Introduction
Atopic dermatitis (AD) is a relapsing skin
inflammatory disease with acute and chronic phases, which is
characterized by acute pruritus and eczema (1). Skin inflammation is caused by complex
interactions between genetic, environmental, pharmacological,
psychological, immunological and skin barrier dysfunction factors
(2). The prevalence of AD is
rapidly increasing in industrialized countries, particularly among
children (3). The immunological
mechanism underlying AD remains to be fully elucidated; however, a
study regarding AD immunopathology have demonstrated that AD is
highly correlated with immune system dysregulation (4).
In human AD, skin inflammation occurs when the skin
is damaged by pruritus-induced scratching, and is followed by
rapidly developing erythema, hemorrhage, scarring, dryness, and
skin lesion hyperplasia (5). This
type of dermatitis is associated with increased production of
proinflammatory cytokines, which activate various types of immune
cell, consequently initiating the AD inflammatory cycle.
Interleukin (IL)-4, IL-5 and IL-13, which are produced by T-helper
(Th)2 cells, may have important roles in the acute phase of AD
(6). Th2 cells mediate
immunoglobulin (Ig)E production via the release of cytokines and
chemical mediators (7). Increased
IgE levels are a hallmark of AD, and increased IL-4 levels are
associated with IgE elevation in B cells. IgE is released from B
cells and binds to mast cells, which release various biological
mediators, particularly histamine, in IgE-mediated AD (2).
Although Th2 cytokines are dominant in the acute
phase of AD, Th1 cytokines, including interferon (IFN)-γ and IL-12,
are expressed and are associated with the pathogenesis of AD in the
chronic phase (8). Recently, T
regulatory (Treg) cells, which are a subtype of T cell, have been
reported to have an important role in the modulation of allergic
and autoimmune responses, and are characterized by the dominant
transcription of forkhead box P3, a forkhead/winged helix
transcription factor gene, which is the fingerprint of native Treg
cells (9). Th1/Th2 polarization is
well-defined in murine models induced by artificial immunization.
Specifically, AD is an allergic disease that results from dermal
inflammation, a hallmark characteristic of which is a disruption in
the immunological balance between Th1 and Th2 cells (10). It has previously been suggested
that IL-17-producing CD4+ T-helper cells (Th17)
participate in the pathogenesis of AD (11). In Th1-mediated chronic inflammatory
disease with epidermal hyperplasia, IL-17 has been reported to be
associated with allergen-specific immune responses (12).
At present, steroid therapy is widely applied for
the treatment of AD; however, since this treatment causes severe
side effects, including immunosuppression, stretch marks, thinning
of the skin, and epidermal barrier dysfunction, it cannot be used
for long periods of time (13).
Therefore, a study investigated the potential of natural substances
for the treatment of patients with AD (14).
Solanum tuberosum L. cv Hongyoung (SH) is a
variety of potato with red skin and flesh. This variety possesses
numerous anthocyanins, which the general potato does not (15); their high anthocyanin content is
due to the pigments that are responsible for their color (16). Anthocyanin concentration varies in
the large range of potatoes, and is correlated with the degree of
pigmentation in colored potato flesh. It has previously been
reported that a high intake of anthocyanin-rich food is associated
with health protective effects, and a reduced risk of diabetes,
arthritis and cancer, partially due to their antioxidant and
anti-inflammatory activities (17). The present study aimed to determine
the effects of a water extract of SH on the skin symptoms of NC/Nga
mice treated with a repeated topical application of
2,4-dinitrochlorobenzene (DNCB). The inhibitory effects of SH
extract were detected on the development of AD in vivo.
Materials and methods
Reagents
RPMI 1640 medium, penicillin, streptomycin,
phosphate-buffered saline (PBS) and fetal bovine serum (FBS) were
purchased from Hyclone (GE Healthcare Life Sciences, Logan, UT,
USA).
DNCB, concanavalin A (Con A) and prednisolone were
obtained from Sigma-Aldrich (St. Louis, MO, USA). Mouse
enzyme-linked immunosorbent assay (ELISA) kit for IgE was obtained
from Shibayagi (Shibukawa, Japan). Mouse ELISA kits for IL-4, IgG1
and IgG2 were purchased from Enzo Life Sciences (UK) Ltd. (Exeter,
UK). IL-13, IFN-γ, and IL-17 ELISA kits were purchased from
eBioscience, Inc. (San Diego, CA, USA). The ELISA kit for IL-12 was
purchased from Bioo Scientific (Austin, TX, USA).
TRIzol® reagent was purchased from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Oligo dT and MMLV
transcriptase were obtained from Promega Corporation (Madison, WI,
USA), and SYBR Green supermix was purchased from Takara Bio Inc.
(Otsu, Japan).
Preparation of SH extracts
SH was supplied by Hamyang-gun Agricultural
Development & Technology Center (Hamyang, South Korea). SH was
cut into slices (1 cm), and treated with 80% aqueous ethanol for 24
h at room temperature. This procedure was repeated twice. After
filtration, the solvent was vaporized under low pressure, and the
filtrate was diluted with distilled water. After freeze-drying, the
resulting extract powder was maintained at −70°C until further
use.
High-performance liquid chromatography
(HPLC) analysis
The HPLC instrument used consisted of Waters HPLC
(separation module 2690), photodiode array detector 2996 running
Empower software, and Atlantis T3 C18 column (4.6×150 mm, 5
μm) (Waters Corporation, Milford, MA, USA). The mobile phase
consisted of acidified acetonitrile with formic acid (0.1%, solvent
A) and acidified water with formic acid (0.1%, solvent B), which
were eluted at a flow rate of 1.0 ml/min. The gradient program for
SH was 0–10 min, 10% solvent A; 60 min, 40% solvent A; 80 min, 80%
solvent A; 81 min, 100% solvent A. Briefly, 30 mg SH extract powder
was dissolved in 1 ml 100% methanol, and was adjusted to pH 2.0
using formic acid. A 10 μl aliquot of the sample solution
was injected into the HPLC system following filtration with a 0.45
μm syringe filter (EMD Millipore, Bedford, MA, USA). HPLC
analysis was performed at 520 nm at room temperature.
Identification of major peaks was performed according to a
HPLC-electrospray ionization (ESI)-mass spectrometry (MS) study.
AccuTOF® single-reflectron time-of-flight mass
spectrometer was equipped with an ESI source (JEOL USA, Inc.,
Peabody, MA, USA) and was operated with MassCenter system version
1.3.7b (JEOL USA, Inc.). MS spectra obtained in the positive ion
mode were more informative than those obtained from the negative
ion mode; therefore, acquisition was performed in the positive ion
mode. The parameters were as follows: Orifice 1=30 V; ring lens and
orifice 2=15 and 10 V, respectively. The ion guide potential and
detector voltage were set to 2,200 V. ESI parameter needle
electrode=1,500 V; nitrogen gas was used as a nebulizer, flow rate
1–3 l/min, desolvating chamber temperature=250°C, orifice 1
temperature=80°C. Mass scale calibration was accomplished using the
YOKUDELNA calibration kit (JEOL Ltd., Tokyo, Japan) for accurate
mass measurements.
Animals
Male NC/Nga mice (age, 4 weeks; weight, 16 g) were
obtained from SLC (SLC, Inc., Shizuoka, Japan) and were stored in
standard cages (individually ventilated cages) at 23±3°C in an
atmosphere containing 55±5% humidity. The mice were maintained
under a 12/12 h light/dark cycle in specific pathogen-free
conditions at the Animal Research Center at Kyung Hee University
(Seoul, North Korea). The mice were allowed ad libitum
access to Purina rodent chow (Raonbion, Seoul, Korea) and tap
water. All mice were acclimated for 7 days prior to commencement of
the experiments. Experimental protocols were performed in
accordance with the Standard Operating Procedure recognized by the
National Institutes of Health Guide for the Care and Animal Welfare
Act and Use of Laboratory Animals (Approval number
KHP-2014-04-1-R1). The study was approved by the ethics committee
of the Department of Life and Nanopharmaceutical Science of
Pharmacy, College of Pharmacy, Kyung Hee University (Seoul,
Korea).
