Introduction
Multiple myeloma (MM), which is also known as plasma
cell myeloma, is a type of cancer characterized by the excessive
proliferation of malignant plasma cells, extensive osteolytic
lesions, and/or osteoporosis (1).
In recent years, among the hematological malignancies, the
incidence of MM in the United States has overtaken acute leukemia,
and is second only to non-Hodgkin's lymphoma (2). In addition, the incidence of MM in
China has been increasing annually (3). MM is the most common primary tumor of
the bone marrow in the USA (4,5).
Matrix metalloproteinases (MMPs), which belong to
the zinc-dependent endopeptidase family, exert proteolytic activity
and are associated with bone remodeling, bone resorption, tumor
invasion and metastasis (6,7).
Previous studies have also demonstrated that in myeloma cells,
elevated levels of MMP-2 may be associated with disease
progression, angiogenesis, and myeloma bone disease (8,9). In
addition, Zdzisińska et al (9) reported that suppression of MMP-2
activity was able to inhibit the proliferation of MM cells.
MicroRNAs (miRs) are small (19–25 nucleotides)
non-coding RNA molecules that are involved in genetic regulation.
miRs are involved in regulating various biological signaling
pathways, and have several roles in the regulation of cell growth
and development (10). miRs
account for 1–2% of the known eukaryotic genome, and have a role in
tumor biology, as either tumor suppressor genes or proto-oncogenes
(11). Overexpression of miR-29b
has previously been shown to reduce the protein expression levels
of myeloid cell leukemia 1 (Mcl-1), thereby inhibiting the growth
of MM cells. miR-29b has also been reported to inhibit the
interleukin-6-induced upregulation of Mcl-1, thus suggesting that
miR-29b may act as a tumor suppressor gene (12,13).
Paeoniflorin exerts numerous functions, including
sedation, spasmolysis, anti-inflammation, memory improvement, blood
glucose-lowering effects, anti-emergency ulcer, coronary vessel
expansion, anti-acute ovarian cancer and inhibition of cardiac
ischemia and platelet aggregation (14). It has previously been demonstrated
that paeoniflorin may effectively modulate the multidrug resistance
of the SGC7901 human gastric cancer cell line, via inhibition of
nuclear factor-κB activation (15). Furthermore, paeoniflorin has been
reported to significantly induce the apoptosis of HeLa human
cervical cancer cells, via the downregulation of B-cell lymphoma-2
(Bcl-2) and the upregulation of caspase-3 (16). However, whether paeoniflorin
affects the proliferation and apoptosis of SKO-007 MM cells via
suppression of MMP-2 expression and upregulation of miR-29b remains
unknown. The present study aimed to investigate the effects and
underlying molecular mechanisms of paeoniflorin on MM cell
proliferation and apoptosis.
Materials and methods
Reagents and chemicals
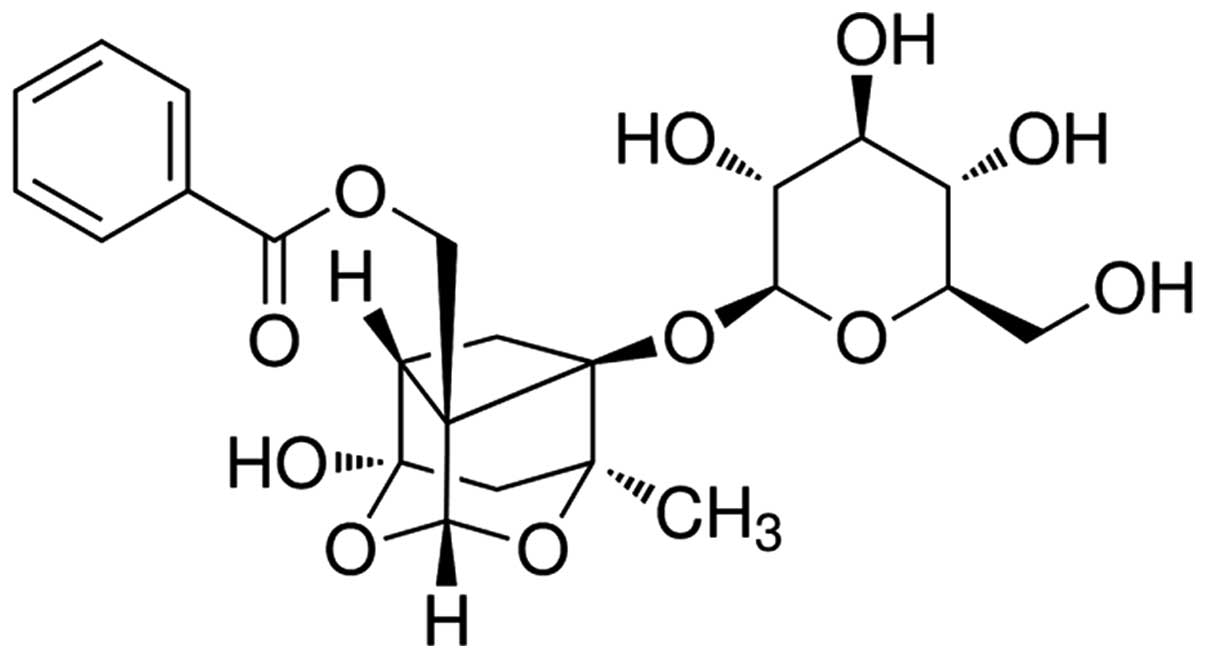
The chemical structure of paeoniflorin
(Sigma-Aldrich, St. Louis, MO, USA; purity >98%) is presented in
Fig. 1. Paeoniflorin was dissolved
in physiological saline, according to the manufacturer's protocol
(17). RPMI-1640 medium and fetal
bovine serum (FBS) were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Caspase-3/9 Activity Assay
kits and Annexin V-Fluorescein Isothiocyanate (FITC)/Propidium
Iodide (PI) Apoptosis Detection kit were purchased from Nanjing
KeyGen Biotech Co., Ltd. (Nanjing, China). TRIzol reagent and
quantitative-polymerase chain reaction (qPCR) assays were purchased
from Invitrogen (Thermo Fisher Scientific, Inc.).
Cell culture
The SKO-007 human MM cell line (Type Culture
Collection of the Chinese Academy of Sciences, Shanghai, China) was
cultured in RPMI-1640 medium supplemented with 10% FBS, 100
units/ml penicillin and 100 mg/ml streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
containing 5% CO2. The culture media were changed every
2–3 days.
Cell viability assay
SKO-007 cells were seeded at a density of
1×104/well into 96-well plates, and were treated with
paeoniflorin (0, 5, 10 and 20 µM) for 48 h. Next, GM60001 (5
μM), was used to suppress the expression of MMP-2 and added
to SKO-007 cells for 48 h. Subsequently, cell viability was
measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Beyotime Institute of Biotechnology, Jiangsu, China) assay.
