Introduction
Previous studies have demonstrated that the oral
ingestion of phytochemicals found in herbs, vegetables and fruits
can safeguard the skin against ultraviolet B (UVB)-induced
photo-damage (1–3). The condition and appearance of the
irradiated skin are improved by dietary supplementation with
phytochemicals. For example, Honeybush (Cyclopia) is rich in
antioxidant polyphenols, and these compounds can protect SKH-1
strain hairless mice from UVB-triggered skin injury (4). In addition, the oral administration
of antioxidant proanthocyanidins and polyphenols derived from grape
seeds and green tea, respectively, can prevent UVB-associated
photocarcinogenesis and oxidative skin damage in SKH-1 mice
(5,6). These and other antioxidants are
essential as scavengers of the intracellular reactive oxygen
species (ROS), the levels of which are elevated by exposure of the
skin to UVB radiation, and include singlet oxygen, superoxide
anions, hydroxyl radicals and hydrogen peroxide (7). The functions of ROS have been
investigated for their contribution to photoaging, inflammation and
numerous disease processes (8–10).
UV exposure frequently provokes skin pigmentation
and wrinkling, and also stimulates the expression and activity of
matrix metalloproteinases (MMPs). The latter degrade all types of
extracellular matrix proteins, thereby damaging connective tissues.
Collagen is the most abundant protein in the dermal connective
tissue, and is enzymatically digested by MMP-1 or interstitial
collagenase. Collagen degradation and damage feature predominantly
in photoaging (11), and thus an
increase in the expression and/or activity of MMP-1 is a hallmark
of UV-mediated skin injury. The exogenous production of free
radicals via ionizing UV radiation further damages the skin at
cellular and tissue levels. Although the body possesses a defense
system to protect against this oxidative damage, excessive levels
of free radicals and ROS can overwhelm this defense system and
incite a state of oxidative stress or immunosuppression, and
potential carcinogenesis. However, antioxidant supplementation
provides additional protection to neutralize ROS (12).
The brown alga, Sargassum muticum, is widely
distributed on the coastline of the southern and eastern areas of
Korea. Extracts derived from S. muticum reportedly
demonstrate antioxidant, antimicrobial and anti-inflammatory
activities (13,14). In previous screening for
anti-photoaging candidates, it was found that an ethyl acetate
extract of S. muticum (SME) exerted cytoprotective activity
against UVB irradiation in cultured human HaCaT keratinocytes by
scavenging free radicals and inducing the expression of antioxidant
enzymes (15). However, to the
best of our knowledge, no previous studies have been performed to
investigate the ability of SME to defend against UVB-induced skin
aging in an animal model. Therefore, the present study examined the
capacity of SME to safeguard HR-1 strain hairless mice against
UVB-provoked photoaging, oxidative stress and wrinkling, and
investigated the potential mechanism underling the action of SME in
the HaCaT cell line. The in vivo and in vitro results
suggest SME as a potential candidate for clinical trials and drug
development. Further study is required in order to fully elucidate
the use of SME in humans.
Materials and methods
Preparation of the SME
Samples of S. muticum were collected from Udo
(Jeju Island, Korea) and identified by Dr Dong Sam Kim (Jeju
Biodiversity Research Institute, Jeju, Republic of Korea). A
voucher specimen (A10-0000107) was deposited at the herbarium of
Jeju Biodiversity Research Institute (Jeju, Korea). S.
muticum was extracted with 80% ethanol under reflux.
Subsequently, the 80% ethanol extract was suspended in distilled
water and fractionated successively with n-hexane,
dichloromethane, ethyl acetate and n-butanol (Merck
Millipore, Darmstadt, Germany). The ethyl vacetate fraction of
S. muticum (SME) was used for further experiments.
Reagents
The primary antibody against MMP-1 was purchased
from Epitomics (Burlingame, CA, USA), and the primary antibody
against actin was obtained from Sigma-Aldrich (St. Louis, MO, USA).
The primary antibodies against c-Jun and phosphorylated
(phospho)-c-Jun were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). All other chemicals and reagents were of
analytical grade, unless otherwise stated.
Experimental animals, oral administration
of SME and UVB radiation
HR-1 strain hairless male mice (6 weeks old; 22–24
g) were purchased from Japan SLC, Inc. (Shizuoka, Japan) and
allowed to acclimate to conditions in the facility for 1 week prior
to experimentation. The animals were housed in climate-controlled
quarters (24°C at 50% humidity) with a 12/12 h light/dark cycle and
free access to standard rodent chow and water. Subsequent to the 1
week acclimation period, the mice were divided into three groups:
An untreated control group (n=5), a UVB-treated vehicle group (n=5)
and a UVB-treated SME group (n=5). The mice in the SME group were
orally administered with SME (100 mg/kg body weight in 0.1 ml of
water per day). SME was administered 5 days/week for 12 weeks.
Exposure to UVB irradiation was then performed using an UVM-225D
Mineralight UV display lamp (UVP, Inc., Phoenix, AZ, USA) emitting
UV light at a wavelength of 302 nm. The UV strength was measured
using a HD2102-2 UV meter (Delta OHM, Padova, Italy). UVB radiation
was applied to the skin on the backs of the animals three times per
week for 12 weeks, with the UVB dose progressively increased
between 60 mJ/cm2 per exposure in week 1 (one minimal
erythematous dose=60 mJ/cm2) and 120 mJ/cm2
per exposure in week 7. The experimental protocol was approved by
the Institutional Animal Care and Use Committee of the Korea
Institute of Oriental Medicine (Daejeon, Korea; approval no.
