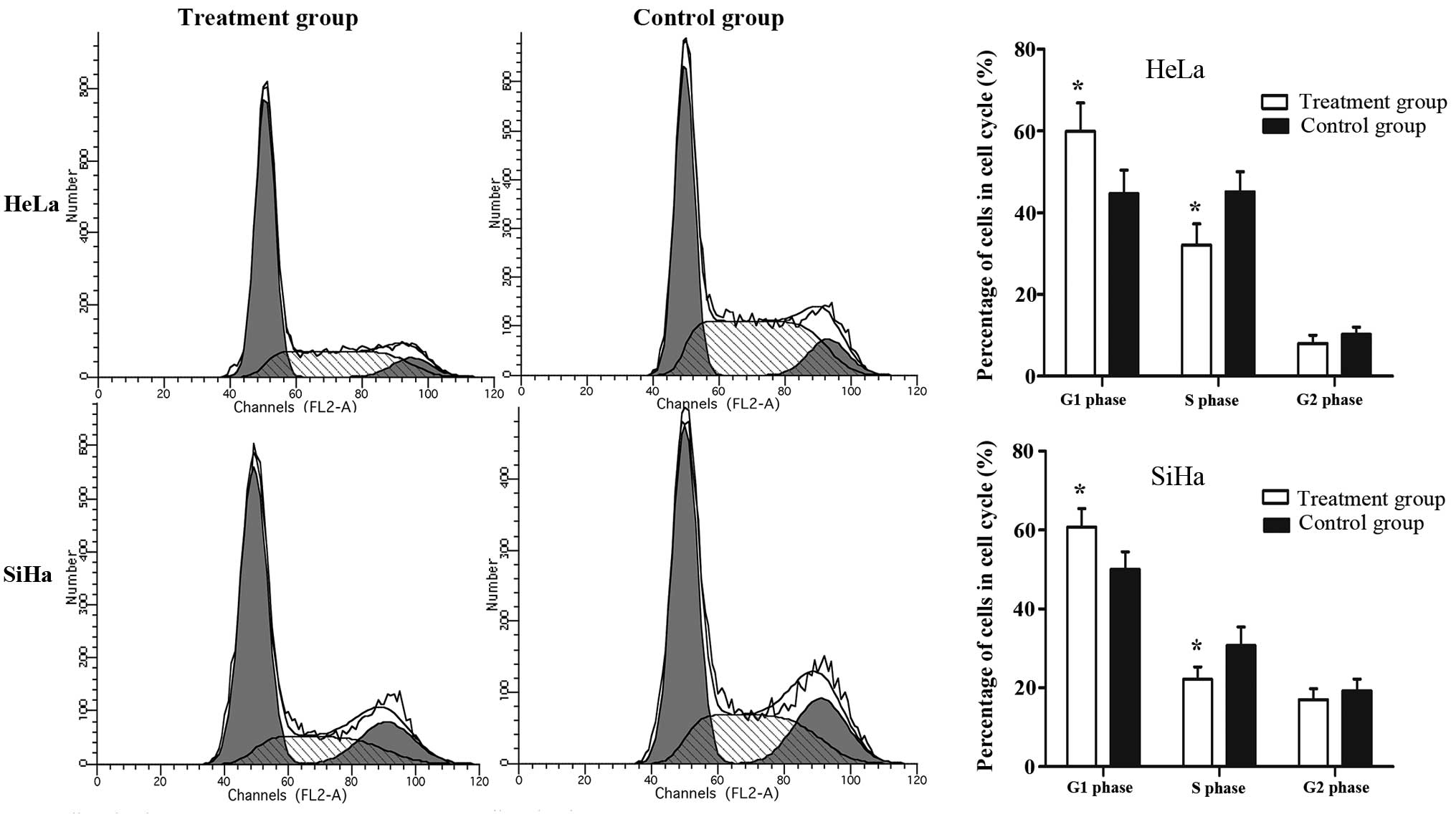
The IRE inhibition of the proliferation of HeLa and
SiHa cells may be due to abnormal cell cycle progression.
Therefore, the cell cycle distribution was examined in each group
using PI staining and flow cytometry. As presented in Fig. 4, HeLa and SiHa cells treated with
IRE presented significantly higher percentages of cells in the G1
phase (59.91±6.99%) compared with the control group (44.63±5.79%)
and significantly lower percentages of cells in the S phase
compared with the control group (32.09±5.2 vs. 45.13±4.89%).
