Introduction
Breast cancer is the most common malignancy observed
amongst women, accounting for nearly a quarter of all cases, and
outcomes vary depending on cancer type, extent of disease the and
patient's age (1). Four main
subtypes of breast tumor have been identified based on patterns of
gene expression, including luminal A/B, human epidermal growth
factor receptor 2 (HER2)+ [estrogen receptor (ER)-, progesterone
receptor (PR)-] and triple negative tumors (ER-, PR- and HER-),
which influence the therapeutic strategies used and the clinical
prognosis (2). Endocrine therapies
have been identified to be effective in treating breast cancer of
luminal A and luminal B subtypes, and when used together with
chemotherapy, the prognosis of patients was observed to be improved
(3,4). Breast cancer in young women is more
likely to be an aggressive triple negative or HER2+ sub type, and
is more likely to be identified at an advanced stage, due to the
aggressive subtype (5).
Trastuzumab has been demonstrated to improve prognosis of patients
with overexpressed HER-2; however, effective treatment strategies
for triple negative breast cancer (TNBC), which is resistant to
common chemo-therapies and does not respond well to endocrine
therapies, remain to be identified. Radiotherapy is used as a
remedial strategy, therefore increasing the sensitization of TNBC
cells to radiation may improve the clinical prognosis and quality
of life of patients (6).
MicroRNAs (miRNAs) are deregulated in the majority
of malignancies, serving roles in tumor development and
progression, and numerous miRNAs have been reported to influence
the therapeutic response of cancer to clinical treatments (7,8). In
breast cancer, miR-17/20, miR-190, miR-200, miR-34, the let-7
family and additional miRNAs have been identified to be involved in
the pathogenesis of tumor biology (7–9). The
let-7 family was the earliest identified family of
non-translational RNAs, of which the functions remain to be fully
elucidated. Let-7 miRNAs act as suppressive genes through directly
binding to the 3′untranslated region (UTR) of Ras, interleukin-6,
cyclin D1, mitogen-activated protein kinase, LIN28-A/B, c-Myc,
DICER1 and numerous other oncogenes in ER-positive breast cancer
(7,10). However, the role of let-7 in TNBC,
and the let-7-induced sensitization of tumor repression remains to
be fully elucidated, which is important for the development of
future clinical treatments. Let-7 miRNAs have been reported to
limit the numbers of stem cells in normal and cancerous tissue
samples, aiding in the maintenance of the differentiation of stem
cells and cancer stem cells (CSCs), thus inhibiting tumor
progression. Previous studies have identified that let-7 is able to
stimulate chemotherapeutic effects, however, the effects of
radiation combined with let-7 remain unclear, which require
elucidation for the improvement in TNBC treatment (11,12).
The current study investigated the roles of let-7 and radiation on
the stem cells of TNBC, and then assessed the combined effects of
let-7d and radiation. The possible mechanisms associated with these
effects were investigated, with the aim of improving treatment
strategies for TNBC by overcoming recurrence via inhibition of the
renewal ability of CSCs.
Materials and methods
Cell culture, transfection and
infection
The human breast cancer cell lines ZR75-1, MCF-7,
BT-20, HS587-T and MDA-MB-231 (MM-231) were purchased from the
American Type Culture Collection (Manassas, VA, USA) and were
cultured in Roswell Park Memorial Institute-1640 medium (Gibco;
Thermo Fisher Scientific China, Beijing, China), containing 10%
fetal bovine serum (Thermo Fisher Scientific China) and 1%
penicillin and streptomycin (Gibco; Thermo Fisher Scientific
China). The mammospheres were cultured in Dulbecco's modified
Eagle's medium/Ham's F-12 medium (Corning Incorporated, Corning,
NY, USA) supplemented with 10 ng/ml human basic fibroblast growth
factor (Sigma-Aldrich, St. Louis, MO, USA), 10 ng/ml epidermal
growth factor (Sigma-Aldrich), 1 µg/ml hydrocortisone
(Sigma-Aldrich), 4 µg/ml insulin and 1% penicillin and
streptomycin (13). All cells were
cultured in 5% CO2 at 37°C. Oligonucleotides encoding
let-7d mature miRNA and scramble control were synthesized and
cloned into the pGLVU6/RFP vector (Shanghai GenePharma Co., Ltd.,
Shanghai, China). Transfections for the luciferase assay were
performed using Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA (1 g) was
prepared using a ReverTra Ace qPCR RT kit from Toyobo Co., Ltd.
(Osaka, Japan). RT-qPCR was performed with the Thunderbird SYBR
qPCR Mix (Toyobo Co., Ltd.) and a LightCycler 480 PCR system (Roche
Diagnostics, Basel, Switzerland). The thermocycling conditions were
as follows: Two repeats of 95°C for 30 sec; and 45 cycles of 95°C
for 5 sec and 60°C for 30 sec. Mature let-7d was quantified using a
predesigned miRCURY LNA™ assay (Exiqon, Inc., Woburn, MA, USA) with
the primer, 5′-AGA GGT AGT AGG TTG CAT AGTT-3′. Expression of the
U6 small nuclear RNA endogenous control was assayed for
normalization. Gene expression determinations were made using the
comparative 2−ΔΔCq method (14). The proteins were harvested in
radioimmunoprecipitation assay lysis buffer (Beijing Biotech Co.,
Ltd., Beijing, China) and then subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel (Sigma-Aldrich) electrophoresis
separation. The rabbit monoclonal antibody against Wnt1 (1:1,000;
cat. no. 2530) was purchased from Cell Signaling Technology
(Shanghai, China), monoclonal cyclin D1 (1:1,000; sc-753) and
phosphorylated-protein kinase B (Akt1) (Ser473; 1:800; sc-7985-R)
were purchased from Santa Cruz Biotechnology, Inc. (Shanghai,
China). Antibodies against viniculin (1:3,000; ab18058) were
obtained from Abcam (Cambridge, MA, USA).