Briefly, 15 µl MTT was added to each well and incubated for
4 h at 37°C in a humidified atmosphere containing 5%
CO2. Subsequently, 150 µl dimethyl sulfoxide was
added to each well, and the plates were agitated for 20 min. The
absorbance of each well was measured at λ=570 nm using a Varioskan
Flash Multi-mode Reader (Thermo Fisher Scientific, Inc.).
Lactate dehydrogenase (LDH) assay
SKO-007 cells were seeded at a density of
1×104/well into 96-well plates, and were treated with
paeoniflorin (0, 5, 10 and 20 µM) for 0, 24, 48 and 72 h.
Subsequently, the cytotoxicity of paeoniflorin to SKO-007 cells was
measured using an LDH assay (Beyotime Institute of Biotechnology).
Briefly, 100 µl LDH was added to each well and incubated for
30 min at room temperature. The absorbance was then measured at 490
nm using a multi-well spectrophotometer (868; BioTek Instruments,
Inc., Winooski, VT, USA).
Annexin V-FITC/PI apoptosis assay
SKO-007 cells were seeded at a density of
1×106/well into 6-well plates, and were treated with
paeoniflorin (0, 5, 10 and 20 µM) for 48 h. The number of
apoptotic cells was measured using an Annexin V-FITC/PI Apoptosis
Detection kit. Briefly, SKO-007 cells were washed twice with
phosphate-buffered saline and resuspended in 1X binding buffer.
Annexin V-FITC (10 µl) was then added and incubated for 10
min at room temperature in the dark. Subsequently, PI (5 µl)
was added and the cells were incubated for a further 10 min at room
temperature in the dark. A flow cytometer (FACScalibur; BD
Biosciences, San Jose, CA, USA) and CellQuest™ Pro software (BD
Biosciences) were used to analyze the rate of apoptosis.
Caspase-3 and caspase-9 activation
assay
SKO-007 cells were seeded at a density of
1×104/well into 96-well plates, and were treated with
paeoniflorin (0, 5, 10 and 20 µM) for 48 h. Caspase-3 and
caspase-9 activities were measured using Caspase-3/9 Activity Assay
kits, according to the manufacturer's protocol. Briefly, 10
µl protein cell lysate (containing 50mM Tris-HCl pH8.0, 5mM
EDTA, 150mM NaCl, 1% Triton-X 100; Beyotime Institute of
Biotechnology) per sample was added to 100 µl reaction
buffer containing 10 µl substrate (Ac-DEVD-pNA for
caspase-3, and Ac-LEHD-pNA for caspase-9) and incubated at 37°C for
4–6 h. Caspase-3 and caspase-9 activities were measured at an
absorbance of 405 nm.
Gelatin zymography
SKO-007 cells were seeded at a density of
1×106/well into 6-well plates, and were treated with
paeoniflorin (0, 5, 10 and 20 µM) for 48 h. The expression
levels of MMP-2 in the SKO-007 cells were determined using
zymographic analysis. Briefly, every sample was subjected to 10%
sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis
containing 0.1% gelatin. Following electrophoresis, the gels were
washed with 50 mM Tris-HCl (pH 7.6) three times for 30 min at room
temperature, in order to remove the SDS. The gels were then
incubated in a reaction buffer (Beyotime Institute of
Biotechnology) for 12 h at 37°C, after which, the gel was stained
with 0.1% Coomassie Brilliant Blue G250 (Aladdin Industrial
Corporation, Shanghai, China) for 1 h and destained in 10% acetic
acid and 10% methanol.. THe resulting gels were analyzed using a
film processor (CP 1000; Agfa-Gevaert N.V, Mortsel, Belgium).
Reverse transcription-qPCR analysis of
miR-29b expression
SKO-007 cells were seeded at a density of
1×106/well into 6-well plates, and were treated with
paeoniflorin (0, 5, 10 and 20 µM) for 48 h. Total RNA was
extracted from the cells using TRIzol reagent, and miRs were
specifically amplified for quantification using individual miRNA
TaqMan Real-Time qPCR analysis (Invitrogen; Thermo Fisher
Scientific, Inc.). The primer sequences were as follows: miR-29b,
forward 5′-GGG GGT ACC CTT CAG GAA GCTG GTT TC-3′, reverse 5′-GGG
GAT ATC TAC ATG TGA GGC AGG TTC TCAC-3′; and U6, forward 5′-CGC TTC
GGC AGC ACA TAT ACTA-3′, and reverse 5′-CGC TTC ACG AAT TTG CGT
GTCA-3′. The PCR conditions were 95°C for 60 sec, 95°C for 30 sec,
60°C for 45 sec, 72°C for 30 min, 55°C for 30 sec, for 35 cycles.
The results were normalized to β-actin. ΔCq was calculated by
subtracting the Ct of U6. The ΔΔCq was then calculated by
subtracting the ΔCq of the negative control from the ΔCq of the
samples using the 2−ΔΔCq method (18).
Transfection of miR-29b and anti-miR-29b
plasmids
miR-29b and anti-miR-29b plasmids and their negative
controls were designed and synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China). Sequences for miR-29b plasmid 5′-GGG GGT ACC CTT
CAG GAA GCT GGT TTC-3′ and 5′-GGG GAT ATC TAC ATG TGA GGC AGG TTC
TCAC-3′ and anti-miR-29b plasmid 5′-ACT GAT TTC AAA TGG TGCT-3′ and
5′-GTG TAA CAC GTC TAT ACG CCCA-3′ Once the SKO-007 cells, which
were seeded into 6-well plates (1×105 cells/well), had
reached 50–60% confluence, the plasmids were transfected into the
cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Statistical analysis
All experiments were repeated three times, in order
to ensure reproducibility. The data are presented as the mean ±
standard deviation, and were analyzed using SPSS 18 (SPSS Inc.,
Chicago, IL. USA). Comparisons between mean values were tested
using Student's unpaired t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
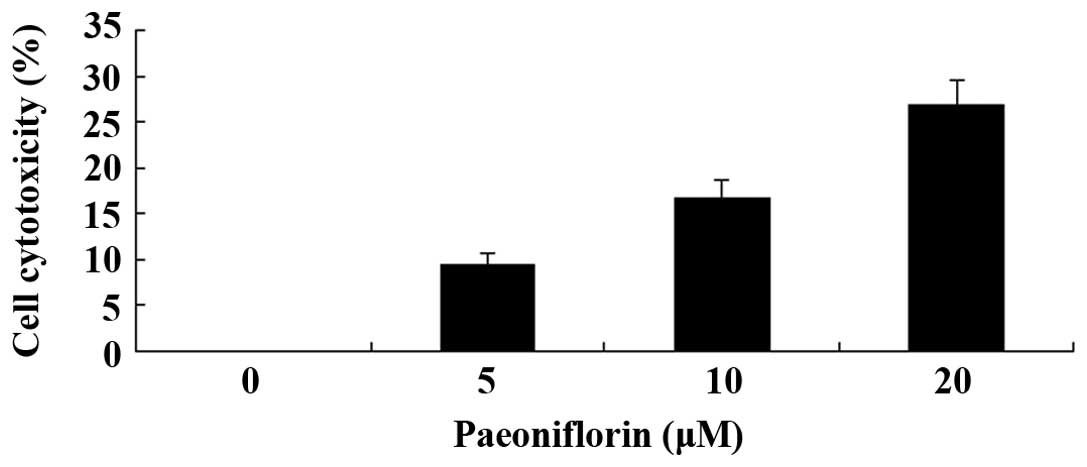
Paeoniflorin inhibits the proliferation
of SKO-007 cells
The effects of paeoniflorin were examined on the
proliferation of the SKO-007 human MM cell line. Treatment with
paeoniflorin inhibited the proliferation of SKO-007 cell in a dose-
and time-dependent manner (Fig.