11-061). All experimental protocols were approved by the Korea
Institute of Oriental Medicine Institutional Animal Care and Use
Committee (11-061).
Cell culture and UVB radiation
The HaCaT keratinocyte cell line was obtained from
Amore Pacific Company (Yongin, Korea). The cells were maintained at
37°C in an incubator with a humidified atmosphere of 5%
CO2, and were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% heat-inactivated fetal calf serum (Gibco; Thermo
Fisher Scientific, Inc.), streptomycin (100 µg/ml) and
penicillin (100 U/ml). For irradiation, the cells were exposed to
UVB light once at a dose of 30 mJ/cm2. The CL-1000 M UV
Crosslinker (UVP, Inc.) was used as the UVB source, which delivered
a UVB energy spectrum of 280–320 nm.
Generation of skin replicas and image
analysis
Mice were anesthetized with intraperitoneal
administration of a diluted solution (1:4 in phosphate-buffered
saline) composed of a 2:1 mixture of 30 mg/kg Zoletil (Virbac,
Carros, France) and 10 mg/kg Rompun (Bayer, Leverkusen, Germany) at
the end of final oral administration. Mice were sacrificed using a
CO2 chamber subsequent to the final oral administration.
Mouse dorsal skin replicas were obtained using a Repliflo Cartridge
kit (CuDerm Corporation, Dallas, TX, USA). An image of the dorsal
skin of each mouse was captured prior to the sacrifice of the
animal. The impression replicas were placed on a horizontal sample
stand, and wrinkle shadows were generated by illumination using a
fixed-intensity optical light at an angle of 35°. Black and white
images were recorded using a charge-coupled device camera and were
analyzed using Skin-Visiometer® VL 650 software (Courage
+ Khazaka Electronic GmbH, Cologne, Germany). The average wrinkle
lengths and depths were measured, and used as parameters to assess
skin wrinkling.
Histological examination of the skin
The thickness of the epidermis was measured via
light microscopy using an eyepiece micrometer (AX-70; Olympus
Corporation, Tokyo, Japan). For this analysis, the dorsal skin (2 ×
2 cm samples) was obtained under anesthesia and was fixed in 10%
neutral-buffered formalin, embedded in paraffin and sectioned at a
thickness of 5 µm. The sections were stained with
hematoxylin and eosin to evaluate tissue morphology, or with
Masson's trichrome stain for collagen fiber analysis.
Western blot analysis
The harvested HaCaT keratinocytes were lysed by
incubating the cells on ice for 10 min in 100 µl PRO-PREP™
protein extraction solution (Intron Biotechnology (Seongnam,
Korea). The cell lysates were centrifuged at 16,000 × g at 4°C for
5 min, and the supernatants were removed from the lysates. Protein
concentrations were then determined using Quant-iT™ Protein Assay
kit (Invitrogen; Thermo Fisher Scientific, Inc.). Aliquots of the
lysates (40 µg of protein) were boiled for 5 min and
electro-phoresed in a 10% sodium dodecyl sulfate-polyacrylamide
gel. The proteins within the gel were then transferred onto
nitrocellulose membranes, and the membranes were subsequently
incubated with the rabbit monoclonal antibodies against MMP-1 (cat.
no. #1973-1; 1:1,000) and phospho-c-Jun (Ser73; cat. no. D47G9;
1:1,000). Following incubation with primary antibodies, the
membranes were further incubated with secondary anti-immunoglobulin
G/horseradish peroxidase conjugates (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Protein bands were visualized using an Enhanced
Chemiluminescence Western Blotting Detection kit (GE Healthcare
Life Sciences, Little Chalfont, UK).
Determination of MMP-1 activity
MMP-1 activity was assessed using a
Fluorokine® E Human Active MMP-1 Fluorescent Assay kit
(R&D Systems, Inc., Minneapolis, MN, USA). This assay uses a
quenched fluorogenic substrate to detect enzyme activity.
Production of the fluorescent cleavage product was measured using a
fluorescence plate reader (BMG Labtech, Ortenberg, Germany) with
excitation and emission wavelengths of 320 and 405 nm,
respectively.
Chromatin immunoprecipitation (ChIP)
assay
The ChIP assay was performed using a SimpleChIP™
Enzymatic Chromatin IP kit (Cell Signaling Technology, Inc.),
according to the manufacturer's protocol with slight modification.
Briefly, the HaCaT keratinocytes were crosslinked by the addition
of 1% formaldehyde, and the prepared chromatin was enzymatically
digested with nuclease for 20 min at 37°C. Primary antibody against
the activator protein-1 (AP-1) family member, c-Jun rabbit
monoclonal antibody (60A8; cat. no. #9165; 1:50; Cell Signaling
Technology, Inc.), and normal rabbit polyclonal IgG (cat. no.
#2729; 1:500; Cell Signaling Technology, Inc.) were added to the
chromatin digest, which was then incubated overnight with constant
rotation at 4°C. To capture the immunoprecipitated DNA/protein
complexes, ChIP-grade protein G magnetic beads were added to the
digest. Pellet protein G agarose was obtained by brief
centrifugation (5,000 × g for 1 min at 4°C) and the supernatant
fraction was removed. The protein G agarose-antibody/chromatin
complex was washed by resuspending the beads in 1 ml each of the
cold buffers in the order listed below, and incubation for 5 min on
a rotating platform followed by brief centrifugation (5,000 × g for
1 min at 4°C) and careful removal of the supernatant fraction. The
immunoprecipitates were eluted with ChIP elution buffer. The
crosslinking was reversed by incubating the eluent at 65°C for 30
min, followed by the addition of proteinase K and further
incubation at 65°C for 2 h.