Sphere formation assays
Cells of different groups were plated in ultra-low
attachment (non-adherent condition) 6-well plates (Corning
Incorporated) to test their ability of forming primary
mammospheres. The mammosphere numbers were counted on day 10 and
the mammosphere formation efficiency (MFE) was calculated as the
percentage ratio between obtained spheres and plated cells
(10,15–17).
Obtained mammospheres from the different groups were disaggregated
then re-suspended to test their ability of self-renewal in the next
generation. Akt1 inhibitors (Akti-1/2; ab142088; Abcam) and
recombinant Wnt1 protein (50 ng/ml; Sigma-Aldrich) were used to
identify the respective roles of Akt1 and Wnt1.
Immunofluorescence and luciferase
assay
The cells were added into the chambers and were
fixed with 10% formalin (Sigma-Aldrich). The cells were blocked
with 2% normal goat serum (ab7481; Abcam), then were incubated with
primary antibodies as above for 1 h at 4°C in phosphate-buffered
saline (PBS; Sigma-Aldrich), then with a secondary antibody for 30
min at room temperature with Alexa Fluor® 488 (goat
anti-mouse IgG; 1:200; Abcam; cat. no. ab150113) or Alexa
Fluor® 633 (goat anti-rabbit IgG; 1:1,000; Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. A-21071) at room
temperature. Cells were then washed in PBS, incubated for 10 min
with PureBlu™ 4′,6-Diamidino-2-Phenylindole Nuclear Staining Dye
[135-1303; Bio-Rad Laboratories (Shanghai) Co., Ltd., China] and
washed in PBS again. The luciferase reporter 3′UTR of cyclin D1,
the T-cell factor (TCF) luciferase reporter and its mutant control,
the promoter of β-catenin and the mutant control were designed and
cloned into miRGLO by Promega Corporation (Madison, WI, USA). Cells
were seeded at 30% confluence in 4-well plates prior to transient
transfection with FuGENE 6 reagent (Roche Diagnostics) (18).