2). When the cells were treated with 5 µM paeoniflorin
for 72 h, 10 µM paeoniflorin for 48 or 72 h, or 20 µM
paeoniflorin for 24, 48 or 72 h, cell proliferation was
significantly reduced, as compared with in the untreated cells
(Fig. 2).
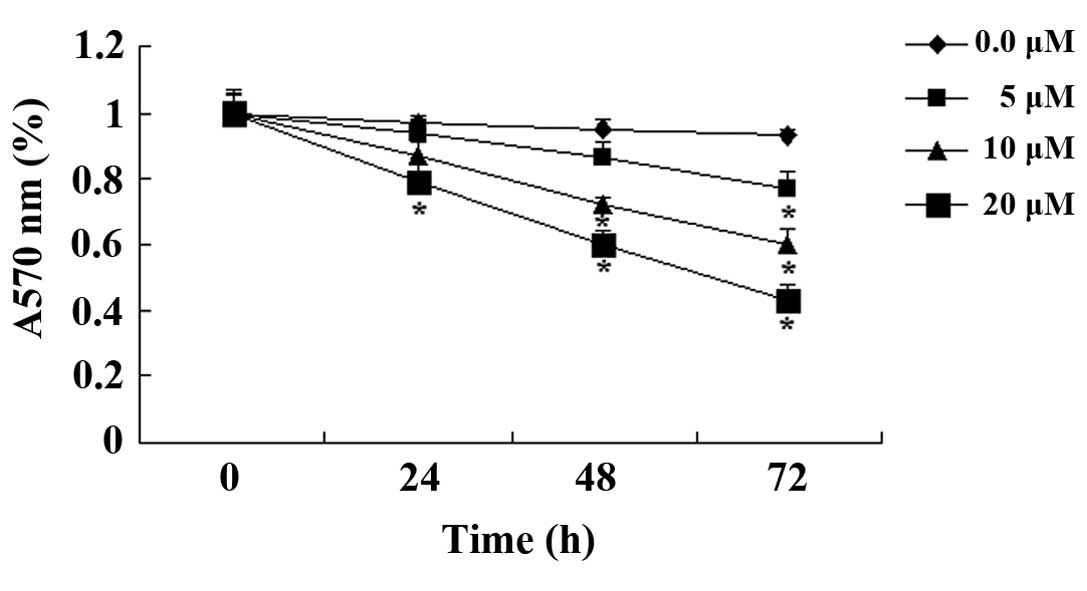
Paeoniflorin exerts cell cytotoxic
effects on SKO-007 cells
To determine whether paeoniflorin was cytotoxic to
SKO-007 cells, an LDH assay was conducted following treatment with
paeoniflorin (0, 5, 10 and 20 µM) for 48 h. As indicated in
Fig. 3, paeoniflorin exerted cell
cytotoxic effects on SKO-007 cells in a dose-dependent manner. The
cell cytotoxic effects of paeoniflorin on SKO-007 cells markedly
increased following treatment with 10 or 20 µM paeoniflorin
for 48 h (Fig. 3).
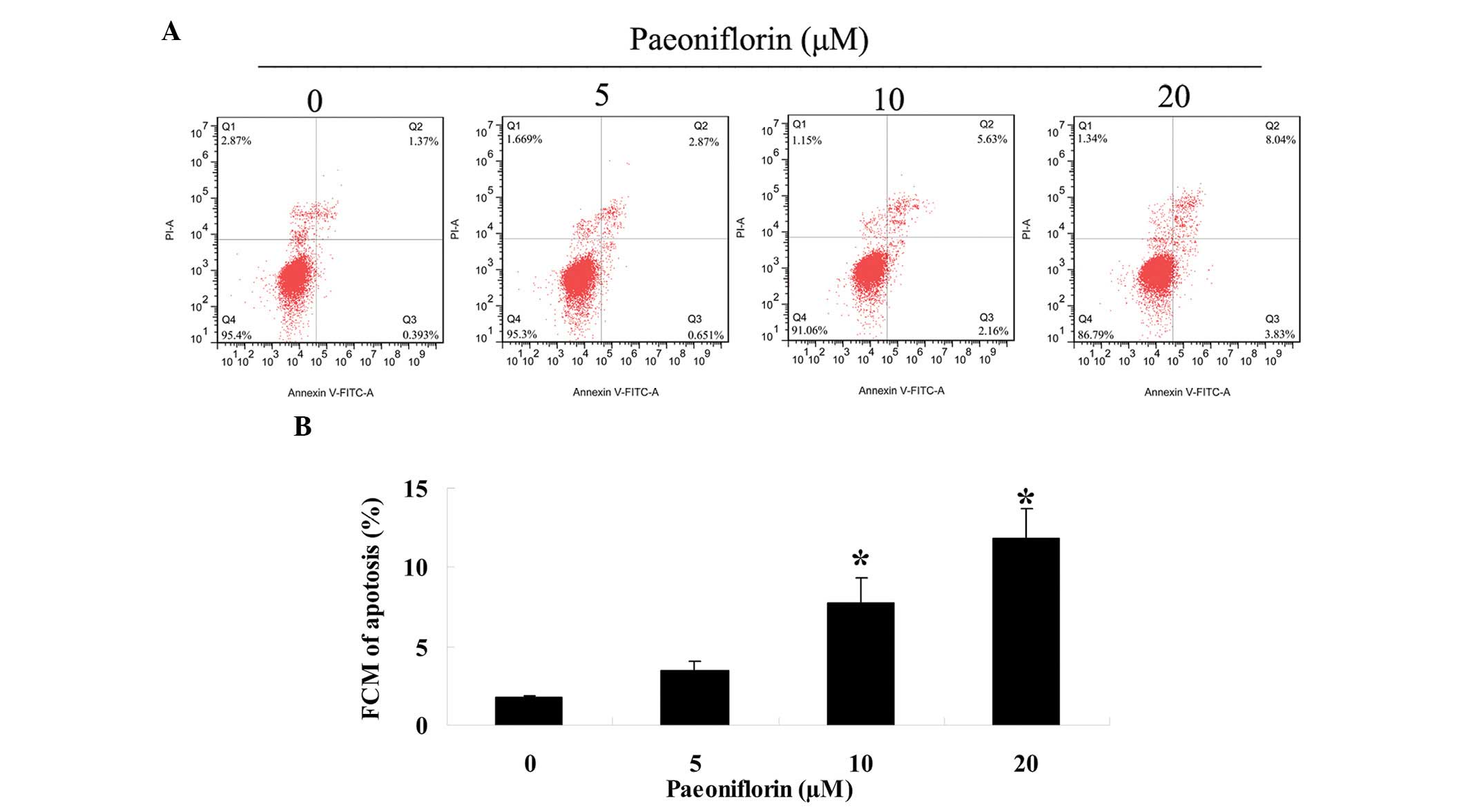
Paeoniflorin induces apoptosis of SKO-007
cells
To investigate whether paeoniflorin was able to
promote SKO-007 cell apoptosis, the number of apoptotic cells was
determined using an Annexin V-FITC/PI apoptosis assay. Treatment
with paeoniflorin (0, 5, 10 and 20 µM) for 48 h induced a
concentration-dependent increase in the rate of SKO-007 cell
apoptosis (Fig. 4A and B).
