Introduction
Oxygen metabolism is a fundamental requirement for
life. However, hypoxia is one of the most common causes of injury,
leading to morbidity and mortality in various pathological
conditions, including anesthetic accidents, shock, myocardial
infarction, stroke, drowning, sleep apnea and acute respiratory
distress syndrome. Hypoxia results in decreased aerobic energy
production and increased production of damaging free radicals that
can lead to cell injury and death. Thus, hypoxia is a clinically
important issue. Tolerance to hypoxia is inherited in some species,
such as estivating and hibernating animals, and enables survival
for long periods even under pure nitrogen/no oxygen conditions.
Species that estivate or hibernate enter steady-state torpor and
are not hypoxic despite tenfold or greater decreases in heart and
respiratory rates, accompanied by hypothermia (1,2). A
hypometabolic state is central to hypoxia tolerance and is
characterized by reduced oxygen consumption (3). Notably, certain toxic gases,
including hydrogen disulfide (4)
and carbon monoxide (5), can
induce a suspended animation-like state and significantly enhance
tolerance to hypoxia in mice and Caenorhab- ditis elegans,
respectively. Previous studies have reported that several small
molecules, such as sevoflurane (6,7) and
magnesium sulfate (8), boost
hypoxia/ischemic tolerance in the brain. However, these agents have
limited use due to their controversial neurotoxicity (9) and cardiodepressive effects at high
serum concentrations (10).
Dexmedetomidine (DEX) is a highly selective
α2 adrenoreceptor agonist that is widely used as an
anesthetic adjuvant, primarily for sedation, anxiolysis,
sympatholysis, and analgesia (11). Increasing evidence suggests that
DEX protects against ischemia-reperfusion injury, including
neuroprotection (12–14), cardioprotection (15,16),
renoprotection (17,18), and intestinal protection (19,20).
Notably, DEX has also been previously demonstrated to markedly
reduce locomotor activity, heart rate (HR), respiratory rate, and
core body temperature (CBT) in mice (21,22),
which appear to be in a suspended animation-like state following
DEX administration. However, whether DEX improves hypoxia tolerance
in mice remains unknown. The current study aimed to elucidate the
effects of different doses of DEX on the survival time of mice
under acute asphyxiating conditions.
Materials and methods
Animals
A total of 68 male Kunming strain mice (weight, 36±3
g; age, 8–12 weeks) were used. All animals were housed under a 12-h
light-dark cycle in a temperature and humidity-controlled
environment with unlimited access to water and food.
This study was approved by the ethics committee of
The First Affiliated Hospital, Sun Yat-sen University (Guangzhou,
China) and was performed in according to the National Institutes of
Health guidelines for use of experimental animals. The experiments
were conducted between 10:00 and 17:30 h. The minimum number of
animals was used in each experiment and all efforts were made to
minimize animal suffering.
Experimental groups and procedures
The mice were randomly allocated into 10 groups
following a 7-day acclimation, with each group contained 6–7
mice/group. The groups received a single dose of 1, 5, 10, 20, 40,
80, 160 or 320 µg/kg DEX-HCl (Jiangsu Hengrui Medicine Co.,
Ltd., Jiangsu, China), 20 mg/kg propranolol-HCl (Sigma-Aldrich, St.
Louis, MO, USA) as a positive control or 0.9% saline by
intraperitoneal injection.
Weight, CBT, and HR of the mice were measured prior
to injection of the drugs or saline. CBT and HR were rerecorded 30
min after the injections, and survival time measurement was
initiated.
CBT measurement
CBTs were recorded using a rectal probe connected to
a digital thermometer. The probe was inserted 2 cm inside the anal
sphincter and the CBT of each animal was recorded twice,
immediately before and 30 min after the drug administration.
Heart rate measurement
HRs were measured noninvasively with a tail-artery
sphygmomanometer (Softron Biotechnology Co., Ltd., Beijing, Chain).
The HR of each mouse was recorded twice, immediately before and 30
min after the drug injections.
