Introduction
Spinal cord injury causes severe neurological
dysfunction, which affects patients and their families as it
requires substantial long-term healthcare expenditure and leads to
permanent deprivation in quality of life (1). Following spinal cord injury, neural
stem cells (NSCs) at the site of damage proliferate and
differentiate into several neural cell types, including neurons,
astrocytes and oligodendrocytes, which are important in
cell-replacement therapy for neurological dysfunction (2). NSCs are considered to be a potential
cell therapy for reconstruction and regeneration of the brain and
spinal cord following injury (3).
The transplantation of NSCs into the injured sites can potentially
replace lost cells and become involved in anatomical regeneration
(4,5). However, which of the cell types the
NSCs differentiate into, and the proportion, determines patient
prognosis, and the poor proliferation rate of NSCs has limited the
practical use of NSC-based therapy (4).
There is increasing evidence that endothelial
progenitor cells (EPCs) contribute to angiogenesis by promoting
migration and proliferation (6).
In addition, EPCs contribute directly and indirectly to
neovascularization, and are incorporated into injured vessels to
become mature endothelial cells in response to tissue injury, which
improves clinical outcomes in patients with ischemic disease
(6,7). It has been shown that patients with
spinal cord injury have high levels of circulating bone
marrow-derived EPCs, and chemoattractive and proangiogenic
cytokines in their blood within the first day of illness (8). A close association between NSCs and
vascular cells in the adult central nervous system has been shown
in the 'vascular niche' (9). A
previous study demonstrated that the transplantation of EPCs
promotes astrogliosis and functional recovery following spinal cord
injury (10). However, the
behavior of EPCs on NSCs and their underlying mechanism remain to
be fully elucidated.
The present study aimed to determine whether
co-culture with bone marrow-derived EPCs affects spinal
cord-derived NSC proliferation and differentiation. The data
obtained in the present study is the first, to the best of our
knowledge, to demonstrate that co-culture with bone marrow-derived
EPCs promoted the proliferation and differentiation of spinal
cord-derived NSC, at least in part, via modulation of the
wingless-type MMTV integration site family, member 3a
(Wnt3a)/β-catenin signaling pathway. In conclusion, the results
provided novel molecular insight into EPC-mediated neurogenesis
during the repair of spinal cord injury.
Materials and methods
Animals
All animal procedures were approved by the Animal
Ethics Committee of the Institutional Animal Care and Use Committee
of Anhui Medical University (Hefei, China), in accordance with the
Guide for the Care and Use of Laboratory Animals in China (11). Bone marrow progenitor cells were
harvested from male Sprague-Dawley (SD) rats (90–120 g; n=25). NPCs
were harvested from newborn SD rats (5–7 days old; n=60). They were
kept in a specific pathogen free environment at 22±1°C under a
14/10 h light-dark cycle with free access to food and access to
water under controlled environmental conditions.
Materials
Endothelial basal medium (EBM)-2 and EGM-2 Single
Quots, containing 10 ml fetal bovine serum (FBS), 0.2 ml
hydrocortisone, 2 ml human fibroblast growth factor (hFGF)-B, 0.5
ml vascular endothelial growth factor (VEGF), 0.5 ml R3-IGF-1, 0.5
ml ascorbic acid, 0.5 ml hEGF, 0.5 ml GA-1000 and 0.5 ml heparin,
were purchased from Clonetics (San Diego, CA, USA). Dulbecco's
modified Eagle's medium/F12 (DMEM/F12) medium, FBS, B27 supplement
and L-glutamic acid (L-glutamine) were purchased from Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Basic (b) FGF and
epidermal growth factor (EGF) were purchased from Peprotech, Inc.
(Rocky Hill, NJ, USA). The rat bone marrow lymphocyte isolation kit
was purchased from Tianjin Hao Yang Biological Products Technology
Co., Ltd. (Tianjin, China). Rat fibronectin was purchased from Gene
Operation, Inc. (Ann Arbor, MI, USA); DiI-labeled acetylated
low-density lipoprotein (Di1-Ac-LDL) and Lipofectamine 2000 were
purchased from Invitrogen; Thermo Fisher Scientific, Inc.
Fluorescein isothiocyanate UEA-1 (FITC-UEA-1), and poly-lysine were
purchased from Sigma-Aldrich; Thermo Fisher Scientific, Inc.
Antibodies against VEGF receptor (VEGFR)-2, β-tubulin III, β-actin
and nestin were purchased from Santa Cruz Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Antibodies against Wnt3a, phosphorylated
(p)-glycogen synthase kinase 3β (GSK-3β), p-β-catenin, GSK-3β and
β-catenin were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Antibody against CD133 was purchased from
Biorbyt, Ltd. (San Fransisco, CA, USA). Rabbit anti-glial
fibrillary acidic protein antibodies were purchased from Abcam
(Cambridge, UK). The recombinant plasmid, pEGFP-short hairpin (sh)
RNA-wnt3a, was purchased from Hefei Hao Xiang Biological Technology
Co., Ltd (Anhui, China).
Isolation and culture of bone
marrow-derived EPCs
The SD rats (90–120 g) were sacrificed with an
excess of 10% chloral hydrate anesthesia, following which both
femurs and tibias were surgically dissected. The bone marrow
mononuclear cell population was isolated using a commercially
available kit (R&D systems, Inc., Minneapolis, MN, USA),
according to the manufacturer's protocol. The bone marrow
mononuclear cells were then re-suspended in EBM-2 complete medium.
To isolate the EPCs, the bone marrow mononuclear cells
(5×105 cells/well) were plated on bovine
fibronectin-coated 24-well plates. The plates were incubated in 5%
CO2 at 37°C. The medium was replaced every 3 days until
the first passage cells were ~70% confluent (14 days). The EPCs
were identified by the expression of cell surface markers, CD133
and VEGFR-2, using fluorescence microscopy. In addition, the uptake
of fluorescent Dil-ac-LDL was evaluated using confocal microscopy.
The binding of UEA-1 was determined using FITC-conjugated
UEA-1.
Isolation and culture of spinal
cord-derived NSCs
Newborn SD rats (5–7 days old) were sacrificed by
cervical dislocation. The thoracolumbar spinal cord, stripped of
soft meninges and blood vessels, were placed in ice-cold DMEM/F12
for further dissection. The spinal cord was cut it into sections
measuring 1 mm3 with ophthalmic scissors, and filtrated
through a 200 mesh cell sieve following repeated pipetting turbid
suspension. The dissociated cell suspension was centrifuged at 800
x g for 5 min at room temperature, and the pellet was seeded
(1×106 cells/ml) into flasks containing DMEM/F-12 with
2% B27, 20 ng/ml EGF, 10 ng/ml bFGF and 0.6 mg/ml L-glutamine. The
flasks were incubated in 5% CO2 at 37°C. After 48 h, the
medium was replaced the remove the non-adherent cells, and was
replaced every 3 days thereafter. All the cells used in the
experiments were obtained from passages 3–10. The NSCs were
identified by positive staining for nestin under a light microscope
(Carl Zeiss Inc., Jena, Germany), a molecular marker for
multi-potent NSCs (12), which is
required for the proliferation and self-renewal of NSCs.