Following treatment with 10 or 20 µM paeoniflorin, the
number of SKO-007 apoptotic cells was significantly increased
(Fig. 4A and B).
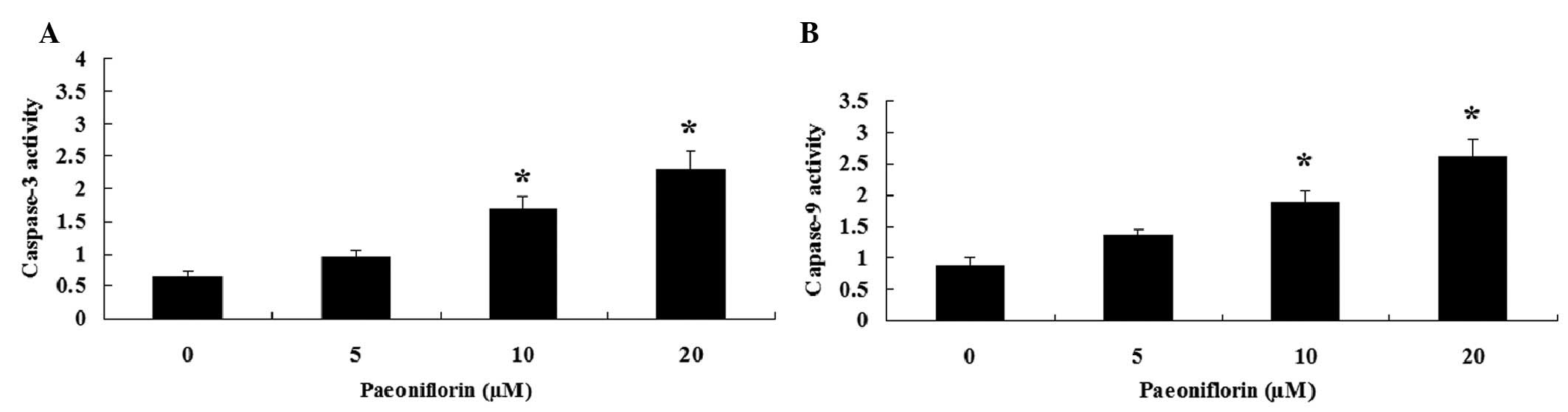
Paeoniflorin induces caspase-3 and
caspase-9 activities in SKO-007 cells
To further investigate the effects of paeoniflorin
on the caspase activity of SKO-007 cells, caspase-3 and caspase-9
activities were measured using Caspase-3/9 Activity Assay kits.
Notably, treatment with paeoniflorin (10 and 20 µM) for 48 h
induced caspase-3 and caspase-9 activities in SKO-007 cells
(Fig. 5A and B).
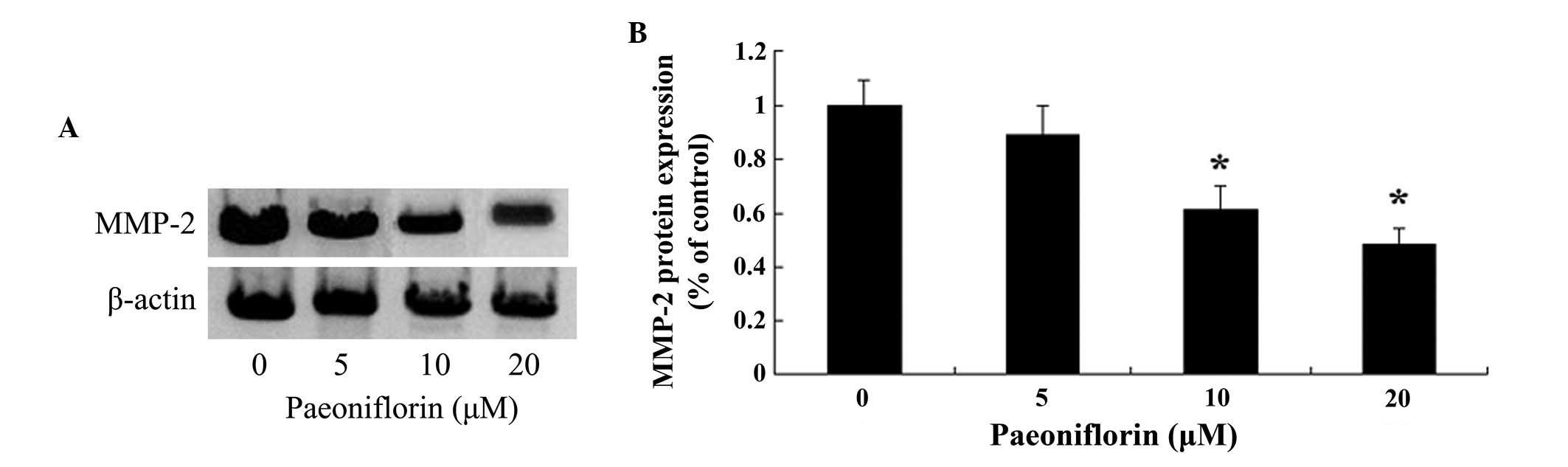
Paeoniflorin inhibits MMP-2 protein
expression levels in SKO-007 cells
The present study aimed to determine whether
paeoniflorin was able to inhibit the expression levels of MMP-2 in
SKO-007 cells. Treatment with paeoniflorin (10 and 20 µM)
for 48 h markedly inhibited the protein expression levels of MMP-2
in the SKO-007 cells (Fig. 6A and
B).