Treatment
To induce AD-like skin lesions, hair on the ears and
dorsal skin of NC/Nga mice was removed using hair removal cream and
an electric shaver twice a week. On the subsequent day, 200
μl 1% DNCB solution (dissolved in 2:3 mixture of acetone and
olive oil) was applied to the shaved dorsal area (~8
cm2) (Fig. 1). On day 4
after the initial sensitization, 200 μl 1% DNCB mixture was
applied to the shaved dorsal area for the second sensitization. On
day 7 after the initial sensitization, the dorsal skin and ears of
the mice were challenged with 150 μl 0.4% DNCB mixture (0.4%
DNCB dissolved in a 3:1 mixture of acetone and olive oil). After
the first challenge, 0.4% DNCB solution was repeatedly applied to
the dorsal skin and ears 3 times a week for 9 weeks. The mice were
allocated to six groups (n=8/group): Normal control group, negative
control group, positive control group, and SH extract-treated
groups, which received 75, 150 or 300 mg/kg·body weight (bw) SH
extract. SH extract was orally administered daily for 4 weeks,
starting 9 weeks after sensitization with 1% DNCB. The normal and
negative control groups were treated with 0.5% carboxymethyl
cellulose (CMC), and the positive control group was administered
prednisolone (3 mg/kg·bw) dissolved into 0.5% CMC (Table I).
 | Table IExperimental design. |
Table I
Experimental design.
| Group (n=8) | Treatment |
|---|
| Normal control | 0.5% CMC |
| Negative
control | 0.4% DNCB + 0.5%
CMC |
| Positive
control | 0.4% DNCB +
Prednisolone 3 mg/kg·bw |
| SH extract 75 | 0.4% DNCB + SH 75
mg/kg·bw |
| SH extract 150 | 0.4% DNCB + SH 150
mg/kg·bw |
| SH extract 300 | 0.4% DNCB + SH 300
mg/kg·bw |
Scoring of skin dermatitis severity and
ear thickness
Following sample treatment, right ear thickness was
gauged three times per week using a thickness gauge (Mitutoyo
Corporation, Tokyo, Japan). Total clinical severity score was
evaluated three times a week. The extent of dryness,
lichenification, excoriation, erythema/edema and erosion was scored
as follows: 0, no symptoms; 1, mild symptoms; 2, moderate symptoms;
and 3, severe symptoms. The total skin score was defined as the sum
of the individual scores (18).
Established scratching behavior
Scratching behavior was observed following
completion of the treatments. Specifically, scratching was counted
three times a week. Mice from each group were placed into a new
cage for 1 h of habituation. The number of times a mouse scratched
the dorsal skin lesion within a period of 30 min was counted
(19). Scratching behavior was
scored from 0 to 4, as follows: 0, no scratching; 2, scratching
shorter than 1.5 sec; and 4, scratching longer than 1.5 sec. The
total scratching behavior number was calculated as the sum of the
individual scores (20).
Plasma Ig analysis
The immunological response during DNCB-induced AD
was monitored by measuring the serum levels of IgE, IgG1 and IgG2a.
The plasma levels of IgE were measured using the mouse IgE ELISA
kit, and IgG1 and IgG2a levels were measured using mouse IgG1 and
IgG2a ELISA kits. The ELISAs were performed according to
manufacturers' protocols.
Cytokine production by splenocytes
Each group of mice was sacrificed by overdose of
diethyl ether, and the spleens from each mouse were obtained.
Spleens isolated from NC/Nga mice were crushed using a cell
strainer (BD Biosciences, Franklin Lakes, NJ, USA) and were then
resuspended in culture medium (RPMI-1640 supplemented with 10% FBS,
50 mg/ml streptomycin and 100 U/ml penicillin). Splenocytes were
cultured in 24-well plates at a cell density of 1×106
cells/ml. Briefly, splenocytes were treated with 5 μg/ml Con
A and were incubated in a 5% CO2 incubator for 72 h at
37°C. Subsequently, the supernatants were harvested to determine
the levels of IL-4, IL-13, IFN-γ, IL-12 and IL-17A using ELISA kits
in accordance with the manufacturers' protocols.
Reverse transcription-quantitative
polymerase chain reaction (PCR) analysis
Tissues were homogenized and total RNA was isolated
from the right ear lesional tissue using TRIzol®
reagent. Isolated RNA underwent reverse transcription and was
amplified by PCR using MMLV reverse transcriptase (Promega
Corporation), 5X buffer, 10 mM deoxyribonucleotide triphosphates
mix, RNase inhibitor and Oligo dT at 42°C for 1 h, 94°C for 5 min
and 4°C for 1 h. Subsequently, equal amounts of cDNA were mixed
with 5 pM primer and SYBR Green supermix and were analyzed using an
ABI StepOnePlus Real-Time PCR machine (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The primer sets used in the PCR
amplification are presented in Table
II. The reactions were set up in a total volume of 20 μl
using 0.5 μl of cDNA and 10 μl of 2X Taqman probe mix
(Applied Biosystems). Amplification was performed under the
following cycling conditions: 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec, 60°C for 1 min, and 72°C for 20 sec. The
mRNA expression levels of each gene were normalized to the levels
of β-actin. According to the comparative Cq method, gene expression
was normalized to the expression of the housekeeping β-actin. The
gene expression level, normalized to the housekeeping gene and
relative to the control sample, was calculated as the
2−ΔΔCq (21).
 | Table IIPrimer sequences and reaction
conditions for quantitative polymerase chain reaction. |
Table II
Primer sequences and reaction
conditions for quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) | Temperature
(°C) |
|---|
| β-actin | (F) CCC AAC TTG ATG
TAT GAA GG | 55 |
| (R) TTG TGT AAG GTA
AGG TGT GC | 55 |
| IL-4 | (F) GTC TGC TGT GGC
ATA TTC TG | 57 |
| (R) GGC ATT TCT CAT
TCA GAT TC | 53 |
| IL-5 | (F) GGC TAC ACA GAG
AAA CCC TGT | 59 |
| (R) CAT GCA TAC ACA
GGT AGT TCA | 55 |
| IL-12 | (F) CAC CAG CAG CTT
CTT CAT CAG A | 60 |
| (R) CAA TGG CTT CAG
CTG CAG GT | 59 |
| IFN-γ | (F) CTC TGA GAC AAT
GAA CGC TAC ACA CT | 61 |
| (R) TGG CAG TAA CAG
CCA GAA ACA G | 60 |
| CCR3 | (F) CCC GTA CAA CCT
GGT TCT CC | 61 |
| (R) AAA GAG CCG AAG
GTG TTT CC | 57 |
| CCR4 | (F) TCG CCT TGT TTC
AGT CAG G | 57 |
| (R) CTT GCC ATG GTC
TTG GTT TT | 55 |
|
Eotaxin-1/CCL11 | (F) CAC CCT GAA AGC
CAT AGT GT | 57 |
| (R) TGT GTA CCT GGG
AAA TTA G | 53 |
| MCP-1 | (F) TTA AGG CAT CAC
AGT CCG AG | 57 |
| (R) TGA ATG TGA AGT
TGA CCC GT | 55 |
| IL-17 | (F) AAG GCA GCA GCG
ATC ATC C | 59 |
| (R) GGA ACG GTT GAG
GTA GTC TGA G | 61 |
Histological analysis
After the mice were sacrificed, the dorsal skin and
one ear from each mouse was fixed in 10% buffered neutral formalin.
Paraffin-embedded dorsal skin samples from the mice were sectioned
into 4 μm slices, which were then stained with hematoxylin
and eosin (H&E) or toluidine blue for 4 h at 2–4°C in order to
measure the number of various inflammatory cells and mast cells,
respectively. The sections were examined by light microscopy to
assess histological alterations.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Differences between groups were detected using SPSS 21
(IBM, Armonk, NY, USA) by one-way analysis of variance followed by
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
HPLC profile of SH
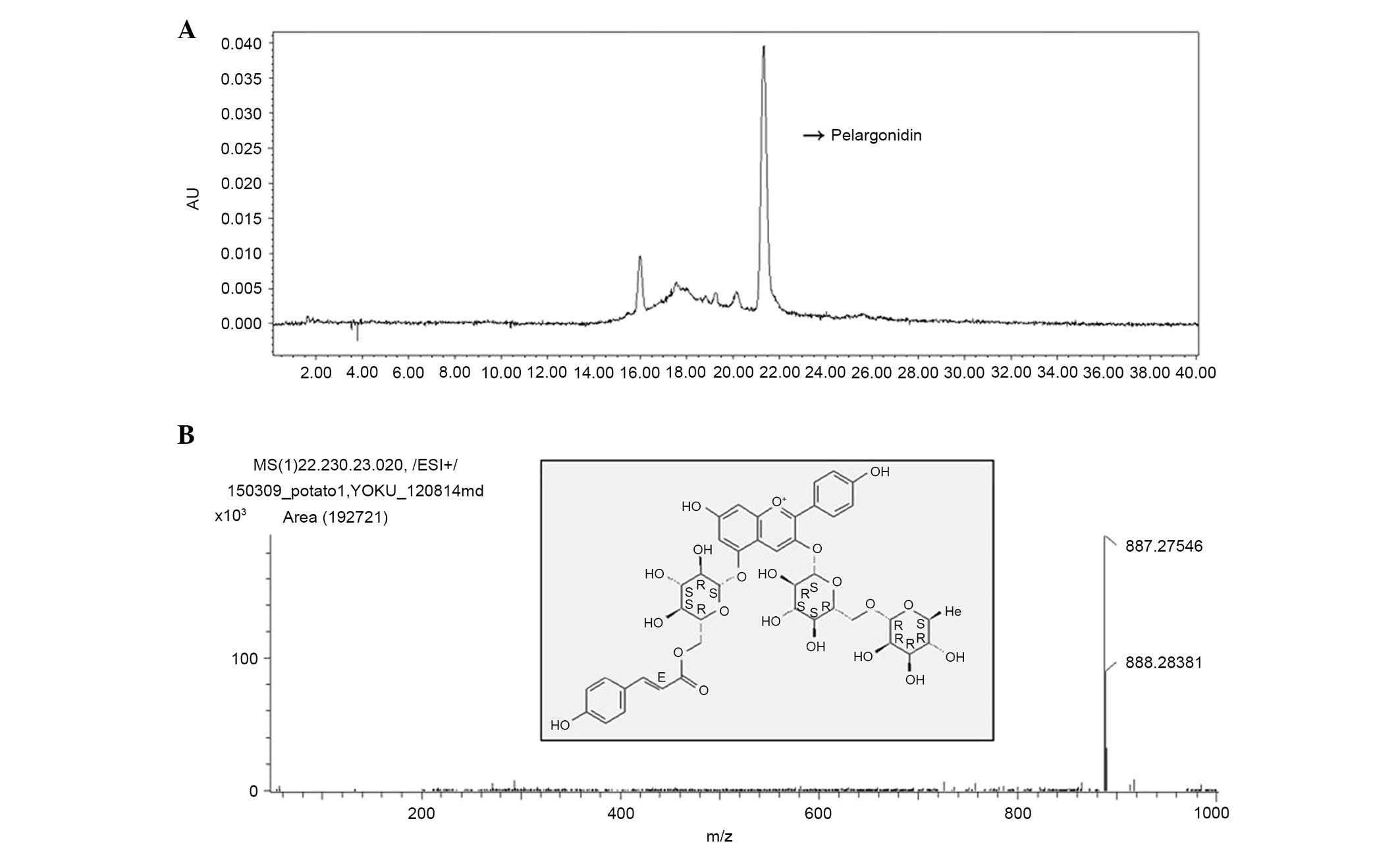
To identify the effective compound from SH, HPLC
analysis was performed at 520 nm. As shown in Fig. 2, the major peak in the total ion
chromatogram at 21 min corresponds with the main peak from the HPLC
chromatogram. The major anthocyanin was identified by HPLC-ESI-MS
and MS/MS using full spectral scan, precursor ion scan, and product
ion scan experiments, demonstrated the molecular and aglycone ions
at m/z 877, 725, 433 and 271, which indicated the presence of
pelargonidin-3-couma-roylrutinoside-5-O-glucoside. The major
anthocyanin peak was identified by HPLC-ESI-MS using a full
spectral scan, precursor ion scan and product ion scan experiments.