Plasmid transfection
The EPCs were transfected with the mouse wnt3a
pEGFP-shRNA plasmid using lipofectamine 2000 and Opti-MEM medium
(Invitrogen; Thermo Fisher Scientific, Inc.). Briefly, the EPCs
were plated on 6-well plates at 70–80% confluence 24 h prior to
transfection. The Wnt3a pEGFP-shRNA plasmid (2 µg),
Lipofectamine 2000 (2 µl) and Opti-MEM were mixed and
incubated at room temperature for 5 min. The plasmid-oligofectamine
complexes were added to the cells for 24 h and the medium was
replaced with fresh serum-free EGM-2 following transfection for 72
h. Transfection efficiency was determined by the percentage of
GFP-positive cells. Knockdown of wnt3a was assessed using western
blot analysis.
Immunofluorescence assessment
For the in vitro experiments, the EPCs or
NSCs were grown on glass slides in 6-well plates. The cells were
fixed in 4% paraformaldehyde for 30 min at room temperature.
Immunostaining was performed using mouse monoclonal anti-VEGR2
(1:150; Biorbyt, Ltd.), polyclonal rabbit anti-CD133 (1:150) and/or
polyclonal rabbit anti-nestin (1:150; Sigma-Aldrich; Thermo Fisher
Scientific, Inc.) and FITC-conjugated anti-rabbit or anti-mouse IgG
(1:200; Sigma-Aldrich; Thermo Fisher Scientific, Inc.) and tetra
methyl rhodamyne iso-thiocyanate anti-rabbit or mouse IgG (1:200;
Sigma-Aldrich; Thermo Fisher Scientific, Inc.) secondary antibodies
were utilized, and counterstaining for nuclei was performed using
2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride.
Immunofluorescence was visualized using a fluorescent microscope
(Olympus Corporation, Tokyo, Japan). The results were based on
three independent analyses.
To determine the uptake of Dil-Ac-LDL and binding of
FITC-UEA-1, the EPCs were incubated with Dil-Ac-LDL overnight at
37°C, and fixed with 4% paraformaldehyde for 20 min. The cells were
then incubated with FITC-UEA-1 for 1 h at 37°C, and examined under
a fluorescent microscope (Olympus Corporation).
EPC/NSC co-culture assay
To investigate the effect of EPC co-culture on the
differentiation and proliferation of NSCs, an EPC/NSC co-culture
assay was performed, as described in a previous report (13) with minor modifications. The EPCs
and NSCs were separately seeded into 24-well (2×105
NSCs/well; 1×105 EPCs/insert) or 6-well
(6×105 NSCs/well; 5×105 EPCs/insert)
Transwell plates (0.4 µm pore-size; Corning Costar, St
Louis, MO, USA). The co-culture system was maintained in culture
medium with DMEM/F12+ serum-free EBM-2 (1:1) in 5% CO2
at 37°C. Following co-culture for 7 days, the cells obtained from
the differentiated NSCs were fixed and labeled with the neuronal
specific marker, β-tubulin III (14), and neuronal proteins were extracted
for western blot analysis. Neuronal viability was measured using a
3-(4,5-dimethyl-thiazol-2-yl)-2,5-di-phenyl-tetrazolium bromide
(MTT) assay.
Cell proliferation assay
Following co-culture with EPCs for different periods
of time, the viability of the NSCs was determined using an MTT Cell
Proliferation and Cytotoxicity Assay kit (Beyotime Institute of
Biotechnology, Haimen, China), according to the manufacturer's
protocol. The cell viability was measured at 490 nm with a
microplate reader (Tecan M200; Tecan Austria GmbH, Salzburg,
Austria). NSC proliferation was measured using a BrdU cell
proliferation kit (Roche, Mannheim, Germany), according to the
manufacturer's protocol. The cell proliferation was measured at 450
nm with a microplate reader (Tecan M200; Tecan Austria GmbH).
Measurement of the numbers of
neurospheres
In order to investigate the proliferation potential
of the NSCs in the presence and absence of EPC co-culture, a
neurosphere growth kinetics assay was performed, as described
earlier. The NSC culture was passaged by gentle trituration, and
the resulting single-cell suspension of NSCs was replated in a
12-well plate at a density of 5×104 cells/well for
co-culture with or without EPCs. The number and diameter of the
neurospheres were measured in all groups using an inverted
phase-contrast microscope (Leica, Mannheim, Germany) and analyzed
using Image J software (v1.50a, National Institutes of Health,
Bethesda, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the differentiated NSCs
using TRIzol (Takara Biotechnology Co., Ltd., Dalian, China). The
total RNA was isolated and purified using an RNeasy minikit (Takara
Biotechnology Co., Ltd.) with the addition of RNase-free DNase I
(Takara Biotechnology Co., Ltd.). The total RNA (1 µg) was
reverse transcribed using a one-step RT kit (Takara Biotechnology
Co., Ltd.), and the resulting complementary DNA was used as a PCR
template for determining the messenger RNA (mRNA) expression levels
using a SYBR-Green Quantitative PCR kit (Takara Biotechnology Co.,
Ltd.) with the iCycler iQ system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was used as the housekeeping gene. Relative expression was
calculated using the ΔΔCq method (15). Quantification was performed using
standard curves derived from the expression of the gene relative to
that of GAPDH. The rat-specific primers for Wnt-3α, β-catenin and
GAPDH were as follows (16):
Wnt-3α, forward 5′-GCT ACT CGG CCT CCT GCT-3′ and reverse 5′-GGC
CAG AGA CGT GTA CTG CT-3′; β-catenin, forward 5′-GAC CAC AAG CAG
AGT GCT GA-3′ and reverse 5′-ACT CGG GTC TGT CAG GTG AG-3′; GAPDH,
forward 5′-AGG TTG TCT CCT GCG ACT TCA and reverse 5′-TGG TCC AGG
GTC CAG GGT TTC TTA CTC C-3′.
Western blot analysis
Western blot analysis was performed, as previously
described (17). Total lysis of
the cells was conducted with RIPA buffers (Thermo Fisher
Scientific, Inc.) and protein concentration was determined with a
bicichoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). Equal quantities (50 µg) of proteins were separated
and transferred onto polyvinylidine difluoride membranes. The
membranes were blocked with 5% non-fat dried milk, following which
the membranes were probed overnight at 4°C with the following
antibodies: Rabbit monoclonal anti-Wnt3a (Ab2721 1:1,000), rabbit
monoclonal anti-p-GSK-3β (Ab5558; 1:2,000), rabbit monoclonal
anti-p-β-catenin (Ab9561; 1:2,000), rabbit monoclonal anti-GSK-3β
(Ab12456; 1:2,000), rabbit monoclonal anti-β-catenin (Ab4176;
1:2,000) or mouse monoclonal anti-β-actin (Ab3700; 1:2,000) (all
from Abcam, Cambridge, MA, USA). This was followed by incubation
with either horseradish peroxidase-conjugated goat anti-rabbit
(ZB-2301) or anti-mouse antibody(ZB-2305) (1:5,000; Zhongshan
Golden Bridge Biotechnology, Beijing, China) for 2 h at room
temperature. Immunoreactive proteins were visualized using enhanced
chemiluminescence, and signal intensity was detected and quantified
using Alpha Imager (Alpha Innotech Corporation, San Leandro, CA,
USA).