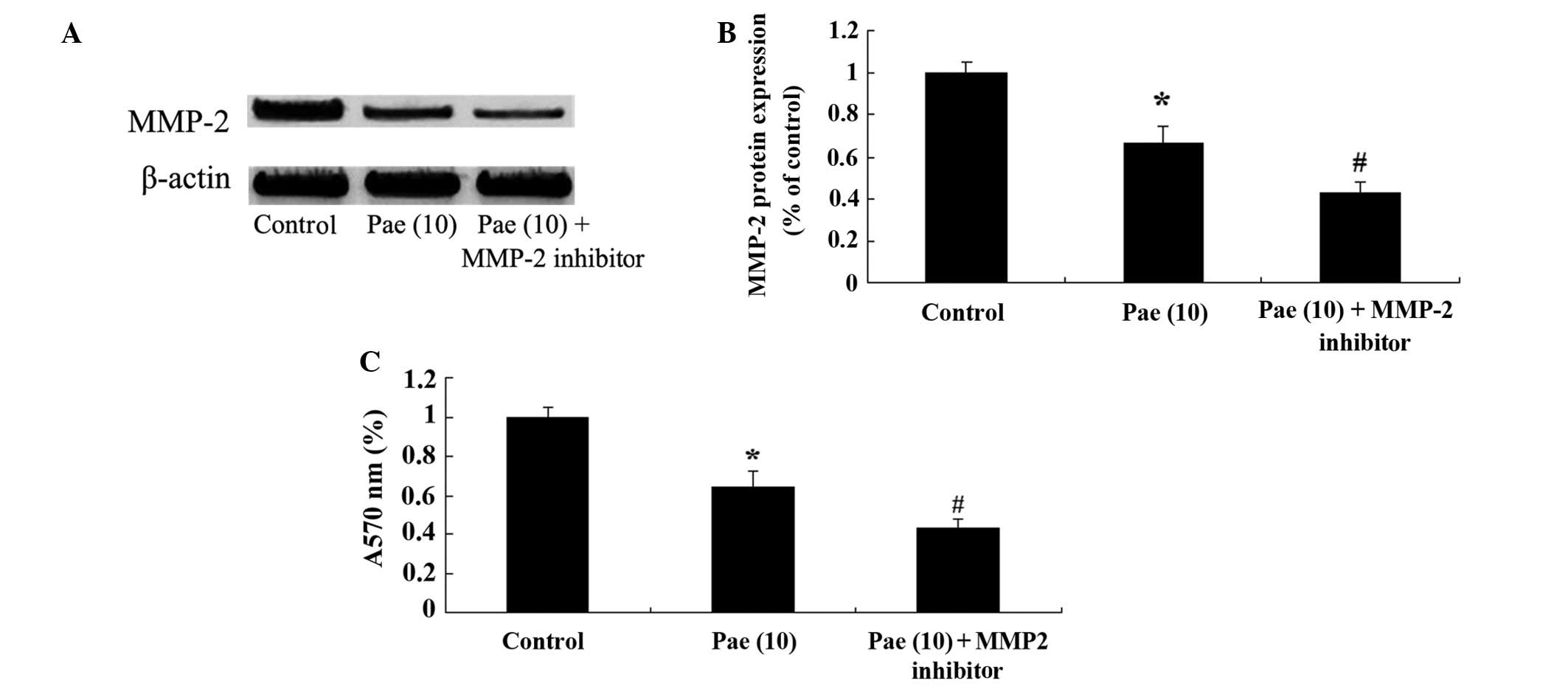
Suppression of MMP-2 reduces the
proliferation of paeoniflorin-treated SKO-007 cells
To determine the potential regulatory mechanisms
underlying the effects of paeoniflorin (10 µM) on the
proliferation of SKO-007 cells after 48 h, the cells were treated
with an MMP-2 inhibitor, GM6001 (5 µM; Chemicon
International, Temecula, CA, USA). Treatment of the cells with the
MMP-2 inhibitor suppressed the protein expression levels of MMP-2,
and further reduced the proliferation of paeoniflorin-treated cells
(Fig. 7A–C).
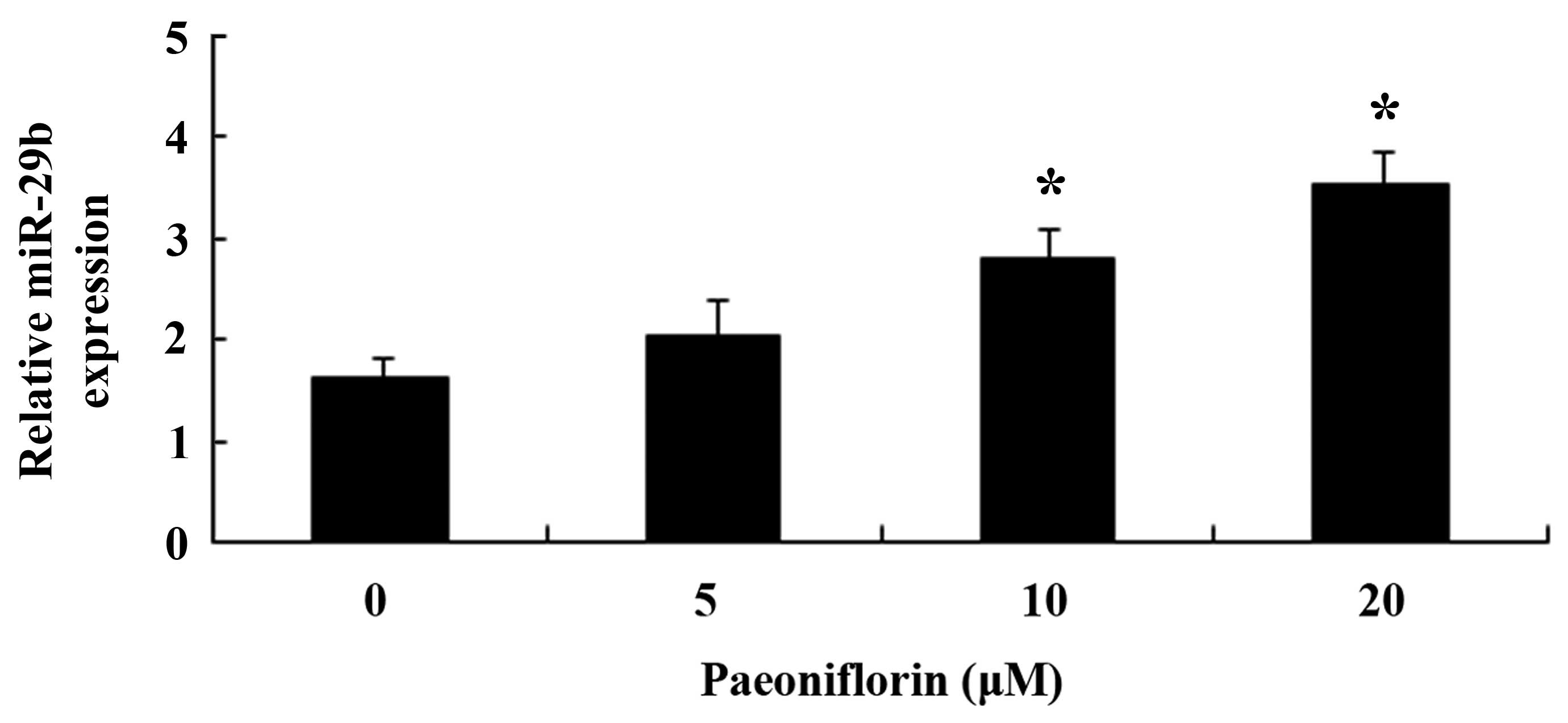
Paeoniflorin upregulates miR-29b
expression in SKO-007 cells
The present study aimed to investigate whether
paeoniflorin was able to activate miR-29b expression in SKO-007
cells. Following treatment with paeoniflorin (10 and 20 µM)
for 48 h, the expression levels of miR-29b were significantly
increased in the SKO-007 cells (Fig.
