Introduction
Primary immune thrombocytopenia (ITP) is a common
acquired autoimmune hemorrhagic disorder, accounting for 30% of the
total hemorrhagic disorders (1).
The annual incidence of ITP in children was approximately
1.9–6.4/100,000 (2). The clinical
manifestations of ITP were predominantly spontaneous skin and
mucosal bleeding, while the complication of intracranial hemorrhage
occurred in severe patients, leading to death. The majority of
children presented acute course, while approximately 20% children
were transformed into chronic ITP (3).
The main mechanism of pathogenesis of this disease
was increased platelet destruction and insufficient thrombo
cytopoiesis due to abnormal cellular and humoral immunity (4,5). The
complicated pathogenesis mechanism of ITP was recently identified
(6). Investigators found apoptosis
of platelets in ITP children, and speculated that platelet
apoptosis was also a pathogenesis mechanism of ITP (7,8).
Currently, there is no uniform 'gold standard' mechanism of
pathogenesis. Clinical diagnosis is mainly an exclusive procedure
and is dependent on bone marrow aspiration, which is traumatic and
not routinely recommended. Previous findings (9,10)
showed that changes of the platelet-associated parameters [mean
platelet volume (MPV), platelet distribution width (PDW),
platelet-large cell ratio (P-LCR), immature platelet fraction
(IPF)%] were used to diagnose thrombocytopenia due to various
causes, and used as indicators of relapse and efficacy. Long-term
clinical diagnosis and treatment also indicated that the
hemorrhagic symptoms of some children were not proportional to the
reduction of the platelet count (PLT), which indicated that
hemorrhage in ITP children was asssociated with the reduction of
platelet count, as well as abnormal platelet function. Therefore,
only platelet count is not sufficient to evaluate disease severity
in children, and monitoring of individual platelet function should
also be considered. Previously employed methods for the detection
of platelet function were limiting and were not optimal to assess
the many characteristics of platelet. Therefore, the development of
a rapid, sensitive and convenient detection method is important to
assess this disease, and understand platelet function.
The development of flow cytometry (FCM) and various
monoclonal antibodies that could be labeled with fluorescein, led
to several tools for the detection of platelet function becoming
available.
Whole blood FCM was used to measure the percentage
or fluorescence intensity of fluorescence-labelled
platelet-specific membrane glycoproteins and activation markers, in
order to assess the activation of circulating platelets and the
in vitro response to activators by platelets, and evaluate
platelet function. This technique simplified the treatment
procedure of samples, avoided the artificial activation of platelet
in vitro and prevented the loss of platelet subset. In
addition, the blood sample volume was small, thus this technique
was especially useful in the evaluation of the platelet function in
the children with thrombocytopenia. Regarding relevant antibodies,
Chen et al (11) compared
the monoclonal antibody-specific immobilization of platelet
antigens (MAIPA) and FCM in the detection of the sensitivity and
specificity of GPIIb/IIIa and GPIIb/IIIa antibodies, and identified
no significant differences. However, the sensitivity of FCM was
higher than that of MAIPA. This observation indicated that FCM
could be used as a new clinical diagnostic method (12–14).
Alterations in platelet function in ITP patients is
controversial. Wang et al (15) demonstrated decreased activation and
lower function of platelet in vitro and in vivo in
ITP patients. Psaila et al (16) compared the expression of membrane
glycoproteins in the platelets in ITP patients and MDS patients,
and found high activation and in vitro response of the
platelet in ITP patients. Therefore, further investigation of the
platelet function change was required. The abovementioned study
detected activation markers of GPIIb/IIIa on the platelet surface,
such as PAC-1, granule membrane glycoprotein (CD62p), CD42b and
IPF, evaluated the function of peripheral platelet and the change
of new platelet in ITP patients prior to and after treatment, and
assessed the change of platelet function in ITP patients after
initial diagnosis and treatment.
The aim of the present study was to detect PAC-1,
CD62p, CD42b and IPF by trace whole blood FCM, evaluate the
function of peripheral platelet and the change of new platelet in
ITP patients before and after treatment, assess the change of
platelet function in ITP patients after initial diagnosis and
treatment, analyze the change of platelet-associated parameters,
such as platelet count (PLT), MPV, plateletcrit (PCT), PDW and
platelet-large cell ratio, and provide the basis of the diagnosis,
and the interpretation of disease course and efficacy.
Materials and methods
Materials
Fluorescein-labeled anti-platelet monoclonal
antibodies used were: fluorescein isothiocyanate (FITC)-labeled
anti-platelet GPIIb/IIIa monoclonal mouse antibody (PAC-1-FITC;
cat. no. 340507); phycoerythrin (PE)-labeled anti-platelet GMP-140
mouse monoclonal antibody (CD62p-PE; cat. no. 348107); peridinin
chlorophyll protein-labeled GPIIIa monoclonal mouse antibody
(CD61-PercP; cat. no. 340506); PE-labeled mouse IgG (MIgG-PE; cat.
no. 349043); phycocyanin-labeled anti-platelet GPIb-IX monoclonal
mouse antibody (CD42b-APC; cat. no. 551061); and
phycocyanin-labeled mouse IgG (MIgG-APC; cat. no. 555751). Thiazole
orange (TO) (1.0 µg/ml; Sigma, St. Louis, MO, USA); RGDS
(Arg-Gly-Asp-Ser) peptide as a blocker of PAC-1, and synthetic
arginyl-glycyl-Asp-serine peptide, (Sigma) were also used. Other
monoclonal antibodies were purchased from Becton-Dickinson
(Franklin Lakes, NJ, USA). The following were also used: 0.2 mol/l
ADP (Sigma); 1% paraformaldehyde solution; phosphate-buffered
saline (PBS); FCM, a BD FACSCanto™ II high-speed sorter
manufactured by Becton-Dickinson; and an automatic hematology
analyzer, BC-6800, Mindray (Shenzhen, China).
