Introduction
Altered expression of nuclear retinoid receptors is
associated with the malignant transformation of human cells
(1). Retinoic acid receptor β
(RARβ) belongs to the RAR superfamily and is frequently silenced
during epithelial cell carcinogenesis, including those of the
breast, lung, esophagus, pancreas, cervix and prostate (2–7).
Thus, RARβ is believed to play a role as a tumor suppressor gene in
human tumorigenesis (8). Of
particular note, esophageal, lung and breast cancer cell lines that
do not express RARβ have been found to be resistant to retinoid
treatment (9,10). This has led to the hypothesis that
RARβ expression may be responsible, in part, for mediating the
efficacy of chemopreventative agents in clinical trials, such as
inhibitors of the epidermal growth factor receptor and
β-cryptoxanthin. An increasing number of studies suggest that
restoration of RARβ expression in tumor cells may enhance their
response to chemotherapeutic agents (11).
Cholangiocarcinoma (CCA) is the second most common
primary liver malignancy, which is highly resistant to available
chemotherapeutic agents and confers a 5-year relative survival rate
of less than 5% (12). Surgery is
the only curative treatment for CCA, however only a very small
percentage of tumors are resectable. This is primarily due to the
majority of patients presenting with late-stage disease, such as
local advanced or metastatic disease (13). Therefore, the identification of new
and effective therapies against CCA is critical. To date, clinical
strategies to enhance drug sensitivity have included efforts that
aim to initiate apoptotic signaling pathways, activate specific
tumor suppressors and determine effective combination regimens
(14). However, expression of the
RARβ tumor suppressor in CCA, together with its role in regulating
sensitivity to chemotherapeutic agents, are currently unknown.
In the present study, the expression of RARβ in CCA
tissues was investigated and the potential mechanisms by which RARβ
confers sensitivity to chemotherapeutic agents within CCA cells
in vitro and in vivo was explored.
Materials and methods
Reagents
5-fluorouracil (5-FU), vincristine sulfate (VCR),
cisplatin (CDDP), mitomycin C (MMC),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
caspase-3 inhibitor (z-VAD-fmk), G418, BMS453 and CoCl2 were all
purchased from Sigma-Aldrich (St. Louis, MO, USA). The RPMI-1640
and fetal bovine serum (FBS) were all purchased from Gibco BRL
(Grand Island, NY). The 100 U/ml penicillin and bicinchoninic acid
protein assay reagent were purchased from Wuhan Boster (Wuhan,
China). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; ab9485)
and RARβ (ab198557) antibodies were purchased from Abcam
(Cambridge, UK). The annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double staining apoptosis detection
kit and TdT-mediated dUTP nick end labeling (TUNEL) kit were
obtained from Roche Diagnostics (Basel, Switzerland). Lipofectamine
2000 was purchased from Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA., USA). The Caspase-3 assay kit was from R&D
Systems, Inc. (Minneapolis, MN, USA). All of other chemicals and
reagents were the highest quality and purchased from standard
commercial sources.
Patients and tumor specimens
Analysis of RARβ expression in clinical CCA tissues
was performed in agreement with the ethical guidelines of the 1975
Declaration of Helsinki, and was approved by the Institute Research
Ethics Committee at The First Affiliated Hospital of Xiamen
University. Between 2009–2013, 33 CCA samples were collected from
patients without metastatic disease that had not received
pre-operative treatment. Tumor tissues were fixed in 10% formalin
and then paraffin-embedded for immunohistochemical (IHC) analysis
and routine histological characterization.
Cell culture and stable transfection
The human CCA cell line QBC939 was a kind gift from
Professor Shu-Guang Wang from Southwest Hospital, the Third
Military Medical University (Chongqing, China). The CCA cell lines
Sk-ChA-1, MZ-ChA-1 and Hccc9810 were a kind gift from Professor
Chun-Dong Yu laboratory of Xiamen University (Xiamen, China).
HIBEpiC human intrahepatic biliary epithelial cells were purchased
from Cell Bank of the Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China). All four human CCA cell lines and
HIBEpiC cells were all cultured in RPMI-1640 supplemented with 10%
FBS and 100 U/ml penicillin in a humidified chamber at 37°C in 5%
CO2. RARβ cDNA was cloned into the expression vector
pBoBi as described previously (15). The RARβ expression vector (4
μg) was stably transfected into QBC939 cells
(1×106) to produce pBoBi-RARβ using 10 μl
Lipofectamine 2000, according to the manufacturer's instructions.