Binding site predication
TargetScan (www.targetscan.org), was used to determine the mRNA
target genes. If no target gene was identified, the existing
binding site between the selected miRNA and target gene may be
frail.
Statistical analysis
All data were obtained from three independent
experiments, and are expressed as the mean ± standard deviation.
Statistical analysis was conducted with Student's t-test and the
χ2 test using SPSS software, version 16.0 for Windows
(SPSS, Inc., Chicago, IL, USA) and Excel 2007 (Microsoft
Corporation, Redmond, WA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
The functions of let-7 miRNAs in TNBC
cells
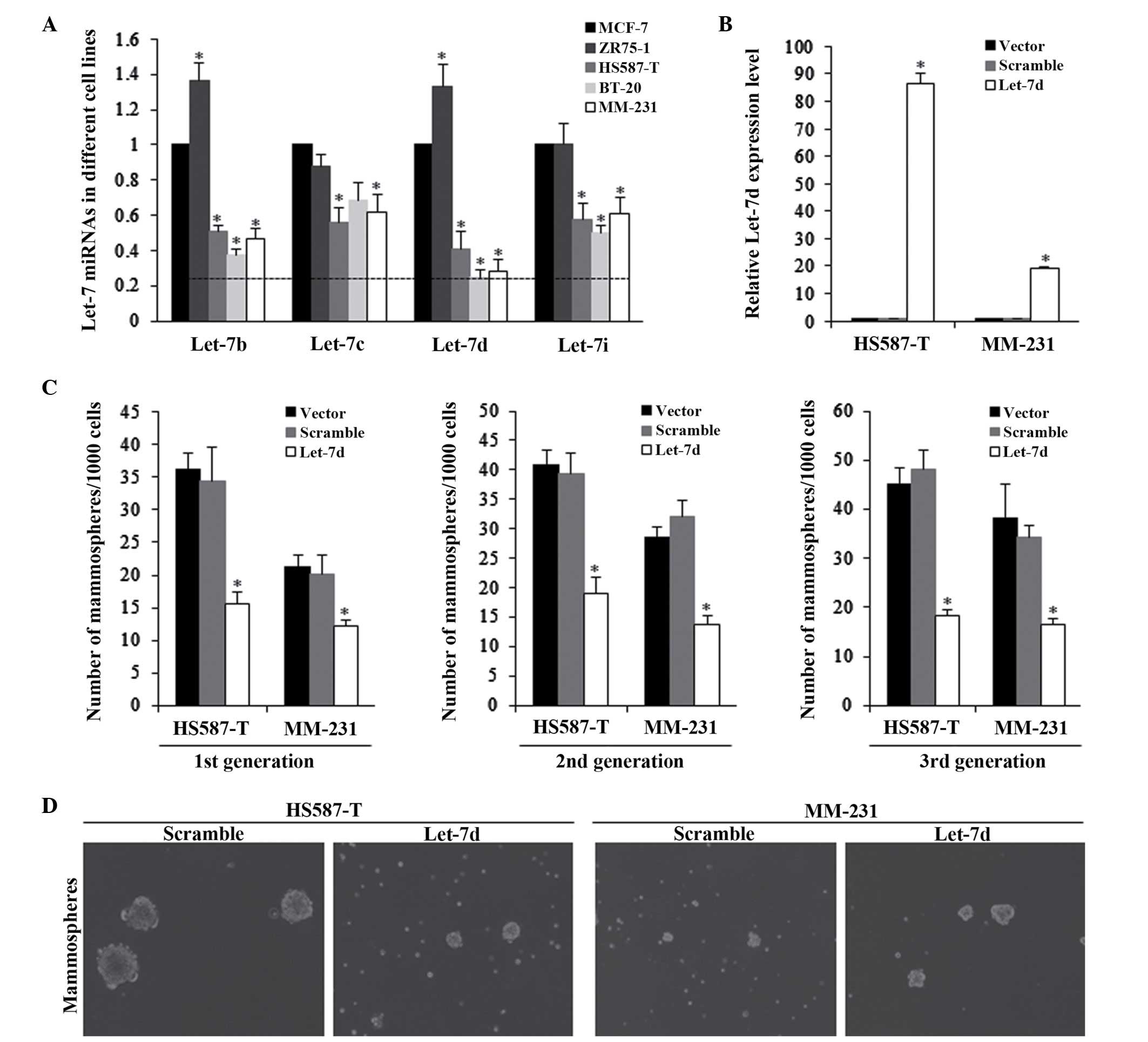
The let-7b, let-7c, let-7d and let-7i expression
levels were assessed in multiple breast cancer cell lines, which
had been previously reported to be significantly deregulated in
breast cancer (19–21). The results indicated that let-7d
was significantly reduced in TNBC in HS587-T and MM-231 cells,
compared with the other cell lines (P<0.01; Fig. 1A). The HS587-T and MM-231 cells
were then infected with lentiviral-based let-7d vectors. The
RFP-based let-7d lentiviral vector was successfully infected into
HS587-T and MDA-MB-231 cells, and the expression levels were
detected and presented in Fig. 1B.
The effects of let-7d on the self-renewal ability of TNBC cells
were evaluated by the mammosphere formation assay, and let-7d was
identified to be able to significantly inhibit the mammosphere
numbers, exerting a continuous repression of the self-renewal
ability (Fig. 1C and D).
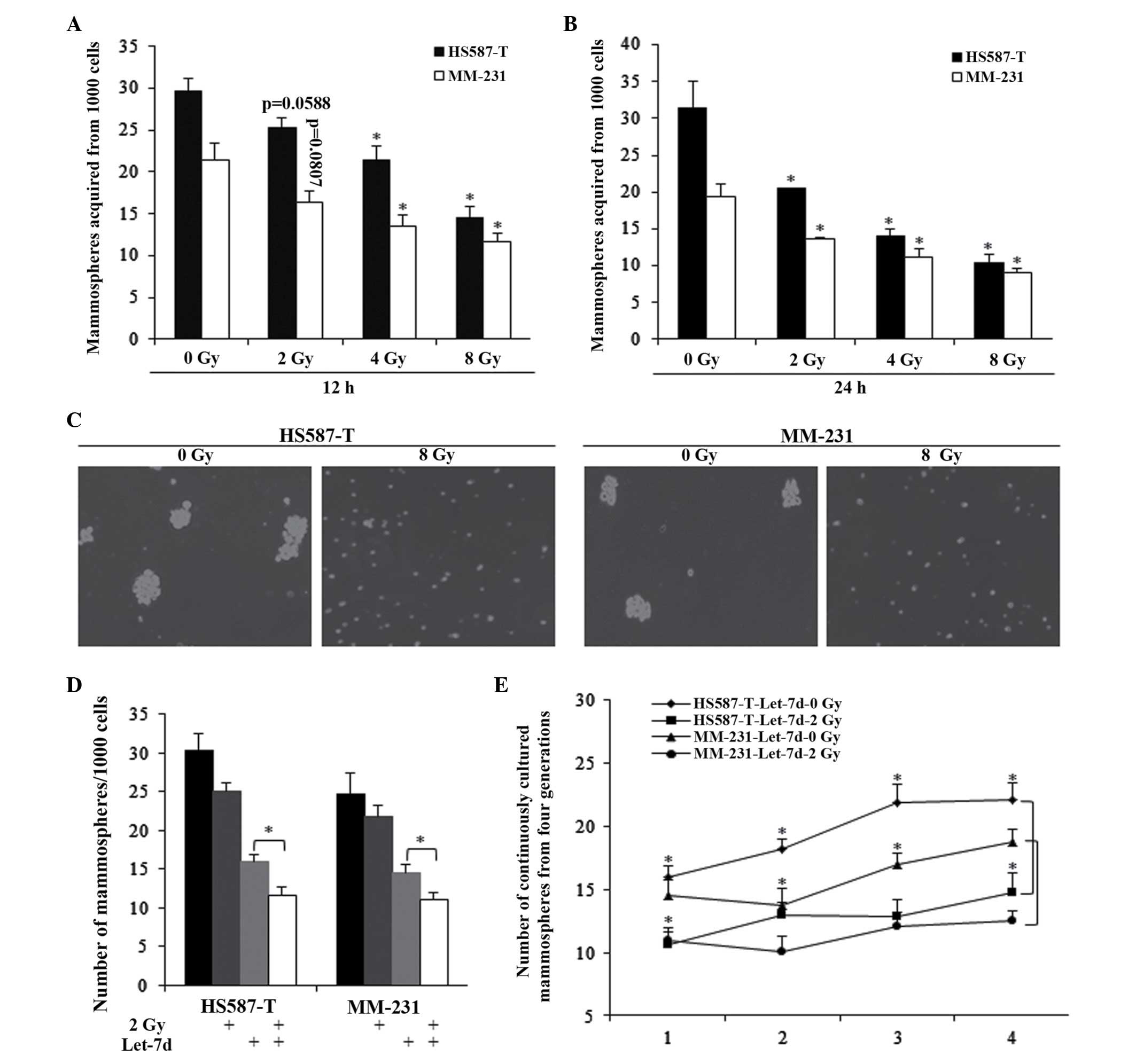
Let-7d sensitized TNBC stem cells to
radiation-induced self-renewal repression
Different doses of radiotherapy and treating
durations were assessed, and the results indicated that radiation
