Introduction
Improvements in quality of living and changes in
diet have been identified as the major issues contributing to the
escalation of obesity in children and adolescents (1). To date, obesity is considered to be
associated with insulin resistance, abnormal glucose metabolism,
dyslipidemia, inflammation and vascular damage (2). The increasing prevalence of
peripubertal obesity has raised the concerns regarding the
prevalence and severity of adolescent metabolic syndrome (3,4).
Epidemiological data indicates a higher incidence of
diabetes mellitus and fatty liver in males than in females, which
may be associated with the expression of endogenous sex hormones.
In males, testosterone is important in the body composition,
particularly of visceral fat (5).
For example, increases in serum triglyceride and total cholesterol
(TC) have been observed in men with testosterone deficiency
(6). In addition, decreased plasma
testosterone has been identified in obese adolescent males,
compared with control individuals of the same Tanner stage,
consistent with data in adult males, associating obesity and
insulin resistance with hypotestosteronemia (7,8).
Furthermore, testosterone replacement treatment can enhance the
effect of insulin and contribute to glucolipid metabolism, whereas
high doses of exogenous testosterone decrease insulin sensitivity
(9,10).
Increasing attention has been focused on the
association between androgen deficiency and a variety of
cardiovascular diseases (11,12).
For example, a decrease in endogenous testosterone is a risk factor
for atherosclerosis in males (11). In addition, short-term
administration of testosterone at physiological concentrations can
ameliorate coronary heart disease and improve endothelial vascular
function (13). To date, the roles
of testosterone in the metabolism of glucolipids and cardiovascular
disease in obesity remain to be fully elucidated. In the present
study, a male rat model subjected to a high-fat diet and castration
was established, based on which the present study aimed to examine
the effect of testosterone in these animals. The results may
determine whether testosterone attenuates vascular injury and
assist in elucidating the underlying mechanism.
Materials and methods
Animals
For the animal model, 40 male Sprague-Dawley rats
(3-week-old, weighing 50–60 g) were provided by the Academy of
Military Medical Sciences (Beijing, China). All rats were housed at
a temperature of 26°C in a 12 h light/12 h dark cycle, and were
provided with free access to water and food. Following adaptive
breeding for 1 week, the animals were randomly divided into the
following groups: Group 1, control group (n=10), in which rats were
fed a high-fat diet containing 57% carbohydrate, 22% protein and 4%
fat; group 2, high-fat-diet + castration group (n=10), which were
fed a high-fat diet following castration; group 3, high-fat-diet +
castration + low dose testosterone group (n=10), which were fed a
high-fat diet following castration, and were administered with
testosterone (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 12.5
µg/kg/day (dissolved in sesame oil); group 4, high-fat-diet
+ castration + high dose testosterone group (n=10), which were fed
a high-fat diet following castration, and received testosterone at
a dose of 25 µg/kg/day. Castration was performed by removal
of the testicles following anesthesia using 10% chloral hydrate
(3.5 ml/kg; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) via intraperitoneal injection. All animals were fed
for 6 weeks, and body weights were monitored weekly. The rats were
sacrificed by cervical dislocation at the age of 10 weeks, and the
thoracic aortas were obtained for subsequent experiments. All
experimental protocols were approved by the Ethics Committee of the
General Hospital of Tianjin Medical University (Tianjin,
China).
Biochemical measurements
Prior to blood collection, the rats were maintained
in a fasting state for 24 h. Blood samples (3–5 ml) were collected
from an angular vein, followed by centrifugation at 1,680 × g and
4°C for 15 min. Subsequently, the levels of TC, high-density
lipoprotein cholesterol (HDL-C), insulin, glucose and testosterone
in the serum were determined using an Olympus AU400 Clinical
Chemistry Analyzer (Olympus Coporation, Tokyo, Japan). Non-HDL
cholesterol was defined as total cholesterol - HDL cholesterol.
Serum glucose was determined using a glucose oxidase kit purchased
from Biosino Bio-Technology & Science Inc. (Beijing, China).
Serum insulin was measured using a rat Insulin ELISA kit purchased
from the China Institute of Atomic Energy (Beijing, China). Plasma
lipids were measured using Hitachi 7170 automatic biochemistry
analyzer (Hitachi, Tokyo, Japan). Homeostatic model
assessment-insulin resistance (HOMA-IR) was calculated according to
the following formula: HOMA-IR = fasting insulin (µU/ml) ×
fasting serum glucose (mmol/l) / 22.5.
Immunohistochemical staining
For immunohistochemical analyses, the
paraffin-embedded sections (8 µm) were dewaxed for 10 min,
followed by immersion in boiling citrate buffer solution (pH 6.4;
Beijing Solarbio Science & Technology Co., Ltd.) for 10 min.
Antigen retrieval was performed by microwave heating at a high heat
for 10 min, a low heat for 10 min and cooled to room temperature.
The sections were then immersed in distilled water in an orbital
shaker for 5 min. Following antigen retrieval, the sections were
incubated in 3% H2O2 solution (Beijing
Solarbio Science & Technology Co., Ltd.) for 5 min at room
temperature, and then blocked with 5% bovine serum albumin (Beijing
Solarbio Science & Technology Co., Ltd.) for 1 h to avoid
nonspecific staining. The sections were then incubated with the
following primary antibodies: Polyclonal rabbit anti-rat IRS-1
(1:100; Abcam, Cambridge, UK; cat. no. ab131487), polyclonal rabbit
anti-rat GLUT-4 (1:400; Abcam; cat. no. ab654), polyclonal rabbit
anti-rat TNF-α (1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA; cat. no. 21926) and polyclonal rabbit anti-rat NF-κB
(1:800; Cell Signaling Technology, Inc.; cat. no. 5970) at 4°C
overnight. Subsequently, the sections were incubated with
biotin-conjugated monoclonal goat anti-rabbit IgG (1:200; Vector
Laboratories, Inc., Burlingame, CA, USA; cat. no. BA-1000) for 2 h
at room temperature, and horseradish peroxidase (HRP) streptavidin
(Vector laboratories, Inc.) for 1 h at room temperature. The images
were analyzed using Image-Pro Plus 6.0 software (Media Cybernetics,
Silver Spring, MD, USA).
TUNEL assay
The tissue sections (5 µm) obtained from the
thoracic aorta were cut on a freezing microtome (Leica RM2255;
Leica Microsystems, Wetzlar, Germany). The numbers and distribution
of apoptotic cells within the aortic tissues were analyzed using an
in situ Cell Death Detection kit (Roche Diagnostics GmbH,
Mannheim, Germany) according to the manufacturer's protocol.
Subsequently, the sections were incubated with 40 µl
reaction mixture containing terminal deoxynucleotidyltransferase
(TdT) and digoxigenin-conjugated dUTP for 1 h at 37°C.