The DNA fragments recovered from the
immunoprecipitated DNA/protein complex were purified on spin
columns. DNA was amplified in a reaction containing primers, dNTPs,
and 0.5 U Taq DNA polymerase (Intron Biotechnology) at a final
volume of 25 µl. The PCR conditions were: 5 min at 95°C for
initial denaturation, followed by 45 cycles of 30 sec at 94°C, 1
min at 49°C and 1 min at 72°C, and a final elongation period of 7
min at 72°C. PCR amplification was conducted in an Applied
Biosystems 2720 thermal cycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primers for the MMP-1 gene promoter were as
follows: Sense 5′-CCTCTT GCTGCTCCAATATC-3′ and antisense
5′-TCTGCTAGGAGTCACCATTTC-3′ (Bioneer Corporation, Daejeon, Korea).
The promoter region of the MMP-1 gene exists between −67 and +94 of
the MMP-1 gene sequence, with reference to the transcription start
site. The PCR products were separated on 2% agarose gels, and the
DNA bands were visualized using RedSafe™ nucleic acid staining
solution (Intron Biotechnology) and an ImageQuant 350 digital
imaging system (GE Healthcare, Wankesha, WI, USA).
Statistical analysis
All measurements were obtained in triplicate, and
all values are presented as the mean ± the standard error of the
mean. The results were subjected to two-way analysis of variance
followed by the F-test using GraphPad Prism 5 (GraphPad Software,
Inc., La Jolla, CA, USA) to analyze differences between conditions.
In all cases, P<0.05 was considered to indicate a statistically
significant difference.
Results
SME inhibits UVB-induced wrinkle
formation in mouse skin
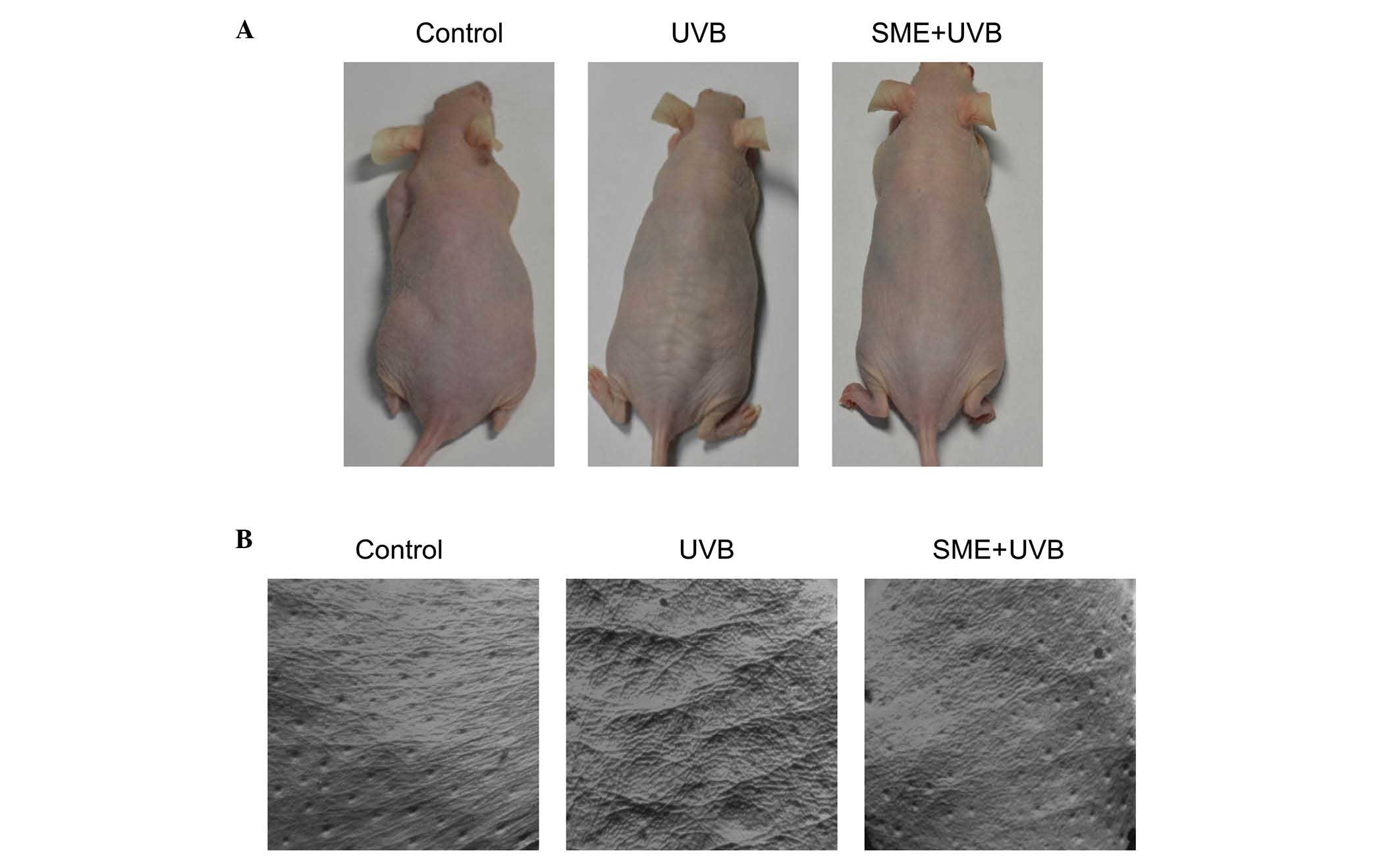
To investigate the effects of SME on UVB-induced
wrinkle formation in vivo, the skin on the backs of HR-1
hairless mice was exposed to UVB light for a period of 12 weeks.