could inhibit the self-renewal ability of TNBC stem cells (Fig. 2A–C). However, it was observed that
2 Gy radiation for 12 h did not significantly affect the MFE of
HS587-T and MM-231 cells (Fig.
2A). In combination with let-7d, 2 Gy radiation exhibited a
markedly stronger inhibition of the self-renewal ability of the two
cell types, which was also significantly stronger than that of
let-7d alone (Fig. 2D).
Furthermore, the combined use of let-7d and 2 Gy radiation was
investigated in continuously cultured mammospheres, with the
results indicating that the combination significantly inhibited the
number of mammospheres, indicating the effects of 2 Gy of radiation
(Fig. 2E).
Let-7d inhibited TNBC stem cells through
the cyclin D1/Akt1/Wnt1 pathway
The cyclin D1/Akt1/Wnt1 pathway, which may
contribute to let-7d and radiation-induced stem cell repression,
was detected using western blotting, a luciferase assay and
immunofluorescence. Let-7d and radiation were observed to suppress
the self-renewal ability of HS587-T and MM-231 cells via the cyclin
D1/Akt1/Wnt1 pathway, and let-7d significantly sensitized the
effects of 2 Gy radiation (Fig.
3A). Through using binding site predication software, no
complementary sequences were recognized between let-7d and Akt1,
TCF-4 and β-catenin (data not shown). Complementary binding
sequences were only identified in the 3′UTR of cyclin D1 and let-7d
(Fig. 3B). Let-7d was observed to
reduce the luciferase activity of cyclin D1 containing the wildtype
3′UTR, with no functions on the mutant 3′UTR (Fig. 3B and C). However, let-7d was not
identified to significantly inhibit TCF-4 and β-catenin (Fig. 3B and C). Immunostaining of TNBC
cells demonstrated that cyclin D1 was reduced in the
let-7d-overexpressed group, while no alterations in TCF-4 were
observed, suggested that let-7d functioned via the cyclin
D1/Akt1/Wnt1 pathway (Fig.
3D).