Survival time testing
Hypoxic conditions were induced as previously
described by Shen et al (23). Briefly, the mice were placed into a
250-ml sealed bottle (one mouse/bottle) containing 15 g soda lime
(Mingxiang Biotechnology, Weihai, China), and survival time was
determined as the time of the last breath as an indication of
death.
Statistical analysis
Parametric data are presented as the means ±
standard deviation and analyzed by one-way repeated-measures
analysis of variance followed by the least significant difference
test. Survival times were analyzed using the Kaplan-Meier method
and differences between the groups were identified using the
log-rank test. Correlations between variables were estimated using
Pearson's correlation coefficient analysis. All statistical
analyses were performed using SPSS software (version. 13.0; SPSS
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
DEX dose-dependently reduces HR in
mice
The physiological data collected prior to the
experiment are presented in Table
I. No significant differences between body weight, CBT and HR
values were observed among the groups.
 | Table IPhysiological values of experimental
mice. |
Table I
Physiological values of experimental
mice.
| Saline | Propranolol | DEX
|
|---|
| 1 µg/kg | 5 µg/kg | 10 µg/kg | 20 µg/kg | 40 µg/kg | 80 µg/kg | 160 µg/kg | 320 µg/kg |
|---|
| Number | 7 | 7 | 7 | 6 | 6 | 7 | 7 | 7 | 7 | 7 |
| Wt (g) | 37.1±1.8 | 37.6±2.2 | 36.9±2.1 | 36.9±1.1 | 36.5±1.7 | 37.0±1.6 | 37.7±1.6 | 37.9±1.5 | 37.2±2.5 | 38.6±1.5 |
| MAP (mmHg) | 81.0±8.3 | 75.3±2.2 | 75.2±10.3 | 76.5±9.1 | 73.2±5.4 | 81.2±8.2 | 84.4±6.3 | 88.0±6.0 | 83.3±8.1 | 80.3±7.1 |
| HR (bpm) | 540.8±57.4 | 521.4±73.1 | 543.5±52.9 | 531.0±79.2 | 507.0±62.4 | 549.6±66.3 | 586.8±81.3 | 547.3±58.0 | 581.5±111.0 | 548.8±89.6 |
| CBT (°C) | 36.9±0.8 | 36.7±0.5 | 36.8±0.7 | 36.9±0.6 | 36.6±0.5 | 36.9±0.8 | 36.4±0.6 | 36.7±0.8 | 37.0±0.7 | 36.6±0.8 |
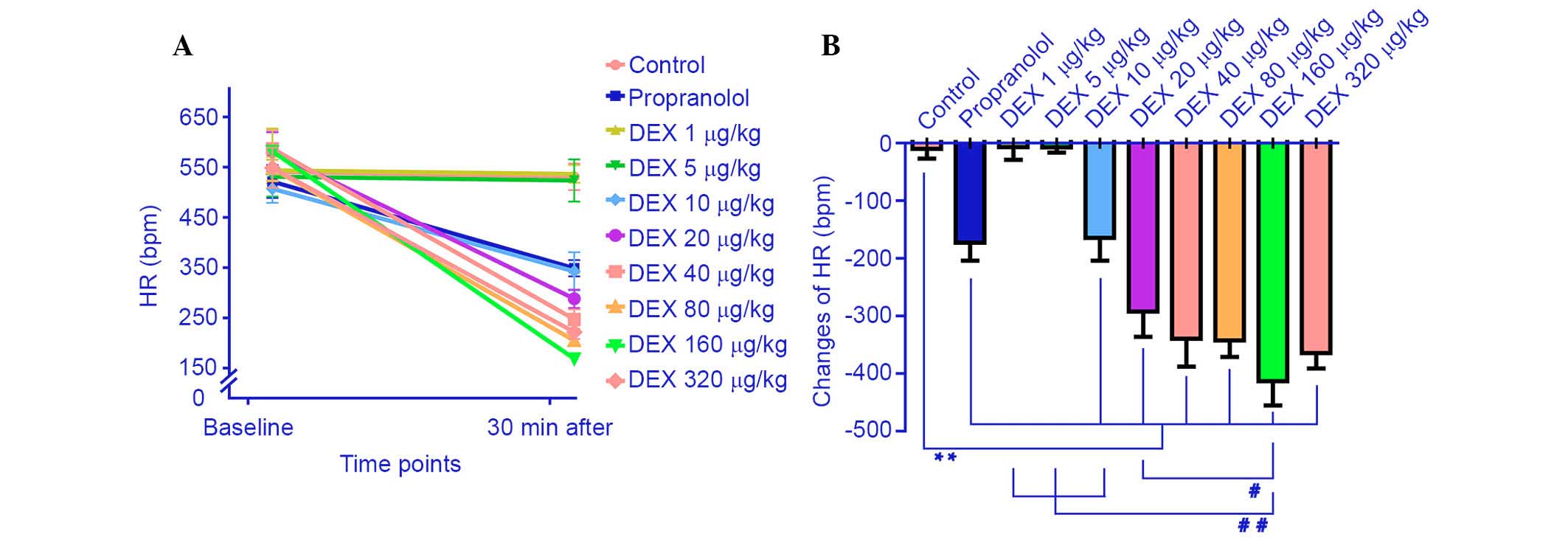
The effect of propranolol and the different doses of
DEX on hemodynamics are demonstrated in Fig. 1. The lower DEX doses (1 and 5
µg/kg) did not significantly affect HR (−7.0±56.4 and
−7.5±28.5 bpm, respectively) compared with the saline group.
However, the higher DEX doses (10, 20, 40, 80, 160 and 320
µg/kg) and 20 mg/kg propranolol significantly reduced the HR
values (342.6±85.1, 288.0±44.9, 247.4±43.5, 209.2±19.1, 168.5±18.0,
221.6±30.4 and 348.8±36.4 bpm, respectively) compared with the
saline group (P=0.003, 0.000, 0.000, 0.000, 0.000, 0.000 and 0.002,
respectively). As the DEX dose was increased further (10, 20, 40,