Subsequently, the sections were stained with DAPI (Roche
Diagnostics GmbH, Mannheim, Germany) to stain the nuclei for 1 min
at room temperature, followed by washing with phosphate-buffered
saline. Finally, the TUNEL (Roche Diagnostics GmbH)-positive cells
were observed under a fluorescent microscope (Olympus BX51TF;
Olympus, Tokyo, Japan) at ×200 magnification.
Western blot analysis
Protein extraction from the aortic tissues was
performed using tissue lysis buffer (Solarbio, Beijing, China)
containing 1% phenylmethanesulfonyl fluoride and protein was
quantified using a BCA protein assay kit. The protein (25
µg) was separated on a 10% SDS-PAGE gel (Beijing Solarbio
Science & Technology Co., Ltd.) and transferred onto
polyvinylidene fluoride membranes (Merck Millipore, Darmstadt,
Germany). Subsequently, the membrane was blocked with 5% nonfat
dried milk for 2 h at room temperature and then incubated with
rabbit primary antibodies against NF-κB, TNF-α and polyclonal
rabbit anti-rat β-actin (1:1,000; Cell Signaling Technology, Inc.;
cat. no. 4967), GLUT4 and IRS1 (1:2,000; Abcam) and polyclonal
rabbit anti-rat PI3K (1:1,000, Sigma-Aldrich; cat. no. 5295)
overnight at 4°C. The membrane was washed with 0.1% PBST three
times for 5 min. Subsequently, the membrane was incubated with
HRP-labeled goat anti-rabbit IgG (cat. no. GGHL-15P; ICL, Inc.,
Portland, OR, USA) secondary antibodies for 1 h at room
temperature. The same membranes probed for β-actin served as an
internal standard. The relative density of protein to β-actin was
analyzed using ImageJ software (version 1.43; National Institutes
of Health, Bethesda, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the aortic tissues
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Synthesis of cDNA was
performed using a TransScript First-Strand cDNA system (TransGen
Biotech, Beijing, China), according to the manufacturer's protocol.
The reaction mixture consisted of 10 µl of 2X TransStart Top
Green qPCR SuperMIX, 0.4 µl forward primer, 0.4 µl
reverse primer, 0.4 µl passive reference dye, 1.0 µl
cDNA template and 20 µl ddH2O. qPCR was performed
using a TransScript Top Green qPCR SuperMix kit (TransGen Biotech)
on a BioRad CFX96 qPCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using the primers listed in Table I. The PCR amplification was
conducted using the following conditions: 94°C for 30 sec, followed