Enzyme-linked immunosorbent (ELISA)
assay
The level of VEGF in the medium of the EPCs was
measured using a commercially available ELISA kit (R&D Systems
Europe, Ltd., Abingdon, UK), according to the manufacturer's
protocol. After 1, 3, 5, 7 and 14 days of culture, the cell
supernatants were collected to measure the levels of VEGF in the
medium. Each assay was repeated at least three times.
Statistical analysis
The results of the experimental investigations are
expressed as the mean ± standard error of the mean. Differences
between the mean values of multiple groups were analyzed using
one-way analysis of variance with Tukey's test for post-hoc
comparisons. All data analysis was performed with the use of
GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation and culture of bone
marrow-derived EPCs and spinal cord-derived NSCs
Bone marrow mononuclear cells were isolated by
density gradient centrifugation; the isolated mononuclear cells
were small and round in shape. After 24 h, a small number of
adherent cells appeared. After 7 days, a proportion of the
mononuclear cells had become spindle-shaped. On day 14, the nearby
colonies had fused with each other, exhibiting a larger cell
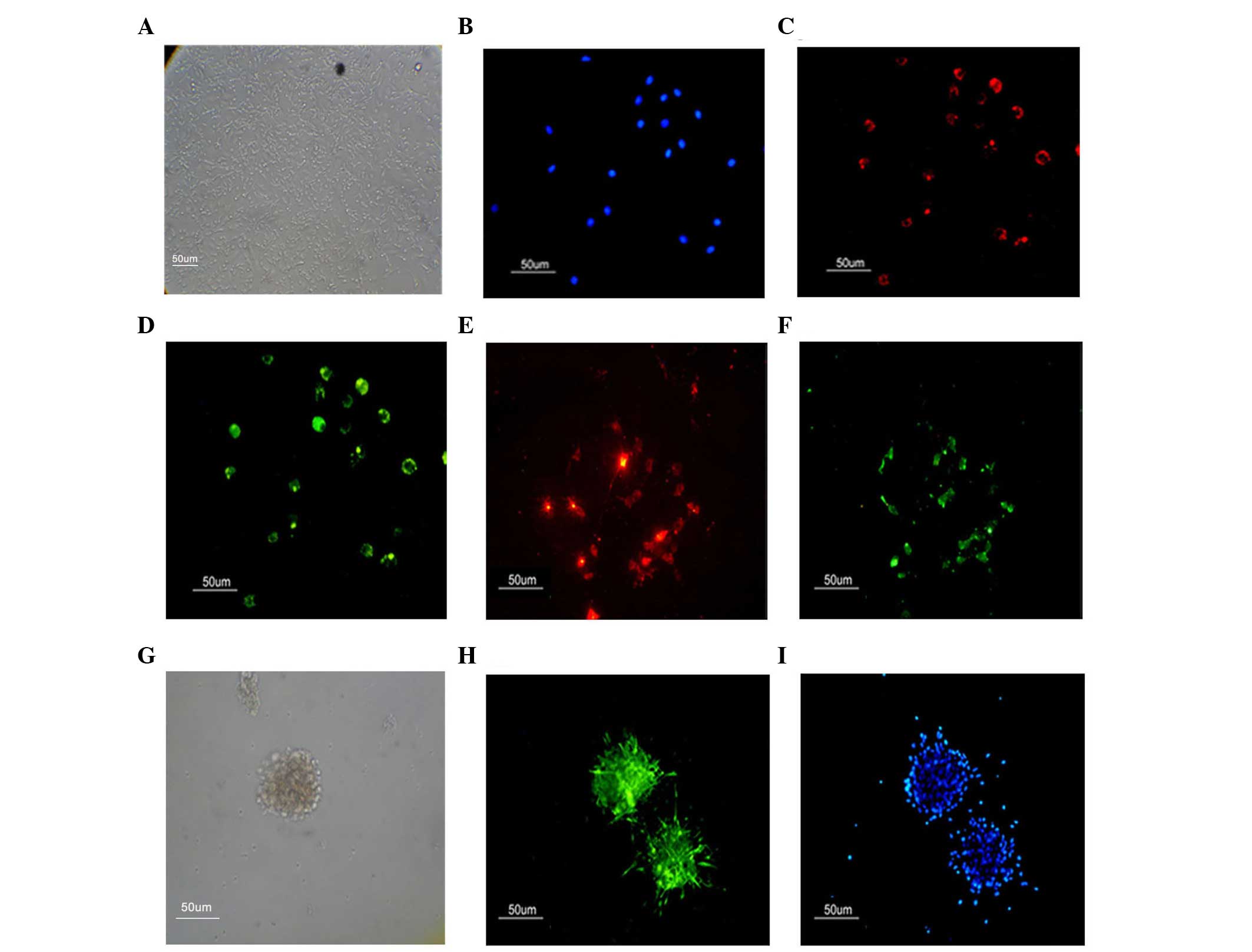
monolayer with a cobblestone-like morphology (Fig. 1A). Certain EPCs formed linear
cord-like structures during cultivation, which is consistent with a
previous report (18). The EPCs
were identified by positive staining for VEGFR-2, an endothelial
cell surface marker (Fig. 1C) and
CD133, a progenitor cell surface antigen (Fig. 1D) (19). The EPCs are further identified by
their ability to take up Dil-Ac-LDL and bind FITC-UEA-l (Fig. 1E and F).
A specific characteristic of spinal cord-derived
NSCs is the ability to self-renew. The ability to passage
neurospheres clonally is an indicator of self-renewal. In the
present study, only individual cells were observed 1 day
post-seeding, with no cell spheres observed. The assembly of the
neurospheres was slow and the spheres were relatively small. After
7–21 days of subculture, phase contrast microscopy indicated that
neurosphere formation had occurred (Fig. 1G). The neurospheres of the NSCs
were identified by positive staining for nestin (Fig. 1H).