The difference in molecular masses of 20 mmu indicated the presence
of pelargonidin-3-coumaroylrutinoside-5-O-glucoside. The chemical
structure of the anthocyanin is shown in Fig. 2.
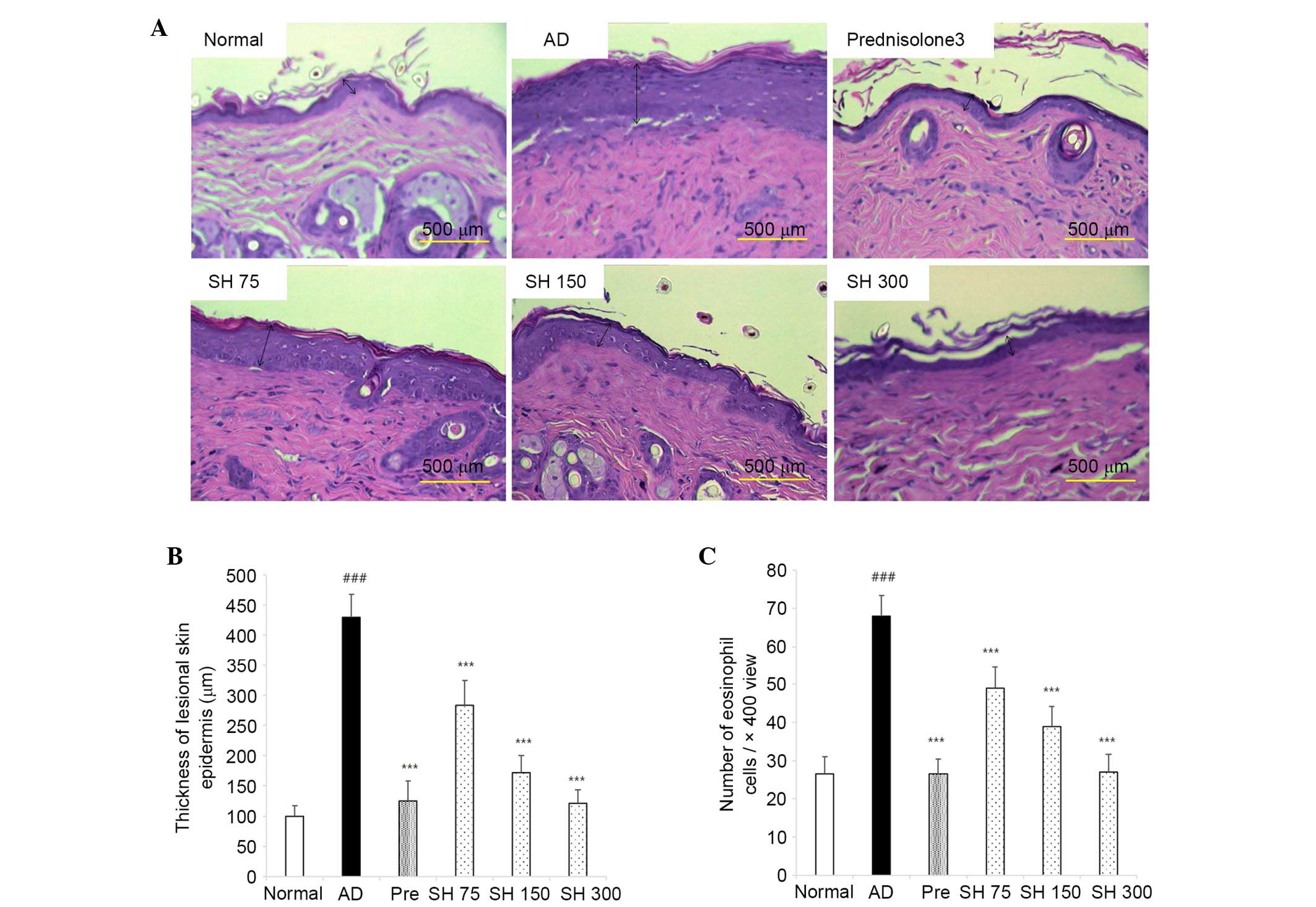
Effects of SH extract on DNCB-induced
AD-like skin lesions and ear thickness in Nc/Nga mice
To evaluate the effectiveness of SH extract against
AD-like skin lesions, post-induction of AD-like skin lesions by
DNCB application, the mice were treated with 75, 150 or 300 mg/kg
SH extract daily for 4 weeks. Skin conditions were investigated
three times a week for 4 weeks according to dermatitis severity
scores. Repeated DNCB application significantly increased
dermatitis severity scores, and induced hemorrhage, edema,
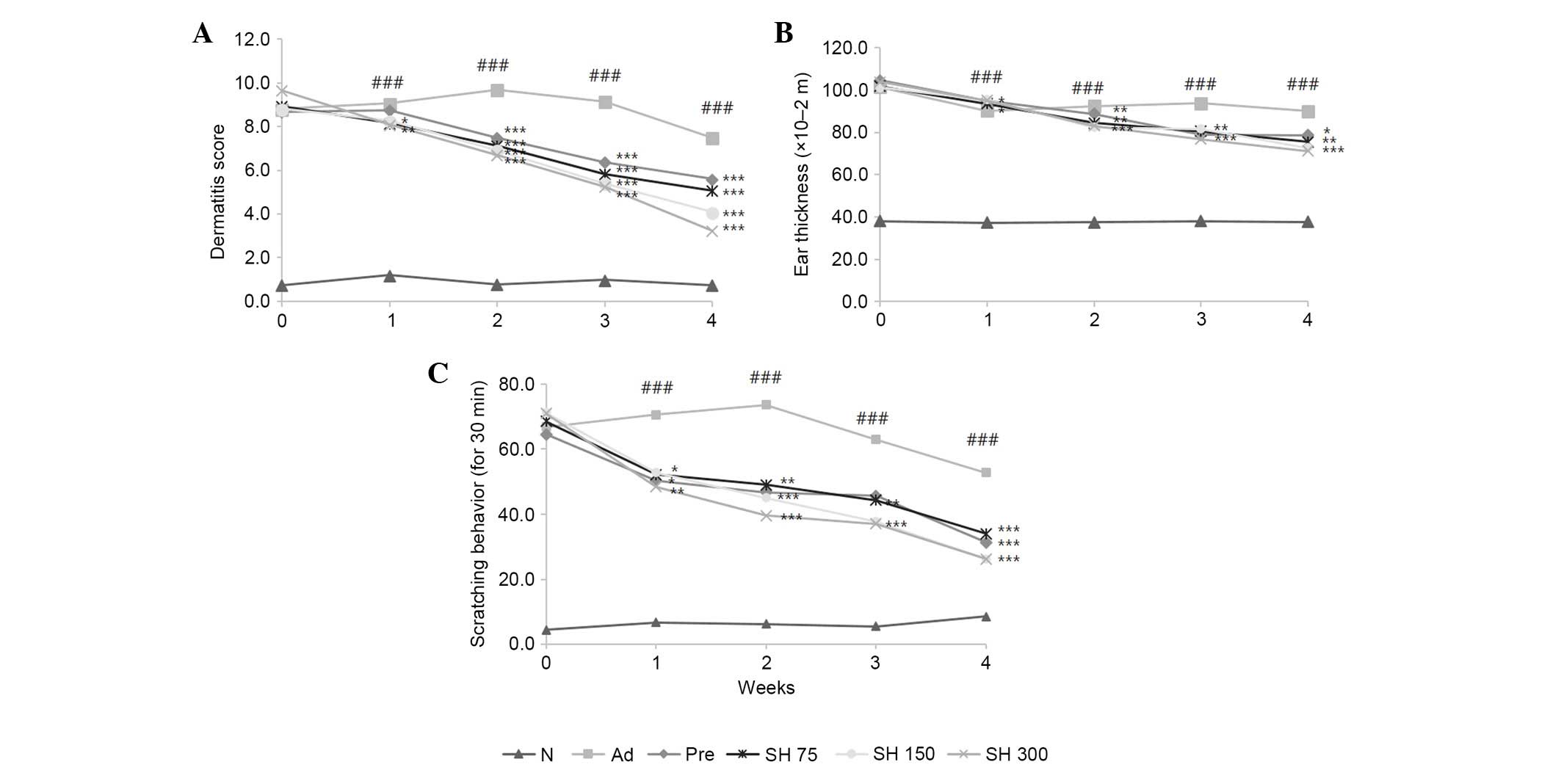
scarring, dryness and erosion in NC/Nga mice (Fig. 3). As shown in Fig. 4A, DNCB-induced AD symptoms were
suppressed by SH extract or prednisolone compared with in the AD
group. Repeated application of DNCB also significantly increased
ear thickness in mice, as compared with in the normal group. In
addition, ear lesions exhibited hyper-keratosis and dermal
thickening in the DNCB-treated group compared with in the control
group. These symptoms were all suppressed by SH extract and
prednisolone treatments (Fig. 4B).