UVB exposure resulted in macroscopic wrinkle formation in the
dorsal skin of the vehicle-treated mice, compared with the
unexposed control mice. However, wrinkling of the UVB-irradiated
skin was markedly inhibited by SME pretreatment (Fig. 1A). To further evaluate the
protective actions of SME against UVB-mediated skin damage,
replicas of the mouse dorsal skin were obtained (Fig. 1B). SME visibly inhibited wrinkle
formation in replica images of UVB-exposed skin, consistent with
the macroscopic observations. Skin replicas were further evaluated
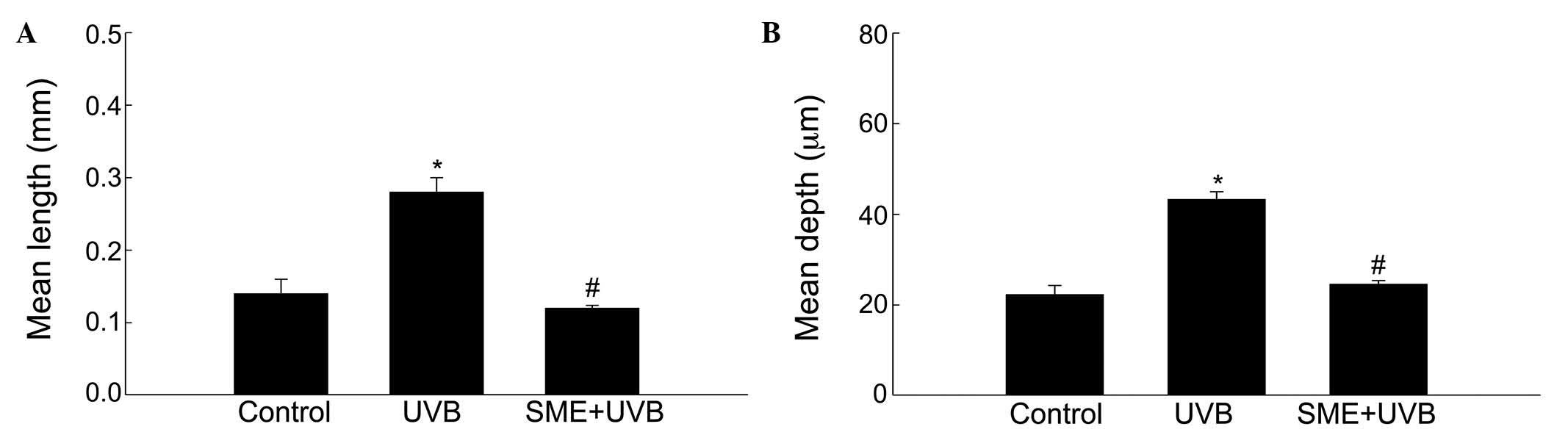
using an image analysis system to quantify the degree of wrinkle
formation. The mean length and depth of the wrinkles were
significantly higher in the UVB-treated vehicle group, compared
with those in the unexposed control group (Fig. 2A and B). However, the mean wrinkle
length and depth were significantly decreased in the UVB-treated
mice pretreated with SME.
SME reduces epidermal thickness in
UVB-irradiated mouse skin
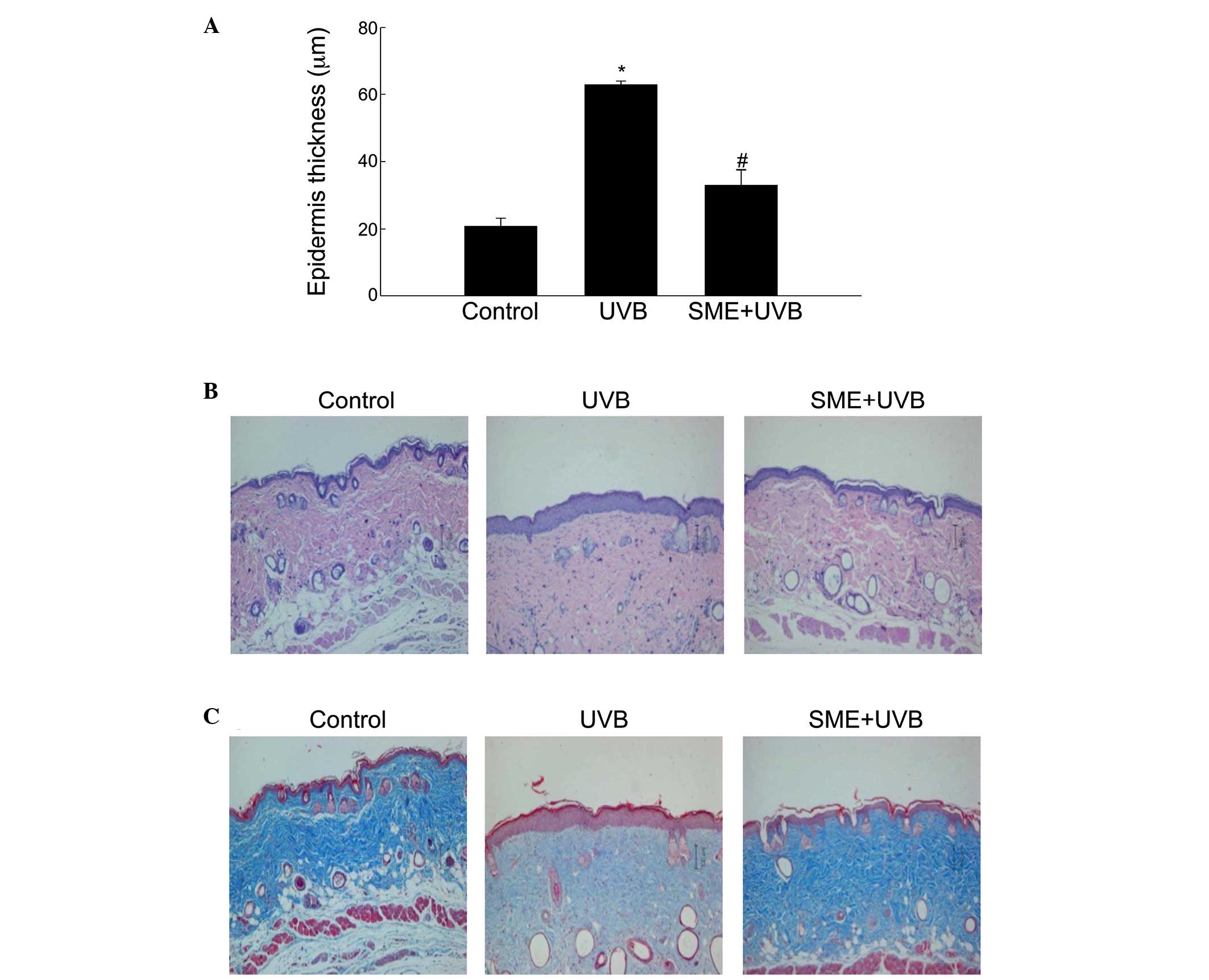
As epidermal thickness is used as a quantitative
parameter to assess inflammation and skin photoaging (16), the present study subsequently
evaluated changes in the dermal thickness of the skin of
UVB-irradiated mice, compared with unexposed control hairless mice.
Measurement of the tissue sections indicated that the epidermal
thickness of the dorsal skin was significantly increased following
UVB irradiation in vehicle-pretreated mice. By contrast, epidermal
hypertrophy was significantly reduced by SME pretreatment (Fig. 3A). Additionally, histological
observations of hematoxylin and eosin-stained sections revealed
that the epidermal thickness in the UVB-irradiated dorsal skin of
the vehicle group was markedly increased. However, SME pretreatment
inhibited the increase in epidermal thickness (Fig. 3B). The tissue sections were then
subjected to Masson's trichrome staining to visualize changes in
collagen fiber formation in the dermal areas of the UVB-exposed
dorsal skin. Notably, the collagen fibers (blue) in the
UVB-treated/SME-pretreated skin showed lower levels of damage and
an increased extent of bundle formation, compared with those in the
UVB-treated/vehicle-pretreated skin (Fig. 3C).
SME inhibits the UVB-induced increase in
the expression and activity of MMP-1 in HaCaT keratinocytes