Therefore, it is possible that IRE treatment leads to cell cycle
arrest in the G1 phase.
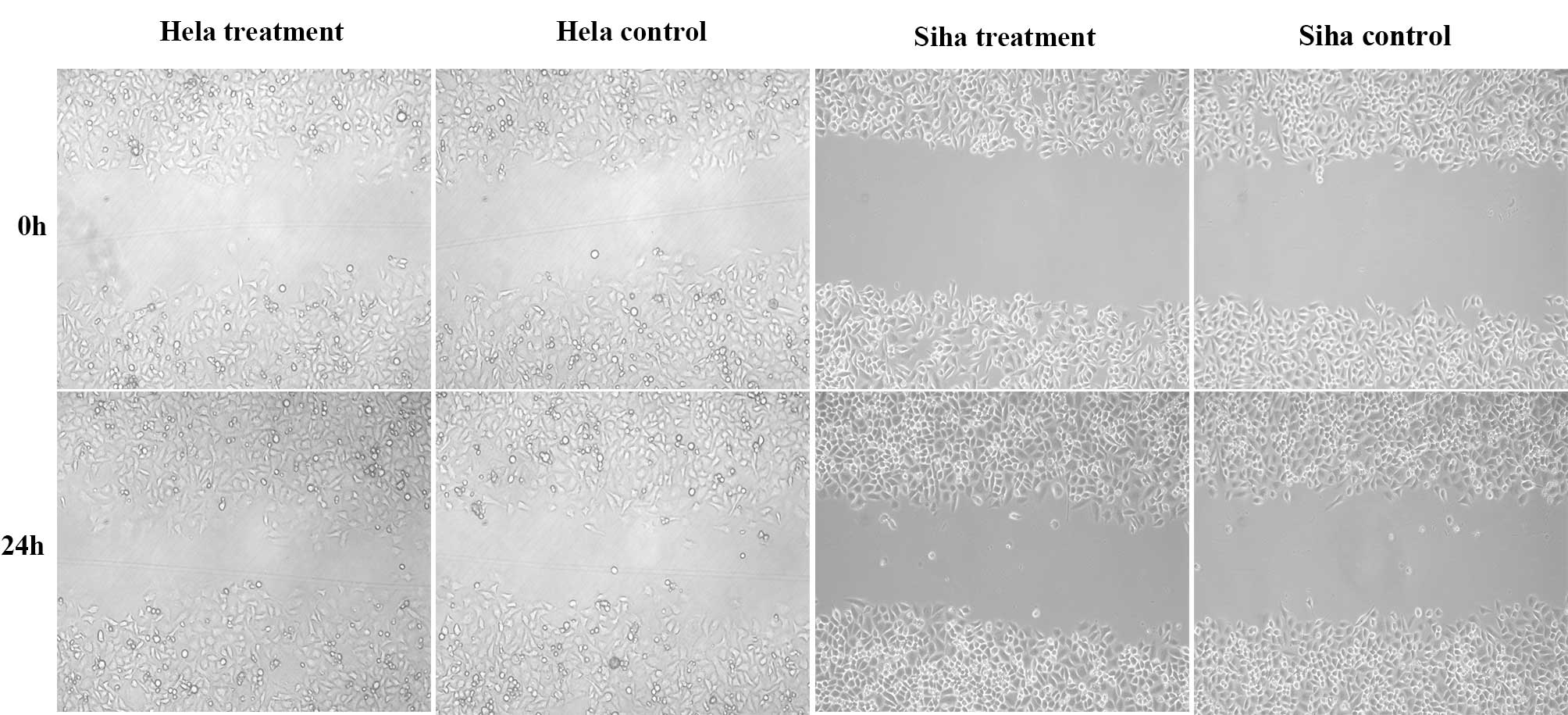
Effect of IRE on cell migration
A wound healing assay was used to examine the effect
of IRE on cell migration. No significant differences were observed
between the control and treatment groups in HeLa and SiHa cells
(Fig. 5). When the counted
respective scrape repair rates were determined, similar results
were obtained (Table I).
