Introduction
As the largest organ of the body, the skin is
frequently exposed to sunlight and ultraviolet rays. The function
of skin is to protect internal organs from environmental harm and
to maintain homeostasis (1), thus,
the skin requires protection from frequent exposure to ultraviolet
radiation, which may cause skin cancer. Skin cancer, the most
common type of cancer worldwide, is a global public health problem
and a burden on healthcare expenditures (2,3).
Therefore, more effective therapeutic strategies are required for
its treatment.
B-cell translocation gene 2 (BTG2) is an important
member of the BTG/TOB family (4,5).
Located on band 2, region 3 of the long arm of chromosome 1, BTG2
encodes a 158-amino acid protein and structural deletions or
changes often result in the occurrence of various tumors in humans
(6–8). Previous studies have determined that
BTG2 is a tumor suppressor of various cancer cells (4,5).
Zhang et al (9) reported
that BTG2 suppressed proliferation and invasion of MDA-MB-231
triple-negative breast cancer cells. Additionally, BTG2 inhibited
bladder cancer invasion via suppression of DNA methyltransferase 1
(10). BTG2 may also induce
cellular differentiation, counteract cellular transformation and
promote activity of pro-apoptotic stimuli (11–13).
Additionally, BTG2 expression is frequently downregulated or
blocked in various human tumors, including gastric and breast
cancer (14,15). However, the function of BTG2 in
skin cancer remains unclear.
The Wnt/β-catenin signaling pathway is important in
the progression of tumors, including skin cancer (16–18).
It was reported that mouse skin tumors demonstrated cytoplasmic and
nuclear accumulation of β-catenin, and upregulation of
β-catenin/Tcf target genes, including v-myc avian myelocytomatosis
viral oncogene homolog and c-jun (19). Thus, inhibiting the Wnt/β-catenin
signaling pathway may prevent the progression of skin cancer.
The present study investigated the expression and
function of BTG2 in skin cancer cells, as well as the underlying
molecular mechanism. The results demonstrated that BTG2 was
downregulated in skin cancer cells lines. Furthermore,
overexpression of BTG2 significantly inhibited cell proliferation,
cell cycle progression, cell invasion and migration of skin cancer
cells via the Wnt/β-catenin signaling pathway.
Materials and methods
Cell lines and culture
The human skin cancer cell lines (A431 and SCC13)
were obtained from Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences (Shanghai, China). All the cells were
cultured as monolayers to 80% confluence in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100
μg/ml penicillin-streptomycin (GE Healthcare, Logan, UT,
USA) in a humidified incubator with 5% CO2 at 37°C.
Plasmids and transfection assays
A431 and SCC13 cell lines were transfected with the
cDNA of the BTG2 gene. For stable transfection, pcDNA3.1 expression
vector (Thermo Fisher Scientific, Inc.) was used for insertion of
BTG2 cDNA between the KpnI and BamHI sites. The
orientation of the insertion was confirmed via restriction
digestion and DNA sequencing. Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to transfect the
pcDNA3.1-BTG2 expression vector or the empty pcDNA3.1 vector into
A431 and SCC13 cells, in accordance with the manufacturer's
protocol. The cells were then cultured in DMEM containing 800
μg/ml G418 for antibiotic selection. After 28 days, positive
clones were collected from the A431 and SCC13 cell cultures in the
pcDNA3.1-BTG2 vector transfection group (A431-BTG2 and SCC13-BTG2)
and the empty pcDNA3.1 vector transfection group (A431-PC and
SCC13-PC). Western blot analysis was performed in order to detect
BTG2 protein expression in the A431-BTG2, A431-PC, SCC13-BTG2 and
SCC13-PC cell groups.
Cell proliferation assay
Transfected and untreated cells were seeded into
96-well culture plates (Corning Life Sciences, MA, USA) at a
density of 1×104 cells/well and cultured for 24 h with
saturated humidity and 5% CO2 at 37°C to form a cell
monolayer. MTT solution (100 μl; Nalge Nunc International,
Roskilde, Denmark) was aliquoted per well and incubated with 5%
CO2 at 37°C for 4 h. Next, the DMEM was discarded and
150 μl dimethyl sulfoxide was added to each well. The
absorbance was measured at 570 nm with a microplate reader (BD
Biosciences, San Jose, CA, USA).
Cell cycle assay
When the cells reached 70–90% confluence, a
serum-free DMEM was applied for synchronization. The cells were
cultured for 24 h, then trypsinized (Sigma-Aldrich, St. Louis, MO,
USA) and fixed with 100% ethanol overnight, followed by propidium
iodide staining. Cell cycle analysis was performed after 30 min
using a flow cytometer (BioVision, Inc., CA, USA). Each experiment
was replicated three times.
