Introduction
Fucoidan, a sulfated polysaccharide, is found in
edible brown algae, such as Undaria pinnatifida, Fucus
vesiculosus and Ecklonia cava (1). A number of previous studies have
shown that fucoidan possesses a wide variety of biological
activities, including anti-coagulant (2), anti-thrombotic (3), anti-tumor (4), anti-viral (5), and anti-inflammatory activity
(6). A previous study indicated
that fucoidan attenuates pulmonary inflammation and reduces
Th2-dominated responses, and may provide a possible strategy for
treating allergic inflammation (7). Additionally, a previous study
reported that fucoidan resulted in the expression of inducible
nitric oxide synthase (iNOS) in macrophage cells. In particular,
low concentration ranges of fucoidan (10 µg/ml) inhibited
lipopolysaccharide (LPS)-induced nitric oxide (NO) via activator
protein-1, which may be associated with anti-inflammatory effects
(8). It has also been reported
that fucoidan suppresses interferon (IFN)-γ-induced NO/iNOS
production in glial cells via the inhibition of Janus kinase
(JAK)/signal transducer and activator (STAT)/isoflavone reductase
homolog 1 (IFR-1) and phospho-p38 (9).
A recent study indicated that fucoidan inhibited the
LPS-induced generation of reactive oxygen species (ROS), which
ultimately alleviated inflammation (10). Additionally, fucoidan-mediated
apoptosis in cancer cells involved the generation of ROS, which are
responsible for the loss of the mitochondrial membrane potential
and the downregulation of Bcl-2 proteins (11). Therefore, fucoidan may be useful as
a dietary supplement due to potential effects on preventing human
disease, additionally, the polysaccharide has no associated
toxicity or irritation (12).
Despite evidence of a number of biological effects of fucoidan, the
inflammatory cytokine combination-induced stimulation on human
keratinocytes has not been investigated previously. In the present
study, the molecular mechanisms of fucoidan against anti-oxidant
activity through the extracellular signal-regulated kinase
(ERK)/nuclear factor erythroid 2-related factor 2 (Nrf2)/heme
oxygenase-1 (HO-1) signaling pathway was investigated.
Materials and methods
Materials
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) and PD98059 were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), and streptomycin-penicillin (100 units/ml) were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Primary antibodies used in the present study included the
following: Mouse anti-HO-1 (cat. no. sc-136960), mouse anti-β-actin
(cat. no. sc-47778), mouse anti-superoxide dismutase-1 (SOD-1; cat.
no. sc-101523), rabbit anti-Nrf2 (cat. no. sc-722), mouse
anti-TATA-box binding protein (TBP; cat. no. sc-74595) and goat
anti-kelch-like ECH-associated protein 1 (Keap1; cat. no. sc-15246)
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA);
and rabbit anti-ERK (cat. no. 4695) and rabbit anti-phospho-ERK
(cat. no. 4370) from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Secondary antibodies included goat anti-rabbit (cat. no. sc
2004) goat anti-mouse (cat. no. sc-2005) and rabbit anti-goat (cat.
no. sc-2768) (Santa Cruz Biotechnology Inc.).
Cell culture
The human keratinocyte cell line (HaCaT) was
purchased from the American Type Culture Collection (Manassas, VA,
USA). Cells were maintained in DMEM supplemented with 10% FBS, 100
units/ml penicillin and 100 µg/ml streptomycin in a
humidified incubator under 37°C, 5% CO2 atmosphere. Cell
counts were performed in a hemocytometer from Hausser Scientific
(Horsham, PA, USA). For the experiments, fucoidan was dissolved in
dimethylsulfoxide (DMSO) at concentrations not exceeding 0.01% and
was then directly applied to the culture medium.
Cell viability assay
The cytotoxic effect of fucoidan on cell viability
was estimated with a colorimetric assay using the MTT method, which
is based on the reduction of a tetrazolium salt by a mitochondrial
dehydrogenase in viable cells (13). Briefly, cells were seeded in
96-well plates and then treated with fucoidan at 1, 5, 10, 30, 50,
70 and 100 µg/ml. After 24 h of incubation, MTT solution was added
to each well at a final concentration of 50 µg/ml. Following
2 h of incubation, the supernatants were aspirated and replaced
with 150 µl of DMSO to dissolve the formazan product. The
absorbance at 540 nm was read on a spectrophotometric plate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Results were
calculated as percentages of the unexposed control.
Preparation of nuclear extracts
Cells were harvested and lysed on ice in 1 ml lysis
buffer (10 mM Tris-HCl, pH 7.9; 10 mM NaCl; 3 mM MgCl2;
and 1% NP-40) for 4 min. Following centrifugation at 3,000 × g for
10 min, the pellets were resuspended in 50 µl extraction buffer (20
mM HEPES, pH 7.9; 20% glycerol; 1.5 mM MgCl2; 300 mM
NaCl; 0.2 mM EDTA, 1 mM dithiothreitol; and 1 mM
phenylmethylsulfonyl fluoride), incubated on ice for 30 min, and
centrifuged at 13,000 × g for 5 min. Supernatants were harvested
and stored at −70°C following measurement of the protein
concentration.
Immunocytochemistry
Cells were plated on coverslips, fixed with 4%
paraformaldehyde for 30 min, and permeabilized with
phosphate-buffered saline (PBS) containing 0.1% Triton X-100 for
2.5 min. They were then treated with blocking medium (PBS
containing 3% bovine serum albumin) for 1 h and incubated with a
primary anti-Nrf2 antibody (dilution, 1:1,000) in blocking medium
for 2 h. The immunoreacted primary antibody was detected by
incubating cells with a fluorescein isothiocyanate-conjugated
secondary antibody (1:500 dilution; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) for 1 h. Following washing
with PBS, stained cells were mounted onto microscope slides in
mounting medium containing DAPI to label nuclei (Vector
Laboratories, Inc., Burlingame, CA, USA). Images were collected
using a Zeiss confocal microscope and Zeiss LSM 510 software
(version 3.2; Zeiss AG, Oberkochen, Germany).