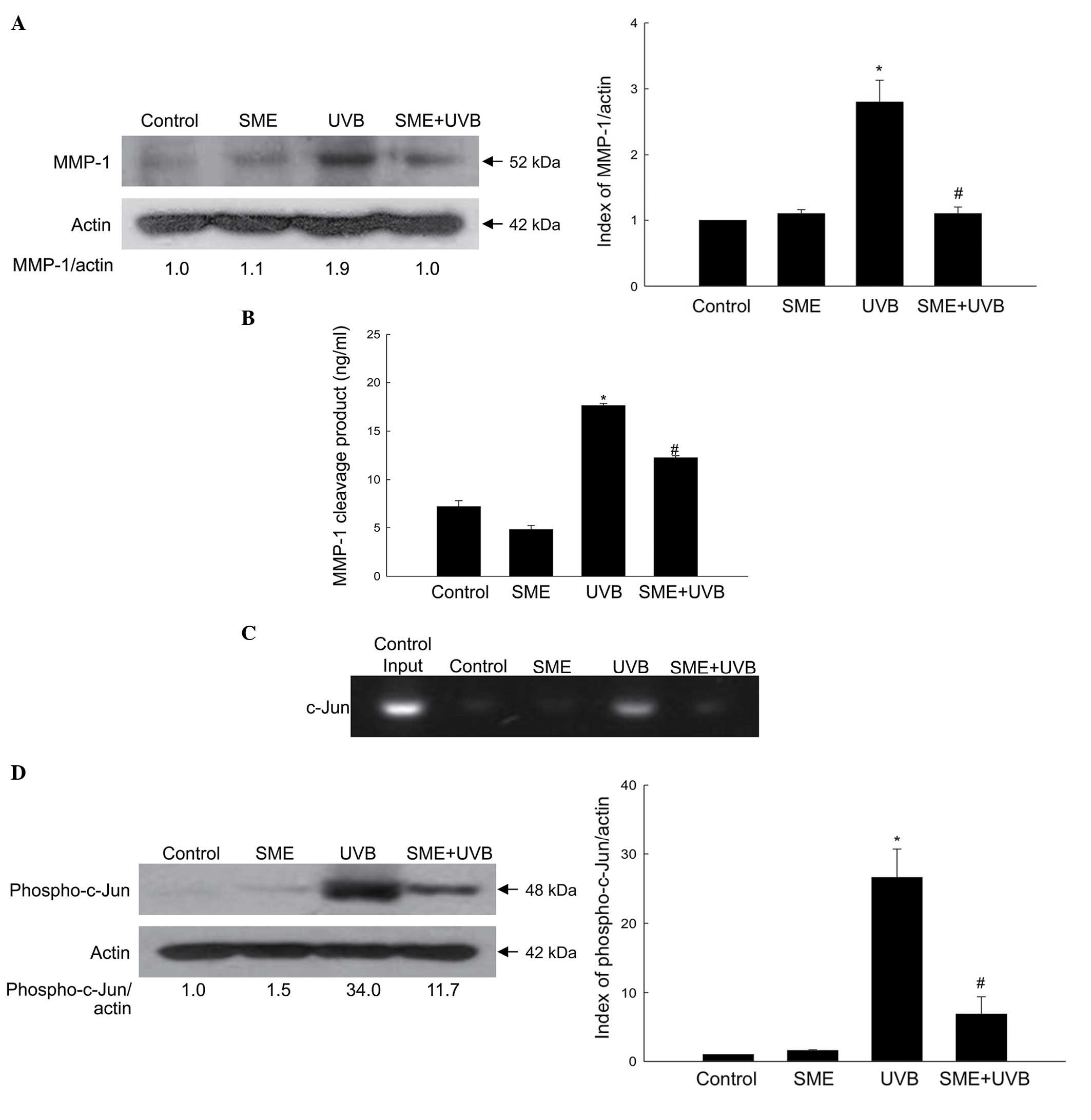
The cultured HaCaT keratinocytes were exposed to UVB
radiation at a dose of 30 mJ/cm2, and the protein
expression level of MMP-1 was measured using western blot analysis.
As shown in Fig. 4A, SME
pretreatment inhibited the accumulation of UVB-induced MMP-1
protein 24 h following exposure. Furthermore, the activity of MMP-1
was significantly enhanced by UVB radiation, but reduced by SME
pretreatment (Fig. 4B).
The MMP-1 promoter contains a binding site for the
AP-1 transcription factor, and activation of AP-1 by UVB exposure
increases the expression of MMP-1 (17). However, SME pretreatment inhibited
UVB-induced binding of the AP-1 family member, c-Jun, to the MMP-1
promoter, as determined using a ChIP assay (Fig. 4C). Furthermore, UVB treatment
markedly stimulated the expression of phospho-c-Jun, whereas SME
treatment decreased its expression (Fig. 4D).
Discussion
Photoprotection refers to a group of mechanisms
developed over the course of evolution to minimize the oxidative
damage that may otherwise occur to organs, including the skin, when
exposed to UV radiation. The skin is a complex organ composed of
multiple layers of tissues and cell types, and serves as a
defensive barrier between the internal organs and external factors,
particularly UV light. Photoprotective mechanisms in the skin can
be stimulated or regulated by naturally occurring compounds or
substances originating from terrestrial and aquatic sources. A
number of photoprotective compounds derived from assorted marine
sources have been developed to prevent photodamage to skin tissues
and cells (18). However, the
source and safety of natural bioactive molecules possessing
anti-photoaging properties has been a major biological concern in
the investigation of photoprotection, culminating in the
identification of safer marine sources over the past few decades
(19). For example, marine brown
algae contain non-cytotoxic phenolic compounds, termed
phlorotannins, with antioxidant activity and the ability to absorb
significant quantities of UV light (20).
The results of numerous investigations performed
over several years has established the family of marine algae as a
principal source of bioactive substances of significant medicinal
and nutritional value (21–23).