80 and 160 µg/kg), HR decreased significantly and reached
the minimum level at 160 µg/kg DEX (−413.0±108 bpm). The HR
of mice treated with 320 µg DEX/kg did not decrease further
but was maintained at a low level (P=0.09) compared with the 160
µg/kg DEX group.
DEX dose-dependently induces hypothermia
in mice
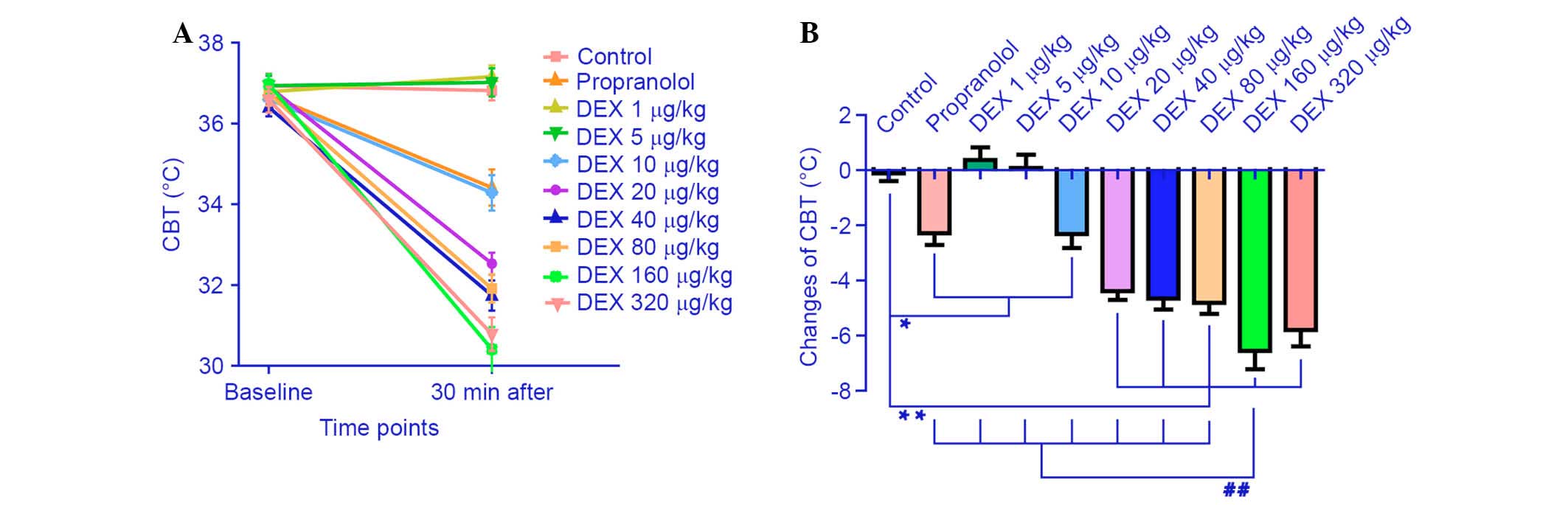
The effect of the different DEX doses and
propranolol on the CBT of the mice is demonstrated in Fig. 2. The CBT was significantly reduced
in the propranolol group (−2.29±1.04°C) compared with in the saline
group (−0.11±0.70°C; P=0.001). Mice administered with the lower
doses of DEX (1 and 5 µg/kg) did exhibit a significant
decrease in CBT. Notably, compared with the control group, CBT was
decreased significantly (P=0.456, P=0.770) in mice treated with ≥10
µg DEX/kg. Additionally, CBT was reduced to a minimum of
−6.56±1.62°C following administration of 160 µg DEX/kg.
Then, compared with the 160 µg/kg group, CBT remained at a
low level without a further significant decrease in mice treated
with 320 µg/kg DEX (−5.8±1.44°C; P=0.247).
DEX increases survival time in mice
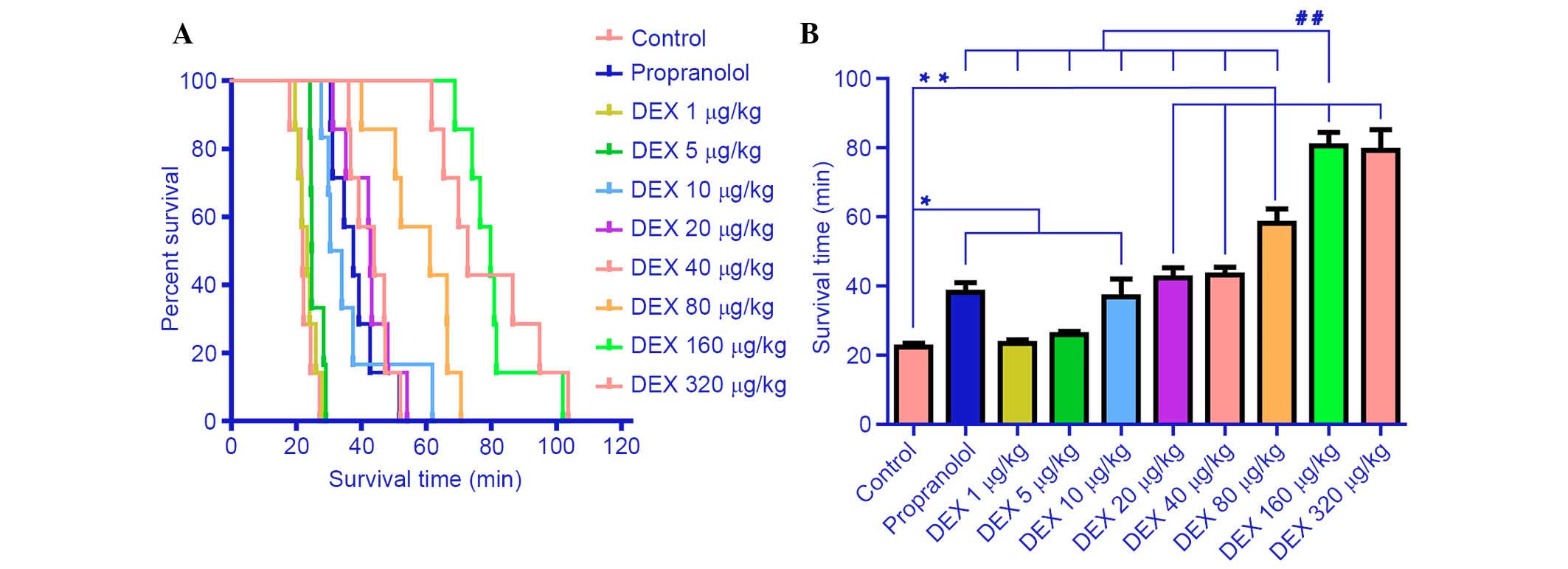
The effects of DEX, propranolol and saline on
survival time are demonstrated in Fig.