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from cells using the
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions and quantified by
spectrophotometry. RT was conducted in a reaction composed of 40
µl 1.25X reacting mix (Invitrogen; Thermo Fisher Scientific,
Inc.), 1 µl enzyme mix (Invitrogen; Thermo Fisher
Scientific, Inc.), 1 µl forward primer and 1 µl
reverse primer. cDNA synthesis was performed using the following
conditions: 94°C for 20 min, followed by denaturation at 94°C for 2
min. PCR was subsequently performed using following conditions:
Initial denaturation at 94°C for 5 min, followed by 40 cycles of
94°C for 15 sec, 52–65°C for 30 sec, and 68°C for 1 min. The final
cycle was followed by an extension step at 72°C for 5 min. The
primer pairs (Bioneer Corporation, Daejeon, Korea) were as follows
(forward and reverse, respectively): HO-1,
5′-CCAGAAAGTGGGCATCAGCT-3′ and 5′-GTCACATTTATGCTCGGCGG-3′; SOD-1,
5′-CAGCATGGGTTCCACGTCCA-3′ and 5′-CACATTGGCCACACCGTCCT-3′; and
β-actin, 5′-CCTCTATGCCAACACAGTGC-3′ and 5′-ATACTCCTGCTTGCTGATCC-3′.
Amplified products were resolved on a 1.2% agarose gel and
visualized with UV light following staining with ethidium bromide.
Densitometry was performed for semi-quantification using Image J
(National Institutes of Health, Bethesda, MD, USA).
Western blotting analysis
Western blot analyses were performed as previously
described (14). The cells were
cultured, harvested, lysed on ice for 30 min in lysis buffer [120
mM NaCl, 40 mM Tris (pH 8.0), and 0.1% NP 40] and centrifuged at
13,000 × g for 15 min. The protein concentration was determined
using a bicinchoninic acid assay. Lysates from each sample were
mixed with 5X sample buffer [0.375 M Tris-HCl, 5% sodium dodecyl
sulfate (SDS), 5% β-mercaptoethanol, 50% glycerol, 0.05%
bromophenol blue (pH 6.8)] and heated to 95°C for 5 min. Equal
amounts of protein (40 µg) were separated by 12% SDS-polyacrylamide
gel electrophoresis and transferred onto a nitrocellulose membrane.
The membranes were washed with Tris-buffered saline (10 mM Tris,
150 mM NaCl) containing 0.05% Tween-20 (TBST) and blocked in TBST
containing 5% non-fat dried milk. The membranes were then incubated
with HO-1, SOD1, Nrf2, Keap1, ERK, phospho-ERK, β-actin and TBP
primary antibodies (dilution, 1:1,000) overnight at 4°C. Following
three washes in TBST, membranes were incubated with appropriate
HRP-conjugated secondary antibodies (dilution, 1,200) for 1 h at