Subjects
All the subjects were inpatients at the Affiliated
Hospital of Luzhou Medical College (Sichuan, China) between March
2014 and February 2015. All the patients agreed to participate in
the current study and signed written informed consent. The patients
were divided into three groups: i) Normal control: 17 children
prior to elective surgery at the Department of Pediatric Surgery,
including 12 male and 5 female patients, with a mean age of
4.05±1.89 years. The children had inguinal hernia, with a mean
platelet count of 309.2±54.06 ×109/l. None of the
children had a previous history of circulatory diseases,
immunological diseases, malignancy or transfusion. None of the
children had any infection signs 1 week prior to hospitalization,
and had no drugs 1 week prior to blood sampling. The children had
normal complete blood count and blood samples were collected prior
to surgery; ii) primary ITP: all the children met the diagnostic
criteria of ITP (17), including
12 male and 6 female patients. The mean age of 3.8±1.4 years, the
mean onset time (from the onset of disease to presentation in
hospital) was 1–6 days and the mean platelet count was 28.61±10.42
×109/l. No children had drugs that could affect platelet
function 3 months prior to admission, such as corticosteroids,
immunosuppressants, heparin, or any relevant treatment. This
disease was not accompanied by significant infectious symptoms, and
required no other anti-infection drugs. ITP was confirmed by the
morphological analysis of bone marrow cells, which were collected
prior to drug administration; and iii) complete response to primary
ITP (ITP-CR): the children met the efficacy criteria (18) with a mean platelet count of
236.9±40.9 ×109/l. The differences in the age and gender
between the treatment and control groups were not statistically
significant (p>0.05).
Approval of the study was obtained from the Ethics
Committee of the Affiliated Hospital of Luzhou Medical College.
Detection methods
Platelet membrane glycoproteins, such as PAC-1,
CD62p and CD42b, were measured. Venous blood (3 ml) was collected
and the middle section of 1.8 ml whole blood was added into an
anticoagulation tube containing sodium citrate, mixed gently and
processed for analysis. Platelet activation was carried out by
adding 50 µl ADP into a Falcon tube (Becton-Dickinson
Company, Franklin Lakes, NJ, USA) followed by 450 µl blood
with anticoagulant, mixed gently by vortexing and incubated at 37°C
for 10 min. Expression of PAC-1, CD62p and CD42b was measured by
three-color fluorescence analysis with FCM. In separate tubes, 20
µl of PE-isotype control antibody, CD61-PercP, PAC-1-FITC
and MIgG-APC, and 10 µl RGDS or 20 µl CD62p-PE,
CD61-PercP, PAC-1-FITC and CD42b-APC were added. Then, 5 µl
non-activated and activated whole blood was added into the tubes of
the treatment groups, and 5 µl non-activated whole blood was
added into the control tube, mixed gently, and incubated at room
temperature (25°C) in the dark for 20 min. Subsequently, 1%
paraformaldehyde was added (2–8°C, 1 ml) into each tube, mixed
completely, and placed at 2–8°C in the dark for 30 min. Using FCM,
signal for 10,000 platelets was acquired, and the positive
expression rate of CD62p, PAC-1 and CD42b was calculated (19).
Platelet-associated parameters (PLT, MPV, PDW, PCT
and P-LCR) were measured. Venous blood (4-ml) was collected into an
anticoagulant tube containing EDTA with a 5-ml syringe, 2-ml whole
blood was reserved, and the remaining blood was used in the
measurement of platelet-associated parameters by an automatic
hematology analyzer. Whole blood (5-µl) was placed into the
treatment tubes, followed by 5 µl CD42b-APC and 50 µl
TO. Whole blood (5-µl) was placed into the control tube,
followed by 5 µl CD42b-APC and 50 µl PBS. The tubes
were mixed gently, incubated at room temperature in the dark for 15
min; and then, 1% paraformaldehyde was added (2–8°C, 1 µl)
into each tube, and mixed completely. The samples were analyzed
within 45 min. Signals for 10,000 platelets were obtained, IPC was
measured and IPF% was calculated.
Statistical analysis
Experimental data were presented as mean ± SD and
analyzed using SPSS 11.5 software (SPSS Inc., Chicago, IL, USA).
The Student's t-test or rank-sum test was used for comparisons
between the two treatment groups. The one-sample t-test or rank-sum
test was used for the comparison between the treatment and control
groups. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Measurement of platelet parameters
As shown in Fig. 1,
PLT and PCT in the ITP group had a lower expression level compared
to those in the normal control and ITP-CR groups (p<0.05), while
the MPV, PDW and P-LCR in ITP group were higher than those in the
normal control and ITP-CR groups (p<0.05). Differences in the
expression of MPV, PDW, PCT and P-LCR between the ITP-CR and normal
control groups were not statistically significant (p>0.05),
whereas PLT was lower in the ITP-CR group (p<0.05).
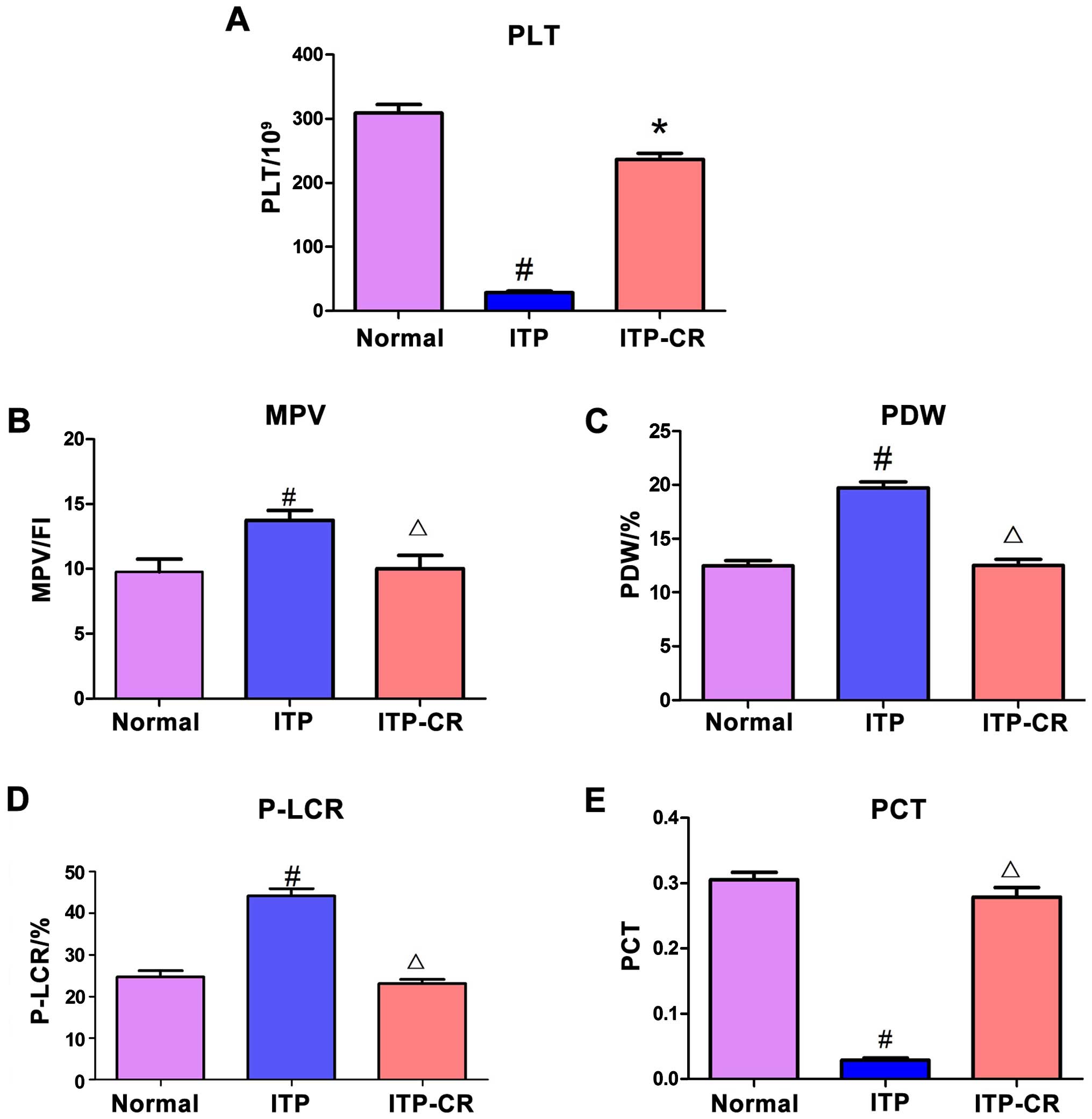 | Figure 1Comparison of platelet parameters. (A)
The expression of PLT in 3 groups, (B) the expression of MPV in 3
groups, (C) the expression of PDW in 3 groups, (D) the expression
of P-LCR in 3 groups, and (E) the expression of PCT in 3 groups.