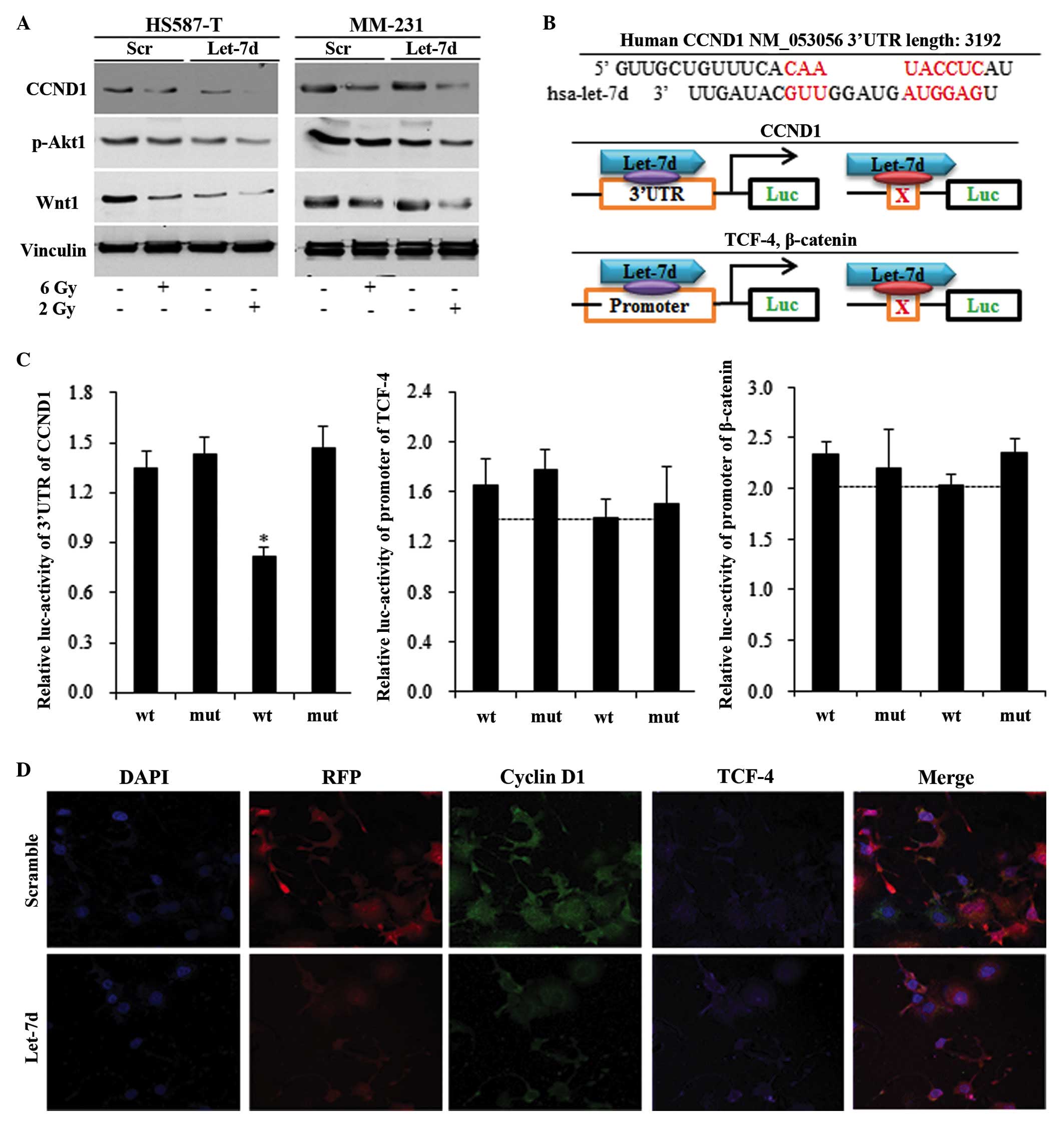 | Figure 3Let-7d inhibited the triple negative
breast cancer stem cells through repressing the CCND1/Akt1/Wnt
pathway. Western blotting, relative luciferase activity and
immunofluorescence were measured in HS587-T and MM-231 cells. (A)
Let-7d alone inhibited the CCND1/Akt1/Wnt pathway, and strengthened
the 2 Gy radiation-induced suppression of the CCND1/Akt1/Wnt
pathway. (B) No binding sites were identified between let-7d and
the 3′UTR of Akt1, TCF-4 or β-catenin, and a common binding site
was only identified between CCND1 and let-7 miRNAs, with the
schematic diagram illustrating the mechanisms by which let-7d
affects the CCND1/Akt1/Wnt pathway. (C) Marked reduction was
observed following insertion of the 3′UTR of wt CCND1 into
let-7d-overexpressed cells, however not with the insertion of mut
CCND1. The reduction of TCF-4 and the β-catenin promoter were not
significant in let-7d-overexpressed HS587-T and MM-231 cells.
*P<0.01 vs. the wt control group. (D) Immunostaining
indicated a reduction in CCND1 in let-7d-overexpressed cells, while
no alterations in TCF-4 were identified. Akt1, protein kinase B;
Wnt, wingless type MMTV integration site family member 1; UTR,
untranslated region; TCF-4, T-cell factor 4; wt, wildtype; mut,
mutant; p-, phosphorylated; Luc, luciferase; DAPI,
4′,6-diamidino-2-phenylindole; RFP, red fluorescent protein (from
let-7 lentivirus); CCND1, cyclin D1. |
Reduced Akt1 phosphorylation and Wnt1
inhibition are required for let-7d sensitization of radiation
through cyclin D1/Akt1/Wnt1 signaling
Let-7d inhibited Akt1/Wnt1 activity through
inhibition of cyclin D1, and the small interfering RNA did not
significantly induce cyclin D1 repression in let-7d-overexpressed
HS587-T and MM-231 cells (Fig.
4A). The inhibition of cyclin D1 in let-7d-overexpressed cells
did not significantly reduce the mammosphere number, when compared
with the let-7d group (Fig. 4B).
Akt1 inhibitors and recombinant Wnt1 protein were used to identify
the respective roles of Akt1 and Wnt1. Let-7d functioned through
Akt inhibition, and the use of Akti-1/2 in the scramble group
significantly inhibited the MFE of HS587-T (Fig. 4C) and MM-231 (Fig. 4D) cells (P<0.01), with no
significant alterations identified between let-7d and
let-7d-Akti-1/2 (Fig. 4C and D).
To elucidate whether inhibition of cyclin D1/Akt1/Wnt1 signaling
accounts for let-7d-induced sensitization of the radiation
response, Akti-1/2 and Wnt1 protein were used in cells treated with
combination let-7d and 2 Gy radiation. Akt phosphorylation
inhibition did not influence the MFE of let-7d and
radiation-treated cells, indicating that let-7d functioned through
Akt phosphorylation inhibition (Fig.