room temperature. The membranes were further washed, and detected
by an enhanced chemiluminescence western blotting detection kit
(Bio-Rad Laboratories, Inc.). β-actin and TBP were used as loading
controls for the nuclear and whole cell extracts, respectively. The
values for the specific protein levels are presented as the
fold-change relative to the control and densitometry was performed
using Image J.
Statistical analysis
All measurements were made in triplicate, and all
values are presented as the mean ± the standard deviation. The
results were subjected to one-way analysis of variance followed by
Tukey's test in order to analyze the differences between
conditions. P<0.05 was considered to indicate a statistically
significant difference.
Results
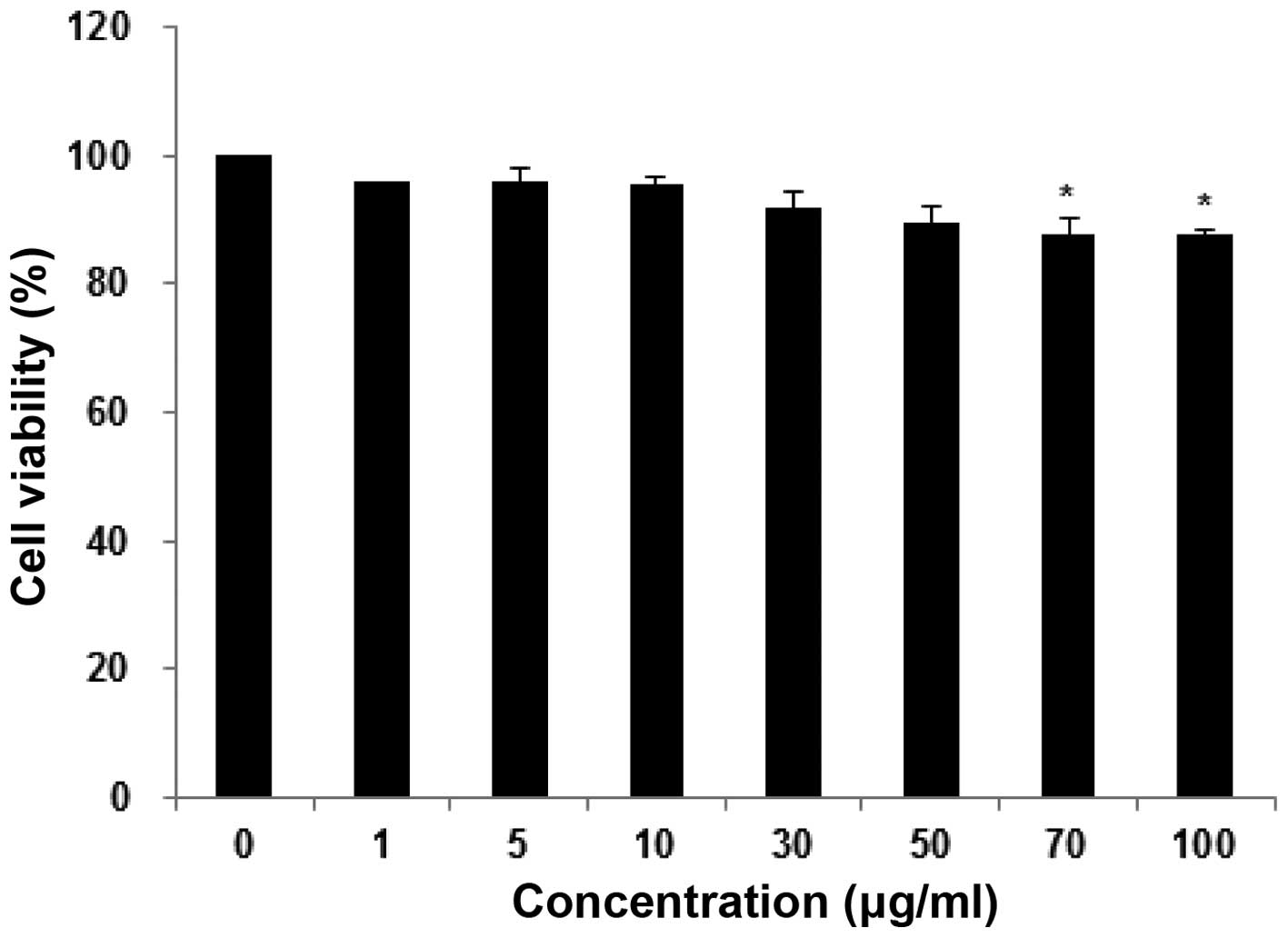
Effects of fucoidan on the viability of
human keratinocytes
To determine whether fucoidan can induce
cytotoxicity in HaCaT cells, an MTT assay was used. As shown in
Fig. 1, cells were incubated with
fucoidan at various concentrations ranging from 1–100 µg/ml
for 24 h. It was demonstrated that, beyond the concentration of 50
µg/ml, no significant alteration in cell viability was
observed. However, fucoidan at 100 µg/ml exhibited
significant cytotoxic effects on these cells. Thus, fucoidan at
1–50 µg/ml was selected for use in subsequent
experiments.
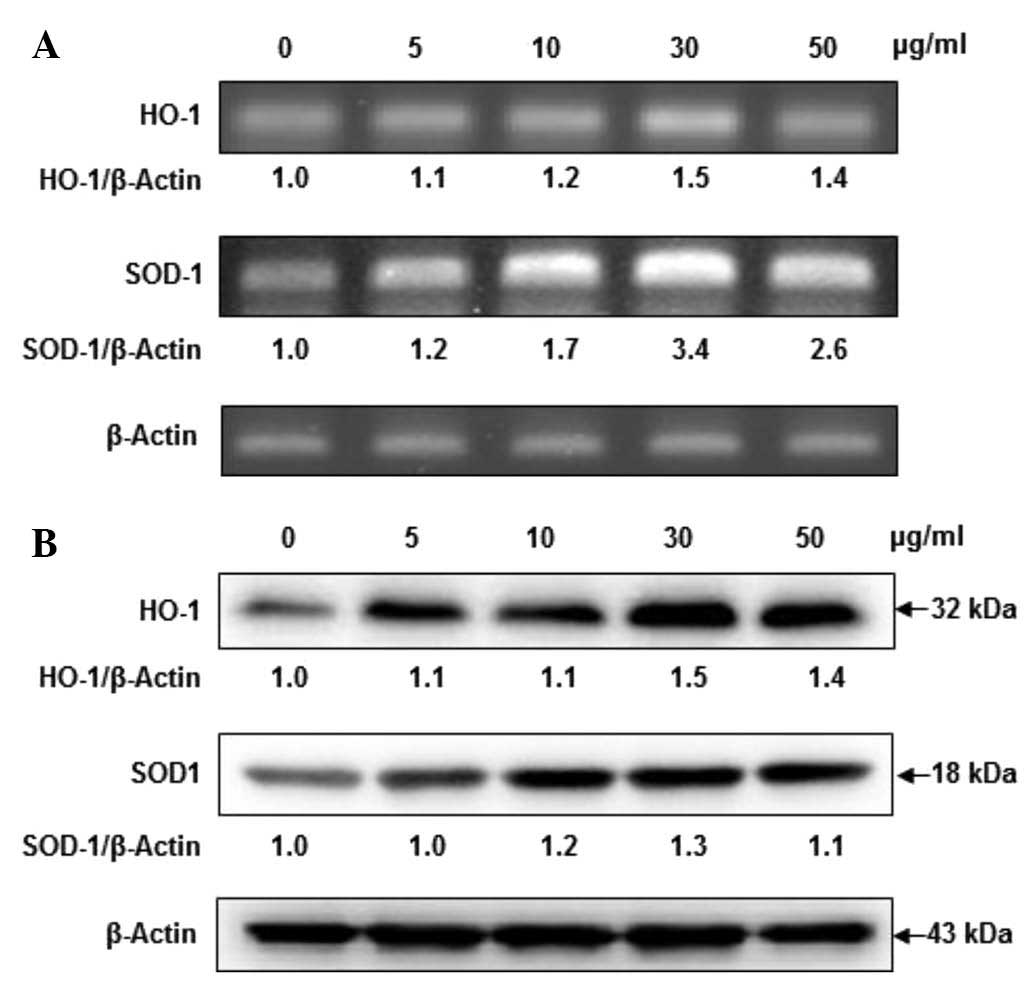
Fucoidan dose-dependently induces HO-1
and SOD-1 expression
When cell viability was assessed by MTT assay,
fucoidan did not exhibit any cytotoxicity at concentrations of 5,
10, 30 and 50 µg/ml. To determine whether fucoidan alters
HO-1 and SOD-1 mRNA and protein expression levels, HaCaT cells were
incubated in the presence of fucoidan. Fucoidan at concentrations
of 5, 10 and 30 µg/ml dose-dependently increased HO-1 mRNA
levels in HaCaT cells, while fucoidan at concentrations of 50
µg/ml slightly reduced expression relative to the levels
induced by 30 µg/ml fucoidan. As presented in Fig. 2, the SOD-1 mRNA levels were
markedly increased in a dose-dependent manner at 30 µg/ml
fucoidan; however, fucoidan at 50 µg/ml exerted a slight
reduction. Furthermore, fucoidan additionally upregulated HO-1 and
SOD-1 protein expression levels in a dose-dependent manner, with a
clear effect at 30 µg/ml of fucoidan. From these results, 30
µg/ml of fucoidan was selected as the optimal concentration
for subsequent experiments.