8).
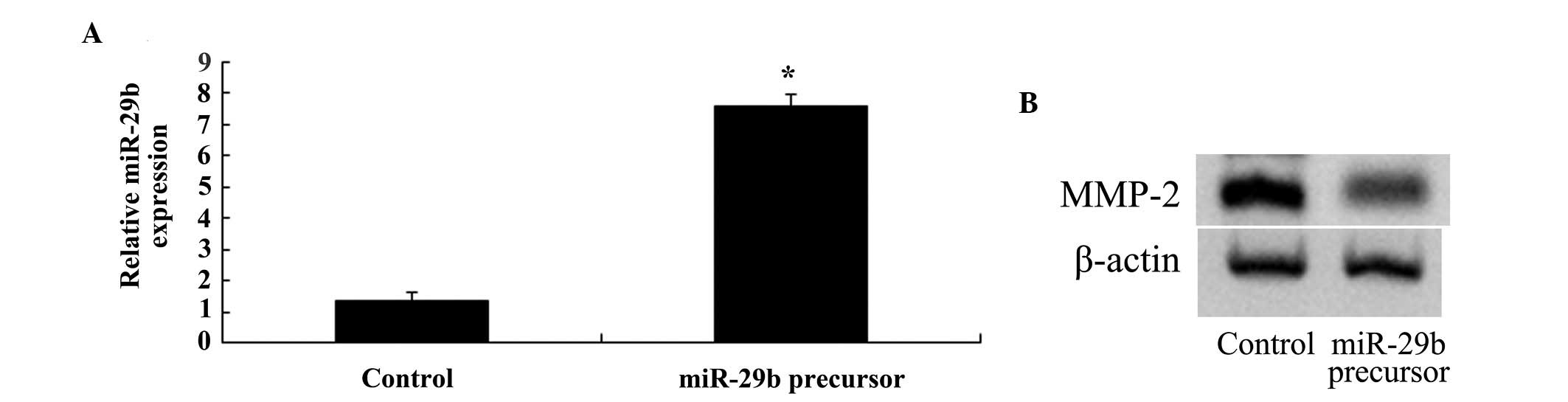
Overexpression of miR-29b inhibits MMP-2
expression in SKO-007 cells
SKO-007 cells were transfected with miR-29b, in
order to determine whether overexpression of miR-29b was able to
inhibit the expression of MMP-2. Transfection of the cells with
miR-29b increased the expression levels of miR-29b and suppressed
the expression of MMP-2 in SKO-007 cells (Fig. 9A and B).
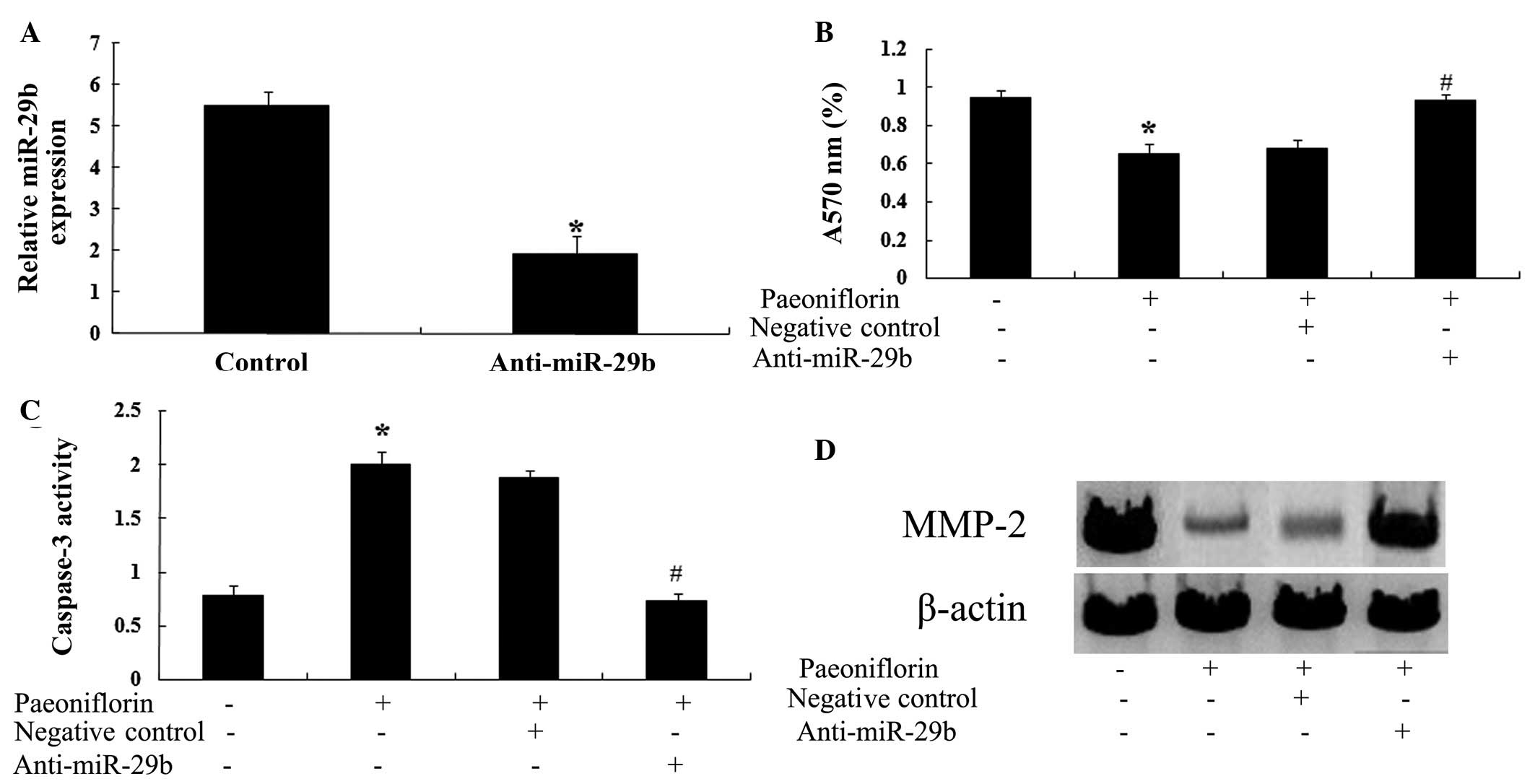
Anti-miR-29b attenuates the effects of
paeoniflorin
To determine whether a correlation exists between
the effects of paeoniflorin and miR-29b expression, the effects of
paeoniflorin on SKO-007 cells transfected with anti-miR-29b were
investigated. Transfection with an anti-miR-29b antibody
significantly reduced the expression levels of miR-29b in SKO-007
cells (Fig. 10A). Notably,
transfection with anti-miR-29b significantly attenuated the effects
of paeoniflorin on cell proliferation (Fig. 10B) and apoptosis (Fig. 10C) in SKO-007 cells. Furthermore,
transfection with anti-miR-29b was able to upregulate the
expression levels of MMP-2 in SKO-007 cells (Fig. 10D).