Cell invasion and migration assays
Twenty-four well Transwell chambers (8 μm;
Corning Life Sciences) were used to detect the invasive and
migratory ability of the skin cancer cell lines. For the invasion
assay, the upper chamber was washed with serum-free DMEM three
times, filled with 20 μl diluted Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA) and incubated for 30 min at 37°C to form
the artificial basement membrane. Subsequently, all the cells were
suspended in the serum-free DMEM and added to the upper chamber.
The lower chamber was filled with DMEM containing 10% FBS. The
cells were incubated for 24 h and those remaining in the upper
chamber surface of the basement membrane were removed with cotton
swabs, cells invading the lower chamber surface were stained with
crystal violet. The number of cells crossing the polycarbonate
membrane was counted under a microscope (CX22; Olympus Corporation,
Tokyo, Japan), at a magnification of ×400. The migration assay was
performed according to the above-mentioned procedure, except that
Matrigel was not used, instead the Transwell chamber was used
alone.
Western blotting
The cells were lysed using a lysis buffer
(Sigma-Aldrich). The total protein (30 μg) was separated
from each group by 10% SDS-PAGE (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and transferred onto polyvinylidene fluoride
membranes (Thermo Fisher Scientific, Inc.). The membrane was
blocked at room temperature for 1 h with Tris-buffered saline (TBS)
containing 5% non-fat milk and then incubated overnight at 4°C with
the mouse primary antibodies (all from Santa Cruz Biotechnology,
Dallas, TX, USA) against BTG2 (1:2,500; cat. no. sc-517187),
β-catenin (1:2,000; cat. no. sc-53484), cyclin D1 (1:3,000; cat.
no. sc-20044), v-myc avian myelocytomatosis viral oncogene homolog
(c-Myc; 1:2,500; cat. no. sc-47694) and β-actin (1:1,500; cat. no.
sc-130300). After washing with TBS Tween-20 (TBST), the membrane
was incubated for 1 h at 37°C with horseradish
peroxidase-conjugated goat anti-mouse polyclonal antibody (1:3,000;
Santa Cruz Biotechnology, Inc.; cat. no. 395760). Subsequent to
another wash with TBST, protein expression was detected using an
enhanced chemiluminescence kit (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis of experimental data was
performed with SPSS 17.0 statistical analysis software (SPSS, Inc.,
Chicago, IL, USA). The Student's t-test was conducted for the
comparison of two groups and one way analysis of variance was
performed for multiple comparisons. Data are presented as the mean
± standard deviation and P<0.05 was considered to indicate a
statistically significant difference.
Results
BTG2 expression in A431 and SCC13 cell
lines and their transfectants
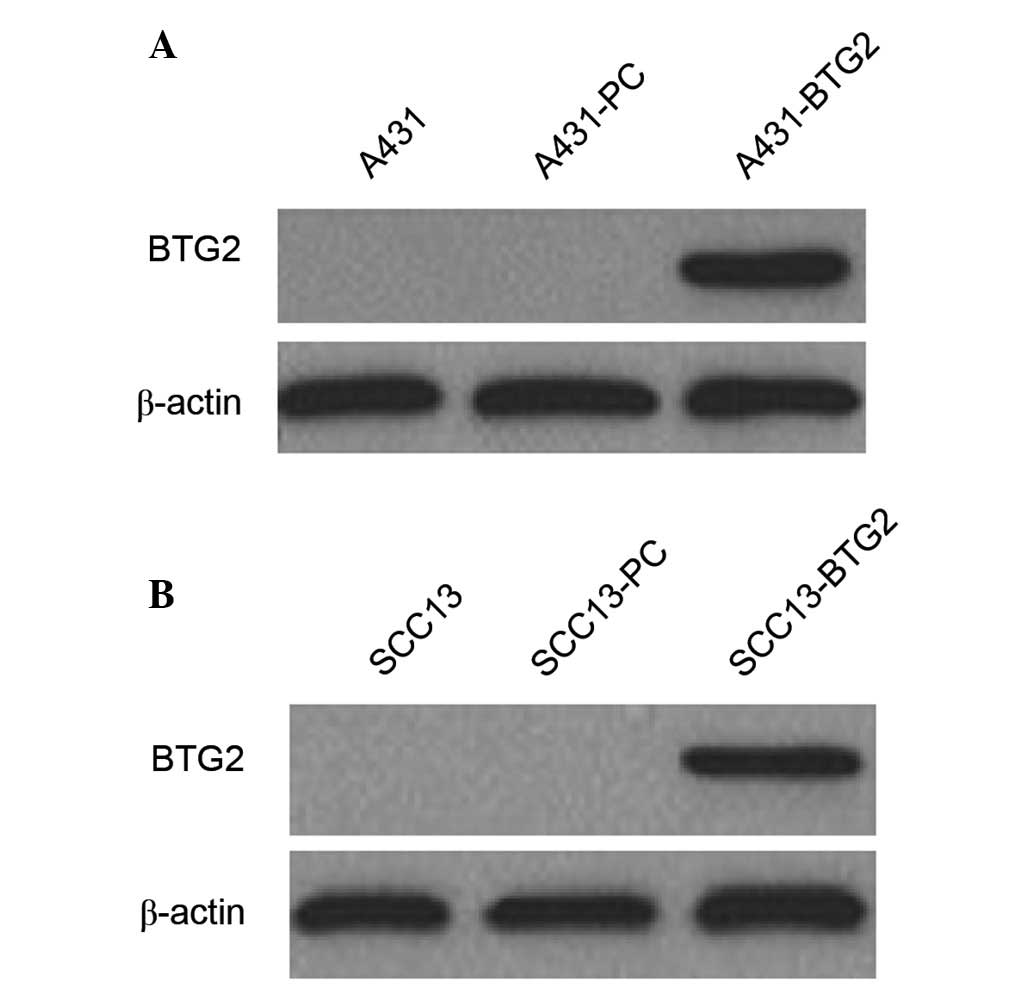
BTG2 protein expression in A431 and SCC13 cells and
their transfectants was detected by western blot analysis. BTG2
protein expression was observed in A431-BTG2 and SCC13-BTG2 cells;
however, it was not detected in A431, A431-PC, SCC13 and SCC13-PC
cells (Fig. 1).