These results indicate that SH extract decreases AD symptoms in
NC/Nga mice.
Effects of SH extract on scratching
behavior
To investigate the effects of SH extract on
scratching behavior, the number of times a mouse scratched the
dorsal skin lesion within a period of 30 min was counted 1 h after
sample treatment. As shown in Fig.
4C, the SH-treated and positive control groups exhibited
reduced scratching behavior compared with the negative control
group. Between weeks 2 and 3, the SH 150 and 300 mg/kg groups
exhibited significantly reduced scratching behavior compared with
the AD control group. These results indicate that SH extract and
prednisolone decrease AD symptom-like scratching.
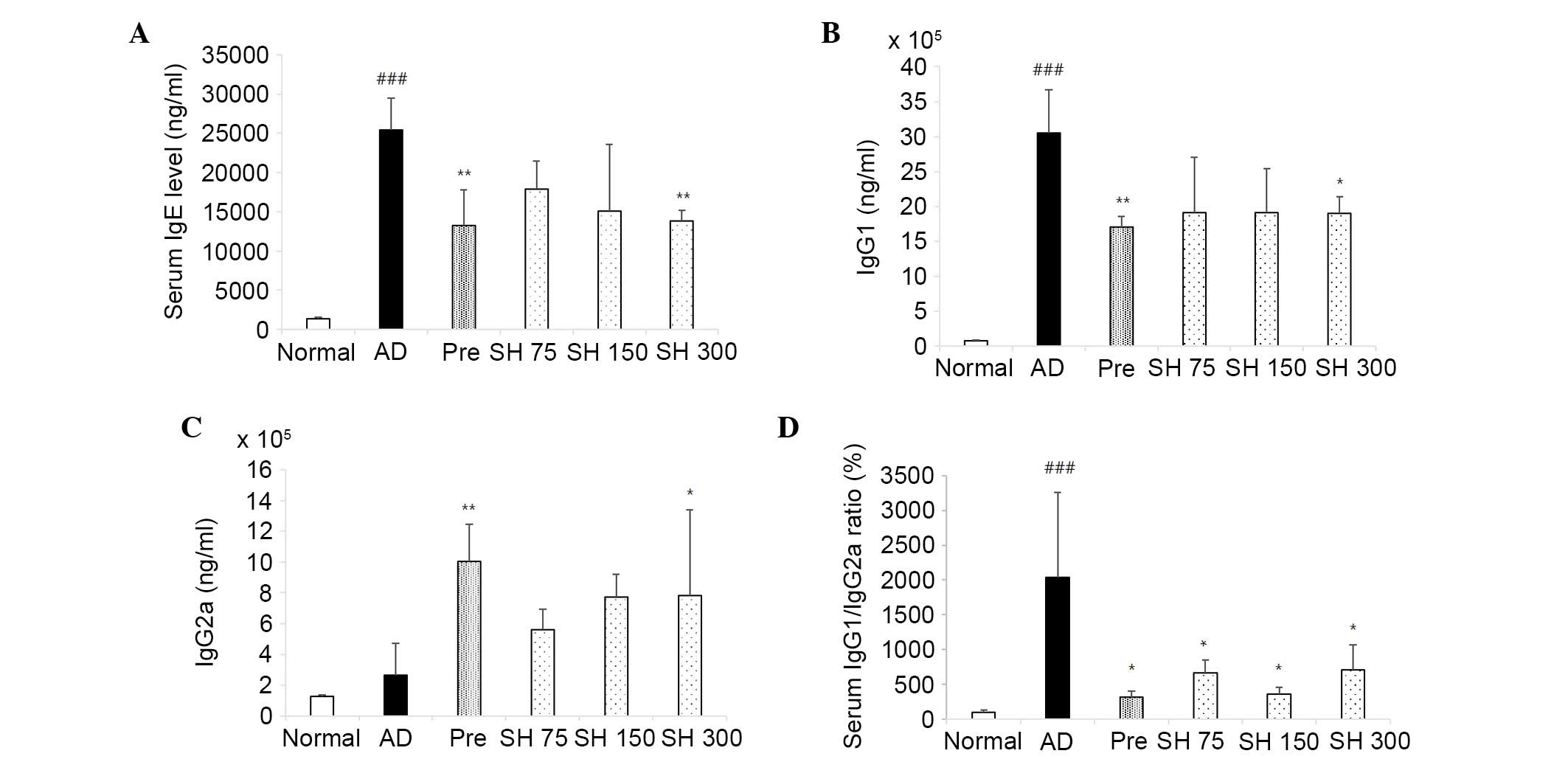
Effects of SH extract on serum Ig
levels
In addition to clinical features, the levels of IgE,
IgG1 and IgG2a were detected in serum samples from NC/Nga mice in
order to characterize the immunological response during disease
progression.
Excessive production of IgE is associated with
disease severity in patients with AD (22). Plasma IgE levels were detected in
the mice treated with SH extract and compared with those of the
control group. Mice were treated with 75, 150 or 300 mg/kg SH
extract and prednisolone daily post-induction of AD-like skin
lesions by DNCB application. As shown in Fig. 5A, repeated topical application of
DNCB significantly increased the secretion of IgE compared with in
the control group. Conversely, oral administration of SH extract
(75, 150 or 300 mg/kg) or prednisolone decreased serum IgE levels
by 29, 40, 45 and 47% for 4 weeks, respectively. AD is associated
with dysregulation of the Th1/Th2 balance (23). SH extract was able to decrease the
serum levels of Th2-mediated IgG1 and increase the levels of
Th1-mediated IgG2a. As shown in Fig.
5B and C, DNCB induced increased IgG1 levels, which were
decreased following treatment with SH extract (75, 150 and 300
mg/kg) and prednisolone. In addition, SH extract increased the
production of IgG2a. As shown in Fig.
5D, the IgG1/IgG2a ratio was decreased in the SH extract and
positive control groups. These results indicate that SH extract may
alleviate AD-like skin symptoms through the upregulation of IgG2a
(Fig. 5) and the concomitant
downregulation of IgE and IgG1.