Therefore, IRE treatment was not observed to have a significant
effect on HeLa and SiHa cell migration.
 | Table IScrape repair rate of HeLa and SiHa
cells subsequent to the pulse treatment at 24 h. |
Table I
Scrape repair rate of HeLa and SiHa
cells subsequent to the pulse treatment at 24 h.
| Repair rate | Treatment group
(%) | Control group
(%) |
|---|
| HeLa cells | 20.53±7.94 | 23.13±3.53 |
| SiHa cells | 14.76±6.30 | 17.39±3.79 |
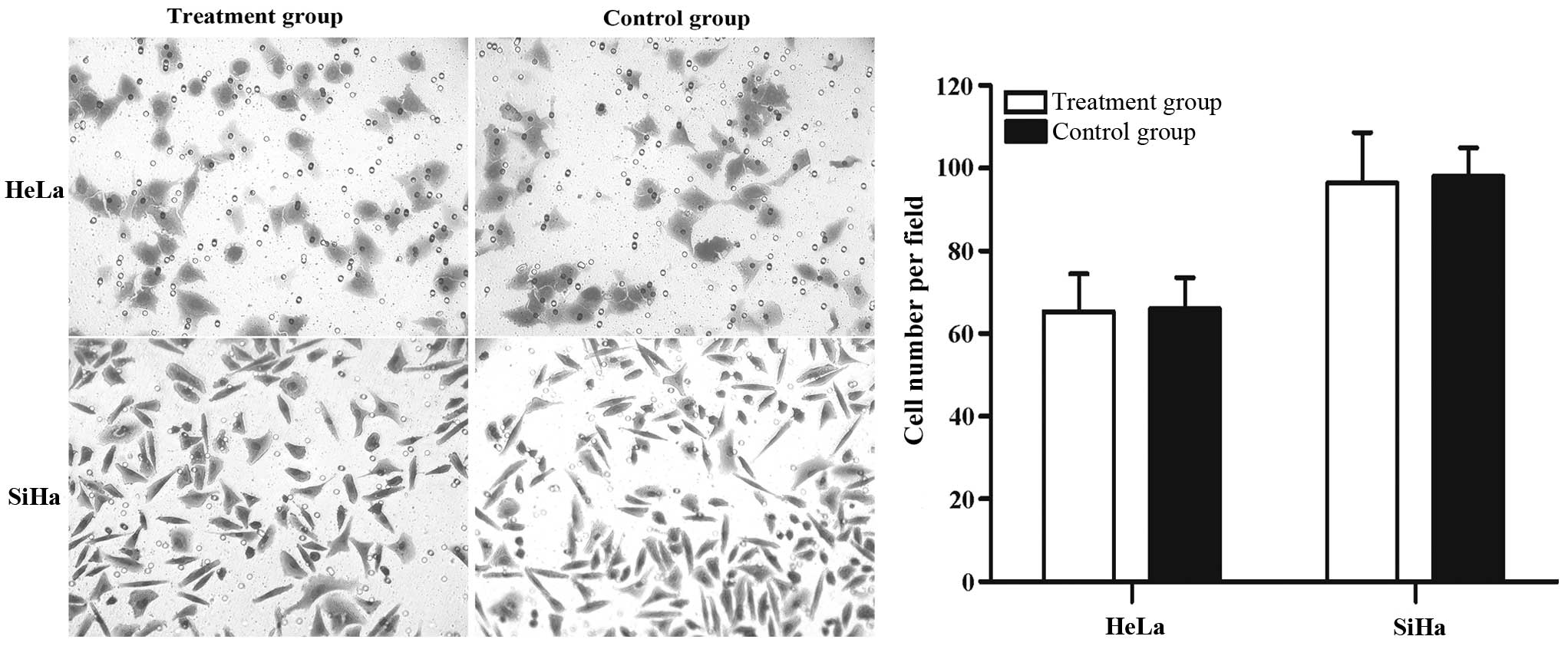
Effect of IRE treatment on cell
invasion
Cell invasion ability is an important factor for
determining tumor malignancy. The effect of IRE on cell invasion
was investigated by Transwell assay. The number of HeLa and SiHa
invading cells did not differ when the treatment group was compared
with the control group (Fig. 6).
Similar to the results of the migration assay, IRE has no clear
effect on HeLa and SiHa cell invasion.