#P<0.05, ITP-CR vs. normal control group;
*P<0.05, in comparison to the normal control group;
ΔP>0.05, in comparison to the normal control group.
PLT, platelet count; MPV, mean platelet volume; PDW, platelet
distribution width; P-LCR, platelet-large cell ratio; PCT,
plateletcrit. |
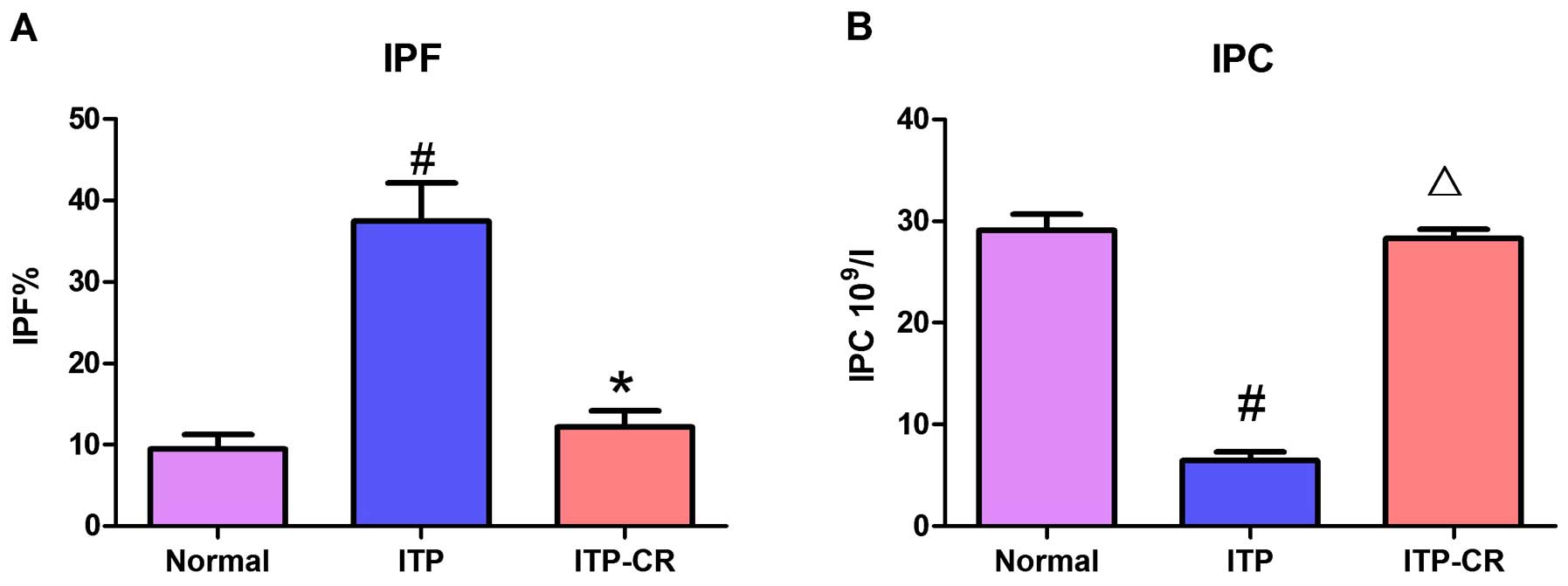
Measurement of IPF% and IPC
As shown in Figs. 2
and 7, IPF% in the ITP group was
higher than that in the ITP-CR and normal control groups
(p<0.05). IPC in the ITP group was lower than that in the ITP-CR
and normal control groups (p>0.05). IPF% was higher (p<0.05)
and IPC was lower in ITP-CR group in comparison to the normal
control group. The differences were not statistically
significant.