by 40 cycles of 94°C for 5 sec, 60°C for 15 sec and 72°C for 10
sec. The relative quantification of each gene was normalized to
β-actin using the 2−ΔΔCq method (14).
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequence
(5′–3′) |
|---|
| NF-κB | Forward:
TGAGGCTGTTTGGTTTGAGA |
| Reverse:
TTATGGCTGAGGTCTGGTCTG |
| TNF-α | Forward:
GCTCCCTCTCATCAGTTCCA |
| Reverse:
GCTTGGTGGTTTGCTACGAC |
| GLUT-4 | Forward:
GTATGTTGCGGATGCTATGG |
| Reverse:
CCTCTGGTTTCAGGCACTCT |
| IRS-1 | Forward:
GGCACCATCTCAACAATCCT |
| Reverse:
GTTTCCCACCCACCATACTG |
| PI3K | Forward:
CAAAGCCGAGAACCTATTGC |
| Reverse:
TTGACTTCGCCATCTACCAC |
| AKT | Forward:
TGGCACCTTTATTGGCTACA |
| Reverse:
CCGCTCTGTCTTCATCAGC |
| β-actin | Forward:
CGTTGACATCCGTAAAGACC |
| Reverse:
AGAGCCACCAATCCACACA |
Statistical analysis
Statistical analysis was performed using SPSS 17
software (SPSS, Inc., Chicago, IL, USA). All data are expressed as
the mean ± standard error of the mean. The inter-group differences
were analyzed using analysis of variance. Student's t-test
was performed to analyze the measurement data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Biochemical parameters
Compared with control (group 1), significant
increases were found in the plasma levels of glucose, insulin,
HOMA-IR, TC and non-HDL in group 2 (P<0.05; Fig. 1). A significant decrease was
revealed in the levels of HDL and testosterone in the plasma of
group 2, compared with the normal control (P<0.05). In the
animals subjected to low dose testosterone replacement (group 3),
significant reductions in plasma TC and non-HDL were observed,
compared with group 2 (P<0.05). The plasma levels of glucose,
insulin, TC and non-HDL were further increased following exposure
to high dose testosterone (group 4), compared with group 2
(P<0.05).
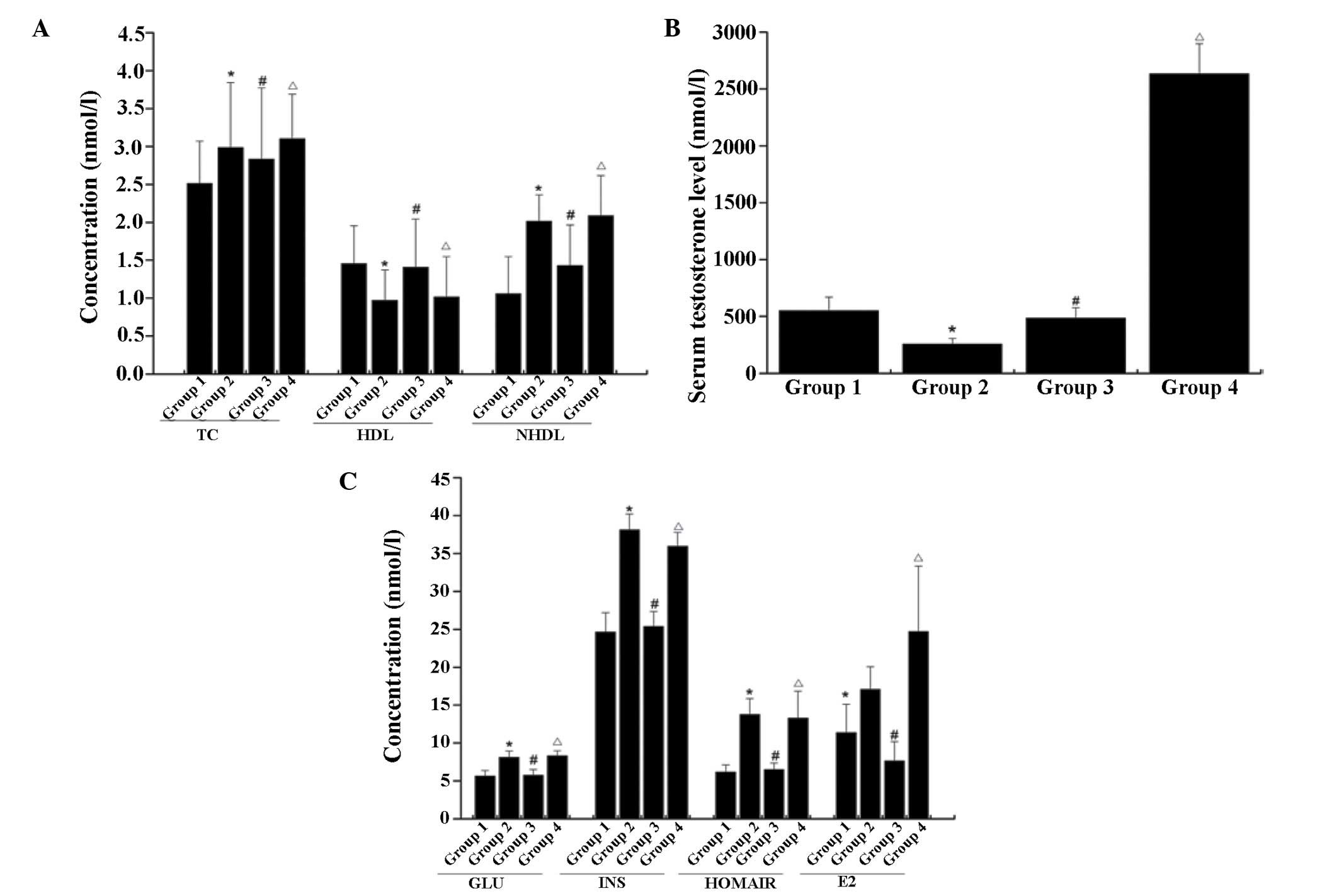 | Figure 1Serum levels of (A) TC, HDL and NHDL,
(B) testosterone, (C) GLU, INS, HOMA-IR and E2 in rats. The data
are presented as the mean ± standard deviation, based on three
independent experiments. *P<0.05, compared with the
control group; #P<0.05, group 3 compared with group
2, ΔP<0.05, group 4 compared with group 2. TC, total
cholesterol; HDL, high density lipoprotein; NHDL, non-high density
lipoprotein; GLU, glucose; INS, insulin; HOMA-IR, homeostatic model
assessment-insulin resistance; E2, estradiol. |
In the scatter diagram of testosterone and the