3. The survival time of mice in the saline group was 22.4±2.6
min. No significant effect on survival time was observed following
administration with the lower DEX doses (1 µg/kg, 23±2.8
min; 5 µg/kg, 26.0±2.3 min; P=0.607) compared with the
saline group. However, treatment with the higher doses of DEX (10,
20, 40, 80 and 160 µg/kg) significantly increased survival
time in a dose-dependent manner compared with the control group
(P=0.006, 0.000, 0.000, 0.000 and 0.000, respectively). The maximal
extension of survival time was 80.5±9.7 min at 160 µg
DEX/kg, which was ~3.6 times that of the control group (P=0.000).
Survival time was not extended further following administration of
320 µg/kg DEX (79.2±14.7 min; P=0.791) compared with the 160
µg/kg group. Survival time of mice treated with 20 mg/kg
propranolol was marginally extended compared with the control group
(38.2±6.8 min; P=0.002), and exerted an equivalent effect compared
with 10, 20 and 40 µg/kg DEX (P=0.792, 0.389 and 0.306).
Correlation analysis
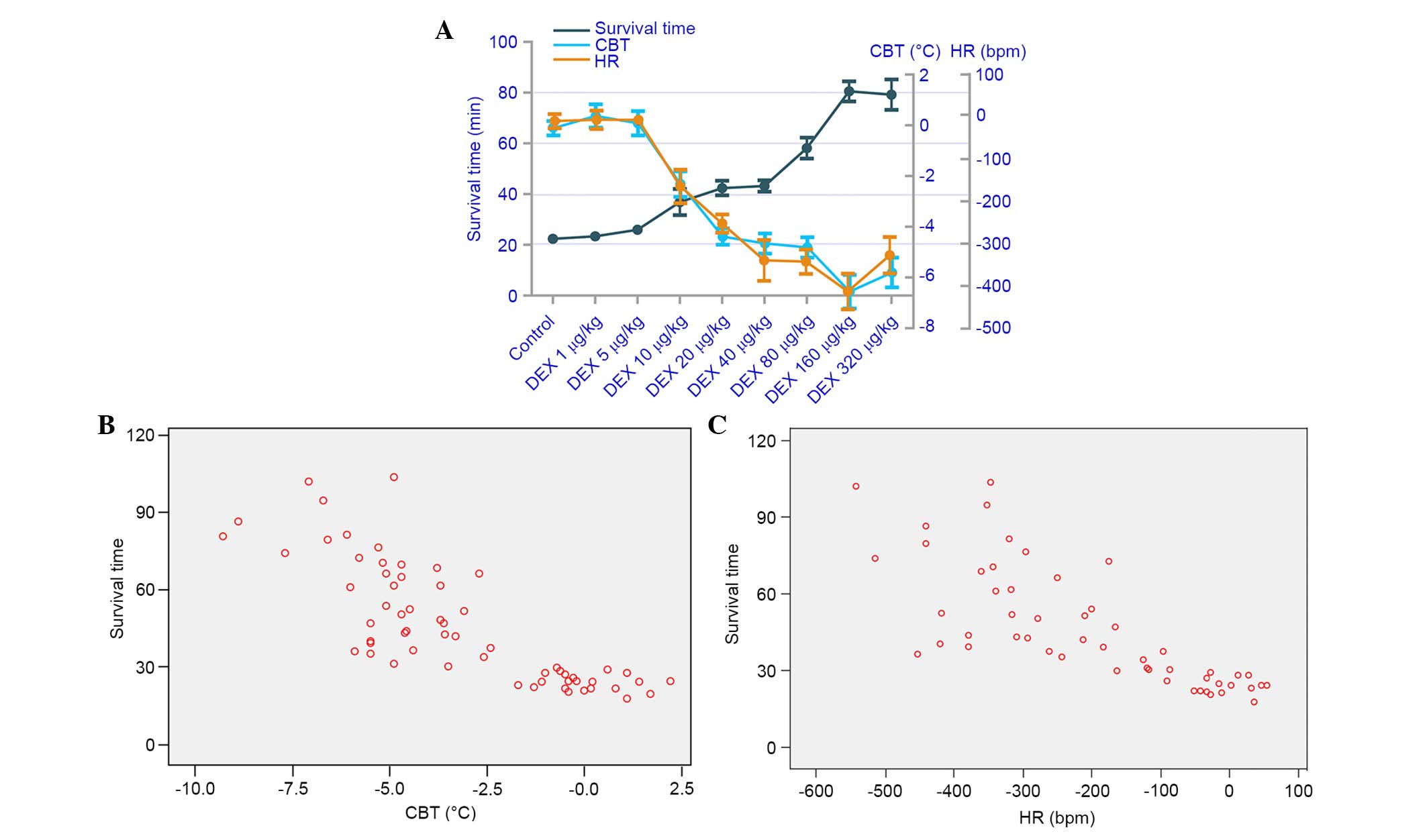
The correlation analysis between survival time and
CBT, and survival time and HR, are presented in Fig. 4. Strong negative correlations were
detected between survival time and CBT (r=−0.802), and survival
time and HR (r=−0.726).
Discussion
Despite recent progress, the main mechanisms
underlying DEX-induced multiorgan protection remain poorly
understood and require further investigation. The current study
demonstrated that DEX dose-dependently improved tolerance to
hypoxia in mice, which may partially explain the mechanism.
Propranolol is a β-adrenoreceptor antagonist that effectively
extends the survival time of mice under limited oxygen conditions
(23). Thus, propranolol was used
as a positive control to determine the efficacy of DEX. The results
of the present study demonstrate that the lower doses of DEX (1 and
5 µg/kg) did not significantly extend survival time of the
mice, but the higher doses (10, 20, 40, 80, 160 and 320