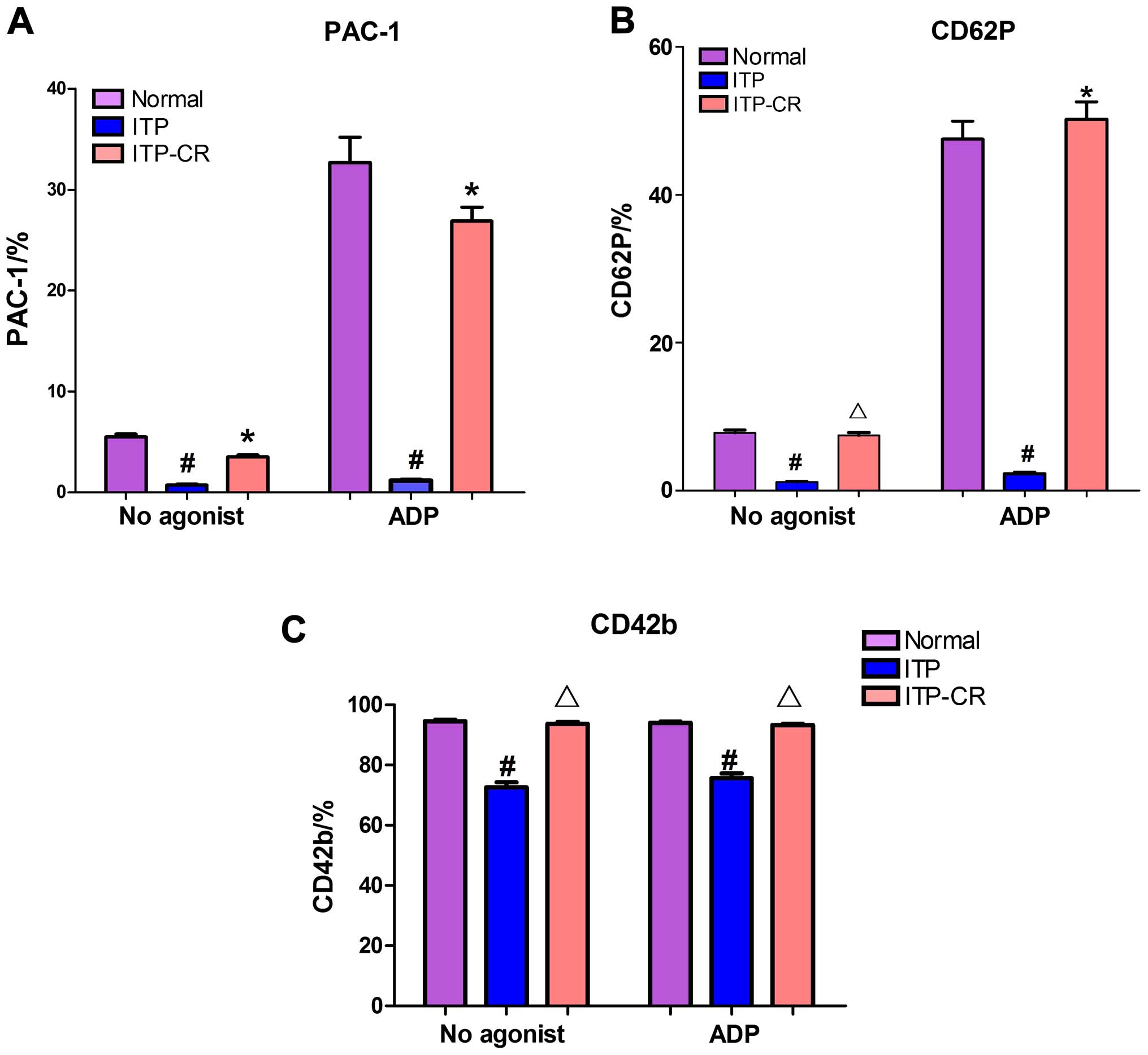
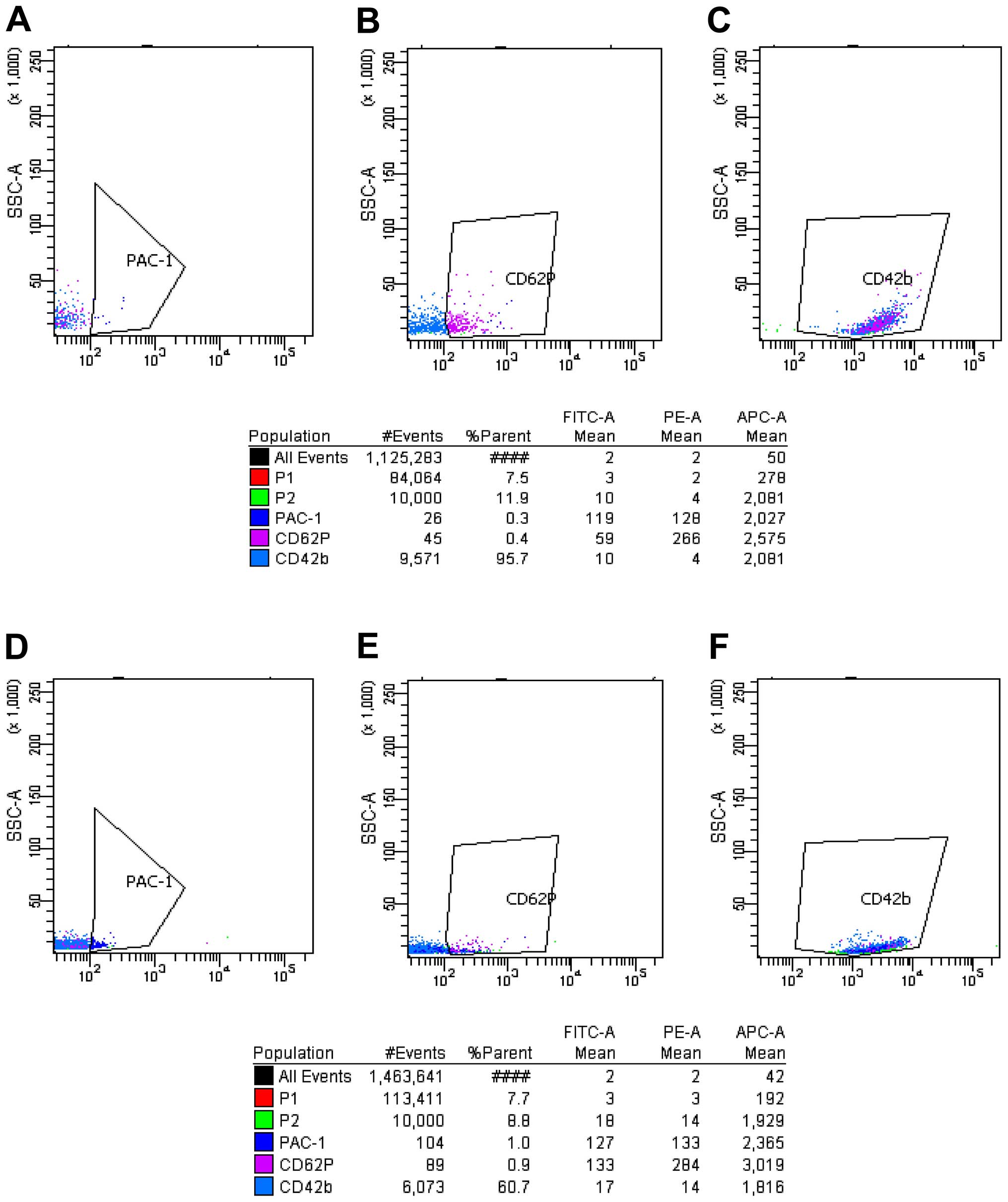
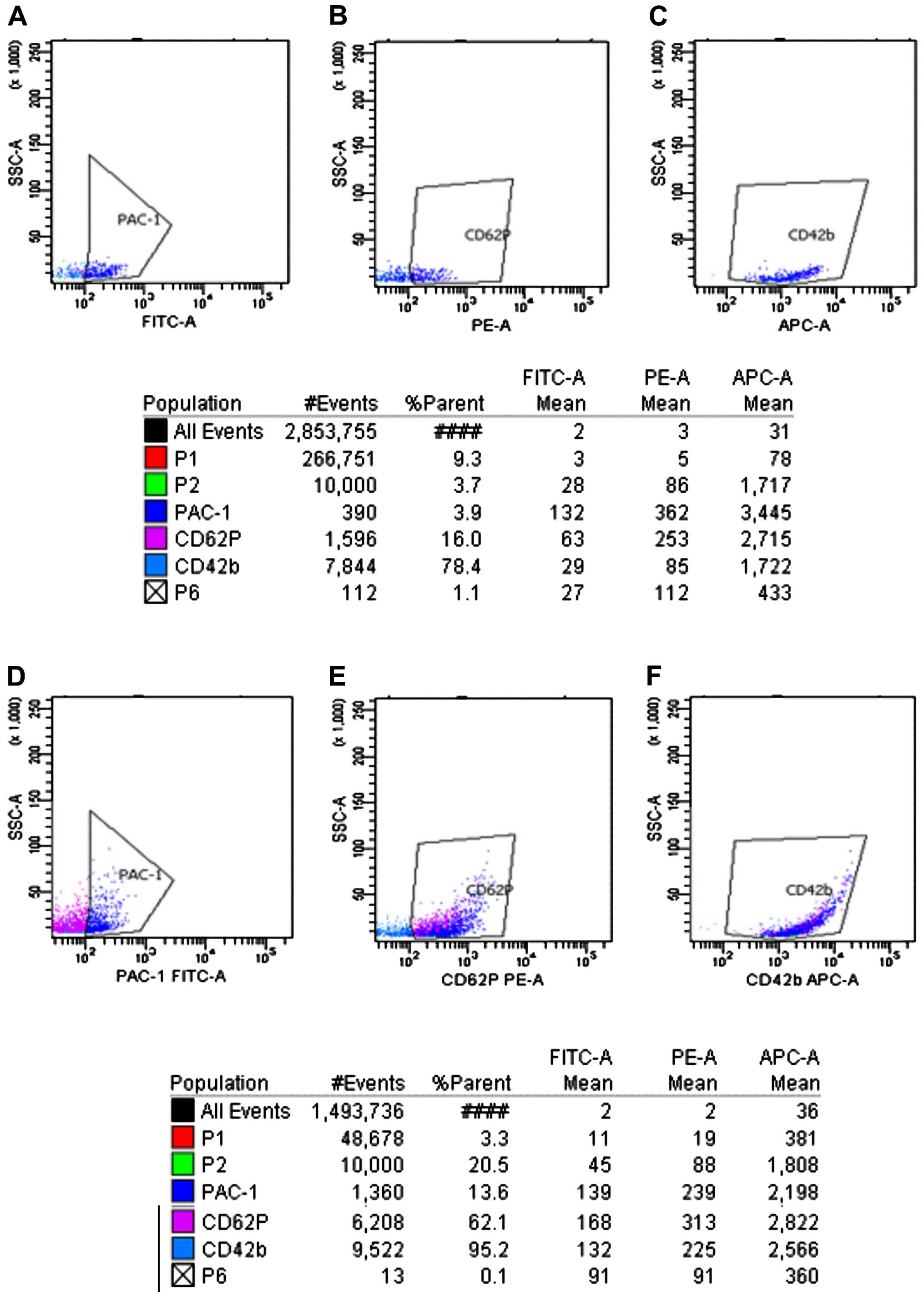
Measurement of platelet membrane
glycoproteins prior to and after platelet activation by ADP
As shown in Figs.
3Figure 4Figure 5–6, prior to platelet activation by ADP,
the expression levels of CD62p, PAC-1 and CD42b in the ITP group
were lower than those in ITP-CR and normal control groups
(p<0.05). PAC-1 was lower in the ITP-CR compared to that in the
normal control group (p<0.05). Differences in CD62p and CD42b
were not statistically significant (p>0.05). After platelet
activation by ADP, the expression levels of CD62p, PAC-1 and CD42b
in the ITP group were lower than those in the ITP-CR and normal
control groups (p<0.05). PAC-1 was lower and CD62p was higher in
the ITP-CR group than that in the normal control group (p<0.05).
Differences in CD42b were not statistically significant
(p>0.05).
Discussion
Platelet is the smallest cell in whole blood cells,
originating from mature megakaryocytes cytoplasm. It has the
functions of adhesion, accumulation and release, and plays an
important role in hemostasis (16). Platelet membrane glycoprotein (GP)
is a main component of platelet membrane proteins, and plays major
roles in the activation of platelet. Platelet GPs can be classified