metabolism of the associated parameters, a continual decrease was
identified in the plasma levels of glucose, insulin, HOMA-IR, TC
and non-HDL as plasma testosterone increased in groups 1, 2 and 3,
respectively (Fig. 2). However, an
increasing trend was noted in these parameters as plasma
testosterone in group 4 increased. Plasma HDL increased as plasma
testosterone in group 2 increased. Plasma HDL increased as plasma
testosterone in group 4 increased.
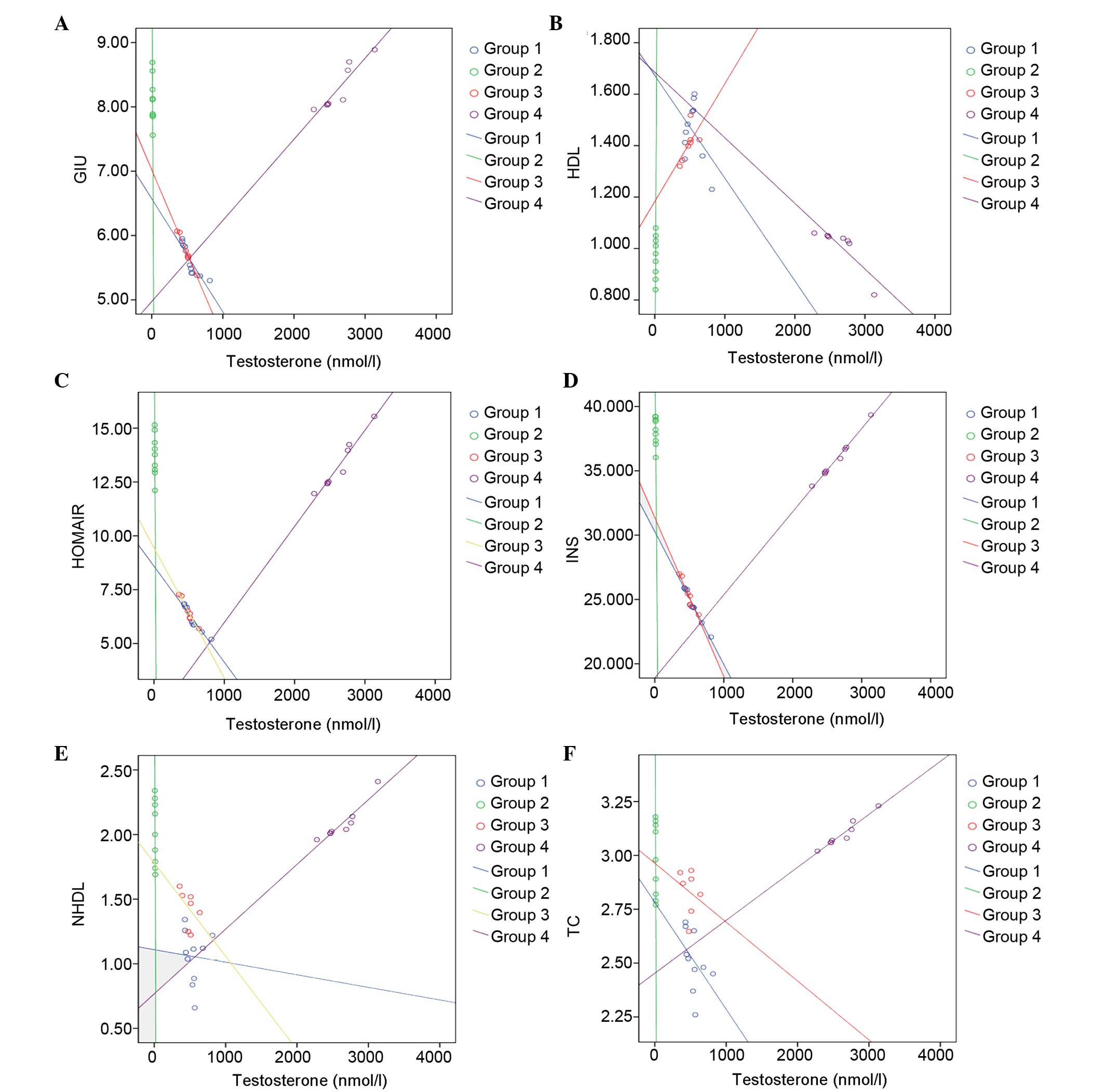 | Figure 2Scatter diagrams of testosterone and
metabolism indices of (A) GLU, (B) HDL, (C) HOMRIR, (D) INS, (E)
NHDL and (F) TC. HDL, high density lipoprotein; NHDL, non-high
density lipoprotein; GLU, glucose; INS, insulin; HOMA-IR,
homeostatic model assessment-insulin resistance; TC, total
cholesterol. |
Morphological changes in thoracic aorta
tissues
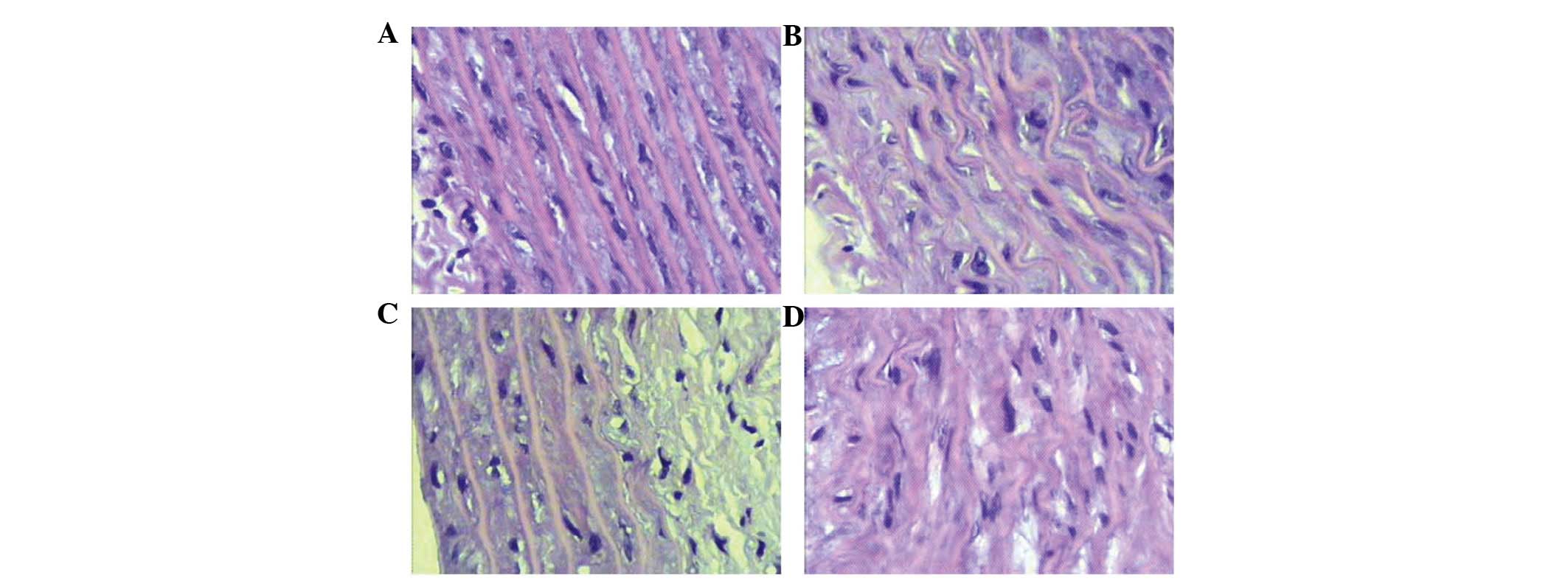
As shown in Fig. 3,
the inner elastic lamina and endothelial cells in the thoracic
aorta tissues were intact in the control group, with no necrosis or
mucoid degeneration (Fig. 3A).
However, an irregular lining, with partial endothelial cell loss,
necrosis and mucoid degeneration was observed in group 2, together
with wrinkling and deformation of the elastic lamina (Fig. 3B). In group 3, a significant
reduction was observed in necrosis and mucoid degeneration,
compared with group 2 (Fig. 3C).
However, in the thoracic aorta tissues obtained from rats treated
with high-dose testosterone replacement, severe necrosis and mucoid
degeneration were noted, together with deformation of the elastic
lamina and endothelium (Fig.
3D).
Immunohistochemical staining
Almost no staining of IRS-1 or GLUT-4 were detected
in group 2 in the immunohistochemical analysis. However, the
expression levels of IRS-1 and GLUT-4 were marked in group 3. Of
note, the expression of IRS-1 in group 3 was significantly higher,
compared with that in the normal group. In group 4, the lowest
levels of IRS-1 and GLUT-4 were detected, as shown in Fig. 4. Castration in combination with a
high-fat diet contributed to increases in the expression levels of
TNF-α and NF-κB. In addition, treatment with a high dose of
testosterone further increased the expression levels of TNF-α and
NF-κB. In the castration + low-dose testosterone group, there was a
decrease in TNF-α and NF-κB staining, compared with the castration
+ high-fat diet group.