Positive selection of stable transfectants was achieved by
supplementing complete medium with 400 mg/ml of G418. Stable
control vector QBC939 transfectants are defined as pBoBi-Ctrl from
herein.
Cell proliferation assay
To determine cell viability in response to
treatment, an MTT assay was used. Briefly, QBC939 cells were seeded
onto 96-well culture plates at a density of 5×103
cells/well and grown in complete RPMI-1640 culture medium.
Following overnight incubation, cells were treated with a variety
of agents (60 μM) including 5-FU, CDDP, VCR and MMC for 48
h. MTT (5 mg/ml) was added to each well and incubated at 37°C for 4
h, before the resulting formazan crystals were dissolved in
dimethyl sulfoxide. Absorbance was read at 490 nm using a
microplate reader (Model 680; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Hypoxic growth conditions
Cells were cultured in a 35 mm dish until 70–80%
confluence was reached. They were then transferred to a hypoxic
incubator with a humidified atmosphere that was flushed with ≤0.1%
O2, 5% CO2 and 95% N2, followed by
incubation at 37°C for 12 h.
Serum starvation
Cells were seeded onto 6-well culture plates at a
density of 2×105 cells/well and grown in complete
RPMI-1640 culture medium overnight. Then the medium was removed and
washed with PBS, and added the RPMI 1640 medium without serum. The
duration of serum starvation was determined according to the
experimental requirements.
Tumor xenograft study
In vivo drug sensitivity experiments were
divided into two groups: RARβ (pBoBi-RARβ) and control
(pBoBi-Ctrl). Female specific pathogen-free BALB/c nude mice
(Shanghai Laboratory Animal Center, Shanghai, China) (age, 4–5
weeks) were injected subcutaneously with 100 μl cells
(2×106). Mice were treated with 70 mg/kg/d 5-FU at day 8
post-transplantation. Tumor volumes were determined using the
following formula: (AxB2)/2, where A is the largest
diameter and B is the perpendicular diameter. After 35 days from
injection, mice were sacrificed by inhalation of carbon dioxide.
All animal procedures were approved by the Animal Care and Use
Committee of the First Affiliated Hospital of Xiamen
University.
Gene and protein expression analyses
mRNA expression levels were determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Protein expression was measured using a western blotting assay.
Both analyses were performed as described previously (15).
Immunohistochemical (IHC) staining
IHC was performed to detect RARβ expression in CCA
tissues as described previously (16). Human CCA tissue sections were
immunostained with an antibody against RARβ (1:100). IHC scoring
was conducted by two independent pathologists. The number of
positively-stained cells per 1,000 cells in randomly selected
fields was recorded, and the mean number from a minimum of five
slides was calculated. Staining intensity was categorized as low
(<50%) or high (>50%).
Analysis of apoptosis
Apoptosis was evaluated using an annexin V-FITC/PI
double-staining assay and a TUNEL assay. The annexin V-FITC/PI
double-staining assay was performed according to the manufacturer's
instructions (Roche Diagnostics) and analyzed using a flow
cytometer (FACSCabilur; BD Biosciences, Franklin Lakes, CA, USA).
TUNEL staining was performed using an in situ cell death
detection kit according to the manufacturer's instructions (Roche
Diagnostics).