Co-culture with EPCs promotes the
proliferation and differentiation of NPCs
To assess the effects of co-culture with EPCs on the
proliferation and viability of the NSCs, the NSCs were co-cultured
with EPCs for different time periods and their viability was
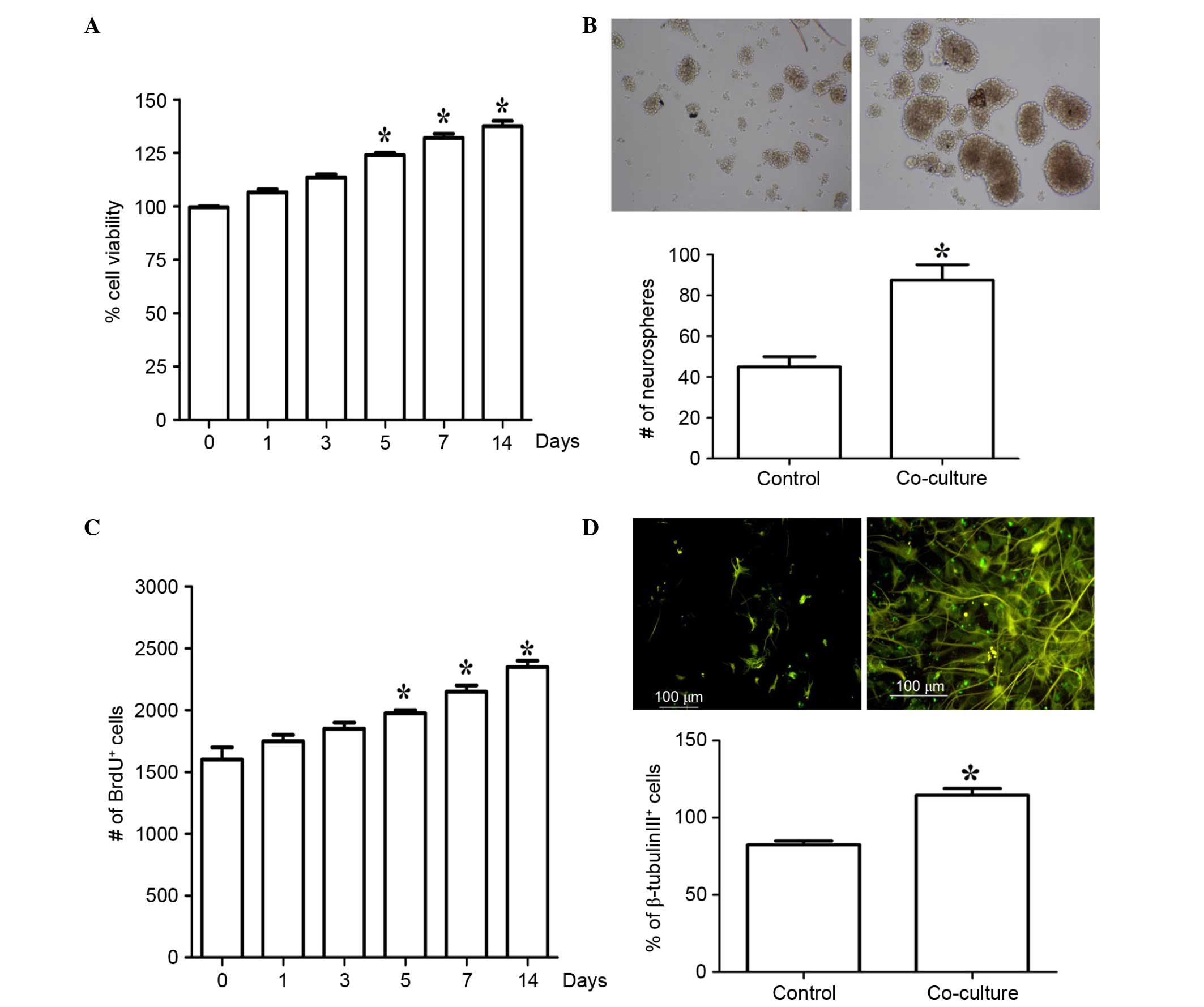
measured using an MTT assay. As shown in Fig. 2A, co-culture with EPCs
significantly increased the proliferation of the NSCs in a
time-dependent manner. The effects of co-culture with EPCs on NSC
proliferation were determined using a BrdU incorporation assay and
neurosphere growth kinetic assay. Following co-culture with EPCs
for 7 days, the number of neurospheres was significantly increased
(Fig. 2B). The co-culture with
EPCs was accompanied by a significant increase in the number of
BrdU+ cells, also in a time-dependent manner (Fig. 2C). In addition, co-culture with
EPCs for 7 days significantly induced differentiation, as evidenced
by the increase of β-tubulin III-positive cells (Fig. 2D). These results suggested that
co-culture with EPCs significantly induced NSC proliferation and
differentiation.
Co-culture with EPCs induces activation
of the Wnt3a/β-catenin pathway in NSCs
Previous studies have identified that the
Wnt/β-catenin pathway may be critical in the regulation of NSC
proliferation and differentiation (20,21).
Therefore, the present study examined the effects of co-culture
with EPCs on the gene and protein expression levels of the
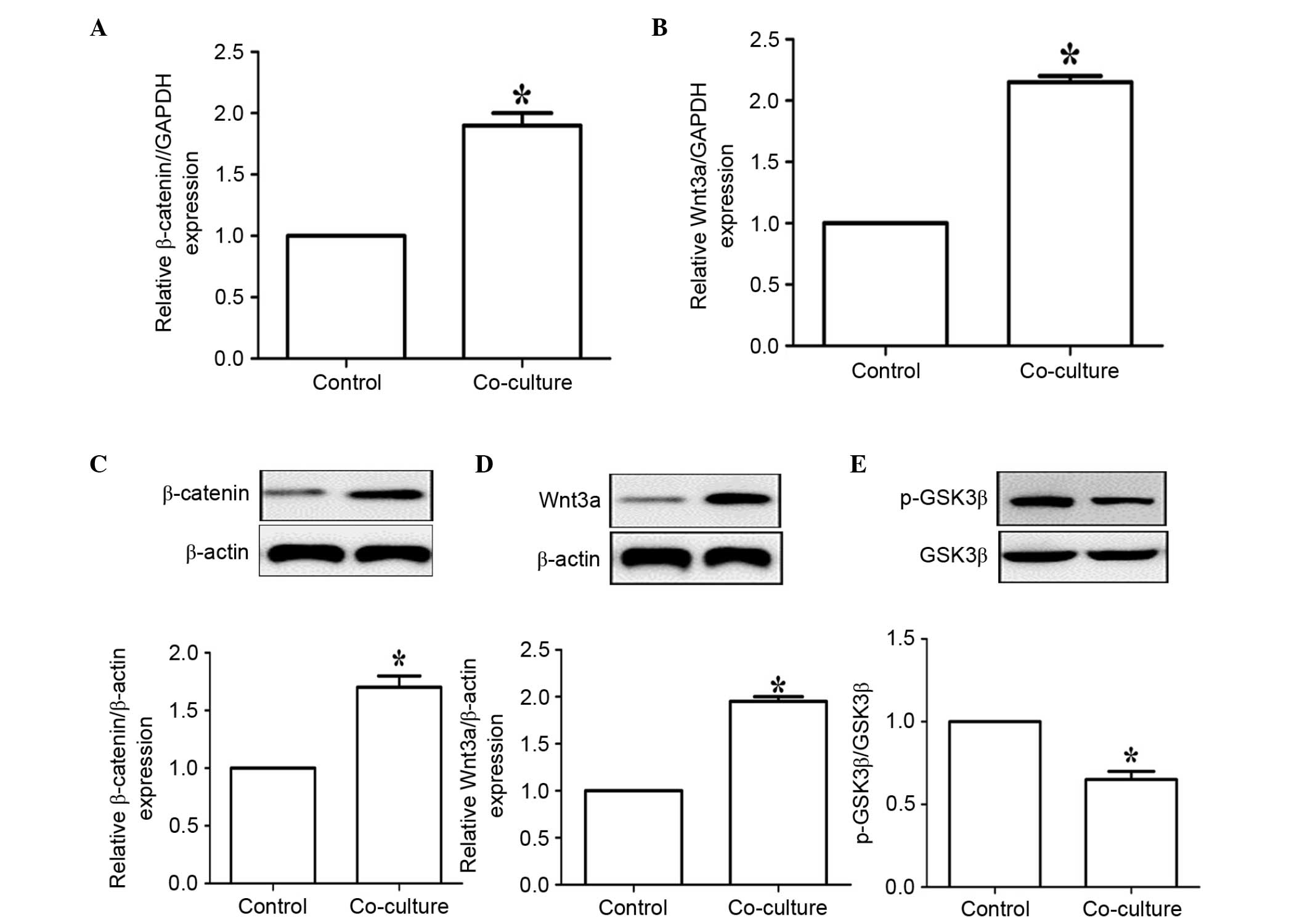
Wnt/β-catenin pathway in NSCs. The results of the RT-qPCR analysis
showed that co-culture with EPCs significantly enhanced the gene
expression levels of β-catenin and Wnt3a in the NSCs (Fig. 3A and B). The results of the western
blot analysis further confirmed that co-culture with EPCs
significantly increased the expression levels of β-catenin and
Wnt3a (Fig. 3C and D). Of note,
co-culture with EPCs also significantly reduced the phosphorylation