µg/kg) significantly prolonged survival time compared with
the control, which peaked at the dose of 160 µg/kg and was
3.6 times longer than that observed in the control group.
Furthermore, CBT and HR decreased similarly in response to DEX.
Propranolol appeared to have a limited effect on extending survival
time and only marginally decreased HR and CBT.
DEX decreases HR in various animals, including
rodents and humans (24,25). The hypothermic effects of DEX
observed in the present study were consistent with previous reports
(21,22), excluding the magnitude of the
changes. For example, body temperature decreased by almost 15°C in
a report by Sallinen et al (22), whereas in the current study the
result was closer to 7°C. It is speculated that multiple factors
may be involved in the difference in the magnitude of the decrease
in CBT; the time points of the measurements differed in the two
studies, the previous study measured body temperature 90 min after
the drug injection, whereas the current study measured CBT 30 min
after drug administration. Additionally, a different mouse strains
were used in the different studies.
Hypoxia/ischemia is a common clinical condition and
is often so critical that treatment must begin immediately to
secure homeostasis. Hypoxia/ischemia injury results from an
imbalance between oxygen supply and demand. Certain natural
phenomena, including hibernation, estivation and a suspended
animation-like state, effectively, and markedly reduce oxidative
metabolism and oxygen demand of an organism. The current study
combined these two events and explored whether these natural
phenomena may be induced, such as a suspended animation-like state,
to apply clinically for a beneficial effect. DEX is a promising
novel type of anesthetic; it causes sedation, hypothermia,
bradycardia and decreases the respiration rate, similar to a state
of suspended animation. Furthermore, DEX protects organs against
ischemia-reperfusion injury (12–20).