Analysis of caspase-3 activity
Caspase-3 activity was determined using the
caspase-3 assay kit (R&D Systems Inc., Minneapolis, MN, USA)
according to the manufacturer's instructions. Briefly, cells were
lysed and centrifuged at 10,000 × g for 20 min at 4°C to obtain
supernatants. The protein concentration of sample supernatants was
determined using a bicinchoninic acid protein assay reagent before
they were added to a dithiothreitol and caspase-3 substrate
reaction mixture and incubated for 2 h at 37°C. Absorbance was
measured at 405 nm using a microplate reader (Model 680; Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard error from
a minimum of three independent experiments. Student's t-test
or one-way analysis of variance was conducted using the SPSS 13.0
software package (SPSS, Inc., Chicago, IL, USA). Dunn test was used
if there was statistical significance after one-way analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Low expression of RARβ is partially
responsible for the drug resistance of CCA
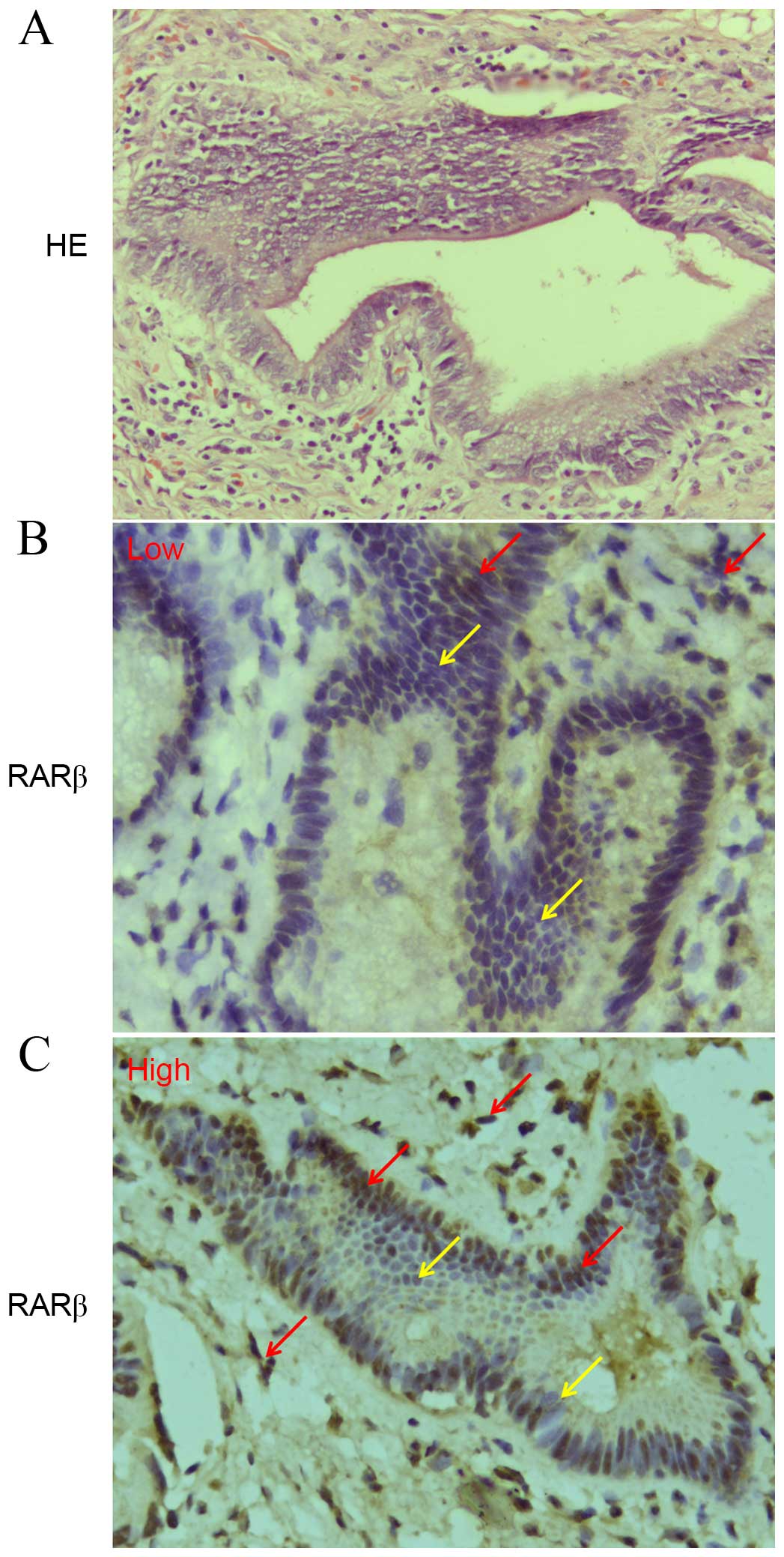
IHC was performed to investigate RARβ protein
expression in a set of 33 paraffin-embedded human CCA tissues. A
total of 28/33 (84.8%) tissues were found to exhibit low RARβ
protein expression (Fig. 1A), only
5 tissues exhibited high RARβ protein expression (Fig. 1B). In addition, RARβ mRNA and
protein expression levels in four CCA cell lines (QBC939, SK-ChA-1,
MZ-ChA-1 and Hccc9810 cells) were found to be significantly lower
than the HBd Epi normal bile duct epithelial cell line (Fig. 2A). RARβ mRNA and protein levels
were markedly reduced within QBC939 cells. Notably, the proportion
of surviving QBC939 cells following exposure to three commonly used
chemotherapeutic agents including 5-FU, VCR and MMC, was found to
be significantly higher compared with the remaining three CCA cell
lines (Fig. 2B). These results
give rise to the hypothesis that the observed resistance of CCA
cells to common therapeutic agents, may be associated with silenced
RARβ expression.