Effect of IRE on cell adhesion
The effect of IRE treatment on the adhesion ability
of cells was verified by adhesion assay. MTT assay was used to
detect the adhesive ability of cells. No significant difference was
observed in the OD values of HeLa and SiHa cells when the treatment
group was compared with the control group (Table II). This indicates that IRE
treatment of cervical cancer cells does not affect the cell
adhesion potential.
 | Table IIOD values of HeLa and SiHa adhesive
cells subsequent to the pulse treatment at 24 h. |
Table II
OD values of HeLa and SiHa adhesive
cells subsequent to the pulse treatment at 24 h.
| OD value | Treatment group | Control group |
|---|
| HeLa cells | 0.234±0.023 | 0.300±0.010 |
| SiHa cells | 0.241±0.037 | 0.283±0.049 |
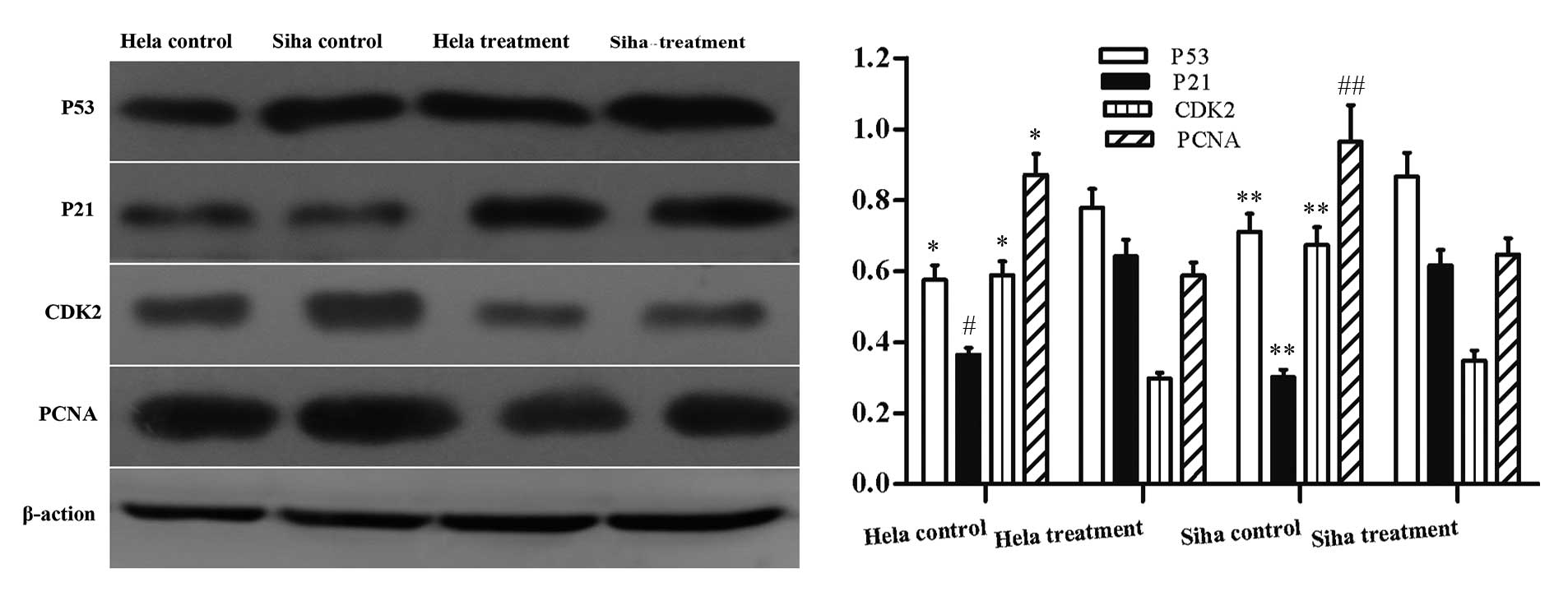
Analysis of the expression level of
proliferation-associated genes
IRE treatment led to the reduction of cell
proliferation and limited cell cycle progression. Therefore, the
expression level of genes associated with cell cycle progression
p53, p21, CDK2 and PCNA was investigated. The protein expression
levels of p53 and p21 were significantly greater 24 h after IRE
treatment. The protein expression levels of CDK2 and PCNA were
significantly reduced compared with the control group (Fig. 7).
Discussion
IRE is a potential novel therapy for the treatment
of tumors. Previous studies have confirmed that the use of pulsed
electric fields in cancer cells and animal xenograft may lead to
the irreversible electroporation, necrosis and apoptosis of tumor
cells (13,14). Additionally, IRE has been used to
treat malignancy, with short-term success (15,16).
Cervical cancer is the most common malignancy of the female
reproductive system. Advance screening technology in cervical
cancer allows for early-stage diagnosis of patients. The specific
anatomical location of the cervical tumor may be exposed fully by a
simple instrument, which provides the necessary basic conditions
required for effective IRE ablation treatment.
Residual tumor tissue often occurs following IRE
treatment. In the case of radio frequency ablation (RFA), it has
been determined that RFA may promote cancer recurrence.