To the best of our knowledge, no other widely used drugs exhibit
these clinically valuable properties.
The hypoxia animal model used in the present study
was simple but novel, as it is completely consistent with the
clinical pathology of hypoxia, with oxygen decreasing gradually,
not immediately. Several potential limitations of this study should
be acknowledged. CBT and HR were only measured before and 30 min
after drugs administration, and thus, are unsure how these values
fluctuated during asphyxia. Further studies are required to probe
the mechanisms underlying the significant effects of DEX.
In summary, the current study demonstrated a novel
effect for DEX in enhancing tolerance to hypoxia in mice by
inducing hypothermia and bradycardia. This finding provides novel
insight for the clinical use of DEX in lethal organ
hypoxia/ischemia and hypothermia therapies.
Acknowledgments
This work was supported by the Science and
Technology Plan projects of Guangdong Province
(2012B031800289).
References
|
1
|
Biorck G, Johansson B and Schmid H:
Reactions of hedgehogs, hibernating and non-hibernating, to the
inhalation of oxygen, carbon dioxide and nitrogen. Acta Physiol
Scand. 37:71–83. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frerichs KU, Kennedy C, Sokoloff L and
Hallenbeck JM: Local cerebral blood flow during hibernation, a
model of natural tolerance to 'cerebral ischemia'. J Cereb Blood
Flow Metab. 14:193–205. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramirez JM, Folkow LP and Blix AS: Hypoxia
tolerance in mammals and birds: From the wilderness to the clinic.
Annu Rev Physiol. 69:113–143. 2007. View Article : Google Scholar
|
|
4
|
Blackstone E, Morrison M and Roth MB: H2S
induces a suspended animation-like state in mice. Science.
308:5182005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nystul TG and Roth MB: Carbon
monoxide-induced suspended animation protects against hypoxic
damage in Caenorhabditis elegans. Proc Natl Acad Sci USA.
101:9133–9136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kapinya KJ, Löwl D, Fütterer C, Maurer M,
Waschke KF, Isaev NK and Dirnagl U: Tolerance against ischemic
neuronal injury can be induced by volatile anesthetics and is
inducible NO synthase dependent. Stroke. 33:1889–1898. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding Q, Wang Q, Deng J, Gu Q, Hu S, Li Y,
Su B, Zeng Y and Xiong L: Sevoflurane preconditioning induces rapid
ischemic tolerance against spinal cord ischemia/reperfusion through
activation of extracellular signal-regulated kinase in rabbits.
Anesth Analg. 109:1263–1272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Westermaier T, Stetter C, Vince GH, Pham
M, Tejon JP, Eriskat J, Kunze E, Matthies C, Ernestus RI, Solymosi
L and Roosen K: Prophylactic intravenous magnesium sulfate for
treatment of aneurysmal subarachnoid hemorrhage: A randomized,
placebo-controlled, clinical study. Crit Care Med. 38:1284–1290.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng H, Dong Y, Xu Z, Crosby G, Culley
DJ, Zhang Y and Xie Z: Sevoflurane anesthesia in pregnant mice
induces neurotoxicity in fetal and offspring mice. Anesthesiology.
118:516–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wester maier T, Zausinger S, Baethmann A
and Schmid-Elsaesser R: Dose finding study of intravenous magnesium
sulphate in transient focal cerebral ischemia in rats. Acta
Neurochir (Wien). 147:525–532; discussion 532. 2005. View Article : Google Scholar
|
|
11
|
Kamibayashi T and Maze M: Clinical uses of
alpha2-adrenergic agonists. Anesthesiology. 93:1345–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jolkkonen J, Puurunen K, Koistinaho J,
Kauppinen R, Haapalinna A, Nieminen L and Sivenius J:
Neuroprotection by the alpha2-adrenoceptor agonist,
dexmedetomidine, in rat focal cerebral ischemia. Eur J Pharmacol.