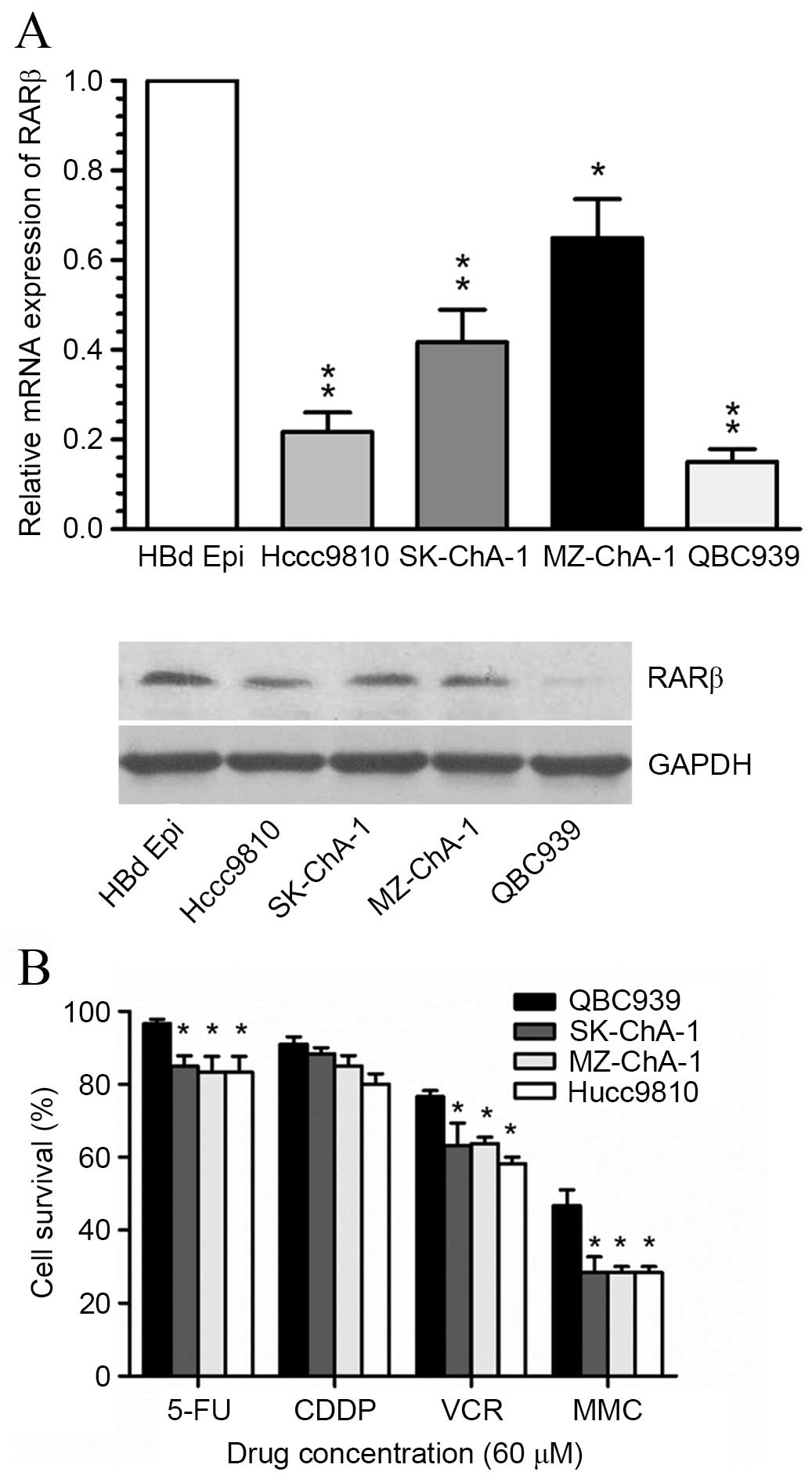 | Figure 2Expression of RARβ and drug
sensitivity in human CCA cell lines. (A) Relative mRNA expression
levels of RARβ in the normal bile duct cells and 4 CCA cell lines,
as detected by reverse transcription-quantitative polymerase chain
reaction. *P<0.05, **P<0.001 vs. HBd
Epi cells. Protein expression detected by western blotting
analysis. (B) Drug sensitivities of four CCA cell lines in response
to 5-FU, CDDP, VCR and MMC chemotherapeutic agents as determined
using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide assay. *P<0.05, **P<0.001 vs.