4E). The addition of Wnt1 significantly reversed
let-7d-mediated induction of the radiation response (P<0.01;
Fig. 4F).
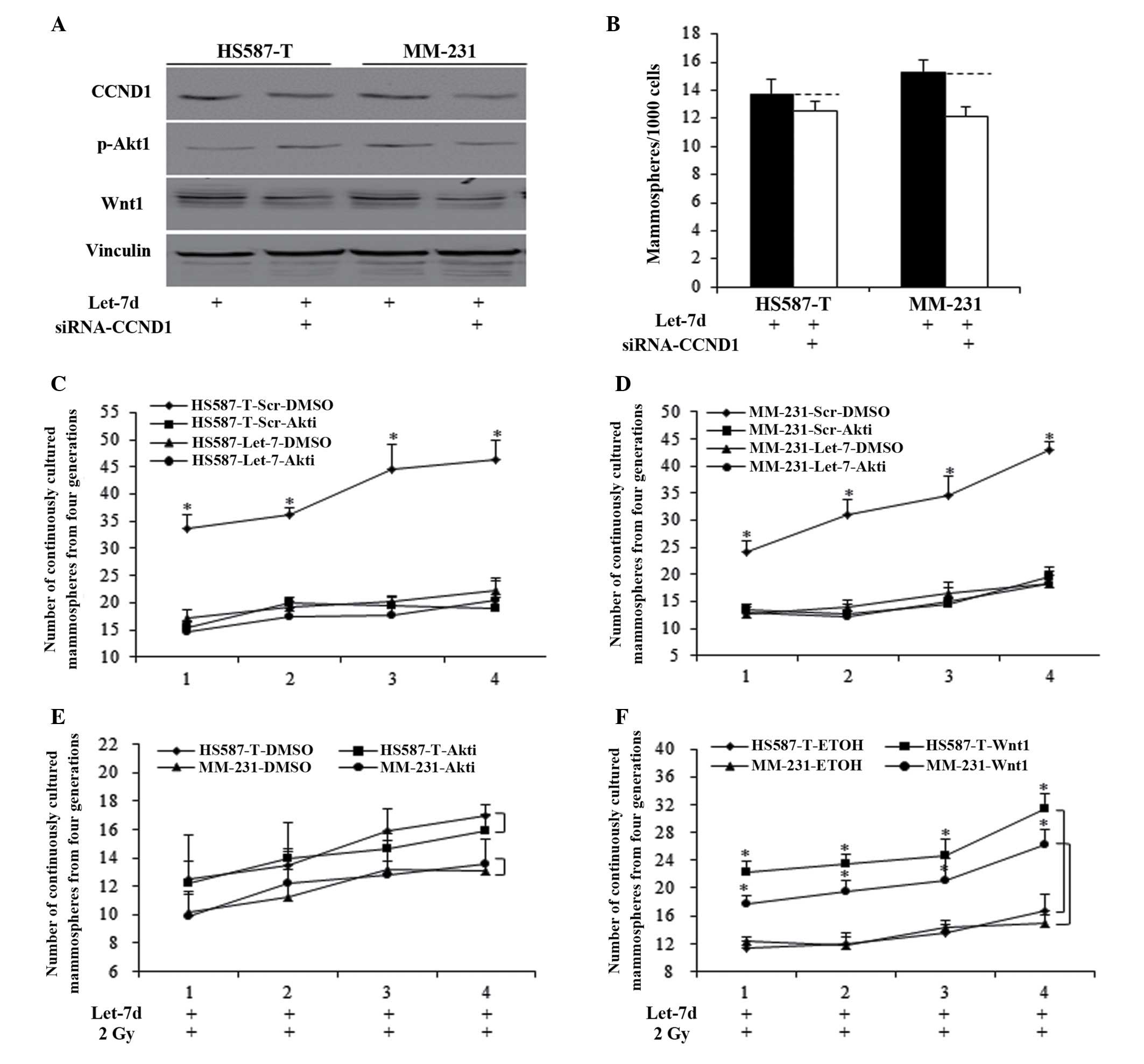 | Figure 4Let-7d sentisized breast cancer cells
to the radiation response through inhibiting cyclin D1/Akt1/Wnt
signaling. (A) Let-7d functioned through inhibition of cyclin D1,
then inhibited the Akt phosphorylation and Wnt1 activation. (B) The
inhibition of cyclin D1 in let-7d-overexpressed cells did not
reduce the mammosphere number significantly, compared with the
let-7d group, P>0.05. Akt inhibitors significantly inhibited the
MFE of (C) HS587-T and (D) MM-231 cells of the scramble group,
*P<0.01 vs. the DMSO control group, with no
significant alterations identified in cells infected with let-7d.
(E and F) Akt phosphorylation inhibition resulting from Akt
inhibitors did not influence the MFE of let-7d- and
radiation-treated cells. (F) The restoration of Wnt1 abolished
let-7d induction of radiation sensitization, *P<0.01.