Effects of BTG2 overexpression on cell
proliferation
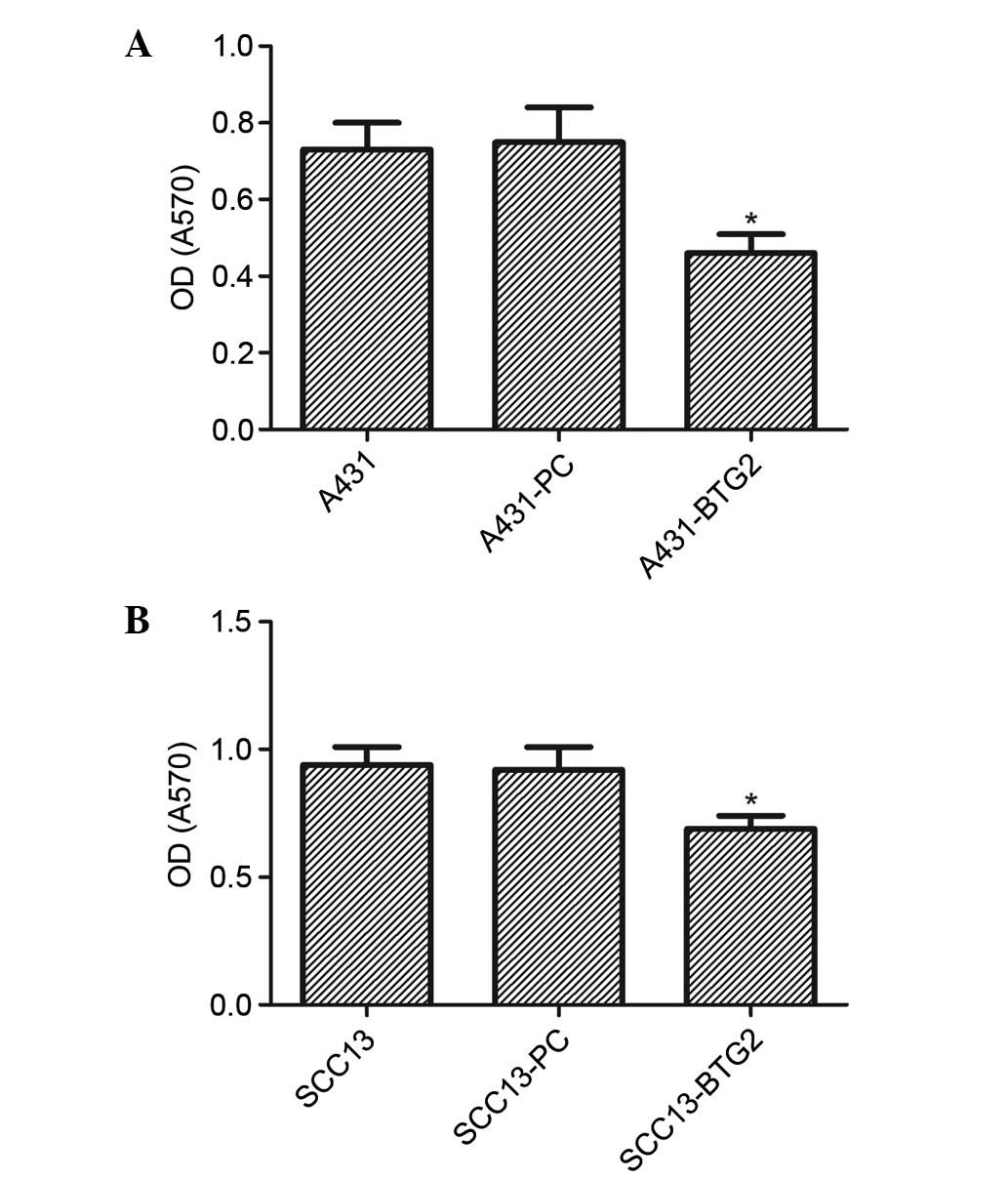
The proliferation of A431-BTG2 cells was
significantly reduced in comparison with the A431-PC and untreated
A431 cells (P<0.05). No significant difference was identified
between the control groups (A431 and A431-PC; Fig. 2A). Cell proliferation in the
SCC13-BTG2 group was significantly reduced when compared with the
control groups (P<0.05; Fig.