Additionally, residual cancer cell proliferation in animal
experiments and clinical treatment of liver cancer has been
observed (17,18). Irreversible electroporation has
therapeutic efficacy in the clinical treatment of prostate cancer
and renal cancer. Although it has not been used clinically in the
treatment of cervical cancer, experimental research has made
significant progress (19).
However it is limited by the lack of long-term follow-up
observation of tumor recurrence and metastasis. Existing physical
therapies, such as use of the electric knife and laser are limited
to the treatment of carcinoma in situ; and are not used for
invasive lesions. This is because these therapies increase residual
tumor cell proliferation and invasion. In the present study, HeLa
and SiHa human cervical carcinoma cell lines were investigated. The
behavior of residual tumor cells treated with sublethal doses of
pulsed electric fields was determined.
Based on previous experimental results (14), a field strength of 1,000 V/cm,
frequency 1 Hz and 16 pulses were selected as pulse parameters. The
change of the proliferative capacity of cells was investigated. The
results indicated that the proliferation of HeLa and SiHa cells was
inhibited 24 h after IRE treatment and cell cycle progression was
arrested at the G1 stage. The expression levels of cell
cycle-associated proteins p53, p21, CDK2 and PCNA were also
determined. The increased expression levels of p53 induce the
expression of p21, which combines with the cyclin E/CDK2 complex to
suppress the activity of CDK2. This leads to a halt of cell cycle
progression at the G1 and S phases (20,21).
The present study confirmed increased expression levels of p53 and
p21, whilst recording decreased expression levels of CDK2. PCNA is
closely associated with DNA synthesis processes in cells and is
frequently used as an evaluation index for tumor cell proliferation
(22). It is possible that the
inhibition of the proliferation of residual tumor cells may be a
result of PCNA expression and the P53-P21-CDK2 signaling pathway.
Invasion and metastasis are important in determining the behavior
of malignant tumor cells and may be one of the factors contributing
to the difficulties in treating cancer. Initially, the invasion of
tumor cells occurs by the adhesion of molecules to the
extracellular matrix (ECM), and matrix metalloproteinases,
including proteolytic enzymes, damage the integrity of the ECM. In
conclusion, local invasion is dependent on tumor cell migration
capacity through the basement membrane. Matrigel mainly contains
laminin and collagen IV, so is able to serve as an in vitro
ECM model. Measuring penetration ability through a membrane coated
by Matrigel is able to indicate the invasion ability of tumor
cells. In the present study, Matrigel adhesion, Transwell chamber
invasion and wound healing assays were used to investigate the
effect of sublethal doses of pulsed electric fields on the invasive
and metastatic properties of HeLa and SiHa cells, and to determine
whether IRE ablation may promote the proliferation of residual
cancer cells. The results indicated that the adhesive, invasive and
migratory properties of tumor cells were not significantly altered.
Therefore, it may be concluded that if complete ablation of the
tumor cells is not achieved, it will not result in proliferation of
the remaining cells, or induce the occurrence of distant
metastases.
In conclusion, the present study confirmed that
sublethal doses of pulsed electric field do not enhance the
proliferation, invasion or metastasis of HeLa and SiHa cervical
cancer cell lines in vitro. It supports the use of IRE
ablation in cervical lesions, in which the growth of residual
disease may be temporarily suppressed, and subsequent treatment by
ablation may be used if required. However, as the present study was
an in vitro investigation, further experiments, including
those using animal models are required in order to confirm the
effects of IRE treatment on the various cell behaviors of cervical
cancer tumors in vivo, in addition to its mechanisms of
action.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81201745), the
Scientific and Technological Research Program of Chongqing
Municipal Education Commission (grant no. KJ1400223) and the
National Natural Science Foundation of China (grant no.
81301928).
References
|
1
|
Motoki Y, Mizushima S, Taguri M, Takahashi
K, Asano R, Kato H, Asai-Sato M, Katayama K, Okamoto N, Hirahara F
and Miyagi E: Increasing trends in cervical cancer mortality among
young Japanese women below the age of 50 years: an analysis using
the Kanagawa population-based Cancer Registry, 1975–2012. Cancer
Epidemiol. 39:700–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ercoli A, Iannone V, Legge F, Fagotti A,
Fanfani F, Carone V, D'Asta M, Scambia G and Ferrandina G: Advances
in surgical management of cervical cancer. Minerva Ginecol.