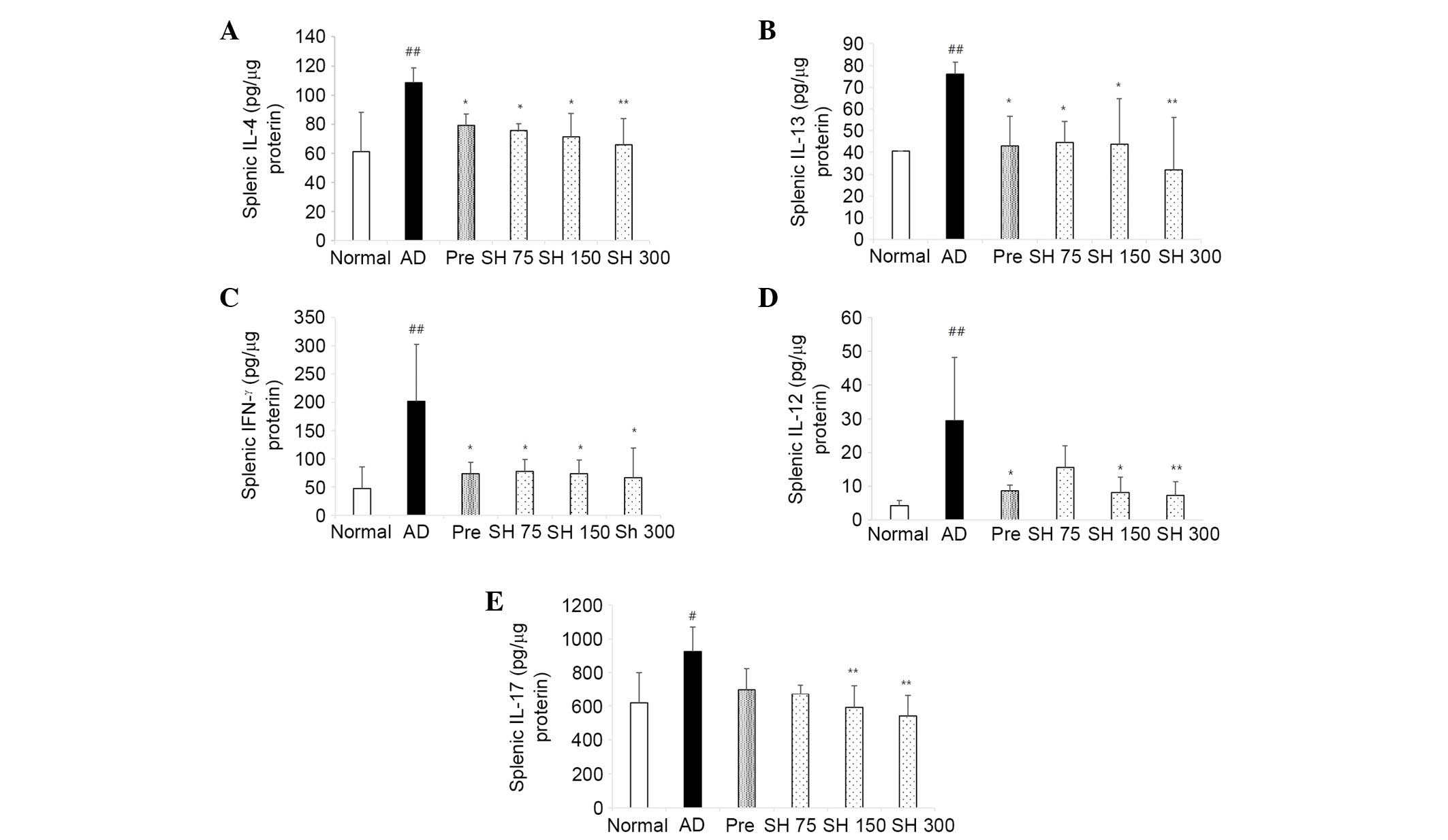
SH extract suppresses cytokine production
in splenocytes
The present study investigated the inhibitory
effects of SH extract on DNCB-induced cytokines produced by
splenocytes. Spleens were collected from the NC/Nga mice treated
with SH extract or prednisolone following sacrifice, and isolated
splenocytes were stimulated with 5 μg/ml Con A and incubated
for 72 h. Following incubation, the supernatants were collected,
and the levels of IL-4 and IL-13 (Th2 cytokines), and IFN-γ and
IL-12 (Th1 cytokines) were measured in the supernatant using ELISA
kits. The Th2 cytokines, IL-4 and IL-13, are expressed in the acute
stage of AD. The Th1 cytokines, IFN-γ and IL-12, are expressed in
the chronic stage of AD. As shown in Fig. 6, repeated application of DNCB
markedly increased the levels of Th1 and Th2 cytokines in the mice.
The cytokine IL-4 was decreased by 26, 30, 34 and 39% in the
prednisolone and SH extract groups (75, 150 and 300 mg/kg),
respectively. IL-13 was decreased by 42, 41, 43 and 57%,
respectively. IFN-γ was decreased by 64, 62, 64 and 67%,
respectively. IL-12 levels were decreased by 70, 47, 72 and 75%,
respectively. These results indicate that SH extract inhibits Th1
and Th2 cytokine levels compared with in the negative control
group. In AD, increased numbers of Th17 cells and enhanced
expression of IL-17 contribute to neutrophil chemotaxis and
increased expression of antimicrobial peptides (24). As shown in Fig. 6, IL-17 was increased in the AD
group, and was decreased by 24, 27, 35 and 42% in the prednisolone
and SH extract groups (75, 150 and 300 mg/kg), respectively.
Effects of SH on DNCB-induced mRNA
expression of IL-4, IL-13, IL-5, IL-12, IFN-γ, eotaxin, C-C
chemokine receptor (CCR)3, CCR4, IL-17 and monocyte chemoattractant
protein (MCP)-1 in NC/Nga mice
The present study investigated the inhibitory
effects of SH extract on the mRNA expression levels of Th1, Th2 and
Th17 cytokines, and chemokines in lesional skin. Repeated
application of DNCB significantly increased the mRNA expression
levels of IL-4, IL-5, IFN-γ, IL-12, eotaxin-1, CCR3, MCP-1, CCR4
and IL-17 in lesional skin; however, SH inhibited DNCB-induced
cytokine and chemokine mRNA expression (Fig. 7). These results indicate that SH
treatment may suppress DNCB-induced cytokine and chemokine
expression, leading to inhibition of skin inflammation caused by
the infiltration of inflammatory cells.
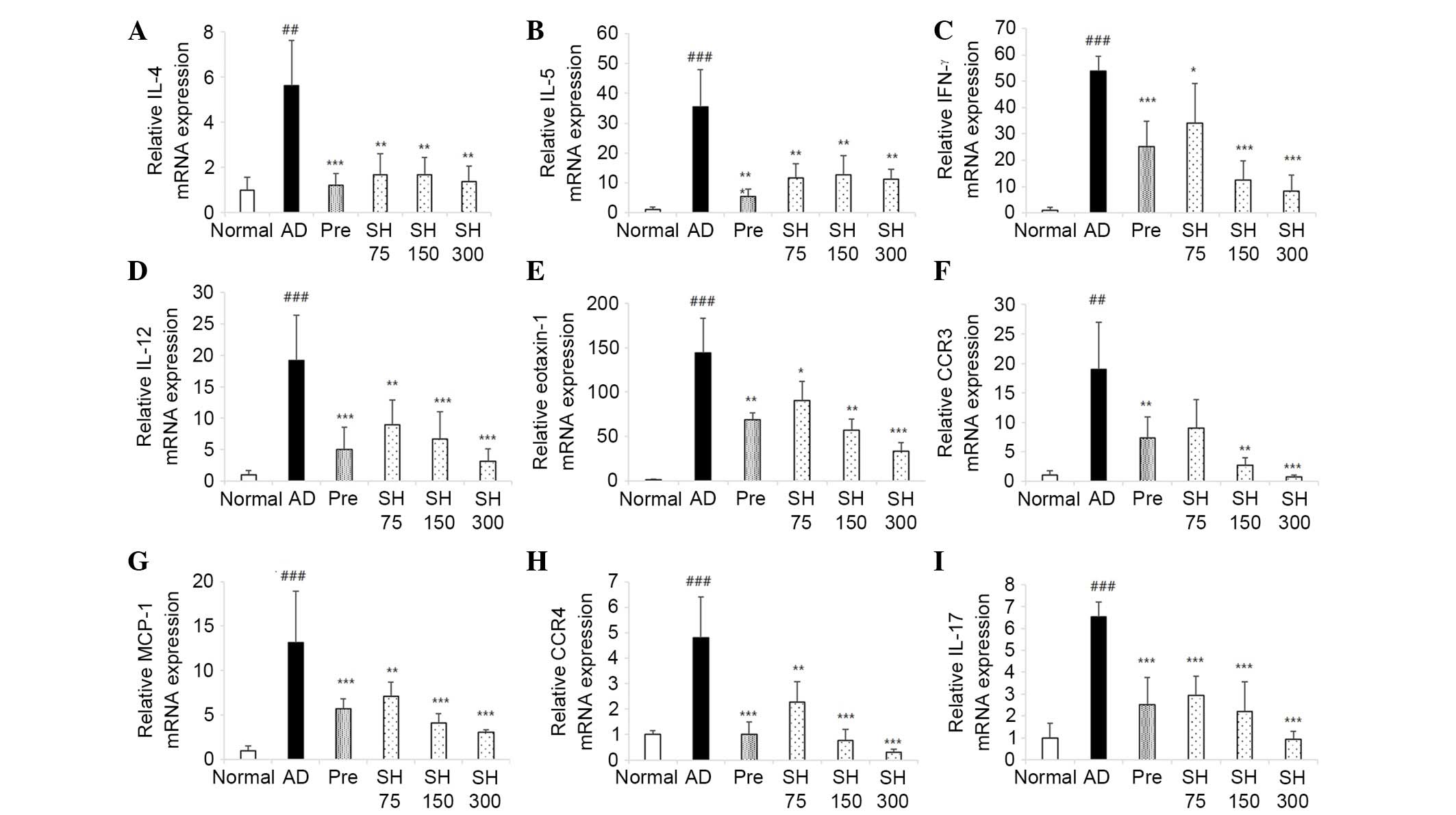 | Figure 7Inhibitory effects of Solanum
tuberosum L. cv Hongyoung extract (SH) on
2,4-dinitrochlorobenzene-induced interleukin (IL)-4, IL-5,
interferon (IFN)-γ, IL-12, eotaxin-1, C-C chemokine receptor
(CCR)3, monocyte chemoattractant protein (MCP)-1, CCR4 and IL-17
mRNA expression in NC/Nga mice. A total of 24 h after the final
treatment, mice were sacrificed and lesional tissues were obtained.