2B).
Effects of BTG2 overexpression on cell
cycle progression
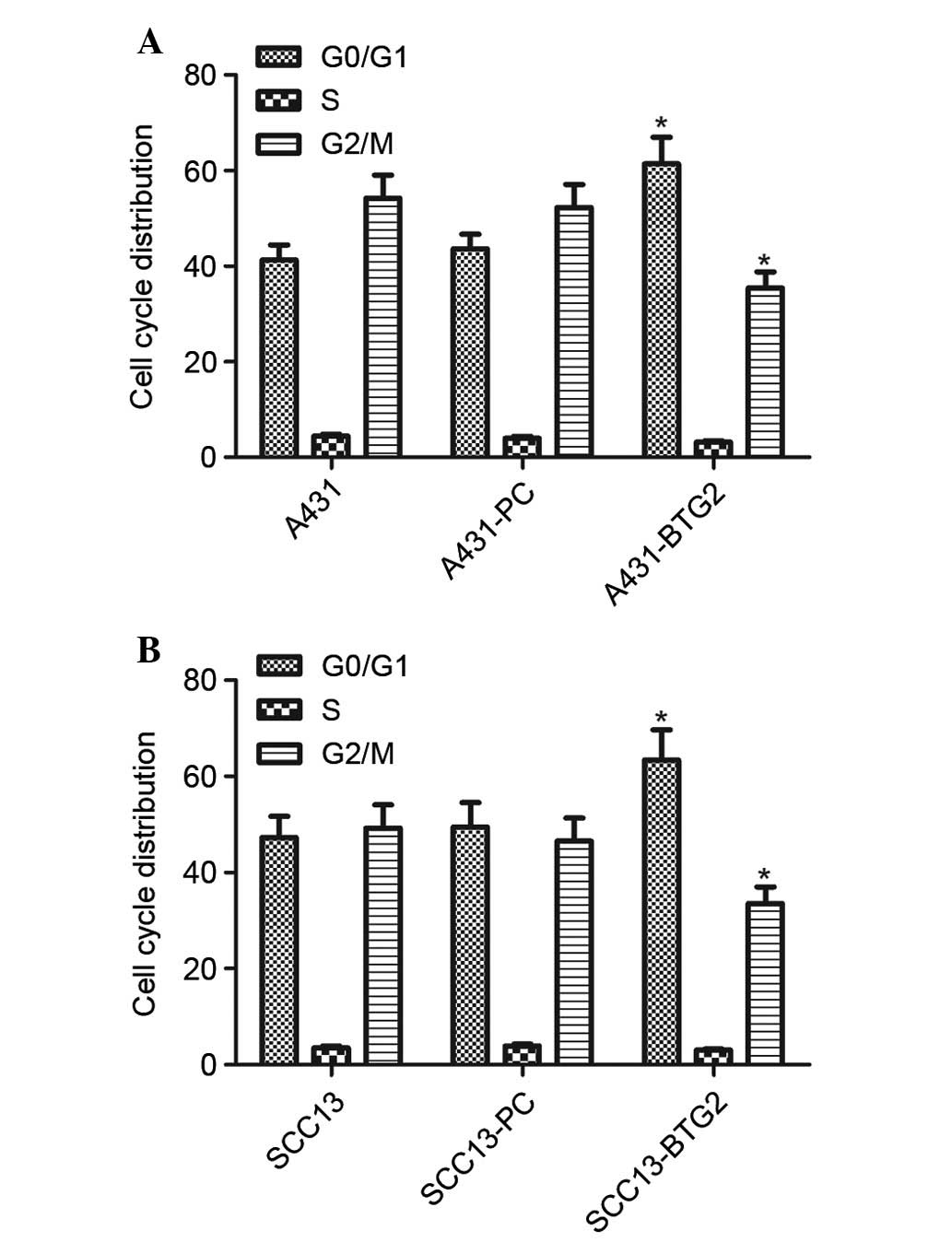
Cell cycle analysis of A431 cells in each group was
performed using flow cytometry. BTG2 overexpression significantly
increased the number of A431 and SCC13 cells in the
G0/G1 phase; however, it decreased the number
of A431 and SCC13 cells in the G2/M phase (P<0.05;
Fig. 3). These results suggested
that cell cycle distribution in A431 and SCC13 cells was affected
by increased BTG2 expression levels and resulted in cell cycle
arrest in the G0/G1 phase in A431 and SCC13
cells.