Marine algae reportedly contain several important secondary
metabolites, including phenols and polyphenols, resulting from
unique linkages known as phenolic couplings (24). These secondary metabolites have a
wide array of skeletal backbones and biological activities
(19). Although exposure of marine
algae to adverse environmental conditions, for example excessive
light and high oxygen concentrations, leads to the formation of
free radicals and other oxidizing molecules, these conditions do
not cause any serious photodynamic damage to the organisms
(25). This suggests that marine
algae, similar to photosynthesizing plants, possess potent
antioxidant mechanisms in addition to compounds that act as
antioxidant agents (26).
Brown algae are a staple of Korean and Japanese
diets, and their use in traditional Chinese medicine has been
documented for >1,000 years (27). As mentioned above, brown algae
contain numerous antioxidant phenolic compounds with the ability to
absorb UV light and, therefore, they have been extensively
investigated for their anti-photoaging qualities (28). The oral administration of
algae-derived dietary antioxidants may protect against chronic
UVB-irradiated skin disorders. In this regard, phytochemicals,
including proanthocyanidin, genistein and daidzein, are potential
photoprotective agents against UVB-induced skin damage (29), and polyphenols and phlorotannins
isolated from brown algae exert substantial antioxidant and
anti-inflammatory actions (30).
The antioxidant activity of these and other compounds associated
with UV protection is currently a focus of investigations due to
the increasing demand from the cosmeceutical market and the
pharmaceutical industry for efficacious anti-aging products.
Exposure to UV light triggers photoaging of the
skin, which is characterized by distinct changes in the components
of the dermal connective tissue extracellular matrix, leading to
wrinkles, laxity/looseness, coarseness, lentigines, pigmentation,
collagen damage and connective tissue modifications (31). Histological studies have revealed
that photodamaged skin is also associated with increased epidermal
thickness (32). The dermal
connective tissue extracellular matrix contains structural proteins
(collagen, keratin and elastin), specialized proteins (fibrillin,
fibronectin and laminin), glycoproteins and proteoglycans.