of GSK-3β (Fig. 3E). Thus,
co-culture with EPCs activated the Wnt/β-catenin pathway and
inhibited the activation of GSK-3β, which contributed to the
promotion of NSC proliferation and differentiation.
Wnt3a knockdown inhibits EPC
co-culture-mediated NSC proliferation and differentiation
NSCs are functionally characterized as cells with
the capacity to proliferate, self-renew and produce populous
progeny, which can differentiate into neurons, astrocytes and
oligodendrocytes (22–24). To determine whether Wnt3a is
essential in EPC co-culture-mediated NSC proliferation and
differentiation, genetic inactivation was performed via the
transfection of EPCs with Wnt3a shRNA. As shown in Fig. 4A, transfection with the Wnt3a shRNA
plasmid markedly reduced the expression of Wnt3a in the EPCs
(P<0.05). EPCs are one of source of angiogenic mediators
(25). To examine the effect of
Wnt3a knockdown on the secretory function of EPCs, the levels of
VEGF were examined in the EPCs following Wnt3a knockdown. The
results showed that Wnt3a knockdown had no effect on the production
of VEGF in the EPCs following culture for different time periods
(Fig. 4B). However, Wnt3a
knockdown in the EPCs significantly reduced EPC-mediated NSC
proliferation, as demonstrated by the BrdU incorporation assay,
following co-culture for 7 days (Fig.
4C). Similarly, Wnt3a knockdown in the EPCs significantly
decreased the number of NSC neurospheres in the co-culture system,
detected using a neurosphere growth kinetic assay (Fig. 4D). In addition, following Wnt3a
knockdown in the EPCs, β-tubulin III-positive staining was reduced
in the co-culture system (Fig.
4E). Collectively, these results suggested that Wnt3a is
critical for EPC-mediated NSC proliferation and differentiation in
this co-culture system.
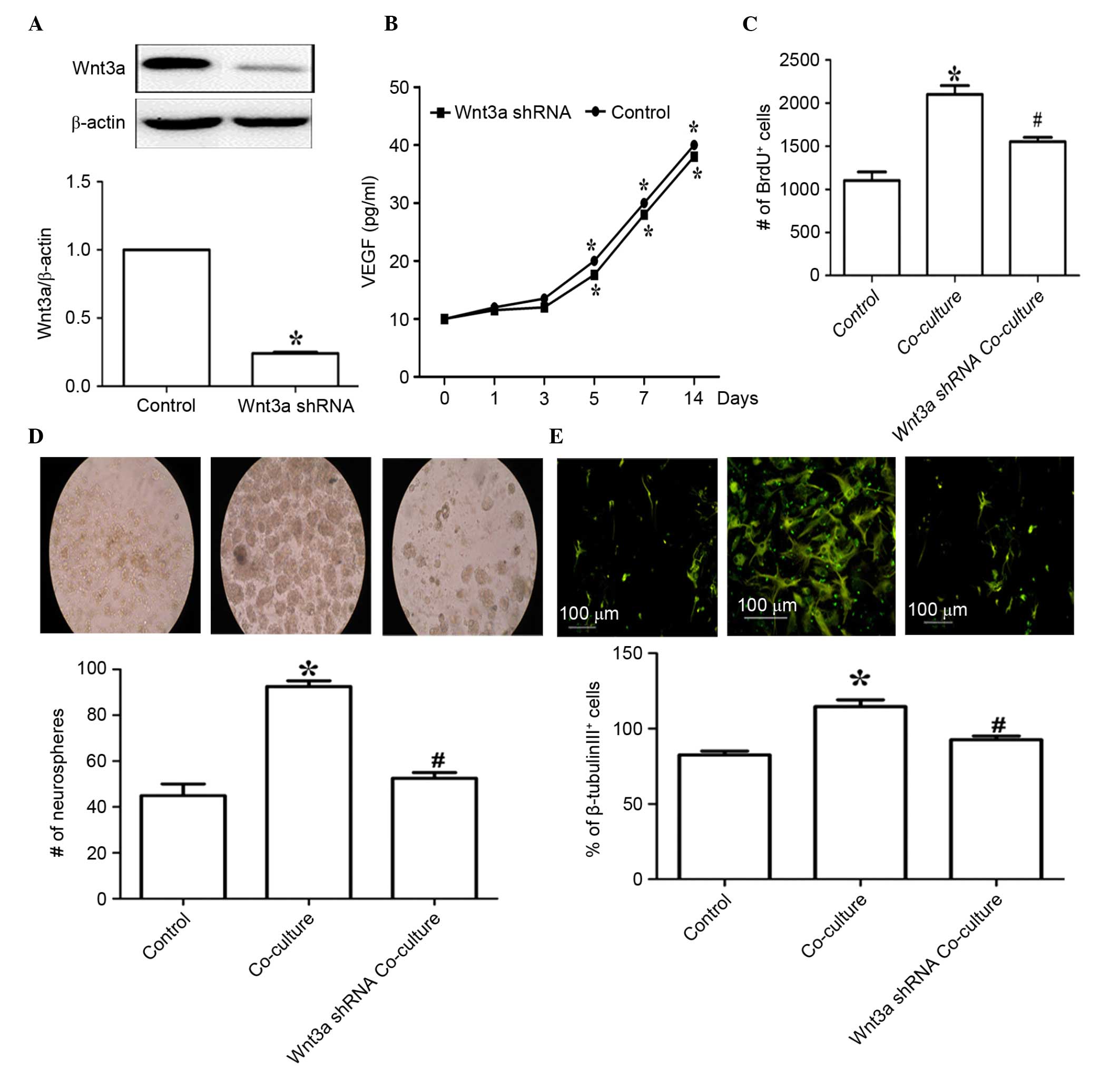 | Figure 4Wnt3a knockdown inhibits EPC
co-culture-mediated NSC proliferation and differentiation. (A)