mRNA expression levels of (A) IL-4, (B) IL-5, (C) IFN-γ, (D) IL-12,
(E) eotaxin-1, (F) CCR3, (G) MCP-1, (H) CCR4 and (I) IL-17 were
measured by quantitative polymerase chain reaction. Data are
presented as the mean ± standard error of the mean from five mice
per group. #P<0.05,
##P<0.01 vs. the normal group;
*P<0.05, **P<0.01,
***P<0.001 vs. the atopic dermatitis (AD)
group. Pre, prednisolone-treated group. |
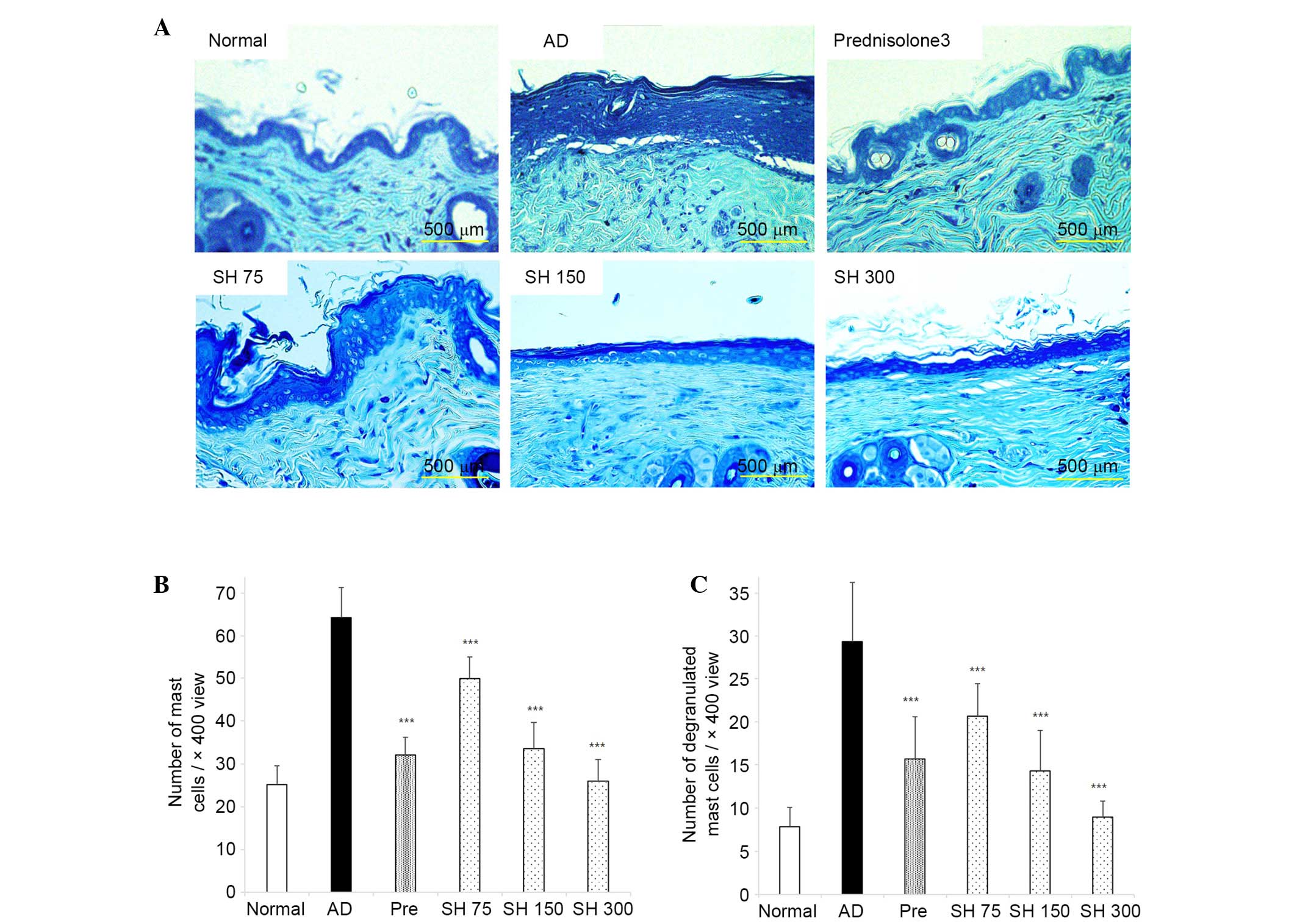
Effects of SH on DNCB-induced AD-like
histopathological alterations in Nc/Nga mice
After the mice were sacrificed, dorsal and ear skin
samples were sectioned, and stained with H&E and toluidine
blue. As shown in Figs. 8 and
9, ear lesions exhibited
hyperkeratosis, thickening of the epidermis, and inflammatory cells
accumulated within the skin lesions. These symptoms were all
suppressed by SH treatment. These results indicate that SH extract
may effectively decrease AD symptoms in NC/Nga mice.
Discussion
AD is a chronic relapsing inflammatory, pruritic
epidemic disease that affects people worldwide (2). Recent studies have markedly improved
our knowledge regarding the immunological mechanisms that underlie
the pathogenesis of AD (25).
However, complex mutual associations between the immune system and
environmental, genetic and immunological factors remain to be
examined (1).
In AD, the existence of high numbers of
CD4+ and CD8+ T cells has been demonstrated
by culturing T cells from skin biopsies and by immunohistochemistry
(26,27). Numerous studies have indicated the
role of activated CD4+ T cells in AD (28,29).
The cutaneous lymphocyte-associated antigen+ T cell
infiltrate according to cytokine and chemokine receptor expression
has disclosed novel T helper cell classification (Th1, Th2, Th17
and Treg cell subsets) that play an important role in AD (30). Reduced numbers of regulatory Treg
cells, influenced by environmental and genetic factors, predispose
individuals to the development of AD in early life (31). AD is characterized by skin lesions
that result from dermal inflammation, a characteristic feature of
which is a disruption in the immunological balance between Th1 and
Th2 cells (9). The Th1/Th2 balance
can be identified through the IgG2/IgG1 ratio, which is a marker
for Th1 and Th2 lymphocytes (32).
The present study investigated whether SH extract was able to
modulate the Th1/Th2 balance and inhibit the development of
DNCB-induced dermatitis in NC/Nga mice, a condition histologically
and clinically similar to human AD. Solanum tuberosum
varieties with intense blue-red colored pulp are well-known and
widely used in the preparation of traditional dishes of local
populations. Potatoes with purple-red flesh are considered good
sources of appreciable amounts of phenolic acids, particularly
acylated anthocyanins (33). The
pigments responsible for the particular color of the tubers reflect
their anthocyanin content (16).
The major pigment present in SH extract is
perelagonidin-3-cou-maroyl-rutinoside-5-O-glucoside. This compound
is also expressed in other red-flashed potatoes (34). Previous studies have reported that
anthocyanins exert anti-inflammatory and anticancer activities
(35,36). In the present study, the
pathogenesis of DNCB-induced contact hypersensitivity resulted from
T cell-mediated immune responses (37). In DNCB-induced AD, Th2 cytokines
are expressed in the acute phase, whereas Th1 cytokines are
expressed and contribute to the pathogenesis of AD in the chronic
phase (38). In the acute phase,
the Th2 cytokines IL-4 and IL-13 mediate the secretion of IgE in B
cells (39). Mast cells are
stimulated in response to active cross-linking of AD-specific IgE
with high affinity cell-surface IgE-receptors, and degranulated
mast cells release active mediators, including histamine (40). In the chronic phase, Th1 cytokines
IL-12 and IFN-γ are produced, inducing a local Th1 response and
tissue alterations, such as dermal thickening (41). Another feature of AD is the
presence of characteristic inflammatory dendritic cell subtypes in
the dermis and epidermis (42).