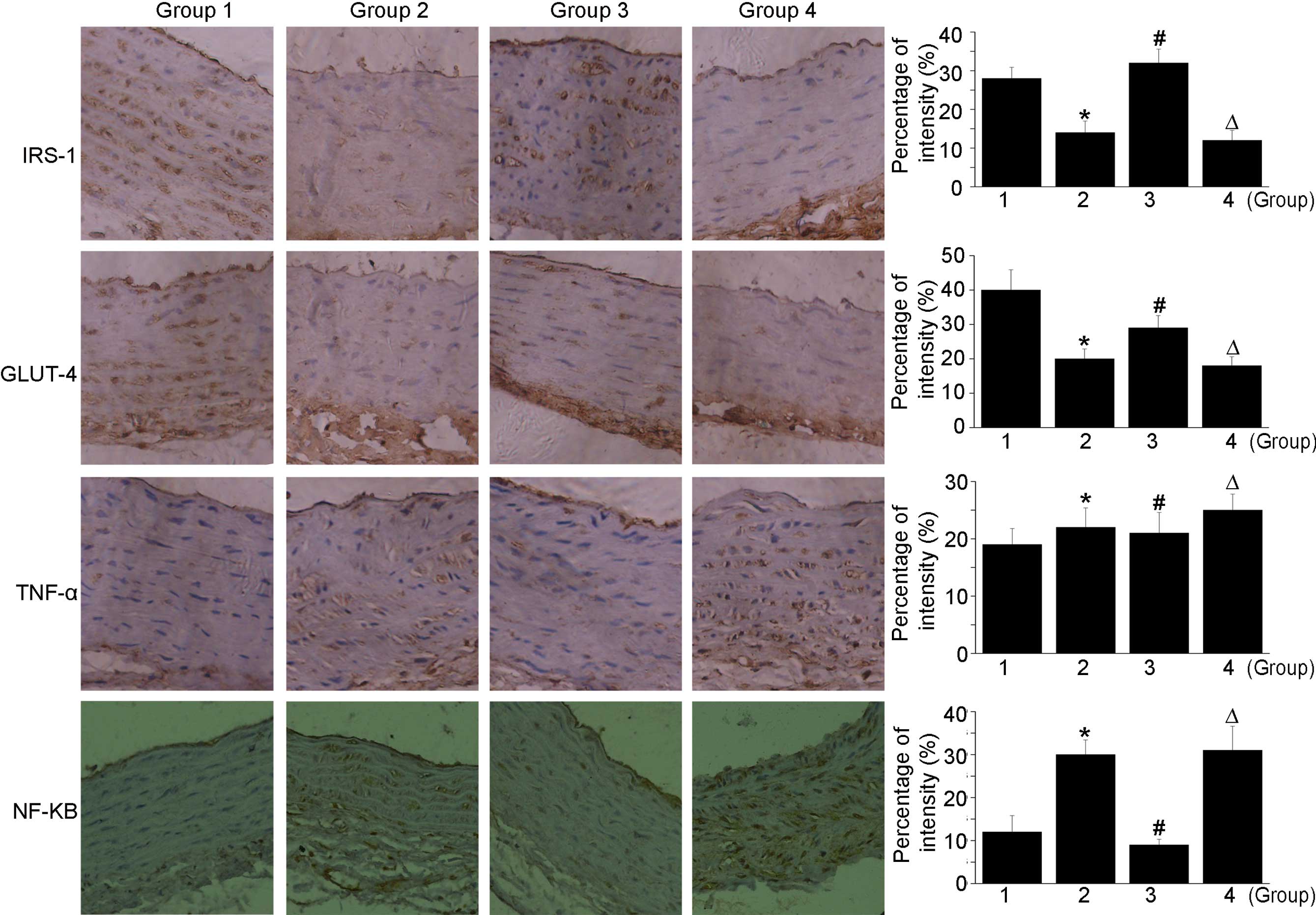 | Figure 4Immunohistochemical staining of
sections obtained from the thoracic aorta. High doses of
testosterone further increase the expression levels of TNF-α and
NF-κB. In the castration + low-dose testosterone group, there were
decreases in TNF-α and NF-κB staining, compared with the castration
+ high-fat diet group. Magnification, ×200 *P<0.05,
group 2 compared with group 1; #P<0.05, group 3
compared with group 2, ΔP<0.05, group 4 compared with
group 2. Group 1, control; group 2, high-fat-diet + castration;
group 3; high-fat-diet + castration + low-dose testosterone group
4, high-fat-diet + castration + high-dose testosterone; IRS-1,
insulin receptor substrate-1; GLUT-4, glucose transporter type 4;
TNF-α tumor necrosis factor-α; NF-κB, nuclear factor-κB. |
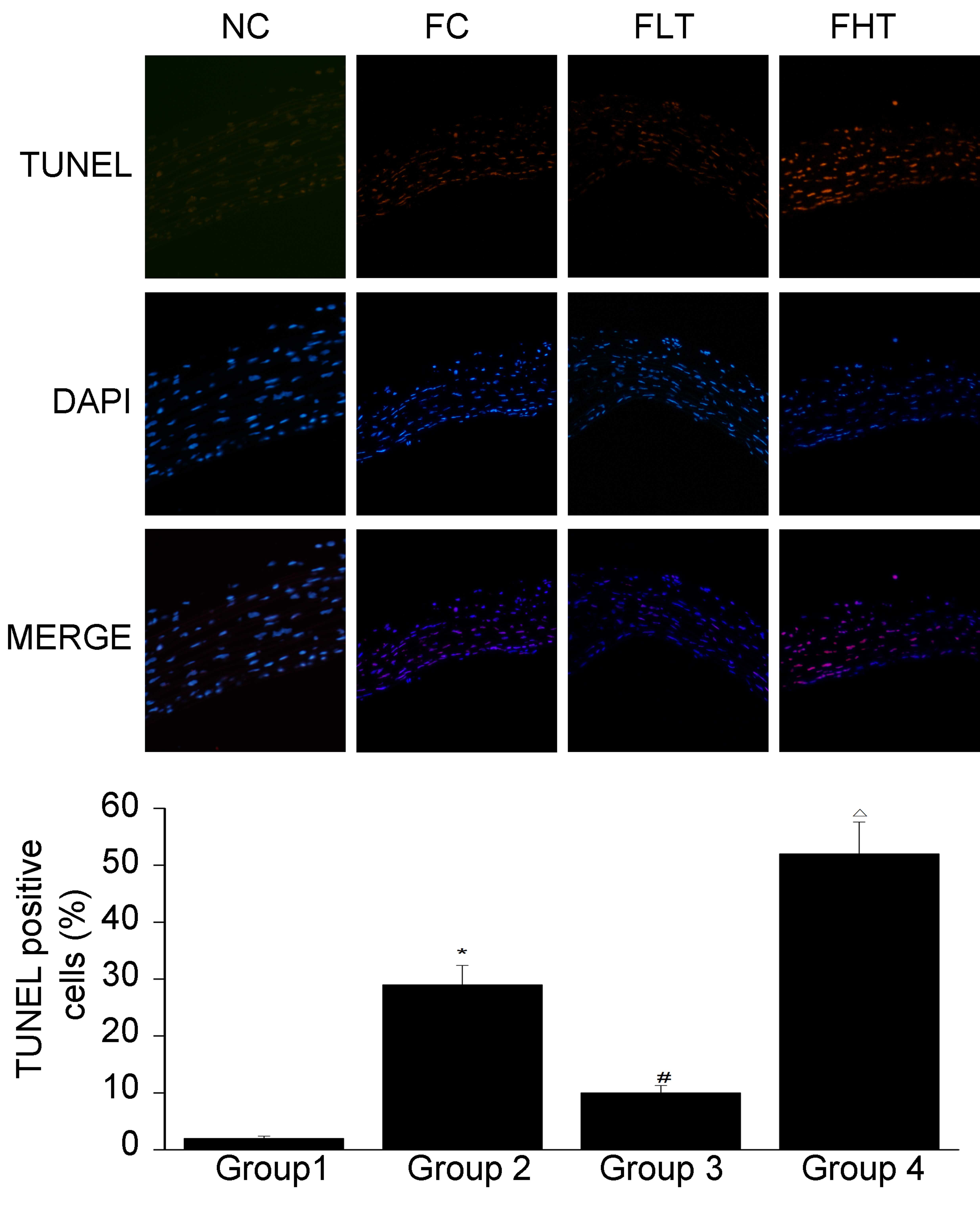
TUNEL staining
The results of the TUNEL staining are shown in
Fig. 5. Compared with group 1, a
significant increase was observed in cell death in group 2. In
addition, a significant decrease in cell death was observed in
group 3, compared with group 2. By contrast, a significant increase
in cell death was observed in group 4, compared with group 2. This
indicated that testosterone at a physiological dose attenuated
cellular apoptosis, whereas a high dose of testosterone aggravated
the apoptosis.
Western blot analysis
As shown in Fig. 6,
the protein levels of IRS-1, AKT and GLUT-4 in group 2 were lower,
compared with the levels in group 1. Following exposure to low-dose
testosterone, the protein expression levels of IRS-1, AKT and
GLUT-4 in group 3 were markedly increased, compared with group 2.
However, their levels of expression remained lower, compared with
the normal control (group 1). By contrast, exposure to a high dose
of testosterone resulted in significant decreases in the levels of
IRS-1, AKT and GLUT-4 in group 4, compared with those in group 2.
In addition, significant upregulation in the levels of NF-κB, TNF-α
and PI3K were observed in group 2, compared with the normal
control. High-dose testosterone caused further increases in the
levels of NF-κB, TNF-α and PI3K, compared with those in group 2,
and a low dose of testosterone caused decreases in the levels of
NF-κB, TNF-α and PI3K in group 3, compared with group 2. However,
the levels of NF-κB and PI3K in group 3 remained higher than in the
normal control.