Effects of BTG2 overexpression on cell
invasion and migration
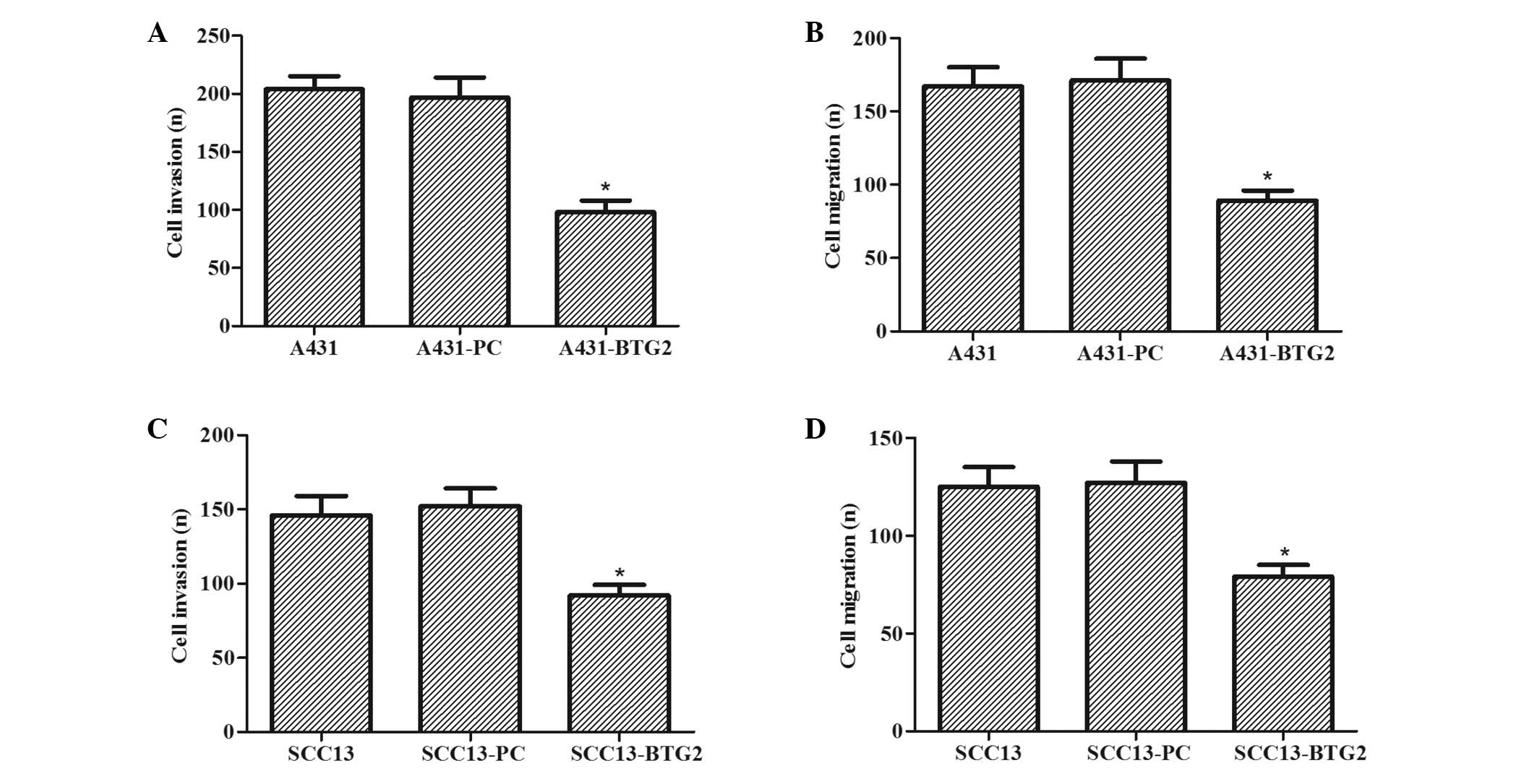
The effects of BTG2 overexpression on invasion and
migration of A431 and SCC13 cells were investigated. As shown in
Fig. 4A and B, the invasive and
migratory abilities of A431-BTG2 cells were significantly reduced
when compared with A431-PC and untreated A431 cells (P<0.05).
The effects of BTG2 overexpression on the invasion and migration of
SCC13 cells were also determined. As shown in Fig. 4C and D, invasive and migratory
abilities of SCC13-BTG2 cells were significantly reduced when
compared with SCC13-PC and untreated SCC13 cells (P<0.05).