Fucoidan time-dependently induces HO-1
and SOD-1 expressions
To investigate the effects of fucoidan on the
expression levels of HO-1 and SOD-1, HaCaT cells were exposed to
fucoidan at times ranging from 3 to 24 h. As shown in Fig. 3, 30 µg/ml fucoidan enhanced
HO-1 mRNA levels in a time-dependent manner. In addition, SOD-1
levels were observed to be upregulated in a time-dependent manner
following fucoidan treatment, however, fucoidan exerted a slight
downregulation at 12 h. Furthermore, increased protein expression
levels of HO-1 and SOD-1 were observed at 3 h, followed by a
sustained increase in expression at 12 h. The time course for the
elevated HO-1 and SOD-1 protein expressions were closely correlated
with the increased HO-1 and SOD-1 mRNA levels. These results
indicate that fucoidan induces the upregulation of HO-1 and SOD-1
in HaCaT cells.
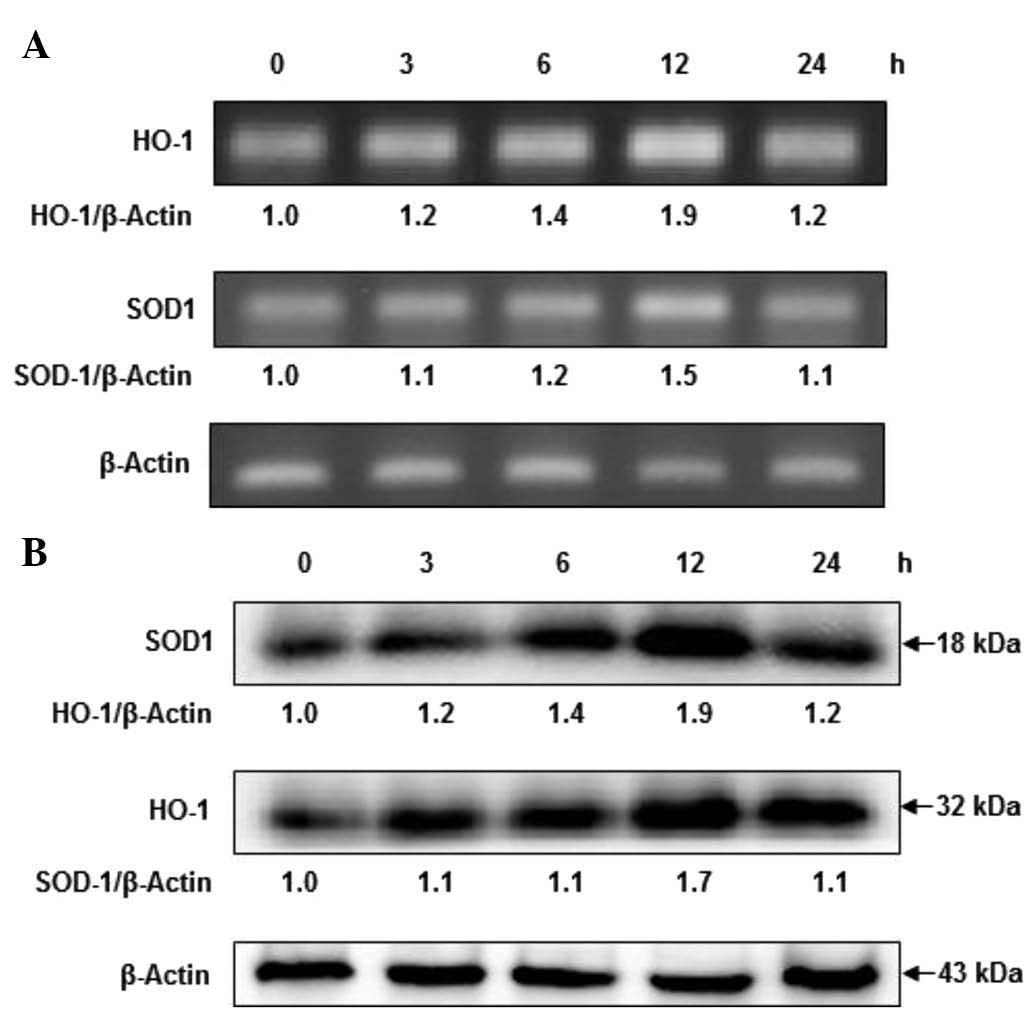 | Figure 3Effects of fucoidan on the mRNA and
protein expression levels of HO-1 and SOD-1 in a time-dependent
manner. (A) Cells were treated with 30 µg/ml fucoidan for 0, 3, 6,
12 and 24 h, and the mRNA levels of HO-1 and SOD-1 analyzed by