into plasma membrane and granule membrane glycoproteins based on
the distribution location (19).
Plasma membrane glycoproteins are predominantly located on the cell
membrane of resting platelets, major GPs including the GPIb
(CD42b)-IX-V complex and GPIIb/IIIa complex. CD42b (GPIb) is an
important platelet adhesion receptor that promotes platelet to
secret platelet-derived growth factors and 5-HT, accelerating the
deformation and aggregation of platelets. GpIIb/IIIa complex is the
most abundant membrane glycoprotein and can mediate the binding of
platelet and fibrinogen (Fg), which is the final pathway of
platelet aggregation (20). PAC-1
is the monoclonal antibody in the exposed Fg-binding site after the
activation of GpIIb/IIIa, and is the specific marker of the
activation of GPIIb/IIIa complex and the early marker of platelet
activation (21). Granule membrane
glycoproteins comprise CD62p and CD63p, with CD62p also known as
P-selectin. Since the increase of CD62p is not altered with time
prolongation, it is considered the 'gold standard' of platelet
activation markers (22).
Therefore, we investigated the change of platelet function by
detecting the expression of PAC-1, CD62p and CD42b (23).
Hemorrhage due to decreased platelets caused by
disrupted platelet autoimmunity is currently considered as the
pathogenesis mechanism of ITP (7).