Disruption of this balanced combination of components has
detrimental consequences for skin integrity. Collagen, one of the
primary components of skin tissue, is essential for structural
maintenance and comprises 70–80% of the dry weight of the skin. At
present, >20 types of collagen have been reported (33). Abnormal changes in skin collagen
metabolism and decreased collagen levels following excessive UV
exposure are largely responsible for skin photo-aging and wrinkle
formation. However, the present study showed that collagen fibers
were restored in UVB-treated/SME-pretreated skin, compared with the
damaged skin of the UVB-treated/vehicle-pretreated mice.
MMP-1 is a critical member of the collagenase
subfamily of MMPs, and is the most important interstitial
collagenase in humans (34). In
the present study, UVB light stimulated the protein expression and
activity of MMP-1 in HaCaT keratinocytes, and the binding of the
AP-1 transcription factor to the MMP-1 promoter. However, treatment
of the cells with SME reversed all the deleterious effects of UVB
exposure.
Considering the correlation between anti-photoaging
and antioxidant activity, the present study hypothesized that
polyphenols and phlorotannins may be central to the protective
effect of SME against UVB-induced skin damage. However, additional
types of SME-derived compounds may also confer anti-photoaging
properties. The precise mechanism by which these compounds
potentially safeguard the skin against photoaging remains to be
fully elucidated, as SME contains several naturally occurring
chemicals, and their concentrations change according to region,
climate, season and other exogenous factors. Accordingly, further
investigations are required regarding the isolation of active
components from SME, and the combinatorial effect of various
chemical constituents of the extract.
In conclusion, the present study evaluated the
anti-photoaging capacity of SME, and its ability to prevent
UVB-induced skin and cell damage in HR-1 strain hairless mice and
HaCaT keratinocytes, respectively. Increased wrinkle formation was
observed following the UVB irradiation of hairless mice, however,
the oral administration of SME protected against skin aging and
injury. Furthermore, SME inhibited UVB-induced increases in
epidermal thickness, wrinkle length and depth, and collagen fiber
loss in hairless mice. SME also inhibited the UVB-induced
upregulated protein expression and activity of MMP-1 in cultured
keratinocytes. Based on these results, it was concluded that SME
may be a suitable candidate as a photoprotective agent with
cosmeceutical and pharmaceutical value.
Acknowledgments
This study was supported by grants (grant nos.
C10010 and K13102) from the Korea Institute of Oriental Medicine
and by the National Research Foundation of Korea Grant funded by
the Korean Government (Ministry of Education, Science and
Technology; grant no. NRF-C1ABA001-2011-0021037).
References
|
1
|
Avila Acevedo JG, Espinosa González AM, De
Maria y Campos DM, Benitez Flores Jdel C, Hernandez Delgado T,
Flores Maya S, Campos Contreras J, Muñoz López JL and García Bores
AM: Photoprotection of Buddleja cordata extract against UVB-induced
skin damage in SKH-1 hairless mice. BMC Complement Altern Med.
14:2812014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hwang E, Park SY, Lee HJ, Lee TY, Sun ZW
and Yi TH: Gallic acid regulates skin photoaging in UVB-exposed
fibroblast and hairless mice. Phytother Res. 28:1778–1788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JE, Song D, Kim J, Choi J, Kim JR,
Yoon HS, Bae JS, Han M, Lee S, Hong JS, et al: Oral supplementation
with cocoa extract reduces UVB-induced wrinkles in hairless mouse
skin. J Invest Dermatol. 136:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrova A, Davids LM, Rautenbach F and
Marnewick JL: Photoprotection by honeybush extracts, hesperidin and
mangiferin against UVB-induced skin damage in SKH-1 mice. J
Photochem Photobiol B. 103:126–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mittal A, Elmets CA and Katiyar SK:
Dietary feeding of proanthocyanidin from grape seeds prevents
photocarcinogenesis in SKH-1 hariless mice: Relationship to
decreased fat and lid peroxidation. Carcinogenesis. 24:1379–1388.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vayalil PK, Mittal A, Hara Y, Elmets CA
and Katiyar SK: Green tea polyphenols prevent ultraviolet
light-induced oxidative damage and matrix metalloproteinases
expression in mouse skin. J Invest Dermatol. 122:1480–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yasui H, Hakozaki T, Date A, Yoshii T and
Sakurai H: Real-time chemiluminescent imaging and detection of
reactive oxygen species generated in the UVB-exposed human skin
equivalent model. Biochem Biophys Res Commun. 347:83–88. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morena M, Delbosc S, Dupuy AM, Canaud B
and Cristol JP: Overproduction of reactive oxygen species in
end-stage renal disease patients: A potential component of
hemodialysis-associated inflammation. Hemodial Int. 9:37–46. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pashkow FJ: Oxidative sress and
inflammation in heart disease: Do antioxidants have a role in
treatment and/or prevention? Int J Inflam. 2011:5146232011.
View Article : Google Scholar
|
|
10
|
Zhang N, Andresen BT and Zhang C:
Inflammation and reactive oxygen species in cardiovascular disease.