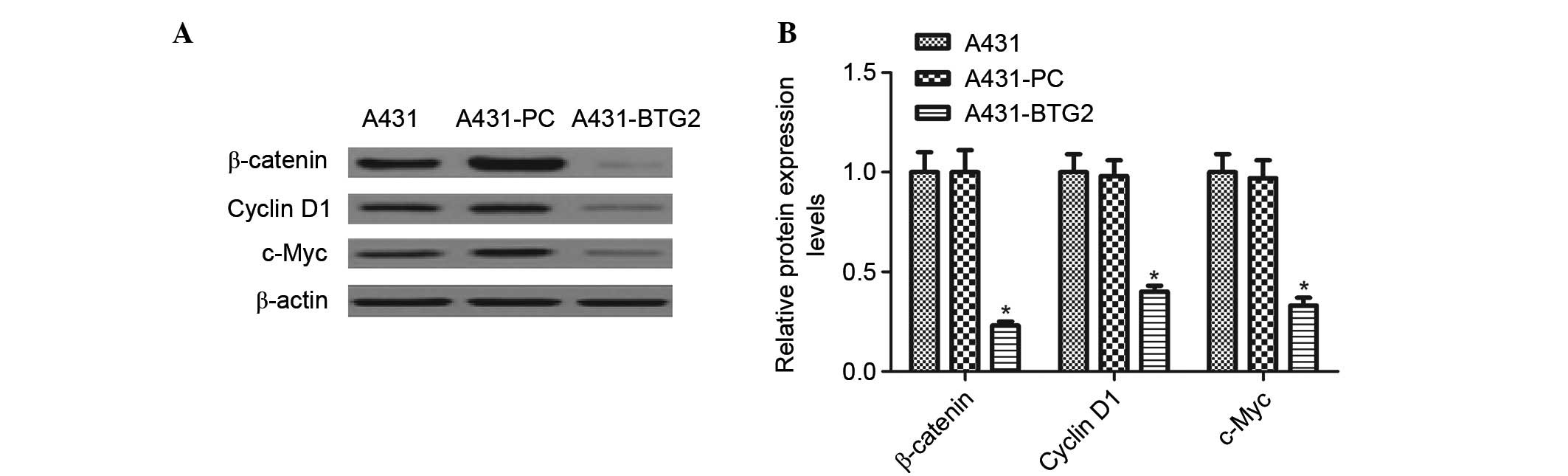
Effects of BTG2 overexpression on
β-catenin, cyclin D1 and c-Myc expression levels
Western blot analysis was performed to detect the
effects of BTG2 overexpression on β-catenin, cyclin D1 and c-Myc. A
significant decrease in the expression levels of β-catenin, cyclin
D1 and c-Myc in A431-BTG2 cells when compared with A431-PC and
untreated A431 cells (P<0.05; Fig.
5).
Discussion
BTG2, a transient early response protein, has been
reported to be a potential tumor suppressor gene (6,12,20,21).
Previous studies have highlighted the importance of BTG2 in tumor
incidence, development, metastasis and invasion (14,22,23).
BTG2 overexpression may suppress proliferation of prostate cancer
cells (24,25). Additionally, BTG2 has frequently
been downregulated or dysfunctional in previous studies of
cancerous tumors (6,26,27).
Takahashi et al (23)
determined that low expression levels of BTG2 in breast cancer may
inhibit tumor metastasis (23).
These studies indicated the association between BTG2 and tumor
progression, and the importance of BTG2 in tumor incidence and
proliferation. However, whether BTG2 is expressed in skin cancer
and the effects BTG2 exerts on skin cancer remained unclear.
The present study investigated the expression and
function of BTG2 in skin cancer progression and BTG2 expression was
not observed in the skin cancer cell lines. Additionally,
upregulation of BTG2 markedly inhibited cell proliferation, cell
cycle progression, cell invasion and migration of skin cancer
cells.
The cell cycle assay performed in the current study
indicated that BTG2 overexpression may significantly increase the
number of cells in the G0/G1 phase and
decrease those in the S and G2 phases. This was
consistent with the results of the cell proliferation assay
performed in the present study, indicating that BTG2 exerted an
inhibitory effect on cell proliferation. These results demonstrated
the possible association between BTG2 overexpression and cell
proliferation inhibition.
The effects of BTG2 on invasion and migration of
skin cancer cells was examined using a Transwell assay. There was a
significant decrease in cells crossing the polycarbonate membrane
of the Transwell chamber, suggesting that BTG2 overexpression may
be associated with the inhibition of skin cancer cell invasion and
migration.