However, platelet function was less investigated. The present study
measured the expression percentage and fluorescence intensity of
three membrane glycoproteins, i.e., PAC-1, CD62p and CD42b with FCM
to analyze the in vitro and in vivo activation of
platelets in ITP patients. The percentage of membrane glycoproteins
indicated the total amount of activated platelets, and the mean
fluorescence intensity (MFI) indicated the expression of membrane
glycoproteins in single-activated platelets. These factors provide
an understanding of the activation capability of individual
platelet. In the current study, the expression levels of PAC-1,
CD62p and CD42b in ITP children were significantly lower those in
the normal control group prior to and after platelet activation by
ADP. This result was consistent with that by Liu and Qian (6), which demonstrated low platelet
activation in vitro and in vivo in ITP children.
Other studies reported that some autoantibodies may affect platelet
function (24–26). As autoantibodies may bind to the
antigenic epitopes on GPIIb/IIIa and GPIb (CD42b), prevent the
binding of added fluorescent mAb, the expression levels of PAC-1
and GPIb (CD42b) were decreased. Furthermore, inhibition of the
initial activation steps prevented the exposure of intracellular
granule membrane glycoproteins on the surface of plasma membrane,
thus decreasing the expression level of CD62p. The present study
also demonstrated normal MFI of CD62p in vivo and lower MFI
of CD62p after platelet activation in vitro. This result
demonstrated low in vivo activation of platelets in ITP
children. Previous findings have shown that the inhibition or
dysfunction of megakaryocytes in ITP children may cause abnormal
membrane glycoproteins in new platelets in a qualitative and
quantitative manner (27).
Platelet activation from the internal to the
external compartment is a series of complicated physiological
process, in which any section affects normal platelet activation.
Further studies are required to determine which section may cause
low platelet activation in ITP children. Our study also
demonstrated that the expression levels of three glycoproteins
gradually increased with the recovery of patients, although the
expression level of PAC-1 in the recovery phase was lower than that
in the normal group. This result may be related to the fact that
platelet activation was enhanced and some antibodies were present
due to the release of new platelets in the recovery phase in ITP
children. The expression level of CD42b in the recovery phase was
similar to that in the normal control group, which may be due to
less membrane glycoprotein GPIb (CD42b) in ITP patients. The
percentage and MFI of platelet membrane glycoprotein CD62p were
higher than those in normal children after activation by ADP. This
result was consistent with Bhoria et al (28), who demonstrated enhanced platelet
function in the ITP children who were in the recovery phase.
Previous studies have demonstrated enhanced platelet
function in ITP children. Wang et al (29) found the positive expression rate of
PAC-1 in ITP patients was significantly higher than that in healthy
and non-ITP patients. Psaila et al (30) observed high intrinsic platelet
activity and intrinsic platelet reactivity due to high IPF,
increased GPIb on the membrane surface of circulating platelets and
increased expression of activated GPIIb/IIIa and GPIb. The finding
was inconsistent with our results due to: i) ITP was a group of
heterogeneous diseases with complicated etiology and pathogenesis
mechanisms. Activation of the individual platelet with different
pathogenesis mechanism may be different. ii) The stage of disease
in the selected subject may be different, as some patients may have
experienced in vitro activation (31); and iii) artificial agitation in the
blood sample collection, the set-up of the instruments and sample
treatment, and the time of sample treatment may have affected the
expression of membrane glycoproteins and the final result. A
limitation of the study is that, the samples collected were
limited. Thus, a larger sample size may be required in future
studies.
Platelet parameter was a simple non-invasive
examination, that was capable of reflecting the proliferation
kinetics of platelets in vivo (32), and was significant for the early
diagnosis, risk assessment and efficacy interpretation of disease.
Platelet parameters included PLT, P-LCR, MPV, PCT and PDW. PLT was
a common indicator for the assessment of treatment protocol and
disease severity. PLT in the ITP children was significantly lower
than that in the normal control group at first visit due to the
disruption of platelets by autoimmunity. MPV reflected the size and
metabolism of platelets and the proliferation capability of
macrophage in bone marrow (33,34).
It was therefore useful in the assessment of platelet function and
the reason for thrombocytopenia. Balduini and Noris (35) identified that the specificity of
MPV in the differentiation of ITP and congenital thrombocytopenia
was up to 91%, and was dependent on the precision of the
instrument. Korkmaz et al (9) demonstrated MPV in a relapsed patient
was significantly higher than that in the patient with a primary
disease, and suggested that MPV could be used to predict the
relapse of ITP, while PDW was a parameter that reflected the
difference of platelet volume and was positively associated with
MPV when marrow proliferation was normal. Ntaios et al
(10) compared the
thrombocytopenia caused by ITP with that by myelosuppression, and
found MPV and PDW were higher when the disruption of peripheral
platelets was increased. PDW and MPV were beneficial to the
diagnosis of ITP. P-LCR refers to the percentage of blood large
platelets (volume of >20 fl). An increase in P-LCR indicates an
elevation in neonatal platelet count, and although the total number
of platelets remained unchanged, it indirectly led to an increase
in platelet destruction (34). PCT
was the product of PLT and MPV, and the change of PCT was
consistent with the change of PLT. The present findings
demonstrated that PLT and PCR in the ITP patients were significant
lower, whereas PDW, MPV and P-LCR in the ITP patients were
significantly higher. Following treatment, PLT and PCR gradually
increased, whereas PDW, MPV and P-LCR gradually decreased. PLT was
negatively related to MPV, while MPV was positively related to PDW
and P-LCR. These results were consistent with those of Fan and Wei
(36), which suggested that the
change of platelet parameters was beneficial to the diagnosis and
efficacy interpretation of ITP patients. However, some factors may
affect the detection of platelet parameters, including the time of
sample storage, the method for blood collection, anticoagulant EDTA
and enlarged platelet volume due to the binding of platelet to
granulocytes or monocytes.
Reticulated platelets (RPs) are immature platelets
that enter the blood from bone marrow, and are in the naïve phase
of the process from megakaryocyte to platelet. RPs are an important
indicator in assessing the ability of bone marrow to produce
platelets. Various indicators can be used to identify
thrombocytopenia (37–40), and be used to predict the recovery
of platelets after hematopoietic stem cell transplantation
(41). Liu et al (42) suggested that IPF% was superior to
PA IgG in the diagnosis of ITP. It was reported that IPF was
associated with the response to ITP treatment (43). The current results have
demonstrated that the IPF% in ITP children prior to treatment was
significantly increased in comparison to the normal control group.