World J Cardiol. 2:408–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong YF, Lee HY, Jung BJ, Jang S, Chung DK
and Kim H: Lipoteichoic acid isolated from Lactobacillus plantarum
down-regulates UV-induced MMP-1 expression and up-regulates type I
procollagen through the inhibition of reactive oxygen species
generation. Mol Immunol. 67:248–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Hu JY and Wang SQ: The role of
antioxidants in photoprotection: A critical review. J Am Acad
Dermatol. 67:1013–1024. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JY, Lee JA, Kim KN, Yoon WJ, Lee WJ
and Park SY: Antioxidative and antimicrobial activities of
Sargassum muticum extracts. J Korean Soc Food Sci Nutr. 36:663–669.
2007. View Article : Google Scholar
|
|
14
|
Yoon WJ, Ham YM, Lee WJ, Lee NH and Hyun
CG: Brown alga Sargassum muticum inhibits proinflammatory
cytokines, iNOS and COX-2 expression in macrophage RAW 264.7 cells.
Turk J Biol. 34:25–34. 2010.
|
|
15
|
Piao MJ, Yoon WJ, Kang HK, Yoo ES, Koh YS,
Kim DS, Lee NH and Hyun JW: Protective effect of the ethyl acetate
fraction of Sargassum muticum against ultraviolet B-irradiated
damage in human keratinocytes. Int J Mol Sci. 12:8146–8160. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gilchrest BA: A review of skin ageing and
its medical therapy. Br J Dermatol. 135:867–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goffin L, Seguin-Estévez Q, Alvarez M,
Reith W and Chizzolini C: Transcriptional regulation of matrix
metalloproteinase-1 and collagen 1A2 explains the anti-fibrotic
effect exerted by proteasome inhibition in human dermal
fibroblasts. Arthritis Res Ther. 12:R732010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sinha RP, Klisch M, Gröniger A and Häder
DP: Ultraviolet-absorbing/screening substances in cyanobacteria,
phytoplankton and macroalgae. J Photochem Photobiol B. 47:83–94.
1998. View Article : Google Scholar
|
|
19
|
Pallela R, Na-Young Y and Kim SK:
Anti-photoaging and photoprotective compounds derived from marine
organisms. Mar Drugs. 8:1189–1202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thomas NV and Kim SK: Potential
pharmacological applications of polyphenolic derivatives from
marine brown algae. Environ Toxicol Pharmacol. 32:325–335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdul QA, Choi RJ, Jung HA and Choi JS:
Health benefit of fucosterol from marine algae: A review. J Sci
Food Agric. 96:1856–1866. 2016. View Article : Google Scholar
|
|
22
|
Fan X, Bai L, Zhu L, Yang L and Zhang X:
Marine algae-derived bioactive peptides for human nutrition and
health. J Agric Food Chem. 62:9211–9222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SK and Karagozlu MZ: Marine algae:
natural product source for gastrointestinal cancer treatment. Adv
Food Nutr Res. 64:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Torres MA, Barros MP, Campos SC, Pinto E,
Rajamani S, Sayre RT and Colepicolo P: Biochemical biomarkers in
algae and marine pollution: A review. Ecotoxicol Environ Saf.
71:1–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiménez-Escrig A, Jiménez-Jiménez I,
Pulido R and Saura-Calixto F: Antioxidant activity of fresh and
processed edible seaweeds. J Sci Food Agric. 81:530–534. 2001.
View Article : Google Scholar
|
|
26
|
Heo SJ, Ko SC, Cha SH, Kang DH, Park HS,
Choi YU, Kim D, Jung WK and Jeon YJ: Effect of phlorotannins
isolated from Ecklonia cava on melanogenesis and their protective
effect against photo-oxidative stress induced by UV-B radiation.
Toxicol In Vitro. 23:1123–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McLellan DS and Jurd KM: Anticoagulants
from marine algae. Blood Coagul Fibrinolysis. 3:69–77. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Henry BE and Alstyne KLV: Effects of UV
radiation on growth and phlorotannins in Focus gardneri
(phaeophyceae) juveniles and embryos. J Phycol. 40:527–533. 2004.
View Article : Google Scholar
|
|
29
|
Kang TH, Park HM, Kim YB, Kim H, Kim N, Do
JH, Kang C, Cho Y and Kim SY: Effects of red ginseng extract on UVB
irradiation-induced skin aging in hairless mice. J Ethnopharmacol.
123:446–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ananthi S, Raghavendran HR, Sunil AG,
Gayathri V, Ramakrishnan G and Vasanthi HR: In vitro antioxidant
and in vivo anti-inflammatory potential of crude polysaccharide
from Turbinaria ornata (Marine Brown Alga). Food Chem Toxicol.
48:187–192. 2010. View Article : Google Scholar
|
|
31
|
Kondo S: The roles of cytokines in
photoaging. J Dermatol Sci. 23(Suppl 1): S30–S36. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rittié L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res. 1:705–720. 2002.
View Article : Google Scholar
|
|
33
|
Son ED, Choi GH, Kim H, Lee B, Chang IS
and Hwang JS: Alpha-ketoglutarate stimulates procollagen production
in cultured human dermal fibroblasts, and decreases UVB-induced
wrinkle formation following topical application on the dorsal skin
of hairless mice. Biol Pharm Bull. 30:1395–1399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ryoo IJ, Moon EY, Kim YH, Lee IS, Choo SJ,
Bae KH and Yoo ID: Anti-Skin Aging effect of Syriacusins from
Hibiscus syriacus on ultraviolet-irradiated human dermal fibroblast
cells. Biomol Ther. 18:300–307. 2010. View Article : Google Scholar
|