Akt, protein kinase B; Wnt, wingless type MMTV integration site
family member 1; MFE, mammosphere formation efficiency; siRNA,
small interfering RNA; DMSO, dimethyl sulfoxide; Scr, scramble;
ETOH, ethanol. |
Discussion
Previous studies have indicated that the restoration
of let-7 in tumors effectively inhibited cell proliferation and
invasion, and sensitized the resistant cancer cells to chemotherapy
(22,23). Studies have previously investigated
the function of let-7 on the self-renewal of CSCs, indicating that
let-7 restoration may be utilized in therapy of breast cancer
(8,21,24).
TNBC is more malignant and responds more poorly to chemotherapy and
endocrine therapy than other subtypes of breast cancer, therefore
it is important to investigate novel therapeutic options that may
improve the prognosis of patients with TNBC. β-catenin controls
cell adhesion and proliferation, stimulating TCF to markedly
upregulate oncogenes (25), thus
serves a major role in breast cancer, including TNBC (26,27).
Wnt1 stimulates the Wnt1/β-catenin/TCF pathway and regulates the
transcription of TCF motif activators, thus stimulating the
self-renewal of CSCs (28–30).
To investigate the role of let-7 in breast cancer,
let-7 miRNA signatures were investigated in multiple breast cancer
cell lines. It was identified that let-7d was significantly reduced
in triple negative HS587-T and MDA-MB-231 cells, indicating a
potentially important role for let-7. Let-7d-overexpressing HS587-T
and MDA-MB-231 were then constructed, and it was demonstrated that
let-7d exhibited a strong inhibition on MFE, continuously
inhibiting the self-renewal ability in up to four generations of
cells. Radiation resulted in self-renewal inhibition. It was
identified that 2 Gy radiation alone resulted in marginal
inhibition on TNBC stem cells; however, when in combination with
let-7d, 2 Gy radiation significantly suppressed the number of
mammospheres. Through bioinformatics analysis and western blotting,
the possible genes accounting for let-7-induced mammosphere
inhibition were assessed. Let-7d was observed to inhibit cyclin D1
expression levels by directly inhibiting the cyclin D1 3′UTR, thus
suppressing cyclin D1/Akt1/Wnt1 activity. The Akt inhibitor and
Wnt1 activator were used to investigate let-7d-induced
sensitization to radiotherapy. It was observed that let-7d
functioned through cyclin D1/Akt1, with no significant alterations
identified between let-7d and the Akt1 inhibitor, however reversal
of Wnt1 inhibition abolished the functions of let-7d.
In conclusion, the results of the current study
indicated that let-7d significantly inhibited the malignant
behaviors of TNBC cells in vitro, and suppressed the
self-renewal abilities of CSCs. Furthermore, in HS587-T and MM-231
cells, it was identified that let-7 functioned through inhibiting
the cyclin D1/Akt1/Wnt1 pathway, and sensitized TNBC to radiation
therapy-induced renewal repression (31). The repression of the Akt1/Wnt1
pathway in CSCs contributed to let-7-induced radiation
sensitization, helping to inhibit the self-renewal of stem cells
and eliminate the tumor group. The results of the current study aid
in the understanding of the mechanisms through which let-7
regulated TNBC, and suggest that restoration of the let-7 family,
particularly let-7d in TNBC, may be a novel therapeutic target.
Acknowledgments
The authors would like to thank the staff of the
Central Laboratory of the First Affiliated Hospital of Zhengzhou
University (Zhengzhou, China), for their technical assistance.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Desmedt C, Haibe-Kains B, Wirapati P,
Buyse M, Larsimont D, Bontempi G, Delorenzi M, Piccart M and
Sotiriou C: Biological processes associated with breast cancer
clinical outcome depend on the molecular subtypes. Clin Cancer Res.
14:5158–5165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tran B and Bedard PL: Luminal-B breast
cancer and novel therapeutic targets. Breast Cancer Res.
13:2212011. View
Article : Google Scholar
|
|
4
|
Hayes EL and Lewis-Wambi JS: Mechanisms of
endocrine resistance in breast cancer: An overview of the proposed
roles of noncoding RNA. Breast Cancer Res. 17:402015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sotiriou C, Neo SY, McShane LM, Korn EL,
Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL and Liu ET: Breast
cancer classification and prognosis based on gene expression
profiles from a population-based study. Proc Natl Acad Sci USA.
100:10393–10398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Assi HA, Khoury KE, Dbouk H, Khalil LE,
Mouhieddine TH and El Saghir NS: Epidemiology and prognosis of
breast cancer in young women. J Thorac Dis. 5(Suppl 1): S2–S8.
2013.PubMed/NCBI
|
|
7
|
Sun X, Jiao X, Pestell TG, Fan C, Qin S,
Mirabelli E, Ren H and Pestell RG: MicroRNAs and cancer stem cells:
The sword and the shield. Oncogene. 33:4967–4977. 2014. View Article : Google Scholar
|
|
8
|
Rothschild SI: microRNA therapies in
cancer. Mol Cell Ther. 2:72014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Costa PM and Pedroso de Lima MC: MicroRNAs
as molecular targets for cancer therapy: On the modulation of
microRNA expression. Pharmaceuticals (Basel). 6:1195–1220. 2013.