QBC939 cells. RARβ, retinoic acid receptor-β; CCA,
cholangiocarcinoma; 5-FU, 5-fluorouracil; CDDP, cisplatin; VCR,
vincristine; MMC, mitomycin C. |
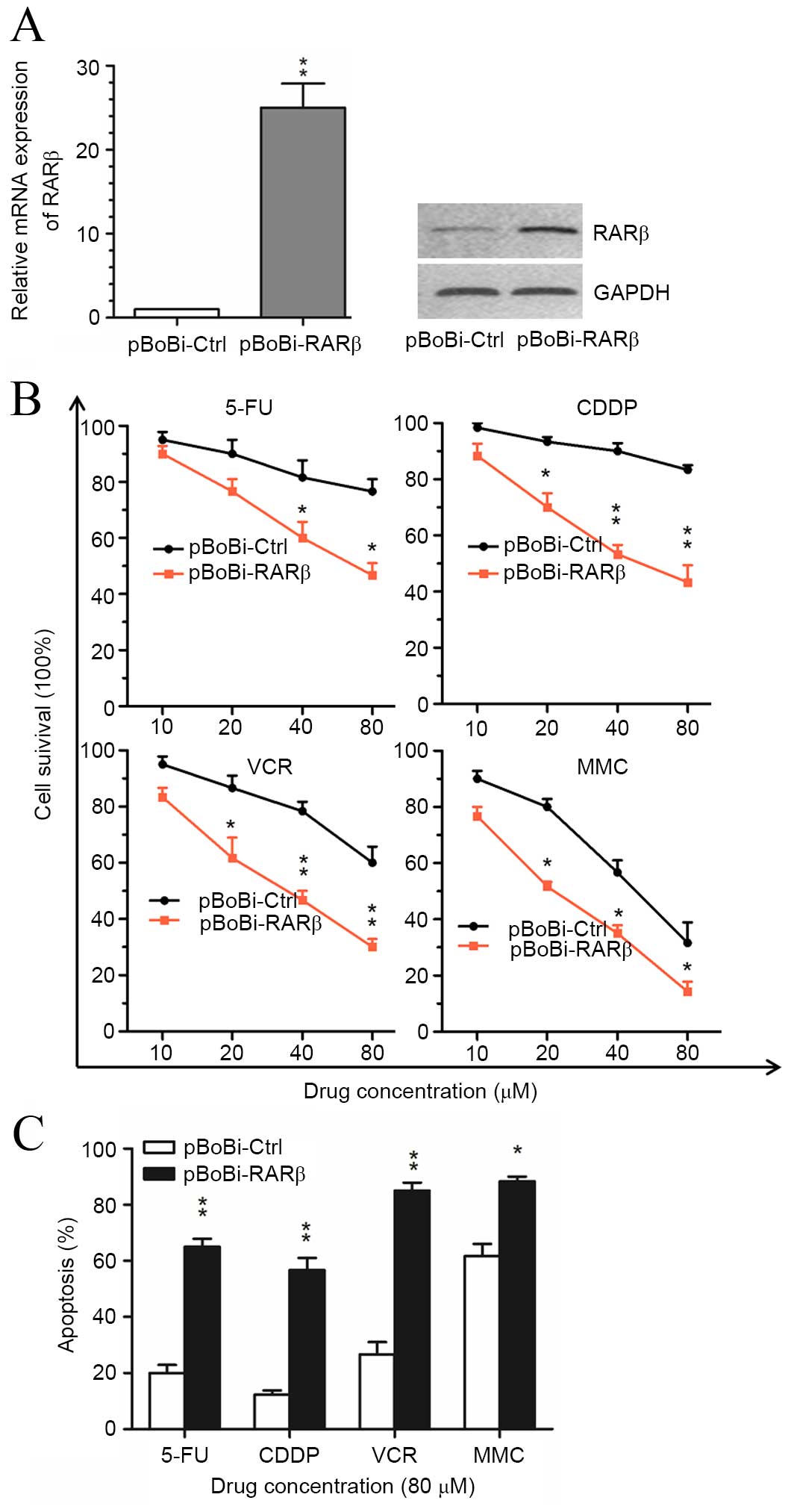
Upregulation of RARβ enhances the
sensitivity of CCA cells to chemotherapeutic agents in vitro
In order to investigate the role of RARβ in the
therapeutic response of CCA cells, RARβ expression vectors were
transfected into QBC939 cells prior to treatment with
chemotherapeutic agents. As shown in Fig. 3A, an increase in RARβ mRNA and
protein levels was observed within stably RARβ-transfected cells
(pBoBi-QBC939) compared with control (pBoBi-Ctrl) cells. Cell
survival analysis results indicated that the sensitivity of
pBoBi-QBC939 cells to common chemotherapeutic agents, including
5-FU, CDDP, VCR and MMC, was significantly enhanced following RARβ
upregulation compared with pBoBi-Ctrl cells (Fig. 3B). Furthermore, the proportion of
apoptotic pBoBi-QBC939 cells generated in response to 5-FU, CDDP,
VCR and MMC, was found to be significantly higher than pBoBi-Ctrl
cells (Fig. 3C). These in
vitro findings suggest that upregulation of RARβ enhances the
sensitivity of CCA cells to chemotherapeutic agents.