IPF% decreased with the relief of disease following treatment. The
results were consistent with those by Adly et al (44), which suggested that the disruption
of peripheral platelets in ITP children was increased, and although
the bone marrow hyperplasia was normal, the compensatory
hyperplasia of megakaryocytes caused the release of a large number
of new platelets, leading to increased IPF% and subsequent
increased PLT. As a result, the stimulation to megakaryocytes was
weak and IPF% gradually decreased. IPC was the product of PLT and
IPF%, and represented the absolute value of reticulated platelets
in unit volume of peripheral blood. The present findings
demonstrated that IPC prior to treatment in the ITP children was
significantly decreased in comparison to the normal control group,
and the response period after ITP treatment was significantly
longer than that prior to treatment. IPC was in inverse proportion
to IPF%. This may be associated with the short life-span of new
platelets or the disruption of new platelets following release into
the blood. Barsam et al (45) suggested that IPC reflected the
balance between the compensatory production of platelets by
megakaryocytes in ITP children and the disruption rate of
peripheral platelets, and could be used as the efficacy indicator
following treatment of thrombopoietin in chronic ITP patients.
Greene et al (46)
suggested that IPC could be used to assess the hemorrhage risk in
ITP patients better than platelet parameters, such as PLT and
MPV.
Currently, physicians tend to focus on the
relationship between the change of platelet count and diseases,
while ignoring the significance of other platelet parameters in the
diagnosis and treatment in diseases. The findings of the current
study have demonstrated that the test of platelet parameters is
convenient and reliable, and valuable to the diagnosis and follow
up in ITP children as it avoids the pain from bone marrow biopsy,
repeated tests, and good patient compliance and dynamic assessment
of the recovery in ITP children is possible. Findings of the
present study indicated that a good platelet count is important for
proper assessment of platelet parameters (47). However, IPF is not affected by
platelet count, thus it is complementary to platelet parameters in
the etiology of thrombocytopenia and the evaluation of
efficacy.
Acknowledgments
The present study was funded by the Sichuan
Department of Education Scientific and Development Funds
(2011ZA150).
References
|
1
|
Min Z and Lin X: The advancement in the
treatment of the children with refractory idiopathic
thrombocytopenic purpura. J Clin Pediatr. 26:727–730. 2008.
|
|
2
|
Terrell DR, Beebe LA, Vesely SK, Neas BR,
Segal JB and George JN: The incidence of immune thrombocytopenic
purpura in children and adults: a critical review of published
reports. Am J Hematol. 85:174–180. 2010.PubMed/NCBI
|
|
3
|
Hu Q: Diagnosis of idiopathic
thrombocytopenic purpura in children. Journal of Applied Clinical
Pediatrics. 25:159–161. 2010.In Chinese.
|
|
4
|
Li P, Shi T, Gao J, Zhao D and Xiao A: The
immunization in the children with acute immune thrombocytopenia.
Chinese Journal of Practical Pediatrics. 29:548–550. 2014.In
Chinese.
|
|
5
|
Provan D and Newland AC: Newl and current
management of primary immune thrombocytopenia. Adv Ther.
32:875–887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu W and Qian X: Interpretation of the
recommendations for the diagnosis and treatment of pediatric
primary immune thrombocytopenia. Chin J Obstet Gynecol Pediatr.
21:245–248. 2015.
|
|
7
|
Winkler J, Kroiss S, Rand ML, Azzouzi I,
Annie Bang KW, Speer O and Schmugge M: Platelet apoptosis in
paediatric immune thrombocytopenia is ameliorated by intravenous
immunoglobulin. Br J Haematol. 156:508–515. 2012. View Article : Google Scholar
|
|
8
|
Yin J, Wang J, Chen XQ, Yu WJ, Liao JY and
Wang DX: Investigation on platelet apoptosis in patients with
chronic idiopathic thrombocytopenic purpura. J Diagn Concepts &
Pract. 9:236–241. 2010.In Chinese.
|
|
9
|
Korkmaz S, Uslu AU, Aydin B, Dogan O and
Sencan M: Pre-treatment and post-treatment changes in platelet
indices in patients with immune thrombocytopenia. Saudi Med J.
34:591–596. 2013.PubMed/NCBI
|
|
10
|
Ntaios G, Papadopoulos A, Chatzinikolaou
A, Saouli Z, Karalazou P, Kaiafa G, Girtovitis F, Kontoninas Z,
Savopoulos C, Hatzitolios A, et al: Increased values of mean
platelet volume and platelet size deviation width may provide a
safe positive diagnosis of idiopathic thrombocytopenic purpura.
Acta Haematol. 119:173–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen JF, Yang LH, Chang LX, Feng JJ and
Liu JQ: The clinical significance of circulating B cells secreting
anti-glycoprotein IIb/IIIa antibody and platelet glycoprotein
IIb/IIIa in patients with primary immune thrombocytopenia.
Hematology. 17:283–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas MR, Wijeyeratne YD, May JA, Johnson
A, Heptinstall S and Fox SC: A platelet P-selectin test predicts
adverse cardiovascular events in patients with acute coronary
syndromes treated with aspirin and clopidogrel. Platelets.
25:612–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He Y, Zhao YX, Zhu MQ, Wu Q and Ruan CG:
Detection of auto-antibodies against platelet glycoproteins in
patients with immune thrombocytopenic purpura by flow cytometric
immunobead array. Clin Chim Acta. 415:176–180. 2013. View Article : Google Scholar
|
|
14
|
Xun L, Zhu WB, Wu JS, Cai XY, Liu X, Han
YS, Yang HZ, Zheng CC and Li Q: Diagnosing ITP by Measuring the CD
61 and PAIg with FCM. Chin J Thromb Hemost. 17:8–12. 2011.In
Chinese.
|
|
15
|
Wang T, Zhang C and Lu J: Platelet
parameter and platelet membrane glycoprotein in childhood acute
lymphoblastic leukemia. J Chin Pediatr Blood Cancer. 11:12–16.
2006.In Chinese.
|
|
16
|
Psaila B, Bussel JB, Frelinger AL, Babula
B, Linden MD, Li Y, Barnard MR, Tate C, Feldman EJ and Michelson
AD: Differences in platelet function in patients with acute myeloid
leukemia and myelodysplasia compared to equally thrombocytopenic
patients with immune thrombocytopenia. J Thromb Haemost.