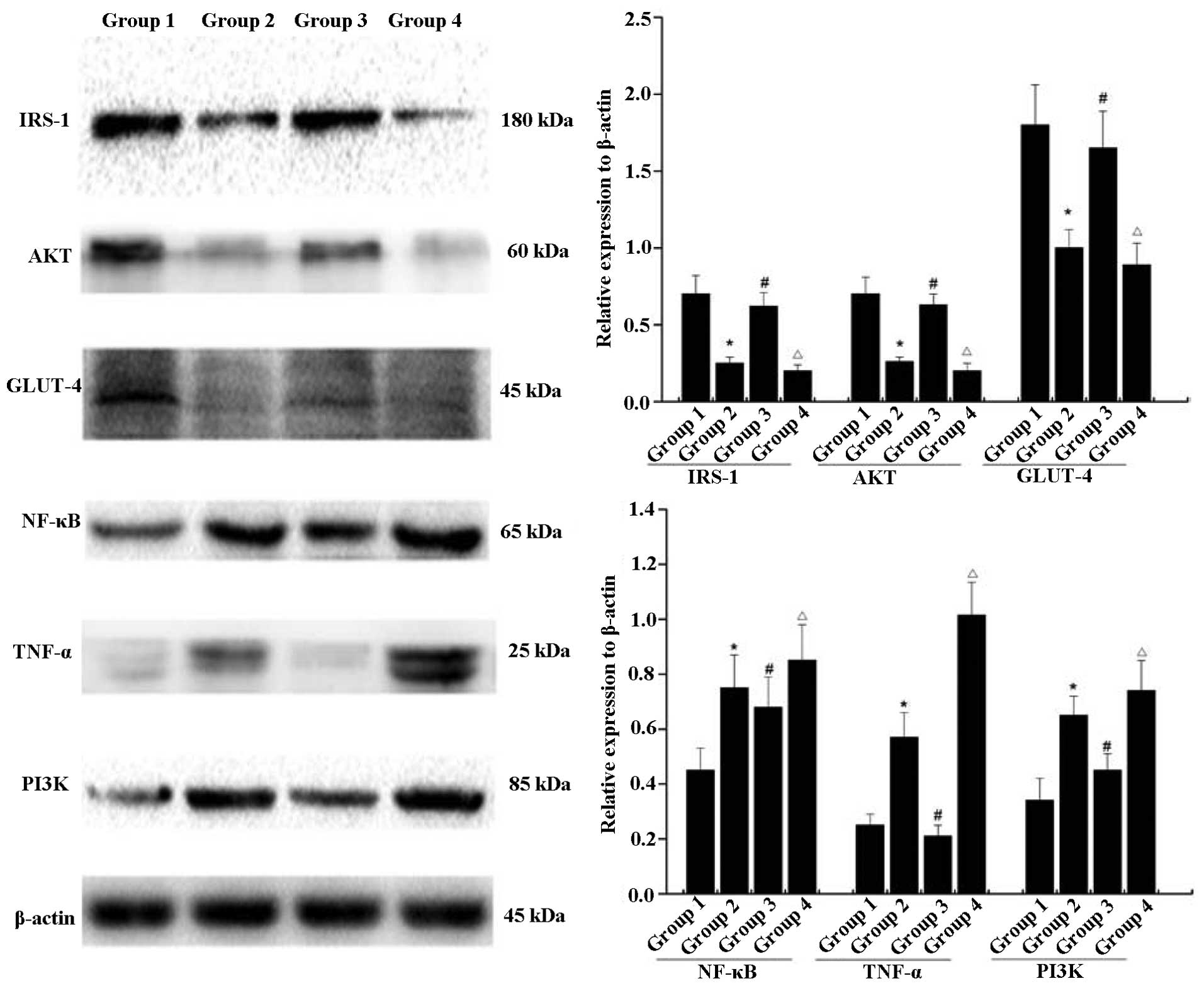 | Figure 6Low-dose testosterone upregulates the
expression levels of IRS-1, AKT, GLUT-4 protein, NF-κB, TNF-α and
PI3K, compared with those of animals on a high-fat diet following
castration. High doses of testosterone resulted in a significant
decrease in the levels of IRS-1, AKT, GLUT-4, NF-κB, TNF-α and
PI3K, compared with animals fed a high-fat diet following
castration. Error bars represent the standard deviation.
*P<0.05, group 2 compared with group 1;
#P<0.05, group 3 compared with group 2;
ΔP<0.05, group 4 compared with group 2. Group 1,
control; group 2, high-fat-diet + castration; group 3;
high-fat-diet + castration + low-dose testosterone; group 4,
high-fat-diet + castration + high-dose testosterone; IRS-1, insulin
receptor substrate-1; GLUT-4, glucose transporter type 4; TNF-α
tumor necrosis factor-α; NF-κB, nuclear factor-κB; PI3K,
phosphoinositide 3-kinase. |
RT-qPCR
As shown in Fig. 7,
the mRNA expression levels of IRS-1, AKT and GlUT-4 in group 2 were
lower than those in the normal control. Following exposure to
low-dose testosterone, the mRNA expression levels of IRS-1, AKT and
GLUT-4 in group 3 were markedly increased, compared with those in
group 2. However, the mRNA levels of IRS-1, AKT and GLUT-4 in group
3 remained lower than those in the normal control group. Exposure
to a high dose of testosterone resulted in significant decreases in
the mRNA levels of IRS-1, AKT and GLUT-4 in group 4, compared with
group 3. Furthermore, upregulation in the mRNA levels of NF-κB,
TNF-α and PI3K were observed in group 2, compared with the normal
control. A further increase was observed in the mRNA expression
levels of NF-κB, TNF-α and PI3K following exposure to high-dose
testosterone, compared with group 2. Whereas, low-dose testosterone
treatment caused a decrease in the mRNA levels of NF-κB, TNF-α and
PI3K mRNA in group 3. This indicated that the loss of testosterone
induced disorder of the PI3K signaling pathway, and contributed to
inflammation. A low dose of testosterone may alleviate disorders of
PI3K and inflammation, whereas, a high dose of testosterone may
aggravate insulin resistance and inflammation.