Antigen-presenting cells, such as CD1a+ epidermal
dendritic cells, dermal dendritic cells, and monocytes appear to
have an important role in AD, and typically express the
high-affinity receptor for IgE (FceRI) (43). Stimulation of FceRI on the surface
of inflammatory dendritic epidermal cells induces the release of
IL-12 and IL-18 (44). These
cytokines may contribute to the switch from the initial Th2 immune
response in acute AD to the Th1 polarization of T cells in the
chronic phase (44). Keratinocytes
of patients with AD release higher amounts of several
proinflammatory cytokines and chemokines, as compared with
keratinocytes from non-AD skin (45). Keratinocytes respond to Th1 and Th2
cytokines from T cells (46). The
Th1 cytokine IFN-γ is one of the most potent
keratinocyte-activating factors (47). In keratinocytes, inflammatory
chemokines, including C-C motif chemokine ligand (CCL)3, CCL4,
CCL11/eotaxin-1 and CCL2/MCP-1 have been reported to be associated
with AD characteristics and may support leukocyte recruitment
(48). The C-C chemokine family
adaptive immune responses are induced via dendritic cells,
basophils, mast cells, lymphocytes and eosinophils (49). CCL11/eotaxin-1 is a member of the
CC chemokines, which is known to be a potent chemoattractant for
eosinophils, and induces the pathological responses typically
associated with AD (50). It has
previously been demonstrated that CCL11/eotaxin-1 receptor CCR3
expression is induced after IL-2 and IL-4 co-incubation (51). In addition, MCP-1 is a member of
the CC chemokine family, with strong chemotactic activity for
lymphocytes (CD45+), eosinophils and
monocytes/macrophages (52). MCP-1
also has an essential role in the recruitment and activation of
mast cells, leukocytes and other cell types at the site of
inflammation (53), and is
presumed to be the functional ligand for CCR4, which is also
expressed at high levels by activated T lymphocytes, particularly
those of the CD4 subset (54).
Therefore, chemokines may have a role in the initiation or
triggering of the immune response by facilitating the interaction
of T cells with antigen-presenting cells at sites of inflammation
(49).
The present study demonstrated that systemic
administration of SH significantly reduced ear thickness, clinical
symptoms, and serum IgE levels in DNCB-induced AD-like skin lesions
of NC/Nga mice, which is comparable to prednisolone. In addition,
oral administration of SH extract reduced IgG2a and IgE levels. In
the serum of SH-treated mice, IgG2a levels were increased and IgG1
levels were decreased, resulting in a reduced IgG2/IgG1 ratio, thus
indicating that the Th1/Th2 ratio was also reduced. SH extract
controls the production of selective Th1, Th2 and Th17-mediated
cytokines in DNCB-induced mice. In addition, the present study
investigated the mRNA expression levels of AD-related cytokines and
chemokines. The skin mRNA expression pattern of Th1, Th2, and
Th17-related cytokines and chemokines in the AD-like mouse model
used in the present study exhibited slight differences compared
with the pattern in human AD skin lesions (55). In the present study, the SH
extract-administrated group exhibited decreased Th1, Th2 and Th17
cytokines, and DNCB-induced chemokines (CCR3, CCR4, MCP-1,
CCL11/eotaxin-1). In AD, inflammatory cell infiltration into the
skin is a major characteristic. Histologically, hypertrophy, and an
accumulation of mast and dendritic cells, occurs in the epidermis
and dermis of patients with AD (56). In the present study, SH extract
reduced hypertrophy and infiltration of inflammatory cells, such as
mast cells and eosinophils, in the skin. Furthermore, the number of
degranulated mast cells was reduced in the SH extract-treated
groups.
In conclusion, the present study demonstrated that
SH extract alleviates atopic symptoms in DNCB-induced NC/Nga mice.
In addition, SH extract reduced AD-related cytokine and chemokine
expression, and inflammatory cell accumulation. These results
indicated that SH extract may exert anti-AD effects, and be
considered a useful treatment for AD.
References
|
1
|
Udompataikul M and Limpa-o-vart D:
Comparative trial of 5% dexpanthenol in water-in-oil formulation
with 1% hydrocortisone ointment in the treatment of childhood
atopic dermatitis: A pilot study. J Drugs Dermatol. 11:366–374.
2012.PubMed/NCBI
|
|
2
|
Amin K: The role of mast cells in allergic
inflammation. Respir Med. 106:9–14. 2012. View Article : Google Scholar
|
|
3
|
Sampson HA: Atopic dermatitis. Ann
Allergy. 69:469–479. 1992.PubMed/NCBI
|
|
4
|
Schneider L, Tilles S, Lio P, Boguniewicz
M, Beck L, Lebovidge J, et al: Atopic dermatitis: A practice
parameter update 2012. J Allergy Clin Immunol. 131:295–299. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hashimoto Y, Takaoka A, Sugimoto M, Honma
Y, Sakurai T, Futaki N and Arai I: Itch-associated scratching
contributes to the development of dermatitis and
hyperimmunoglobulinaemia E in NC/Nga mice. Exp Dermatol.
20:820–825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leung DY and Soter NA: Cellular and
immunologic mechanisms in atopic dermatitis. J Am Acad Dermatol.
44(Suppl 1): S1–S12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miraglia del Giudice M, Decimo F, Leonardi
S, Maioello N, Amelio R, Capasso A, Capristo C and Capristo AF:
Immune dysregulation in atopic dermatitis. Allergy Asthma Proc.
27:451–455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wierenga EA, Snoek M, Jansen HM, Bos JD,
van Lier RA and Kapsenberg ML: Human atopen-specific types 1 and 2
T helper cell clones. J Immunol. 147:2942–2949. 1991.PubMed/NCBI
|
|
9
|
Szegedi A, Baráth S, Nagy G, Szodoray P,
Gál M, Sipka S, Bagdi E, Banham AH and Krenács L: Regulatory T
cells in atopic dermatitis: epidermal dendritic cell clusters may
contribute to their local expansion. Br J Dermatol. 160:984–993.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oyoshi MK, He R, Kumar L, Yoon J and Geha
RS: Cellular and molecular mechanisms in atopic dermatitis. Adv
Immunol. 102:135–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eyerich K, Pennino D, Scarponi C, Foerster
S, Nasorri F, Behrendt H, Ring J, Traidl-Hoffmann C, Albanesi C and
Cavani A: IL-17 in atopic eczema: Linking allergen-specific
adaptive and microbial-triggered innate immune response. J Allergy
Clin Immunol. 123:59–66.e4. 2009. View Article : Google Scholar
|
|
12
|
Nakae S, Komiyama Y, Nambu A, Sudo K,
Iwase M, Homma I, Sekikawa K, Asano M and Iwakura Y:
Antigen-specific T cell sensitization is impaired in
IL-17-deficient mice, causing suppression of allergic cellular and
humoral responses. Immunity. 17:375–387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hengge UR, Ruzicka T, Schwartz RA and Cork
MJ: Adverse effects of topical glucocorticosteroids. J Am Acad
Dermatol. 54:1–15; quiz 16–18. 2006. View Article : Google Scholar
|
|
14
|
Yang G, Choi CH, Lee K, Lee M, Ham I and
Choi HY: Effects of Catalpa ovata stem bark on atopic
dermatitis-like skin lesions in NC/Nga mice. J Ethnopharmacol.
145:416–423. 2013. View Article : Google Scholar
|
|
15
|
Ieri F, Innocenti M, Andrenelli L, Vecchio
V and Mulinacci N: Rapid HPLC/DAD/MS method to determine phenolic
acids, glycoalkaloids and anthocyanins in pigmented potatoes
(Solanum tuberosum L.) and correlations with variety and
geographical origin. Food Chemistry. 125:750–759. 2011. View Article : Google Scholar
|
|
16
|
Mulinacci N, Ieri F, Giaccherini C,
Innocenti M, Andrenelli L, Canova G, Saracchi M and Casiraghi MC:
Effect of cooking on the anthocyanins, phenolic acids,
glycoalkaloids, and resistant starch content in two pigmented
cultivars of Solanum tuberosum L. J Agric Food Chem.
56:11830–11837. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Afaq F, Malik A, Syed D, Maes D, Matsui MS
and Mukhtar H: Pomegranate fruit extract modulates UV-B-mediated
phosphorylation of mitogen-activated protein kinases and activation
of nuclear factor kappa B in normal human epidermal keratinocytes
paragraph sign. Photochem Photobiol. 81:38–45. 2005. View Article : Google Scholar
|
|
18
|
Suto H, Matsuda H, Mitsuishi K, Hira K,
Uchida T, Unno T, Ogawa H and Ra C: NC/Nga mice: A mouse model for
atopic dermatitis. Int Arch Allergy Immunol. 120(Suppl 1): S70–S75.
1999. View Article : Google Scholar
|
|
19
|
Takano N, Arai I and Kurachi M: Analysis
of the spontaneous scratching behavior by NC/Nga mice: A possible
approach to evaluate antipruritics for subjects with atopic
dermatitis. Eur J Pharmacol. 471:223–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mihara K, Kuratani K, Matsui T, Nakamura M
and Yokota K: Vital role of the itch-scratch response in
development of spontaneous dermatitis in NC/Nga mice. Br J
Dermatol. 151:335–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Wesolowski J and Paumet F: The impact of
bacterial infection on mast cell degranulation. Immunol Res.