9:2302–2310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Subspecialty Group of Hematology, The
Society of Pediatrics Chinese Medical Association; Editorial Board,
Chinese Journal of Pediatrics: Recommendations for diagnosis and
treatment of primary immune thrombocytopenia in children. Zhonghua
Er Ke Za Zhi. 51:382–384. 2013.In Chinese.
|
|
18
|
Xu Q and Liu WJ: Advances in the diagnosis
and treatment of primary immune thrombocytopenia in children. Chin
J Pract Pediatr. 28:646–652. 2013.In Chinese.
|
|
19
|
Huang Z, Liu WJ, Guo QL and Liu CY:
Platelet parameters and expression of platelet membrane
glycoprotein in childhood acute lymphoblastic leukemia. Genet Mol
Res. 14:16074–16089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarratt KL, Chen H, Zutter MM, Santoro SA,
Hammer DA and Kahn ML: GPVI and alpha2beta1 play independent
critical roles during platelet adhesion and aggregate formation to
collagen under flow. Blood. 106:1268–1277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Z and Liu W: Progress in research on
platelet glycoproteins of thrombocytopenia in children. J Appl Clin
Pediatr. 29:227–230. 2014.In Chinese.
|
|
22
|
Mutlu A, Gyulkhandanyan AV, Freedman J and
Leytin V: Concurrent and separate inside-out transition of platelet
apoptosis and activation markers to the platelet surface. Br J
Haematol. 163:377–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Serebruany V, Malinin A, Aradi D,
Kuliczkowski W, Norgard NB and Boden WE: The in vitro effects of
niacin on platelet biomarkers in human volunteers. Thromb Haemost.
104:311–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cines DB and Blanchette VS: Immune
thrombocytopenic purpura. N Engl J Med. 346:995–1008. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong NZ, Cui RJ and Ruan CG: Generation of
human Fab antibody against platelet membrane glycoprotein iib/iia
and its effect on platelet aggregation. Chin J Cell Mol Immunol.
25:65–67. 2009.In Chinese.
|
|
26
|
Yang G: research and application of
platelet glycoprotein iib/iiia acceptor antagonist. Clin Med Eng.
17:149–151. 2010.
|
|
27
|
Kashiwagi H and Tomiyama Y:
Pathophysiology and management of primary immune thrombocytopenia.
Int J Hematol. 98:24–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhoria P, Sharma S, Varma N, Malhotra P,
Varma S and Luthra-Guptasarma M: Effect of steroids on the
activation status of platelets in patients with Immune
thrombocytopenia (ITP). Platelets. 26:119–126. 2015. View Article : Google Scholar
|
|
29
|
Wang C, Niu A, Lu Z, Yang X, Wang L and
Zou X: The clinical significances of PAIgG, PAC-1 and CD62P in
patients with idiopathic thrombocytopenic purpura. Zhejiang J Lab
Med. 3:7–10. 2005.In Chinese.
|
|
30
|
Psaila B, Bussel JB, Linden MD, Babula B,
Li Y, Barnard MR, Tate C, Mathur K, Frelinger AL and Michelson AD:
In vivo effects of eltrombopag on platelet function in immune
thrombocytopenia: no evidence of platelet activation. Blood.
119:4066–4072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vinholt PJ, Hvas AM and Nybo M: An
overview of platelet indices and methods for evaluating platelet
function in thrombocytopenic patients. Eur J Haematol. 92:367–376.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y and Gang W: Study on clinical
significance of platelet relevant parameters in diagnosis of
thrombocytopenia. J Clin Exp Med. 12:1533–1535. 2013.
|
|
33
|
Bai J and Liu W: Changes of platelet
parameters and platelet function in primary immune
thrombocytopenia. Chin J Pract Pediatr. 30:150–153. 2015.In
Chinese.
|
|
34
|
Adly AA, Ragab IA, Ismail EA and Farahat
MM: Evaluation of the immature platelet fraction in the diagnosis
and prognosis of childhood immune thrombocytopenia. Platelets.
26:645–650. 2015. View Article : Google Scholar
|
|
35
|
Balduini CL and Noris P: Mean platelet
volume for distinguishing between inherited thrombocytopenias and
immune thrombocytopenia - response to Beyan. Br J Haematol.
163:413–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan L and Wei W: Platelet parameters in
children with idiopathic thrombocytopenic purpura clinical
application analysis. J Clin Exp Med. 13:124–126. 2014.
|
|
37
|
Strauss G, Vollert C, von Stackelberg A,
Weimann A, Gaedicke G and Schulze H: Immature platelet count: A
simple parameter for distinguishing thrombocytopenia in pediatric
acute lymphocytic leukemia from immune thrombocytopenia. Pediatr
Blood Cancer. 57:641–647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pons I, Monteagudo M, Lucchetti G, Muñoz
L, Perea G, Colomina I, Guiu J and Obiols J: Correlation between
immature platelet fraction and reticulated platelets. Usefulness in
the etiology diagnosis of thrombocytopenia. Eur J Haematol.
85:158–163. 2010.PubMed/NCBI
|
|
39
|
Jiang W, Jiang H, Wu Y, Zhang Z and Chen
X: The variation of immature platelet fraction in patients with
thrombocytopenic diseases. Lab Med. 28:47–50. 2013.
|
|
40
|
Yang J, Zhao Y, Wu W, Hua B, Zhu Z, Yang R
and Wang S: Value of Reticulated Platelet Countsin Identifying
Thrombocytopenia Aetiology. J Exp Hematol. 18:482–485. 2010.In
Chinese.
|
|
41
|
Michur H, Maślanka K, Szczepiński A and
Mariańska B: Reticulated platelets as a marker of platelet recovery
after allogeneic stem cell transplantation. Int J Lab Hematol.
30:519–525. 2008.PubMed/NCBI
|
|
42
|
Liu Y, Wang Z, Yuan B, Wang X, Wu Q and
Yuan H: Role of reticulated platelets and platelet-associated
antibody in differential diagnosis of idiopathic thrombocytopenic
purpura. J Exp Hematol. 19:979–982. 2011.In Chinese.
|
|
43
|
Xiao M, Liu J, Wu P and Yuan Q:
Significance of platelet parameters measurement in thrombocytopenia
disease. Int J Lab Med. 34:418–420. 2013.In Chinese.
|
|
44
|
Adly AA, Ragab IA, Ismail EA and Farahat
MM: Evaluation of the immature platelet fraction in the diagnosis
and prognosis of childhood immune thrombocytopenia. Platelets.
26:645–650. 2015. View Article : Google Scholar
|
|
45
|
Barsam SJ, Psaila B, Forestier M, Page LK,
Sloane PA, Geyer JT, Villarica GO, Ruisi MM, Gernsheimer TB, Beer
JH, et al: Platelet production and platelet destruction: assessing
mechanisms of treatment effect in immune thrombocytopenia. Blood.
117:5723–5732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Greene LA, Chen S, Seery C, Imahiyerobo AM
and Bussel JB: Beyond the platelet count: Immature platelet
fraction and thromboelastometry correlate with bleeding in patients
with immune thrombocytopenia. Br J Haematol. 166:592–600. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Banfi G and Germagnoli L: Preanalytical
phase in haematology. J Med Biochem. 27:348–353. 2008. View Article : Google Scholar
|