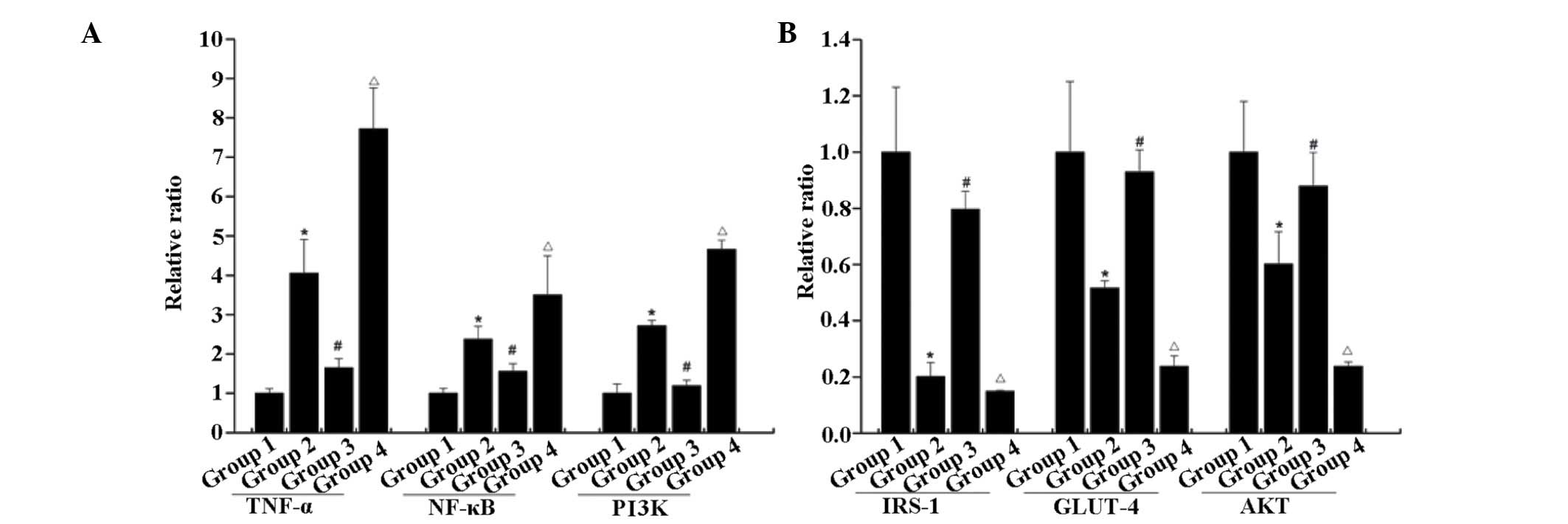 | Figure 7mRNA levels of (A) NF-κB, TNF-α and
PI3K, and (B) IRS-1, AKT and GLUT-4. Compared with the rats
subjected to castration and fed a high-fat diet, low-dose
testosterone induced upregulation of the mRNA levels of IRS-1, AKT
and GLUT-4, and downregulation of the mRNA levels of NF-κB, TNF-α
and PI3K. However, high-dose testosterone resulted in a significant
decrease in the levels of IRS-1, AKT and GLUT-4, and a significant
increase in the mRNA levels of NF-κB, TNF-α and PI3K, compared with
the low dose group. Error bars represent the standard deviation.
*P<0.05, group 2 compared with group 1;
#P<0.05, group 3 compared with group 2;
ΔP<0.05, group 4 compared with group 2. Group 1,
control; group 2, high-fat-diet + castration; group 3;
high-fat-diet + castration + low-dose testosterone group 4,
high-fat-diet + castration + high-dose testosterone; IRS-1, insulin
receptor substrate-1; GLUT-4, glucose transporter type 4; TNF-α
tumor necrosis factor-α; NF-κB, nuclear factor-κB; PI3K,
phosphoinositide 3-kinase. |
Discussion
Testosterone, the most abundant androgen in males,
is synthesized and secreted by leydig cells and regulated by
pituitary-derived luteinizing hormone (15). To date, testosterone has been used
in clinical practice due to its biological properties in the
maintenance of normal sexual differentiation, puberty development
and anabolism (16). In the
present study, the roles of testosterone in glucolipid metabolism
and cardiovascular diseases were investigated.
In the early stage of puberty, testosterone is
regularly secreted by the testis under the stimulation of
luteinizing hormone. The association between endogenous androgen
and lipids is complex as it involves various factors, including as
age, gender, body mass index and waist-hip ratio. For example,
Botella-Carretero et al found that the plasma levels of
testosterone of obese patients were higher following bariatric
surgery, compared with baseline levels (17). In addition, a linear trend towards
lower TC, low triglycerides and higher HDL-C have been reported
with the increase of serum testosterone (18). However, other studies have shown
that testosterone replacement induces a decrease in plasma HDL and
an increase in TC (19,20). In the present study, testosterone
was negatively correlated with TC and non-HDL, and was positively
correlated with HDL. Adverse effects were observe from the
supraphysiological testosterone concentration on plasma lipids. On
this basis, an appropriate dose of testosterone is required in
clinical practice.
Previously, testosterone was considered to be
associated with the occurrence of coronary heart disease (21). However, recent data has revealed
that testosterone is negatively correlated with the occurrence of
coronary heart disease (22). In
addition, testosterone is important in anti-atherosclerosis. It is
known that the vascular endothelium is the barrier between
circulating blood and vascular smooth muscle, and it is the major
component of endocrine organs. It has been reported to be involved
in the synthesis and secretion of several vasoactive substances
associated with the regulation of vasomotor function. Testosterone
can promote the secretion of nitric oxide from vascular endothelial
cells, which affects vasomotor function (23). The results of the present study
revealed that testosterone alleviated injury to the morphology of
the thoracic aorta induced by hypoandrogenism.
Increasing attention has been focussed on the roles
of insulin resistance in metabolic syndrome (22). For example, epidemiological data
indicates that the incidence of type 2 diabetes in males is higher
than in females, which may be affected by endogenous hormones
(24). Increasing evidence has
revealed that hypotestosteronemia is associated with increased
risks of developing metabolic syndrome and diabetes, as well as
insulin resistance (25). Previous
studies have shown that testosterone replacement can ameliorate the
pathological components of metabolism syndrome, however, adverse
effects may occur in the presence of excessive administration
(26,27). To investigate the potential
mechanism underlying how testosterone is involved in insulin
resistance, the present study investigated the activity of
PI3K/AKT, a major component in the insulin signaling pathway for
glucose transport. In the present study, rats subjected to
castration exhibited upregulated expression of PI3K and
down-regulated expression levels of IRS-1, GLUT-4 and AKT. In the
animals treated with testosterone, the levels of IRS-1, GLUT-4 and
AKT were upregulated, together with downregulation in the levels of
NF-κB and TNF-α. On this basis, the present study hypothesized that
testosterone may regulate glucolipid metabolism through modulation
of the PI3K signaling pathway.
In conclusion, the present study demonstrated that
castration induced marked disorder of glucolipid metabolism and
vascular injuries in male pubescent rats. Low-dose testosterone
replacement treatment ameliorated the damage caused by castration
via the PI3K/AKT signaling pathway. However, high-dose testosterone
induced severe adverse effects, indicating an appropriate dose of
testosterone is necessary. Testosterone deficiency and overdose
induced disorder of glucolipid metabolism and vascular injuries in
male pubescent rats. Thus, the dosage of testosterone used must be
appropriate for the treatment of patients with testosterone
deficiency and testosterone overdose should be avoided.
Acknowledgments
This study was supported by The Neurological
Institute of Tianjin Medical University General Hospital and the
Tianjin High Education Development Fund (grant no. 20140126).