Following treatment with a Wnt3a shRNA plasmid, western blot and
densitometric analyses were performed to determine the level of
Wnt3a, β-actin was used as loading control. (B) Following Wnt3a
knockdown for the indicated time periods, the levels of VEGF
released in the EPCs were determined. After 7 days co-culture with
the EPCs, the (C) proliferation of the NSCs, the (D) number of
neurospheres (magnification, ×100) and (E) differentiation of NSCs
were determined. Scale bar=100 µm. Values are expressed as
the mean ± standard error of the mean (n=3). *P<0.05,
vs. NSCs alone; #P<0.05, vs. EPC co-culture. EPCs,
endothelial progenitor cells; NSCs, neural stem cells; Wnt3a,
wingless-type MMTV integration site family, member 3a; shRNA, short
hairpin RNA. |
Wnt3a knockdown inhibits the activation
of β-catenin signaling in the co-culture system
To further assess the function of Wnt3a signaling,
the NSCs were co-cultured with EPCs with Wnt3a knockdown. As shown
in Fig. 5A and B, the cellular
gene and protein levels of β-catenin were significantly increased
in the NSCs co-cultured with EPCs for 7 days (P<0.05), and were
significantly decreased in the co-culture system containing EPCs
with Wnt3a knockdown. Wnt3a knockdown in the EPCs also markedly
reduced the phosphorylation and nuclear translocation of β-catenin
(Fig. 5C and D). Wnt3a knockdown
in the EPCs markedly enhanced the phosphorylation of GSK-3β
following co-culture for 7 days (Fig.
5E). These results demonstrated that Wnt3a/β-catenin signaling
was responsible for the EPC-mediated NSC proliferation and
differentiation.
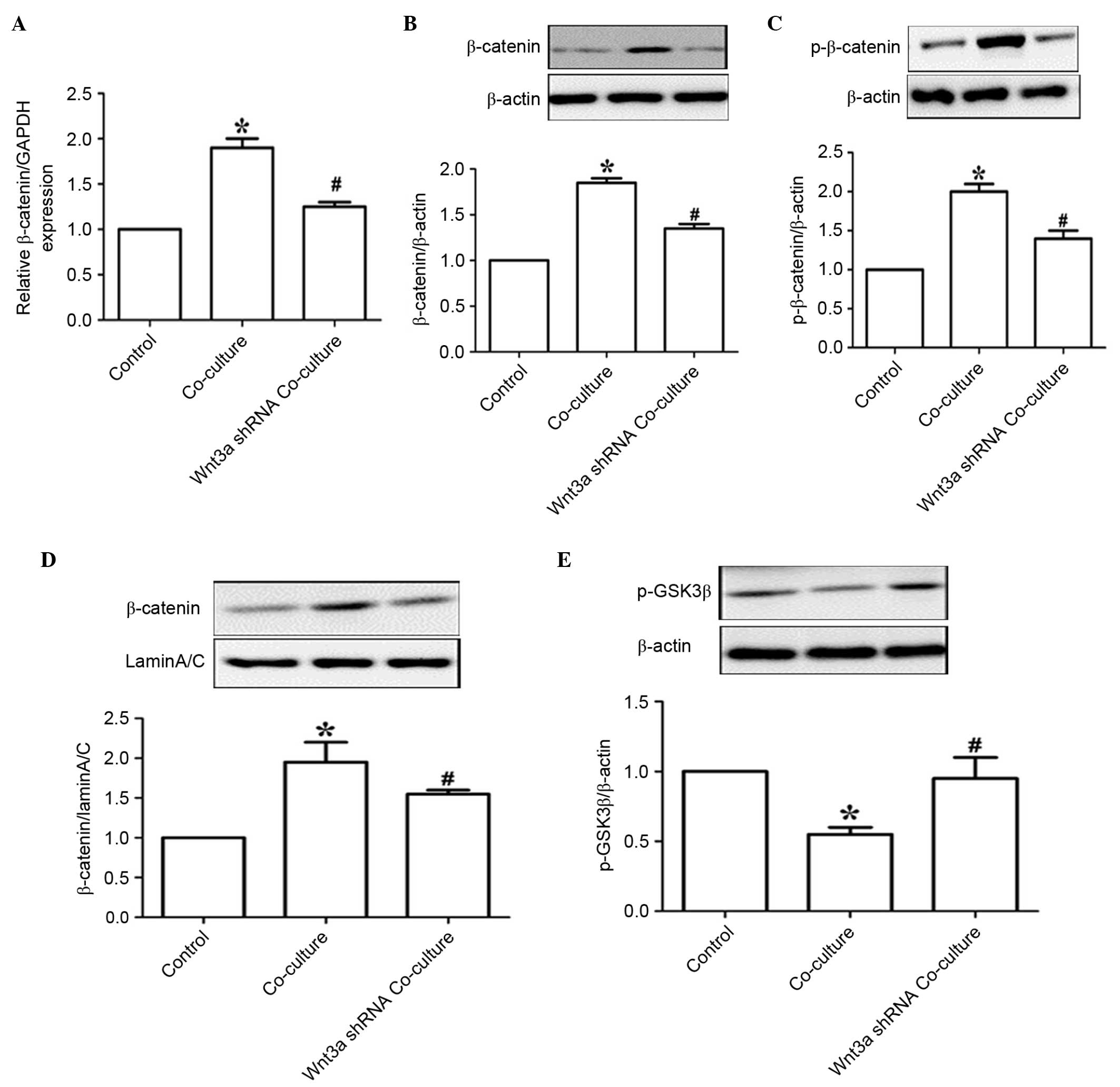 | Figure 5Wnt3a knockdown inhibits β-catenin
signaling activation in the co-culture system. (A) Following
co-culture with EPCs for 7 days, reverse transcription-quantitative
polymerase chain reaction analysis was used to determine the mRNA
levels of β-catenin. GAPDH was used as the housekeeping gene.