reverse trancription-polymerase chain reaction. Relative abundance
of proteins was calculated for the HO-1/β-Actin and SOD-1/β-Actin
ratios. (B) Cells were treated with 30 µg/ml fucoidan for 0, 3, 6,
12 and 24 h, and HO-1 and SOD-1 protein expression were analyzed by
western blotting. Relative abundance of proteins was calculated for
the HO-1/β-Actin and SOD-1/β-Actin ratios. HO-1, heme oxygenase-1;
SOD-1, superoxide dismutase 1. |
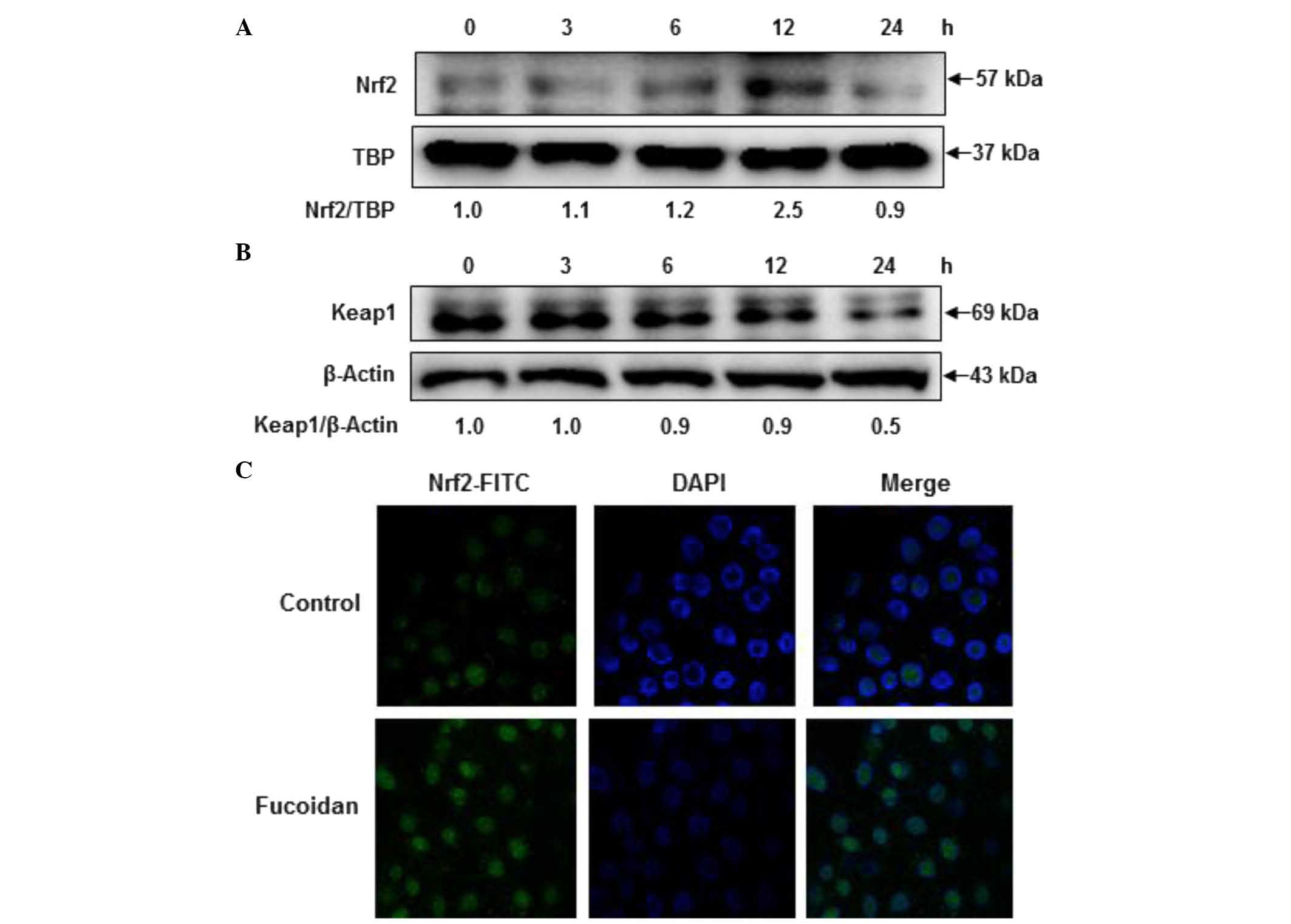
Fucoidan induces Nrf2 and Keap1
expression
To explore the effects of fucoidan on the expression
of Nrf2, HaCaT cells were treated with fucoidan at different time
intervals, and western blotting was performed. As shown in Fig. 4A and B, treating HaCaT cells with
fucoidan induced the phosphorylation of Nrf2 in a time-dependent
manner, whereas Keap1 expression was reduced. Additionally, the
nuclear localization of Nrf2 by fucoidan treatment was observed by
immunocytochemistry. As shown in Fig.
4C, treatment with fucoidan increased the translocation of Nrf2
from the cytosol into the nucleus. These results suggest that Nrf2
protein expression and nuclear localization is induced by fucoidan
treatment.