61:227–237. 2009.PubMed/NCBI
|
|
3
|
Schoenbach KH, Joshi RP, Kolb JF, Chen N,
Stacey M, Blackmore PF, Buescher ES and Beebe SJ: Ultrashort
electrical pulses open a new gateway into biological cells. Proceed
IEEE. 92:1122–1137. 2004. View Article : Google Scholar
|
|
4
|
Yao C, Mo D, Li C, Sun C and Mi Y: Study
of transmembrane potentials of inner and outer membranes induced by
pulsed-electric-field model and simulation. IEEE Trans Plasma Sci.
35:1541–1549. 2007. View Article : Google Scholar
|
|
5
|
Weaver JC: Electroporation of cells and
tissues. IEEE Trans Plasma Sci. 28:24–33. 2000. View Article : Google Scholar
|
|
6
|
Rubinsky B: Irreversible electroporation
in medicine. Technol Cancer Res Treat. 6:255–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller L, Leor J and Rubinsky B: Cancer
cells ablation with irreversible electroporation. Technol Cancer
Res Treat. 4:699–705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rubinsky J, Onik G, Mikus P and Rubinsky
B: Optimal parameters for the destruction of prostate cancer using
irreversible electroporation. J Urol. 180:2668–2674. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
José A, Sobrevals L, Ivorra A and Fillat
C: Irreversible electro-poration shows efficacy against pancreatic
carcinoma without systemic toxicity in mouse models. Cancer Lett.
317:16–23. 2012. View Article : Google Scholar
|
|
10
|
Davalos RV, Mir IL and Rubinsky B: Tissue
ablation with irreversible electroporation. Ann Biomed Eng.
33:223–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Phillips MA, Narayan R, Padath T and
Rubinsky B: Irreversible electroporation on the small intestine. Br
J Cancer. 106:490–495. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee EW, Chen C, Prieto VE, Dry SM, Loh CT
and Kee ST: Advanced hepatic ablation technique for creating
complete cell death: Irreversible electroporation. Radiology.
255:426–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou W, Xiong Z, Liu Y, Yao C and Li C:
Low voltage irreversible electroporation induced apoptosis in HeLa
cells. J Cancer Res Ther. 8:80–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wanda KN and John CN: Irreversible
electroporation. 1st edition. Springer-Verlag; Berlin, Germany: pp.
85–86. 2010
|
|
15
|
Gary O and Rubinsky B: Irreversible
electroporation. 1st edition. Springer-Verlag; Berlin, Germany: pp.
235–247. 2010
|
|
16
|
Pech M, Janitzky A, Wendler JJ, Strang C,
Blaschke S, Dudeck O, Ricke J and Liehr UB: Irreversible
electroporation of renal cell carcinoma: A first-in-man phase I
clinical study. Cardiovasc Intervent Radiol. 34:132–138. 2011.
View Article : Google Scholar
|
|
17
|
von Breitenbuch P, Köhl G, Guba M,
Geissler E, Jauch KW and Steinbauer M: Thermoablation of colorectal
liver metastases promotes proliferation of residual intrahepatic
neoplastic cells. Surgery. 138:882–887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruzzenente A, Manzoni GD, Molfetta M,
Pachera S, Genco B, Donataccio M and Guglielmi A: Rapid progression
of hepatocellular carcinoma after radiofrequency ablation. World J
Gastroenterol. 10:1137–1140. 2004.PubMed/NCBI
|
|
19
|
Liu XY, Xiong ZA, Li HS and Li CX:
Alterations in the mortality and growth cycle of cervical cancer
cells treated with electro-poration at different electric
strengths. Eur J Gynaecol Oncol. 33:79–85. 2012.
|
|
20
|
Chae HD, Kim SY, Park SE, Kim J and Shin
DY: p53 and DNA-dependent protein kinase catalytic subunit
independently function in regulating actin damage-induced
tetraploid G1 arrest. Exp Mol Med. 44:236–240. 2012. View Article : Google Scholar :
|
|
21
|
Warfel NA and El-Deiry WS: p21WAF1 and
tumourigenesis: 20 years after. Curr Opin Oncol. 25:52–58. 2013.
View Article : Google Scholar
|
|
22
|
Tsai WC, Cheng JW, Chen JL, Chen CY, Chang
HN, Liao YH, Lin MS and Pang JH: Low-level laser irradiation
stimulates tenocyte proliferation in association with increased NO
synthesis and upregulation of PCNA and cyclins. Lasers Med Sci.
29:1377–1384. 2014. View Article : Google Scholar : PubMed/NCBI
|