The present study demonstrated that overexpression
of BTG2 significantly decreased the expression levels of β-catenin,
cyclin D1 and c-Myc. The Wnt signaling pathway is fundamental for
the determination of cell fate, polarity, proliferation and death
(28). Additionally, environmental
and genetic perturbations of the Wnt signaling pathway may lead to
a variety of human diseases including various types of cancer
(29). β-catenin, and its
accumulation within the nucleus and cytoplasm, is associated with
various types of cancer and is important in the successful
functioning of Wnt signaling (30). In the current study, β-catenin was
highly expressed in skin cancer cells and BTG2 overexpression
significantly inhibited its expression levels. As target genes of
β-catenin, cyclin D1 and c-Myc are powerful proto-oncogenes with
similar downstream effects (31)
and the two are associated with cell cycle control (32,33).
The present study identified a significant decrease in their
expression levels, which may explain the inductive effect of BTG2
on the cell cycle arrest at the G0/G1 phase
in skin cancer cells.
In conclusion, the present study investigated the
effects of BTG2 overexpression on skin cancer cells and evaluated
the underlying molecular mechanism. The results demonstrated that
BTG2 was not expressed in skin cancer cells lines and its
upregulation may suppress cell proliferation, cell cycle
progression, cell invasion and migration of skin cancer cells via
the Wnt/β-catenin signaling pathway. Therefore, BTG2 may serve as a
potential target for the treatment of skin cancer.
References
|
1
|
Kumar A, Shrestha PR, Pun J, Thapa P,
Manandhar M and Sathian B: Profile of skin biopsies and patterns of
skin cancer in a tertiary care center of Western Nepal. Asian Pac J
Cancer Prev. 16:3403–3406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang YJ and Bikle DD: LncRNA: A new
player in 1α, 25(OH)(2) vitamin D(3)/VDR protection against skin
cancer formation. Exp Dermatol. 23:147–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh T and Katiyar SK: Green tea
polyphenol, (-)-epigallocatechin-3-gallate, induces toxicity in
human skin cancer cells by targeting β-catenin signaling. Toxicol
Appl Pharmacol. 273:418–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikematsu N, Yoshida Y, Kawamura-Tsuzuku J,
Ohsugi M, Onda M, Hirai M, Fujimoto J and Yamamoto T: Tob2, a novel
anti-proliferative Tob/BTG1 family member, associates with a
component of the CCR4 transcriptional regulatory complex capable of
binding cyclin-dependent kinases. Oncogene. 18:7432–7441. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buanne P, Corrente G, Micheli L, Palena A,
Lavia P, Spadafora C, Lakshmana MK, Rinaldi A, Banfi S, Quarto M,
et al: Cloning of PC3B, a novel member of the PC3/BTG/TOB family of
growth inhibitory genes, highly expressed in the olfactory
epithelium. Genomics. 68:253–263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim IK: TIS21 (/BTG2/PC3) as a link
between ageing and cancer: Cell cycle regulator and endogenous cell
death molecule. J Cancer Res Clin Oncol. 132:417–426. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winkler GS: The mammalian
anti-proliferative BTG/Tob protein family. J Cell Physiol.
222:66–72. 2010. View Article : Google Scholar
|
|
8
|
Duriez C, Moyret-Lalle C, Falette N,
El-Ghissassi F and Puisieux A: BTG2, its family and its tutor. Bull
Cancer. 91:E242–E253. 2004.PubMed/NCBI
|
|
9
|
Zhang Y-J, Wei L, Liu M, Li J, Zheng Y-Q,
Gao Y and Li X-R: BTG2 inhibits the proliferation, invasion, and
apoptosis of MDA-MB-231 triple-negative breast cancer cells. Tumor
Biol. 34:1605–1613. 2013. View Article : Google Scholar
|
|
10
|
Devanand P, Kim SI, Choi YW, Sheen SS, Yim
H, Ryu MS, Kim SJ, Kim WJ and Lim IK: Inhibition of bladder cancer
invasion by Sp1-mediated BTG2 expression via inhibition of DNA
methyltransferase 1. FEBS J. 281:5581–5601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
el-Ghissassi F, Valsesia-Wittmann S,
Falette N, Duriez C, Walden PD and Puisieux A: BTG2 (TIS21/PC3)
induces neuronal differentiation and prevents apoptosis of
terminally differentiated PC12 cells. Oncogene. 21:6772–6778. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boiko AD, Porteous S, Razorenova OV,
Krivokrysenko VI, Williams BR and Gudkov AV: A systematic search
for downstream mediators of tumor suppressor function of p53
reveals a major role of BTG2 in suppression of Ras-induced
transformation. Genes Dev. 20:236–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lim YB, Park TJ and Lim IK: B cell
translocation gene 2 enhances susceptibility of HeLa cells to
doxorubicin-induced oxidative damage. J Biol Chem. 283:33110–33118.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Huang H, Wu K, Wang M and Wu B:
Impact of BTG2 expression on proliferation and invasion of gastric
cancer cells in vitro. Mol Biol Rep. 37:2579–2586. 2010. View Article : Google Scholar
|
|
15
|
Möllerström E, Kovács A, Lövgren K, Nemes
S, Delle U, Danielsson A, Parris T, Brennan DJ, Jirström K,
Karlsson P and Helou K: Up-regulation of cell cycle arrest protein
BTG2 correlates with increased overall survival in breast cancer,
as detected by immunohistochemistry using tissue microarray. BMC
Cancer. 10:2962010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Ji L, Chen J, Zhang W and Ye Z:
Wnt/β-catenin signaling pathway in skin carcinogenesis and therapy.