372:31–36. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuhmonen J, Pokorný J, Miettinen R,
Haapalinna A, Jolkkonen J, Riekkinen P Sr and Sivenius J:
Neuroprotective effects of dexmedetomidine in the gerbil
hippocampus after transient global ischemia. Anesthesiology.
87:371–377. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maier C, Steinberg GK, Sun GH, Zhi GT and
Maze M: Neuropro-tection by the alpha 2-adrenoreceptor agonist
dexmedetomidine in a focal model of cerebral ischemia.
Anesthesiology. 79:306–312. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ibacache M, Sanchez G, Pedrozo Z, Galvez
F, Humeres C, Echevarria G, Duaso J, Hassi M, Garcia L, Díaz-Araya
G and Lavandero S: Dexmedetomidine preconditioning activates
pro-survival kinases and attenuates regional ischemia/reperfusion
injury in rat heart. Biochim Biophys Acta. 1822:537–545. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wijeysundera DN, Naik JS and Beattie WS:
Alpha-2 adrenergic agonists to prevent perioperative cardiovascular
complications: A meta-analysis. Am J Med. 114:742–752. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu J, Sun P, Zhao H, Watts HR, Sanders RD,
Terrando N, Xia P, Maze M and Ma D: Dexmedetomidine provides
reno-protection against ischemia-reperfusion injury in mice. Crit
Care. 15:R1532011. View
Article : Google Scholar
|
|
18
|
Si Y, Bao H, Han L, Shi H, Zhang Y, Xu L,
Liu C, Wang J, Yang X, Vohra A and Ma D: Dexmedetomidine protects
against renal ischemia and reperfusion injury by inhibiting the
JAK/STAT signaling activation. J Transl Med. 11:1412013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao
X, Huang WQ and Liu KX: Dexmedetomidine administration before, but
not after, ischemia attenuates intestinal injury induced by
intestinal ischemia-reperfusion in rats. Anesthesiology.
116:1035–1046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang ZX, Huang CY, Hua YP, Huang WQ, Deng
LH and Liu KX: Dexmedetomidine reduces intestinal and hepatic
injury after hepatectomy with inflow occlusion under general
anaesthesia: A randomized controlled trial. Br J Anaesth.
112:1055–1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lähdesmäki J, Sallinen J, MacDonald E,
Sirviö J and Scheinin M: Alpha2-adrenergic drug effects on brain
monoamines, locomotion, and body temperature are largely abolished
in mice lacking the alpha2A-adrenoceptor subtype.
Neuropharmacology. 44:882–892. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sallinen J, Link RE, Haapalinna A,
Viitamaa T, Kulatunga M, Sjöholm B, Macdonald E, Pelto-Huikko M,
Leino T, Barsh GS, et al: Genetic alteration of alpha
2C-adrenoceptor expression in mice: Influence on locomotor,
hypothermic, and neurochemical effects of dexmedetomidine, a
subtype-nonselective alpha 2-adrenoceptor agonist. Mol Pharmacol.
51:36–46. 1997.PubMed/NCBI
|
|
23
|
Shen ZB, Yin YQ, Tang CP, Yan CY, Chen C
and Guo LB: Phar-macodynamic screening and simulation study of
anti-hypoxia active fraction of xiangdan injection. J
Ethnopharmacol. 127:103–107. 2010. View Article : Google Scholar
|
|
24
|
Salmenperä MT, Szlam F and Hug CC Jr:
Anesthetic and hemodynamic interactions of dexmedetomidine and
fentanyl in dogs. Anesthesiology. 80:837–846. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hogue CW Jr, Talke P, Stein PK, Richardson
C, Domitrovich PP and Sessler DI: Autonomic nervous system
responses during sedative infusions of dexmedetomidine.
Anesthesiology. 97:592–598. 2002. View Article : Google Scholar : PubMed/NCBI
|