View Article : Google Scholar
|
|
10
|
Sun X, Tang SC, Xu C, Wang C, Qin S, Du N,
Liu J, Zhang Y, Li X, Luo G, et al: Dicer regulated let-7
expression levels in p53-induced cancer repression requires cyclin
D1. J Cell Mol Med. 19:1357–1365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan
J, Wu J and Li M: MicroRNA-374a activates Wnt/β-catenin signaling
to promote breast cancer metastasis. J Clin Invest. 123:566–579.
2013.PubMed/NCBI
|
|
12
|
Laezza C, d'Alessandro A, Malfitano AM and
Bifulco M: Anandamide inhibits the Wnt/β-catenin signalling pathway
in human breast cancer MDA MB 231 cells. Eur J Cancer.
49:2066–2067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malanchi I, Santamaria-Martinez A, Susanto
E, Peng H, Lehr HA, Delaloye JF and Huelsken J: Interactions
between cancer stem cells and their niche govern metastatic
colonization. Nature. 481:85–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Cicalese A, Bonizzi G, Pasi CE, Faretta M,
Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP and Pelicci
PG: The tumor suppressor p53 regulates polarity of self-renewing
divisions in mammary stem cells. Cell. 138:1083–1095. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oliveras-Ferraros C, Cufi S,
Vazquez-Martin A, Torres-Garcia VZ, Del Barco S, Martin-Castillo B
and Menendez JA: Micro (mi) RNA expression profile of breast cancer
epithelial cells treated with the anti-diabetic drug metformin:
Induction of the tumor suppressor miRNA let-7a and suppression of
the TGFβ-induced oncomiR miRNA-181a. Cell Cycle. 10:1144–1151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Wang Y, Fan C, Gao P, Wang X, Wei G
and Wei J: Estrogen promotes stemness and invasiveness of
ER-positive breast cancer cells through Gli1 activation. Mol
Cancer. 13:1372014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Wang C, Wang Z, Dampier W, Wu K,
Casimiro MC, Chepelev I, Popov VM, Quong A, Tozeren A, et al:
Attenuation of Forkhead signaling by the retinal determination
factor DACH1. Proc Natl Acad Sci USA. 107:6864–6869. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson CD, Esquela-Kerscher A, Stefani G,
Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J,
Shingara J, et al: The let-7 microRNA represses cell proliferation
pathways in human cells. Cancer Res. 67:7713–7722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsang WP and Kwok TT: Let-7a microRNA
suppresses therapeutics-induced cancer cell death by targeting
caspase-3. Apoptosis. 13:1215–1222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saridaki Z, Weidhaas JB, Lenz HJ,
Laurent-Puig P, Jacobs B, De Schutter J, De Roock W, Salzman DW,
Zhang W, Yang D, et al: A let-7 microRNA-binding site polymorphism
in KRAS predicts improved outcome in patients with metastatic
colorectal cancer treated with salvage cetuximab/panitumumab
monotherapy. Clin Cancer Res. 20:4499–4510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Worringer KA, Rand TA, Hayashi Y, Sami S,
Takahashi K, Tanabe K, Narita M, Srivastava D and Yamanaka S: The
let-7/LIN-41 pathway regulates reprogramming to human induced
pluripotent stem cells by controlling expression of
prodifferentiation genes. Cell Stem Cell. 14:40–52. 2014.
View Article : Google Scholar
|
|
23
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar
|
|
24
|
Ohno S, Takanashi M, Sudo K, Ueda S,
Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, et
al: Systemically injected exosomes targeted to EGFR deliver
antitumor microrna to breast cancer cells. Mol Ther. 21:185–191.
2013. View Article : Google Scholar :
|
|
25
|
Laezza C, D'Alessandro A, Paladino S,
Maria Malfitano A, Chiara Proto M, Gazzerro P, Pisanti S, Santoro
A, Ciaglia E and Bifulco M; Endocannabinoid Research Group:
Anandamide inhibits the Wnt/β-catenin signalling pathway in human
breast cancer MDA MB 231 cells. Eur J Cancer. 48:3112–3122. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taurin S, Sandbo N, Yau DM, Sethakorn N
and Dulin NO: Phosphorylation of beta-catenin by PKA promotes
ATP-induced proliferation of vascular smooth muscle cells. Am J
Physiol Cell Physiol. 294:C1169–C1174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Midwood KS and Orend G: The role of
tenascin-C in tissue injury and tumorigenesis. J Cell Commun
Signal. 3:287–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi AR, Park JR, Kim RJ, Kim SR, Cho SD,
Jung JY and Nam JS: Inhibition of Wnt1 expression reduces the
enrichment of cancer stem cells in a mouse model of breast cancer.
Biochem Biophys Res Commun. 425:436–442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He B and Jablons DM: Wnt signaling in stem
cells and lung cancer. Cancer Stem Cells. Wiestler OD, Haendler B
and Mumberg D: Springer-Verlag; Berlin: pp. 27–58. 2007, View Article : Google Scholar
|
|
31
|
Sun X, Qin S, Fan C, Xu C, Du N and Ren H:
Let-7: A regulator of the ERalpha signaling pathway in human breast
tumors and breast cancer stem cells. Oncol Rep. 29:2079–2087.
2013.PubMed/NCBI
|