Biomed Res Int. 2015:9648492015.
|
|
17
|
Kuphal S and Bosserhoff AK:
Phosphorylation of β-catenin results in lack of β-catenin signaling
in melanoma. Int J Oncol. 39:235–243. 2011.PubMed/NCBI
|
|
18
|
Kang MI, Baker AR, Dextras CR, Cabarcas
SM, Young MR and Colburn NH: Targeting of noncanonical Wnt5a
signaling by AP-1 blocker dominant-negative Jun when it inhibits
skin carcinogenesis. Genes Cancer. 3:37–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhatia N and Spiegelman VS: Activation of
Wnt/beta-catenin/Tcf signaling in mouse skin carcinogenesis. Mol
Carcinog. 42:213–221. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Z, Chen C, Wang G, Yang Z, San J,
Zheng J, Li Q, Luo X, Hu Q, Li Z and Wang D: Aberrant expression of
the p53-inducible antiproliferative gene BTG2 in hepatocellular
carcinoma is associated with overexpression of the cell
cycle-related proteins. Cell Biochem Biophys. 61:83–91. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horvilleur E, Bauer M, Goldschneider D,
Mergui X, de la Motte A, Bénard J, Douc-Rasy S and Cappellen D:
p73alpha isoforms drive opposite transcriptional and
post-transcriptional regulation of MYCN expression in neuroblastoma
cells. Nucleic Acids Res. 36:4222–4232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang CH, Yue J, Pfeffer SR, Handorf CR and
Pfeffer LM: MicroRNA miR-21 regulates the metastatic behavior of
B16 melanoma cells. J Biol Chem. 286:39172–39178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi F, Chiba N, Tajima K, Hayashida
T, Shimada T, Takahashi M, Moriyama H, Brachtel E, Edelman E,
Ramaswamy S and Maheswaran S: Breast tumor progression induced by
loss of BTG2 expression is inhibited by targeted therapy with the
ErbB/HER inhibitor lapatinib. Oncogene. 30:3084–3095. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Segev DL, Kucirka LM, Oberai PC, Parekh
RS, Boulware LE, Powe NR and Montgomery RA: Age and comorbidities
are effect modifiers of gender disparities in renal
transplantation. J Am Soc Nephrol. 20:621–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu M, Wu H, Liu T, Li Y, Wang F, Wan H,
Li X and Tang H: Regulation of the cell cycle gene, BTG2, by miR-21
in human laryngeal carcinoma. Cell Res. 19:828–837. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hagan S, Al-Mulla F, Mallon E, Oien K,
Ferrier R, Gusterson B, García JJ and Kolch W: Reduction of Raf-1
kinase inhibitor protein expression correlates with breast cancer
metastasis. Clin Cancer Res. 11:7392–7397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawakubo H, Brachtel E, Hayashida T, Yeo
G, Kish J, Muzikansky A, Walden PD and Maheswaran S: Loss of B-cell
translocation gene-2 in estrogen receptor-positive breast carcinoma
is associated with tumor grade and overexpression of cyclin d1
protein. Cancer Res. 66:7075–7082. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito-Diaz K, Chen TW, Wang X, Thorne CA,
Wallace HA, Page-McCaw A and Lee E: The way Wnt works: Components
and mechanism. Growth Factors. 31:1–31. 2013. View Article : Google Scholar :
|
|
29
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Wang X, Gong W, Mi B, Liu S and
Jiang B: Increased expression of beta-catenin, phosphorylated
glycogen synthase kinase 3beta, cyclin D1, and c-myc in laterally
spreading colorectal tumors. J Histochem Cytochem. 57:363–371.
2009. View Article : Google Scholar :
|
|
31
|
Ripple MJ, Parker Struckhoff A,
Trillo-Tinoco J, Li L, Margolin DA, McGoey R and Del Valle L:
Activation of c-Myc and Cyclin D1 by JCV T-Antigen and β-catenin in
Colon Cancer. PLoS One. 9:e1062572014. View Article : Google Scholar
|
|
32
|
Baldin V, Lukas J, Marcote M, Pagano M and
Draetta G: Cyclin D1 is a nuclear protein required for cell cycle
progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dang CV: c-Myc target genes involved in
cell growth, apoptosis, and metabolism. Mol Cell Biol. 19:1–11.
1999. View Article : Google Scholar
|