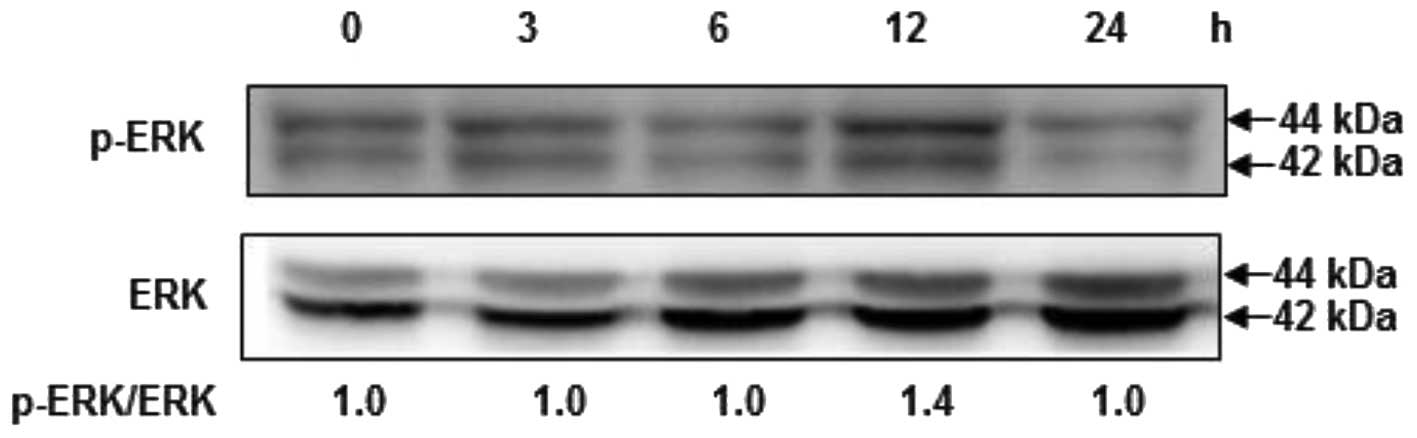
Fucoidan induces the phosphorylation of
ERK
To examine the upstream signaling pathways involved
in fucoidan-induced Nrf2 and HO-1 expression, cells were exposed to
fucoidan for various times, and then ERK activation was analyzed by
western blotting analysis using phospho-specific antibodies against
ERK proteins. As shown in Fig. 5,
treatment of cells with fucoidan significantly induced the
phosphorylation of ERK at 12 h, compared with the control,
suggesting that fucoidan may modulate cell proliferation by the
activation of the ERK signaling pathway.
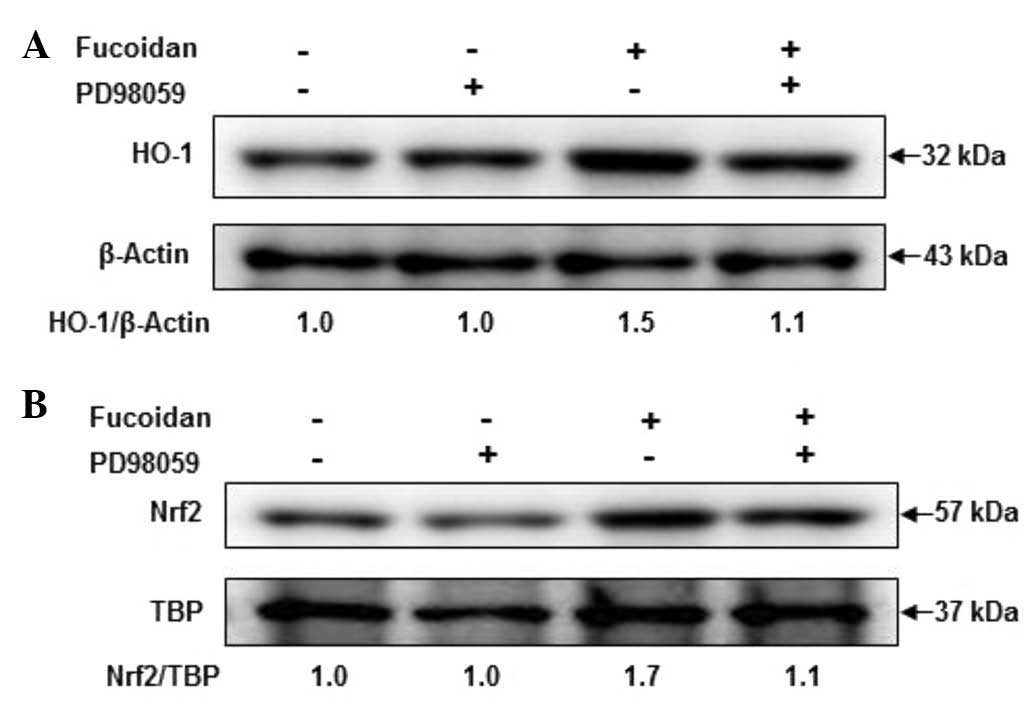
Role of ERK in fucoidan-induced HO-1 and
Nrf2 expression
To investigate the role of ERK in fucoidan-induced
HO-1 and Nrf2 induction, cells were pretreated with 10 µM PD98059
for 1 h prior to incubation with fucoidan at 30 µg/ml for 12
h, and then HO-1 and Nrf2 activation were analyzed by western
blotting. As shown in Fig. 6,
activation of HO-1 was apparent following treatment with fucoidan.
The increase in HO-1 expression levels by fucoidan was markedly
attenuated in PD98059 and fucoidan-treated keratocytes. These
results suggest that fucoidan increased the expression levels of
HO-1 and Nrf2 via ERK signaling pathways.
Discussion
In previous studies, antioxidants activated HO-1 and
SOD-1 expression by targeting the antioxidant response element
(ARE) sequence within the HO-1 and SOD-1 promoter region (15,16).