Western blot analysis was used to determine the protein levels of
(B) β-catenin, (C) p-β-catenin, (D) nuclear β-catenin and (E)
p-GSK-3β, β-actin was used as a loading control. Values are
expressed as the mean ± standard error of the mean (n=3).
*P<0.05, vs. NSCs alone; #P<0.05, vs.
EPC co-culture. EPCs, endothelial progenitor cells; NSCs, neural
stem cells; GSK3β, glycogen synthase kinase 3β; p-, phosphorylated;
shRNA, short hairpin RNA. |
Discussion
The present study demonstrated that co-culture with
EPCs promoted spinal cord-derived NSC proliferation and
differentiation, and that these effects were not observed following
Wnt3a knockdown in the EPCs. The data further demonstrated that
Wnt3a was critical for EPC-mediated NSC proliferation and
differentiation through modulation of the β-catenin and GSK-3β
signaling pathway.
Spinal cord injury is a serious threat to human
health and quality of life. One option for treating spinal tissue
damage includes the replacement of lost neuronal cells, and NSC
transplantation therapy offers the potential to promote the repair
of neuronal loss and functional recovery of spinal cord-transected
rats (26,27), as they have the capacity to
differentiate into neurons and oligodendrocytes. However, the
survival of transplanted NSCs is the primary problem associated
with this therapy. The microenvironment may also affect the
mechanism by which transplanted NSCs induce proliferation and
differentiation to promote repair following spinal cord injury.
Previous studies have shown that co-culture with certain cells,
including glial cells, olfactory ensheathing cells (28) and mesenchymal stromal cells
(29) can promote the neurogenesis
of NSCs. Vascular endothelial cells also promote NSC self-renewal
and neurogenesis (30).
Endothelial cells secrete numerous factors, several of which have
been implicated in regulating the germinal niche (31). In the present study, co-culture
with EPCs was found to significantly induce cell proliferation and
the expression of nestin in the neurospheres, suggesting that
co-culture with EPCs may have an effect on the microenvironment and
enhance the proliferation of NSCs. In addition, the co-culture with
EPCs markedly induced the differentiation of NSCs, which was
consistent with previous reports that endothelial cells induce the
differentiation of N0SCs into neurons and astrocytes (32,33).
Taken together, the results of the present study indicated that
co-culture with EPCs may stimulate NSC proliferation and
differentiation via cell-cell communication.
The activation of Wnt/β-catenin signaling is a key
factor in initiating and promoting neurogenesis due to its ability
to selectively trigger the expression of a panel of
neuronal-associated genes for NSC proliferation and differentiation
(34). However, inhibition of the
Wnt/β-catenin pathway reduces myelination and neurogenesis, which
leads to cognitive dysfunction in rats (17). Therefore, to elucidate the
mechanisms by which co-culture with EPCs promotes NSC proliferation
and differentiation, the present study investigated the signaling
pathway of the co-culture system. Several findings confirmed that
the activation of Wnt3a/β-catenin signaling by co-culture with EPCs
was critical for EPC-mediated NSC proliferation and
differentiation: i) Co-culture with EPCs markedly promoted NSC
proliferation, the number of neurospheres and NSC differentiation,
which was accompanied by increased mRNA and protein expression
levels of β-catenin and Wnt3a; ii) Wnt3a knockdown in the EPCs
decreased the proliferation of NSCs, the number of neurospheres and
the differentiation of NSCs in the co-culture system. Wnt3a
knockdown in the EPCs eliminated the EPCs-mediated increase in the
mRNA and protein expression levels of β-catenin and Wnt3a, and
reversed the EPC-mediated phosphorylation and nuclear translocation
of β-catenin, which was in accordance with a previous report
(35); iii) co-culture with EPCs
reduced the phosphorylation of GSK-3β in the NSCs, and Wnt3a
knockdown in the EPCs significantly increased the phosphorylation
of GSK-3β in the co-culture system. GSK-3β, a multifunctional
protein kinase, acts as a key and negative regulator of the
classical Wnt/β-catenin signaling pathway, and is responsible for
the phosphorylation and downregulation of β-catenin (36,37).
Taken together, the results of the present study demonstrated that
Wnt3a/β-catenin was critical in EPC-mediated NSC proliferation and
differentiation.
In conclusion, the results of the present study
suggested that co-culture with EPCs promoted the proliferation and
differentiation of NSCs through modulation of the Wnt3a/β-catenin
and GSK-3β signaling pathway. The present study provided molecular
insight into the EPC-mediated effects of neurogenesis during the
process of repair following spinal cord injury.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant no 81171173) andt the
Anhui Provincial Natural Science Foundation (grant no
11040606Q25).
References
|
1
|
La Spada A and Ranum LP: Molecular genetic
advances in neurological disease: Special review issue. Hum Mol
Genet. 19:R1–R3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moyse E, Segura S, Liard O, Mahaut S and
Mechawar N: Microenvironmental determinants of adult neural stem
cell proliferation and lineage commitment in the healthy and
injured central nervous system. Curr Stem Cell Res Ther. 3:163–184.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin Y, Huang P, Han Z, Wei G, Zhou C, Wen
J, Su B, Wang X and Wang Y: Collagen nanofibers facilitated
presynaptic maturation in differentiated neurons from
spinal-cord-derived neural stem cells through MAPK/ERK1/2-Synapsin
I signaling pathway. Biomacromolecules. 15:2449–2460. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ogawa Y, Sawamoto K, Miyata T, Miyao S,
Watanabe M, Nakamura M, Bregman BS, Koike M, Uchiyama Y, Toyama Y
and Okano H: Transplantation of in vitro-expanded fetal neural
progenitor cells results in neurogenesis and functional recovery
after spinal cord contusion injury in adult rats. J Neurosci Res.
69:925–933. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brustle O and McKay RD: Neuronal
progenitors as tools for cell replacement in the nervous system.
Curr Opin Neurobiol. 6:688–695. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu K, Wang Z, Lin XL, Zhang K, He XL and
Zhang H: MicroRNAs: Key regulators of endothelial progenitor cell
functions. Clin Chim Acta. 448:65–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zampetaki A, Kirton JP and Xu Q: Vascular
repair by endothelial progenitor cells. Cardiovasc Res. 78:413–421.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paczkowska E, Rogińska D, Pius-Sadowska E,
Jurewicz A, Piecyk K, Safranow K, Dziedziejko V, Grzegrzółka R,
Bohatyrewicz A and Machaliński B: Evidence for proangiogenic
cellular and humoral systemic response in patients with acute onset
of spinal cord injury. J Spinal Cord Med. 38:729–744. 2015.