51:215–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee TY, Kim DJ, Won JN, Lee IH, Sung MH
and Poo H: Oral administration of poly-gamma-glutamate ameliorates
atopic dermatitis in Nc/Nga mice by suppressing Th2-biased immune
response and production of IL-17A. J Invest Dermatol. 134:704–711.
2014. View Article : Google Scholar
|
|
24
|
Koga C, Kabashima K, Shiraishi N,
Kobayashi M and Tokura Y: Possible pathogenic role of Th17 cells
for atopic dermatitis. J Invest Dermatol. 128:2625–2630. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bieber T: Atopic dermatitis. N Engl J Med.
358:1483–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akdis M, Simon HU, Weigl L, Kreyden O,
Blaser K and Akdis CA: Skin homing (cutaneous lymphocyte-associated
antigen-positive) CD8+ T cells respond to superantigen and
contribute to eosinophilia and IgE production in atopic dermatitis.
J Immunol. 163:466–475. 1999.PubMed/NCBI
|
|
27
|
Akdis CA, Akdis M, Simon D, Dibbert B,
Weber M, Gratzl S, Kreyden O, Disch R, Wüthrich B, Blaser K and
Simon HU: T cells and T cell-derived cytokines as pathogenic
factors in the nonallergic form of atopic dermatitis. J Invest
Dermatol. 113:628–634. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim GD, Kim TH, Park YS, Ahn HJ, Cho JJ
and Park CS: Immune response against
2,4-dinitrofluorobenzene-induced atopic dermatitis-like clinical
manifestation is suppressed by spermidine in NCNga mice. Scand J
Immunol. 81:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin YT, Wang CT, Chao PS, Lee JH, Wang LC,
Yu HH, Yang YH and Chiang BL: Skin-homing CD4+
Foxp3+ T cells exert Th2-like function after
staphylococcal superantigen stimulation in atopic dermatitis
patients. Clin Exp Allergy. 41:516–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Werfel T: The role of leukocytes,
keratinocytes, and allergen-specific IgE in the development of
atopic dermatitis. J Invest Dermatol. 129:1878–1891. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hinz D, Bauer M, Röder S, Olek S, Huehn J,
Sack U, Borte M, Simon JC and Lehmann I: Cord blood Tregs with
stable FOXP3 expression are influenced by prenatal environment and
associated with atopic dermatitis at the age of one year. Allergy.
67:380–389. 2012. View Article : Google Scholar
|
|
32
|
Mountford AP, Fisher A and Wilson RA: The
profile of IgG1 and IgG2a antibody responses in mice exposed to
Schistosoma mansoni. Parasite Immunol. 16:521–527. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
N'Dri D, Mazzeo T, Zaupa M, Ferracane R,
Fogliano V and Pellegrini N: Effect of cooking on the total
antioxidant capacity and phenolic profile of some whole-meal
African cereals. J Sci Food Agric. 93:29–36. 2013. View Article : Google Scholar
|
|
34
|
Eichhorn S and Winterhalter P:
Anthocyanins from pigmented potato (Solanum tuberosum L.)
varieties. Food Research International. 38:943–948. 2005.
View Article : Google Scholar
|
|
35
|
Han KH, Sekikawa M, Shimada K, Hashimoto
M, Hashimoto N, Noda T, Tanaka H and Fukushima M: Anthocyanin-rich
purple potato flake extract has antioxidant capacity and improves
antioxidant potential in rats. Br J Nutr. 96:1125–1133. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Afaq F, Saleem M, Krueger CG, Reed JD and
Mukhtar H: Anthocyanin- and hydrolyzable tannin-rich pomegranate
fruit extract modulates MAPK and NF-kappaB pathways and inhibits
skin tumorigenesis in CD-1 mice. Int J Cancer. 113:423–433. 2005.
View Article : Google Scholar
|
|
37
|
Zhang EY, Chen AY and Zhu BT: Mechanism of
dinitrochlorobenzene-induced dermatitis in mice: Role of specific
antibodies in pathogenesis. PLoS One. 4:e77032009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vestergaard C, Yoneyama H, Murai M,
Nakamura K, Tamaki K, Terashima Y, Imai T, Yoshie O, Irimura T,
Mizutani H and Matsushima K: Overproduction of Th2-specific
chemokines in NC/Nga mice exhibiting atopic dermatitis-like
lesions. J Clin Invest. 104:1097–1105. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kishimoto T and Hirano T: Molecular
regulation of B lymphocyte response. Annu Rev Immunol. 6:485–512.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hussain Z, Katas H, Mohd Amin MC and
Kumolosasi E: Efficient immuno-modulation of TH1/TH2 biomarkers in
2,4-dinitroflu-orobenzene-induced atopic dermatitis:
Nanocarrier-mediated transcutaneous co-delivery of
anti-inflammatory and antioxidant drugs. PLoS One. 9:e1131432014.
View Article : Google Scholar
|
|
41
|
Niebuhr M and Werfel T: Innate immunity,
allergy and atopic dermatitis. Curr Opin Allergy Clin Immunol.
10:463–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wollenberg A, Kraft S, Hanau D and Bieber
T: Immunomorphological and ultrastructural characterization of
Langerhans cells and a novel, inflammatory dendritic epidermal cell
(IDEC) population in lesional skin of atopic eczema. J Invest
Dermatol. 106:446–453. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bieber T: The pro- and anti-inflammatory
properties of human antigen-presenting cells expressing the high
affinity receptor for IgE (Fc epsilon RI). Immunobiology.
212:499–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Novak N, Valenta R, Bohle B, Laffer S,
Haberstok J, Kraft S and Bieber T: FcepsilonRI engagement of
Langerhans cell-like dendritic cells and inflammatory dendritic
epidermal cell-like dendritic cells induces chemotactic signals and
different T-cell phenotypes in vitro. J Allergy Clin Immunol.
113:949–957. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kasraie S, Niebuhr M, Baumert K and Werfel
T: Functional effects of interleukin 31 in human primary
keratinocytes. Allergy. 66:845–852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Meyer N, Zimmermann M, Bürgler S, Bassin
C, Woehrl S, Moritz K, Rhyner C, Indermitte P, Schmid-Grendelmeier
P, Akdis M, et al: IL-32 is expressed by human primary
keratinocytes and modulates keratinocyte apoptosis in atopic
dermatitis. J Allergy Clin Immunol. 125:858–865.e810. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Klunker S, Trautmann A, Akdis M, Verhagen
J, Schmid-Grendelmeier P, Blaser K and Akdis CA: A second step of
chemotaxis after transendothelial migration: Keratinocytes
undergoing apoptosis release IFN-gamma-inducible protein 10,
monokine induced by IFN-gamma, and IFN-gamma-inducible
alpha-chemoattractant for T cell chemotaxis toward epidermis in
atopic dermatitis. J Immunol. 171:1078–1084. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Homey B, Steinhoff M, Ruzicka T and Leung
DY: Cytokines and chemokines orchestrate atopic skin inflammation.
J Allergy Clin Immunol. 118:178–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sokol CL and Luster AD: The chemokine
system in innate immunity. Cold Spring Harb Perspect Biol. 7:pii:
a016303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nakatani T, Kaburagi Y, Shimada Y, Inaoki
M, Takehara K, Mukaida N and Sato S: CCR4 memory CD4+ T lymphocytes
are increased in peripheral blood and lesional skin from patients
with atopic dermatitis. J Allergy Clin Immunol. 107:353–358. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Amerio P, Frezzolini A, Feliciani C,
Verdolini R, Teofoli P, De Pità O and Puddu P: Eotaxins and CCR3
receptor in inflammatory and allergic skin diseases: Therapeutical
implications. Curr Drug Targets Inflamm Allergy. 2:81–94. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Esnault S, Benbernou N, Lavaud F, Shin HC,
Potron G and Guenounou M: Differential spontaneous expression of
mRNA for IL-4, IL-10, IL-13, IL-2 and interferon-gamma (IFN-gamma)
in peripheral blood mononuclear cells (PBMC) from atopic patients.
Clin Exp Immunol. 103:111–118. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Conti P, Pang X, Boucher W, Letourneau R,
Reale M, Barbacane RC, Thibault J and Theoharides TC: Impact of
Rantes and MCP-1 chemokines on in vivo basophilic cell recruitment
in rat skin injection model and their role in modifying the protein
and mRNA levels for histidine decarboxylase. Blood. 89:4120–4127.
1997.PubMed/NCBI
|
|
54
|
Imai T, Baba M, Nishimura M, Kakizaki M,
Takagi S and Yoshie O: The T cell-directed CC chemokine TARC is a
highly specific biological ligand for CC chemokine receptor 4. J
Biol Chem. 272:15036–15042. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schröder JM and Mochizuki M: The role of
chemokines in cutaneous allergic inflammation. Biol Chem.
380:889–896. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Galli SJ, Nakae S and Tsai M: Mast cells
in the development of adaptive immune responses. Nat Immunol.
6:135–142. 2005. View
Article : Google Scholar : PubMed/NCBI
|