It has additionally been reported that the expression of HO-1 is
regulated by various transcription factors, such as Nrf2 (17). Nrf2, a redox-sensitive
basic-leucine zipper transcription factor, serves an important role
in the transactivation of genes encoding cytoprotective proteins.
In unstressed cells, Nrf2 remains inactive and blocked in the
cytoplasm by forming a complex with its inhibitory protein, Keap1,
a negative regulator of Nrf2. However, upon exposure of cells to
mild oxidative stress, Nrf2 is phosphorylated at specific serine
and/or threonine residues of Nrf2, dissociates from Keap1 and
translocates to the nucleus (18,19).
ERK is an important signaling molecule that is involved in HO-1
expression in various types of cells (20). It has been demonstrated that ERK
signaling induces Nrf2 activation and regulates cellular protection
against oxidative stress (21).
HaCaT cells are a spontaneously immortalized human adult skin
keratinocytes line, however, these cells are not tumorigenic. These
cells retain normal keratinocyte morphology and epidermal
differentiation capacity and thus offer a suitable model for in
vitro studies of skin diseases (17,22).
The possible molecular mechanism of fucoidan against
mild oxidative stress was investigated, and the Nrf-2/HO-1
signaling pathway was focused on. The current study has indicated
that fucoidan significantly augmented the antioxidants HO-1 and
SOD-1 via the upregulation of Nrf2 and markedly reduced the
cytoplasmic stability of Keap1. Additionally, Nrf2 was only present
in the cytoplasm of cells in the control group, however, was
observed to accumulate in the nucleus following fucoidan treatment,
indicating that fucoidan facilitated the Nrf2 translocation from
the cytoplasm to the nucleus.
Nrf2 is a ubiquitously expressed transcription
factor that is present in a wide range of tissues and cells,
including keratinocytes (23).
Normally, Nrf2 is sequestered in the cytoplasm by Keap1. However
under mild oxidative stress, disruption of the Keap1-Nrf2 complex
results in the nuclear translocation of Nrf2 and its subsequent
binding to promoter regions of antioxidant enzymes, including HO-1
and SOD-1 (24,25).
Accumulating evidence supports a role for the ERK
pathway in HO-1 expression. In a previous study,
7,8-dihydroxyflavone regulated HO-1 expression through the
activation of Nrf2 and Keap1 degradation in an ERK-dependent
pathway in HaCaT cells (15). In
addition, specific inhibitors of ERK reduced the accumulation of
HO-1 and phospho-Nrf2 through the inhibition of ERK
phosphorylation, indicating that Nrf2 is a direct downstream target
of ERK (26). It has also been
reported that fucoidan suppresses IFN-γ-induced NO/iNOS production
in glial cells via the inhibition of JAK/STAT/IFR-1 and phospho-p38
(9). Therefore, fucoidan may be
hold potential as a dietary supplement for the prevention of human
diseases, as its polysaccharide has no toxic or irritating side
effects (12).
In the present study, the molecular mechanisms of
fucoidan anti-oxidant activity via the ERK/Nrf2/HO-1 signaling
pathway were investigated. The results indicated that Nrf2 protein
expression levels and the accumulation of Nrf2 in the nucleus was
increased, and that Keap1 protein expression was reduced in the
fucoidan-treated cells. The upregulation of HO-1 and SOD-1 detected
in the fucoidan-treated cells may be responsible for the increased
resistance to mild oxidative stress, indicating that fucoidan may
augment the activities of antioxidant enzymes via stimulating Nrf2.
These results clearly indicate that fucoidan is an effective
component in food supplements used for skin protection. To the best
of our knowledge, this is the first report, to demonstrate that
fucoidan attenuates oxidative stress by regulating the gene
expression of SOD-1 and HO-1 via the Nrf2/ERK signaling
pathway.
Abbreviations:
|
HaCaT
|
human keratinocyte cell line
|
|
HO-1
|
heme oxygenase-1
|
|
SOD-1
|
superoxide dismutase-1
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
Keap1
|
kelch-like ECH-associated protein
1
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
iNOS
|
inducible nitric oxide synthase
|
|
LPS
|
lipopolysaccharide
|
|
ROS
|
induced reactive oxygen species
|
|
ARE
|
antioxidant response element
|
References
|
1
|
Patankar MS, Oehninger S, Barnett T,
Williams RL and Clark GF: A revised structure for fucoidan may
explain some of its biological activities. J Biol Chem.
268:21770–21776. 1993.PubMed/NCBI
|
|
2
|
Durig J, Bruhn T, Zurborn KH, Gutensohn K,
Bruhn HD and Béress L: Anticoagulant fucoidan fractions from Fucus
vesiculosus induce platelet activation in vitro. Thromb Res.
85:479–491. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soeda S, Kozako T, Iwata K and Shimeno H:
Oversulfated fucoidan inhibits the basic fibroblast growth
factor-induced tube formation by human umbilical vein endothelial
cells: Its possible mechanism of action. Biochim Biophys Acta.
1497:127–134. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teruya T, Konishi T, Uechi S, Tamaki H and
Tako M: Anti-proliferative activity of oversulfated fucoidan from
commercially cultured Cladosiphon okamuranus TOKIDA in U937 cells.
Int J Biol Macromol. 41:221–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson KD and Dragar C: Antiviral
activity of Undaria pinnatifida against herpes simplex virus.
Phytotherapy Res. 18:551–555. 2004. View
Article : Google Scholar
|
|
6
|
Cumashi A, Ushakova NA, Preobrazhenskaya
ME, D'Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE,
Berman AE, Bilan MI, et al: A comparative study of the
anti-inflammatory, anticoagulant, antiangiogenic and antiadhesive
activities of nine different fucoidans from brown seaweeds.