Discussion
MM is a debilitating and incurable malignant
disease, which originates in B cells. MM is characterized by the
malignant proliferation and abnormal accumulation of bone marrow
clonal plasma cells, and is associated with increased monoclonal
immunoglobulin levels (19,20).
In the present study, treatment with paeoniflorin was able to
inhibit the proliferation of SKO-007 cells in a dose- and
time-dependent manner. Paeoniflorin is a promising agent in the
treatment of liver cancer via the downregulation of prostaglandin
E2 receptor 2 expression and the increased activation of caspase-3
(21). In addition, in the present
study paeoniflorin significantly enhanced cell cytotoxicity,
increased apoptosis, and accelerated caspase-3 activation in
SKO-007 cells. Zhang and Zhang (17) reported that paeoniflorin was able
to significantly induce the apoptosis of HeLa cells, via the
downregulation of Bcl-2 and the upregulation of caspase-3 (22). Hung et al (23) demonstrated that the
antiproliferative activity of paeoniflorin promoted the apoptosis
of A549 human non-small cell lung cancer cells. However, further
research regarding the underlying molecular mechanisms of
paeoniflorin on MM cell proliferation and apoptosis is
required.
MMP-2 is usually located in the cytoplasm of tumor
cells, and is often detected in the endothelial cells of the
vascular basement membrane, thus suggesting that during the
invasion of astrocytoma, MMP-2 and vascular endothelial growth
factor have a synergistic role in regulating the angiogenesis of
tumor vessels in MM (24,25). The structure of newly formed tumor
vasculature is incomplete, and easily leaks, thus affecting the
integrity of the blood-brain barrier (BBB); plasma and other
macromolecules may penetrate into the cells through the damaged BBB
and gather around the tumor, forming extensive peritumoral edema
(26). Furthermore, MMP-2 may
directly degrade the extracellular matrix of the basement membrane
of blood vessels, loosening the structure, which is conducive to
the invasion of tumor cells along the basement membrane (27). The results of the present study
revealed that paeoniflorin was able to inhibit the expression
levels of MMP-2 in SKO-007 cells. In addition, downregulation of
MMP-2 further reduced the proliferation of paeoniflorin-treated
SKO-007 cells. A previous study demonstrated that paeoniflorin
inhibits the MMP-2 activity of splenocytes from picryl
chloride-induced ear contact sensitivity mice (28).
In MM, tumor cells proliferate in the surrounding
bone marrow, promoting the activities and inhibiting the function
of osteoclasts. In experiments on peripheral blood samples, the
expression of miR-29b is decreased during osteoclast
differentiation, and is therefore considered a negative regulator
of human osteoclast differentiation and activity (29). Furthermore, miR-29b inhibits the
activity of tartrate-resistant acid phosphatase on osteoclasts and
affects osteoclast induced bone resorption through reducing MMP-9
expression (30,31). The present study demonstrated that
paeoniflorin significantly increased the expression levels of
miR-29b in SKO-007 cells. Simultaneously, overexpression of miR-29b
was capable of inhibiting the expression of MMP-2 in SKO-007 cells.
These results indicated that the expression of miR-29b may regulate
and control MMP-2 expression in SKO-007 cells; therefore, the
miR-29b/MMP-2 signaling pathway may be considered a novel
pharmaceutical target for the treatment of MM using
paeoniflorin.
In conclusion, the key observation of the present
study was that paeoniflorin was able to inhibit proliferation and
promote apoptosis of MM cells via inhibition of MMP-2 and
upregulation of miR-29b. Understanding the precise role of
paeoniflorin may advance knowledge regarding MM, and may be
beneficial for future treatment.
References
|
1
|
Fujisawa M, Seike K, Fukumoto K, Suehara
Y, Fukaya M, Sugihara H, Takeuchi M and Matsue K: Oligoclonal bands
in patients with multiple myeloma: Its emergence per se could not
be translated to improved survival. Cancer Sci. 105:1442–1446.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma Y, Li Z, Wang Y and Feng J: Brucine
induces the apoptosis of U266 multiple myeloma cells by
phosphorylation of c-Jun. Mol Med Rep. 7:481–484. 2013.
|
|
3
|
Qiao M, Wu D, Carey M, Zhou X and Zhang L:
Multi-scale agent-based multiple myeloma cancer modeling and the
related study of the balance between osteoclasts and osteoblasts.
PLoS One. 10:e01432062015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He X, Yang K, Chen P, Liu B, Zhang Y, Wang
F, Guo Z, Liu X, Lou J and Chen H: Arsenic trioxide-based therapy
in relapsed/refractory multiple myeloma patients: A meta-analysis
and systematic review. Onco Targets Ther. 7:1593–1599. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, Ai L, Lv T, Jiang X and Liu F:
Asiatic acid, a triterpene, inhibits cell proliferation through
regulating the expression of focal adhesion kinase in multiple
myeloma cells. Oncol Lett. 6:1762–1766. 2013.PubMed/NCBI
|
|
6
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao L, Wang H and Wang F:
Amyloid-β-induced matrix metalloproteinase-9 secretion is
associated with retinal pigment epithelial barrier disruption. Int
J Mol Med. 31:1105–1112. 2013.PubMed/NCBI
|
|
8
|
Vacca A, Ribatti D, Presta M, Minischetti
M, Ria R, Albini A, Bussolono F and Dammacco F: Bone marrow
neovascularization, plasma cell angiogenic potential, and matrix
metalloproteinase-2 secretion parallel progression of human
multiple myeloma. Blood. 93:3064–3073. 1999.PubMed/NCBI
|
|
9
|
Zdzisinska B, Walter-Croneck A and
Kandefer-Szerszen M: Matrix metalloproteinases-1 and -2, and tissue
inhibitor of metal-loproteinase-2 production is abnormal in bone
marrow stromal cells of multiple myeloma patients. Leuk Res.