View Article : Google Scholar
|
|
9
|
Ottone C, Krusche B, Whitby A, Clements M,
Quadrato G, Pitulescu ME, Adams RH and Parrinello S: Direct
cell-cell contact with the vascular niche maintains quiescent
neural stem cells. Nat Cell Biol. 16:1045–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamei N, Kwon SM, Ishikawa M, Ii M,
Nakanishi K, Yamada K, Hozumi K, Kawamoto A, Ochi M and Asahara T:
Endothelial progenitor cells promote astrogliosis following spinal
cord injury through Jagged1-dependent Notch signaling. J
Neurotrauma. 29:1758–1769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang Y, Jia X, Wei F, Wang C, Sun X, Xu
S, Yang X, Zhao Y, Chen J, Wu H, Zhang L and Wei W: CP-25, a novel
compound, protects against autoimmune arthritis by modulating
immune mediators of inflammation and bone damage. Sci Rep.
6:262392016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lendahl U, Zimmerman LB and McKay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Liu L, Barger SW and Griffin WS:
Interleukin-1 mediates pathological effects of microglia on tau
phosphorylation and on synaptophysin synthesis in cortical neurons
through a p38-MAPK pathway. J Neurosci. 23:1605–1611.
2003.PubMed/NCBI
|
|
14
|
Dráberová E, Del Valle L, Gordon J,
Marková V, Smejkalová B, Bertrand L, de Chadarévian JP, Agamanolis
DP, Legido A, Khalili K, et al: Class III beta-tubulin is
constitutively coexpressed with glial fibrillary acidic protein and
nestin in midgestational human fetal astrocytes: Implications for
phenotypic identity. J Neuropathol Exp Neurol. 67:341–354. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Wang L, Liu Y, Li S, Long ZY and Wu YM:
Wnt signaling pathway participates in valproic acid-induced
neuronal differentiation of neural stem cells. Int J Clin Exp
Pathol. 8:578–585. 2015.PubMed/NCBI
|
|
17
|
Tiwari SK, Agarwal S, Chauhan LK, Mishra
VN and Chaturvedi RK: Bisphenol-A impairs myelination potential
during development in the hippocampus of the rat brain. Mol
Neurobiol. 51:1395–1416. 2015. View Article : Google Scholar
|
|
18
|
Shintani S, Murohara T, Ikeda H, Ueno T,
Sasaki K, Duan J and Imaizumi T: Augmentation of postnatal
neovascularization with autologous bone marrow transplantation.
Circulation. 103:897–903. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garlanda C and Dejana E: Heterogeneity of
endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol.
17:1193–1202. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gould TD and Manji HK: The Wnt signaling
pathway in bipolar disorder. Neuroscientist. 8:497–511. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ille F and Sommer L: Wnt signaling:
Multiple functions in neural development. Cell Mol Life Sci.
62:1100–1108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borrell V and Reillo I: Emerging roles of
neural stem cells in cerebral cortex development and evolution. Dev
Neurobiol. 72:955–971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chojnacki A, Cusulin C and Weiss S: Adult
periventricular neural stem cells: Outstanding progress and
outstanding issues. Dev Neurobiol. 72:972–989. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rossi F and Cattaneo E: Opinion: Neural
stem cell therapy for neurological diseases: Dreams and reality.
Nat Rev Neurosci. 3:401–409. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kupatt C, Horstkotte J, Vlastos GA,
Pfosser A, Lebherz C, Semisch M, Thalgott M, Büttner K, Browarzyk
C, Mages J, et al: Embryonic endothelial progenitor cells
expressing a broad range of proangiogenic and remodeling factors
enhance vascularization and tissue recovery in acute and chronic
ischemia. FASEB J. 19:1576–1578. 2005.PubMed/NCBI
|
|
26
|
Guo JS, Zeng YS, Li HB, Huang WL, Liu RY,
Li XB, Ding Y, Wu LZ and Cai DZ: Cotransplant of neural stem cells
and NT-3 gene modified Schwann cells promote the recovery of
transected spinal cord injury. Spinal Cord. 45:15–24. 2007.
View Article : Google Scholar
|
|
27
|
Uchida N, Chen K, Dohse M, Hansen KD, Dean
J, Buser JR, Riddle A, Beardsley DJ, Wan Y, Gong X, et al: Human
neural stem cells induce functional myelination in mice with severe
dysmyelination. Sci Transl Med. 4:155ra1362012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang G, Ao Q, Gong K, Zuo H, Gong Y and
Zhang X: Synergistic effect of neural stem cells and olfactory
ensheathing cells on repair of adult rat spinal cord injury. Cell
Transplant. 19:1325–1337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Habisch HJ, Liebau S, Lenk T, Ludolph AC,
Brenner R and Storch A: Neuroectodermally converted human
mesenchymal stromal cells provide cytoprotective effects on neural
stem cells and inhibit their glial differentiation. Cytotherapy.
12:491–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen Q, Goderie SK, Jin L, Karanth N, Sun
Y, Abramova N, Vincent P, Pumiglia K and Temple S: Endothelial
cells stimulate self-renewal and expand neurogenesis of neural stem
cells. Science. 304:1338–1340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Daneman R, Zhou L, Agalliu D, Cahoy JD,
Kaushal A and Barres BA: The mouse blood-brain barrier
transcriptome: A new resource for understanding the development and
function of brain endothelial cells. PLoS One. 5:e137412010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Imura T, Tane K, Toyoda N and Fushiki S:
Endothelial cell-derived bone morphogenetic proteins regulate glial
differentiation of cortical progenitors. Eur J Neurosci.
27:1596–1606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai B, Mao XO, Greenberg DA and Jin K:
Endothelium-induced proliferation and electrophysiological
differentiation of human embryonic stem cell-derived neuronal
precursors. Stem Cells Dev. 17:565–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tiwari SK, Agarwal S, Tripathi A and
Chaturvedi RK: Bisphenol-A mediated inhibition of hippocampal
neurogenesis attenuated by curcumin via canonical Wnt pathway. Mol
Neurobiol. 53:3010–3029. 2016. View Article : Google Scholar
|
|
35
|
Flentke GR, Garic A, Amberger E, Hernandez
M and Smith SM: Calcium-mediated repression of beta-catenin and its
transcriptional signaling mediates neural crest cell death in an
avian model of fetal alcohol syndrome. Birth Defects Res A Clin Mol
Teratol. 91:591–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Willert K and Nusse R: Beta-catenin: A key
mediator of Wnt signaling. Curr Opin Genet Dev. 8:95–102. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khiari M, Arfaoui A, Kriaa L, Chaar I,
Amara S, Lounis MA, Sammoud S, Dhraeif M, Gharbi L, Mzabi-Regaya S
and Bouraoui S: The prognostic value of the immunohistochemical
expression and mutational pattern of the key mediator of Wnt
signaling: Beta-catenin in Tunisian patients with colorectal
carcinoma. Appl Immunohistochem Mol Morphol. 20:62–70. 2012.
View Article : Google Scholar
|