References
|
1
|
Karoutsou E and Polymeris A: Environmental
endocrine disruptors and obesity. Endocr Regul. 46:37–46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boodai SA, Cherry LM, Sattar NA and Reilly
JJ: Prevalence of cardiometabolic risk factors and metabolic
syndrome in obese Kuwaiti adolescents. Diabetes Metab Syndr Obes.
7:505–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kursawe R and Santoro N: Metabolic
syndrome in pediatrics. Adv Clin Chem. 65:91–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Özer S, Yılmaz R, Özlem Kazancı N,
Sönmezgöz E, Karaaslan E, Altuntaş B and Emre Kuyucu Y: Higher HDL
levels are a preventive factor for metabolic syndrome in obese
Turkish children. Nutr Hosp. 31:307–312. 2014.
|
|
5
|
Denzer C, Thiere D, Muche R, Koenig W,
Mayer H, Kratzer W and Wabitsch M: Gender-specific prevalences of
fatty liver in obese children and adolescents: Roles of body fat
distribution, sex steroids and insulin resistance. J Clin
Endocrinol Metab. 94:3872–3881. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shelton JB and Rajfer J: Androgen
deficiency in aging and metabolically challenged men. Urol Clin
North Am. 39:63–75. 2012. View Article : Google Scholar
|
|
7
|
Dhindsa S, Prabhakar S, Sethi M,
Bandyopadhyay A, Chaudhuri A and Dandona P: Frequent occurrence of
hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol
Metab. 89:5462–5468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moriarty-Kelsey M, Harwood JE, Travers SH,
Zeitler PS and Nadeau KJ: Testosterone, obesity and insulin
resistance in young males: Evidence for an association between
gonadal dysfunction and insulin resistance during puberty. J
Pediatr Endocrinol Metab. 23:1281–1287. 2010. View Article : Google Scholar
|
|
9
|
Janjgava S, Zerekidze T, Uchava L,
Giorgadze E and Asatiani K: Influence of testosterone replacement
therapy on metabolic disorders in male patients with type 2
diabetes mellitus and androgen deficiency. Eur J Med Res.
19:562014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sattler F, He J, Chukwuneke J, Kim H,
Stewart Y, Colletti P, Yarasheski K and Buchanan T: Testosterone
supplementation improves carbohydrate and lipid metabolism in some
older men with abdominal obesity. J Gerontol Geriatr Res.
3:10001592014.PubMed/NCBI
|
|
11
|
Yeap BB, Alfonso H, Chubb SA, Handelsman
DJ, Hankey GJ, Golledge J, Flicker L and Norman PE: Lower plasma
testosterone or dihydrotestosterone, but not estradiol, is
associated with symptoms of intermittent claudication in older men.
Clin Endocrinol (Oxf). 79:725–732. 2013.
|
|
12
|
Srinath R, Hill Golden S, Carson KA and
Dobs A: Endogenous testosterone and its relationship to preclinical
and clinical measures of cardiovascular disease in the
atherosclerosis risk in communities (ARIC) study. J Clin Endocrinol
Metab. 100:1602–1608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu J, Akishita M, Eto M, Ogawa S, Son BK,
Kato S, Ouchi Y and Okabe T: Androgen receptor-dependent activation
of endothelial nitric oxide synthase in vascular endothelial cells:
Role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology.
151:1822–1828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li F, Li L, Zhong Y, Xie Q, Huang J, Kang
X, Wang D, Xu L and Huang T: Relationship between LTR methylation
and gag expression of HIV-1 in human spermatozoa and sperm-derived
embryos. PLoS One. 8:e548012013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beaudin G: Men and low testosterone.
Diabetes Self Manag. 32:24–27. 2015.PubMed/NCBI
|
|
16
|
Jockenhövel F: Testosterone therapy -
what, when and to whom? Aging Male. 7:319–324. 2004. View Article : Google Scholar
|
|
17
|
Botella-Carretero JI, Balsa JA,
Gómez-Martin JM, Peromingo R, Huerta L, Carrasco M, Arrieta F,
Zamarron I, Martin-Hidalgo A and Vazquez C: Circulating free
testosterone in obese men after bariatric surgery increases in
parallel with insulin sensitivity. J Endocrinol Invest. 36:227–232.
2013.
|
|
18
|
Zhang N, Zhang H, Zhang X, Zhang B, Wang
F, Wang C, Zhao M, Yu C, Gao L, Zhao J and Guan Q: The relationship
between endogenous testosterone and lipid profile in middle-aged
and elderly Chinese men. Eur J Endocrinol. 170:487–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu YQ, Wang XH, Xu D, Peng L, Cheng LF,
Huang MK, Huang ZJ and Zhang Y: A multicenter contraceptive
efficacy study of injectable testosterone undecanoate in healthy
Chinese men. J Clin Endocrinol Metab. 88:562–568. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gårevik N, Rane A, Björkhem-Bergman L and
Ekström L: Effects of different doses of testosterone on
gonadotropins, 25-hydroxyvitamin D3 and blood lipids in healthy
men. Subst Abuse Rehabil. 5:121–127. 2014. View Article : Google Scholar
|
|
21
|
Rosano GM, Sheiban I, Massaro R, Pagnotta
P, Marazzi G, Vitale C, Mercuro G, Volterrani M, Aversa A and Fini
M: Low testosterone levels are associated with coronary artery
disease in male patients with angina. Int J Impot Res. 19:176–182.
2007. View Article : Google Scholar
|
|
22
|
Shores MM, Matsumoto AM, Sloan KL and
Kivlahan DR: Low serum testosterone and mortality in male veterans.
Arch Intern Med. 166:1660–1665. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duckles SP and Miller VM: Hormonal
modulation of endothelial NO production. Pflugers Arch.
459:841–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lapauw B, Ouwens M, 't Hart LM, Wuyts B,
Holst JJ, T'Sjoen G, Kaufman JM and Ruige JB: Sex steroids affect
triglyceride handling, glucose-dependent insulinotropic polypeptide
and insulin sensitivity: A 1-week randomized clinical trial in
healthy young men. Diabetes Care. 33:1831–1833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kupelian V, Page ST, Araujo AB, Travison
TG, Bremner WJ and McKinlay JB: Low sex hormone-binding globulin,
total testosterone and symptomatic androgen deficiency are
associated with development of the metabolic syndrome in nonobese
men. J Clin Endocrinol Metab. 91:843–850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jones TH, Arver S, Behre HM, Buvat J,
Meuleman E, Moncada I, Morales AM, Volterrani M, Yellowlees A,
Howell JD and Channer KS; TIMES2 Investigators: Testosterone
replacement in hypogonadal men with type 2 diabetes and/or
metabolic syndrome (the TIMES2 study). Diabetes Care. 34:828–837.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guay AT: The emerging link between
hypogonadism and metabolic syndrome. J Androl. 30:370–376. 2009.
View Article : Google Scholar
|