32:1763–1769. 2008. View Article : Google Scholar
|
|
10
|
Bolkun L, Lemancewicz D, Sobolewski K,
Mantur M, Semeniuk J, Kulczynska A, Kloczko J and Dzieciol J: The
evaluation of angiogenesis and matrix metalloproteinase-2 secretion
in bone marrow of multiple myeloma patients before and after the
treatment. Adv Med Sci. 58:118–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YP, Jin X, Kong M and Li YM: Pattern
of microRNA expression associated with different stages of
alcoholic liver disease in rat models. Mol Med Rep. 10:1195–1204.
2014.PubMed/NCBI
|
|
12
|
Vandenboom Ii TG, Li Y, Philip PA and
Sarkar FH: MicroRNA and cancer: Tiny molecules with major
implications. Curr Genomics. 9:97–109. 2008. View Article : Google Scholar
|
|
13
|
Ru P, Steele R, Newhall P, Phillips NJ,
Toth K and Ray RB: miRNA-29b suppresses prostate cancer metastasis
by regulating epithelial-mesenchymal transition signaling. Mol
Cancer Ther. 11:1166–1173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang YK, Wang H, Leng Y, Li ZL, Yang YF,
Xiao FJ, Li QF, Chen XQ and Wang LS: Overexpression of microRNA-29b
induces apoptosis of multiple myeloma cells through down regulating
Mcl-1. Biochem Biophys Res Commun. 414:233–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu ZY, Xu L, Yan R, Huang Y, Liu G, Zhou
WX and Zhang YX: Advance in studies on effect of paeoniflorin on
nervous system. Zhongguo Zhong Yao Za Zhi. 38:297–301. 2013.In
Chinese. PubMed/NCBI
|
|
16
|
Fang S, Zhu W, Zhang Y, Shu Y and Liu P:
Paeoniflorin modulates multidrug resistance of a human gastric
cancer cell line via the inhibition of NF-κB activation. Mol Med
Rep. 5:351–356. 2012.
|
|
17
|
Zhang L and Zhang S: Modulating Bcl-2
family proteins and caspase-3 in induction of apoptosis by
paeoniflorin in human cervical cancer cells. Phytother Res.
25:1551–1557. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Chen YF, Wu KJ and Wood WG: Paeonia
lactiflora extract attenuating cerebral ischemia and arterial
intimal hyperplasia is mediated by paeoniflorin via modulation of
VSMC migration and Ras/MEK/ERK signaling pathway. Evid Based
Complement Alternat Med. 2013:4824282013.PubMed/NCBI
|
|
20
|
Wang XS, Shi Q, Williams LA, Shah ND,
Mendoza TR, Cohen EN, Reuben JM, Cleeland CS and Orlowski RZ:
Longitudinal analysis of patient-reported symptoms post-autologous
stem cell transplant and their relationship to inflammation in
patients with multiple myeloma. Leuk Lymphoma. 56:1335–1341. 2015.
View Article : Google Scholar
|
|
21
|
Trepel M, Martens V, Doll C, Rahlff J,
Gösch B, Loges S and Binder M: Phenotypic detection of clonotypic B
cells in multiple myeloma by specific immunoglobulin ligands
reveals their rarity in multiple myeloma. PLoS One. 7:e319982012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu S, Sun W, Wei W, Wang D, Jin J, Wu J,
Chen J, Wu H and Wang Q: Involvement of the prostaglandin E
receptor EP2 in paeoniflorin-induced human hepatoma cell apoptosis.
Anticancer Drugs. 24:140–149. 2013. View Article : Google Scholar
|
|
23
|
Hung JY, Yang CJ, Tsai YM, Huang HW and
Huang MS: Anti-proliferative activity of paeoniflorin is through
cell cycle arrest and the Fas/Fas ligand-mediated apoptotic pathway
in human non-small cell lung cancer A549 cells. Clin Exp Pharmacol
Physiol. 35:141–147. 2008. View Article : Google Scholar
|
|
24
|
Yang JL, Lin JH, Weng SW, Chen JC, Yang
JS, Amagaya S, Funayana S, Wood WG, Kuo CL and Chung JG: Crude
extract of Euphorbia formosana inhibits the migration and invasion
of DU145 human prostate cancer cells: The role of matrix
metalloproteinase-2/9 inhibition via the MAPK signaling pathway.
Mol Med Rep. 7:1403–1408. 2013.PubMed/NCBI
|
|
25
|
Han X, Jin D, Zheng G, Luo Y and Cai Z:
Astrocytoma development following complete multiple myeloma
remission in a 49-year-old patient: A case report. Exp Ther Med.
6:509–512. 2013.PubMed/NCBI
|
|
26
|
Harford-Wright E, Lewis KM, Ghabriel MN
and Vink R: Treatment with the NK1 antagonist emend reduces blood
brain barrier dysfunction and edema formation in an experimental
model of brain tumors. PLoS One. 9:e970022014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang JH, Wu MY, Chen MJ, Chen SU, Yang YS
and Ho HN: Increased matrix metalloproteinase-2 and tissue
inhibitor of metalloproteinase-1 secretion but unaffected
invasiveness of endometrial stromal cells in adenomyosis. Fertil
Steril. 91(5 Suppl): 2193–2198. 2009. View Article : Google Scholar
|
|
28
|
Zhang L, Dong Y, Sun Y, Chen T and Xu Q:
Role of four major components in the effect of Si-Ni-San, a
traditional Chinese prescription, against contact sensitivity in
mice. J Pharm Pharmacol. 58:1257–1264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kagiya T and Nakamura S: Expression
profiling of microRNAs in RAW264.7 cells treated with a combination
of tumor necrosis factor alpha and RANKL during osteoclast
differentiation. J Periodontal Res. 48:373–385. 2013. View Article : Google Scholar
|
|
30
|
Sugio A, Iwasaki M, Habata S, Mariya T,
Suzuki M, Osogami H, Tamate M, Tanaka R and Saito T: BAG3
upregulates Mcl-1 through downregulation of miR-29b to induce
anticancer drug resistance in ovarian cancer. Gynecol Oncol.
134:615–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rossi M, Pitari MR, Amodio N, Di Martino
MT, Conforti F, Leone E, Botta C, Paolino FM, Del Giudice T,
Iuliano E, et al: miR-29b negatively regulates human osteoclastic
cell differentiation and function: Implications for the treatment
of multiple myeloma-related bone disease. J Cell Physiol.
228:1506–1515. 2013. View Article : Google Scholar
|