Glycobiol. 17:541–552. 2007. View Article : Google Scholar
|
|
7
|
Maruyama H, Tamauchib H, Hashimotoc M and
Nakano T: Suppression of Th2 immune responses by Mekabu fucoidan
from Undaria pinnatifida Sporophylls. Int Arch Allergy Immunol.
137:289–294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang JW, Yoon SY, Oh SJ, Kim SK and Kang
KW: Biofunctional effects of fucoidan on the expression of
inducible nitric oxide synthase. Biochem Biophys Res. 346:345–350.
2006. View Article : Google Scholar
|
|
9
|
Do H, Kang NS, Pyo SK, Billiar TR and Sohn
EH: Differential regulation by fucoidan of IFN-γ-induced NO
production in glial cells and macrophages. J Cell Biochem.
111:1337–1345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SH, Ko CL, Jee Y, Jeong Y, Kim M, Kim
JS and Jeon YJ: Anti-inflammatory effect of fucoidan extracted from
Ecklonia cava in zebrafish model. Carbohydr Polym. 92:84–89. 2013.
View Article : Google Scholar
|
|
11
|
Zhang H, Teruya K, Eto H and Shirahata S:
Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism
involving the ROS-dependent JNK activation and
mitochondria-mediated pathways. PLoS One. 6:e274412011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto S, Nagaoka M, Hara T,
Kimura-Takagi I, Mistuyama K and Ueyama S: Fucoidan derived from
Cladosiphon okamuranus Tokida ameliorates murine chronic colitis
through the down-regulation of Interleukin-6 production on colonic
epithelial cells. Clin Exp Immunol. 136:432–439. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: Assessment of chemosensitivity testing. Cancer
Res. 47:936–942. 1987.PubMed/NCBI
|
|
14
|
Ryu MJ, Kim AD, Kang KA, Chung HS, Suh IS,
Chang WY and Hyun JW: The green algae Ulva fasciata Delile extract
induces apoptotic cell death in human colon cancer cells. In Vitro
Cell Dev Biol Anim. 49:74–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryu MJ, Kang KA, Piao MJ, Kim KC, Zheng J,
Yao CW, Cha JW, Chung HS, Kim SC, Jung E, et al:
7,8-Dihydroxyflavone protects human keratinocytes against oxidative
stress-induced cell damage via the ERK and PI3K/Akt-mediated
Nrf2/HO-1 signaling pathways. Int J Mol Med. 33:964–970.
2014.PubMed/NCBI
|
|
16
|
Kim KC, Lee IK, Kang KA, Piao MJ, Ryu MJ,
Kim JM, Lee NH and Hyun JW: Triphlorethol-A from Ecklonia cava
up-regulates the oxidant sensitive 8-oxoguanine DNA glycosylase 1.
Mar Drugs. 12:5357–5371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kundu J, Kim DH, Kundu JK and Chun KS:
Thymoquinone induces heme oxygenase-1 expression in HaCaT cells via
Nrf2/ARE activation: Akt and AMPKα as upstream targets. Food Chem
Toxicol. 65:18–26. 2014. View Article : Google Scholar
|
|
18
|
Dinkova-Kostova AT, Holtzclaw WD, Cole RN,
Itoh K, Wakabayashi N, Katoh Y, Yamamoto M and Talalay P: Direct
evidence that sulfhydryl groups of Keap1 are the sensors regulating
induction of phase 2 enzymes that protect against carcinogens and
oxidants. Proc Natl Acad Sci USA. 99:11908–11913. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wakabayashi N, Dinkova-Kostova AT,
Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW and
Talalay P: Protection against electrophile and oxidant stress by
induction of the phase 2 response: Fate of cysteines of the Keap1
sensor modified by inducers. Proc Natl Acad Sci USA. 101:2040–2045.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X and Darzynkiewicz Z: Cleavage of
poly(ADP-ribose) polymerase measured in situ in individual cells:
Relationship to DNA fragmentation and cell cycle position during
apoptosis. Exp Cell Res. 255:125–132. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu R, Chen C, Mo YY, Hebbar V, Owuor ED,
Tan TH and Kong AN: Activation of mitogen-activated protein kinase
pathways induces antioxidant reponse element-mediated gene
expression via a Nrf2-dependent mechanism. J Biol Chem.
275:39907–39913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boukamp P, Petrussevska RT, Breitkreutz D,
Hornung J, Markham A and Fusenig NE: Normal keratinization in a
spontaneously immortalized aneuploidy human keratinocyte cell line.
J Cell Biol. 106:761–771. 1998. View Article : Google Scholar
|
|
23
|
Baraun S, Hanselmann C, Gassmann MG, auf
dem Keller U, Born-Berclaz C, Chan K, Kan YW and Werner S: Nrf2
transcription factor, a novel target of keratinocyte growth factor
action which regulates gene expression and inflammation in the
healing skin wound. Mol Cell Biol. 22:5492–5505. 2002. View Article : Google Scholar
|
|
24
|
Jain AK, Mahajan S and Jaiswal AK:
Phosphorylation and dephosphorylation of tyrosine 141 regulate
stability and degradation of INrf2: A novel mechanism in Nrf2
activation. J Biol Chem. 283:17712–17720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee OH, Jain AK, Papusha V and Jaiswal AK:
An auto-regulatory loop between stress sensors INrf2 and Nrf2
controls their cellular abundance. J Biol Chem. 282:36412–36420.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cullinan SB and Diehl JA: PERK-dependent
activation of Nrf2 contributes to reox homeostasis and cell
survival following endoplasmic reticulum stress. J Biol Chem.
279:20108–20117. 2004. View Article : Google Scholar : PubMed/NCBI
|