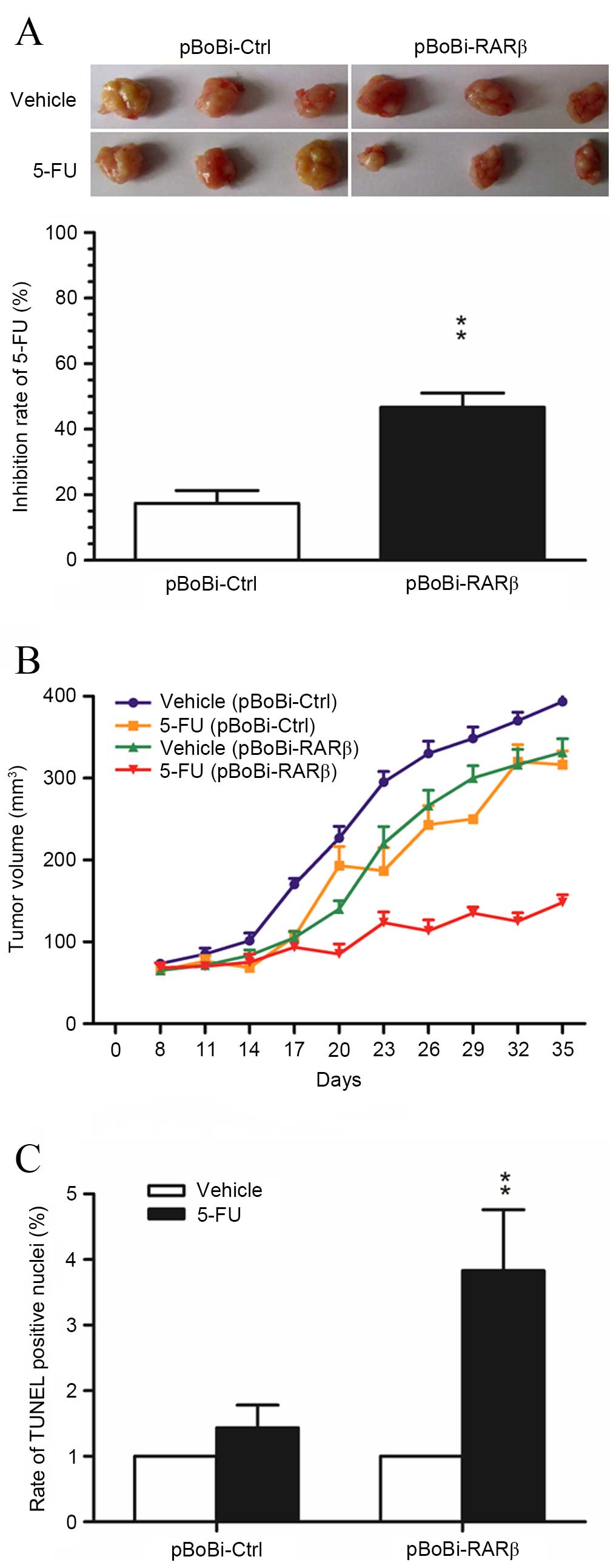
Upregulation of RARβ increases the
cytotoxic effect of 5-FU on xenografted CCA tumors
5-FU is a common chemotherapeutic agent used for the
treatment of hepatobiliary tumors. Therefore, this agent was
selected to investigate the contribution of RARβ upregulation in
the sensitivity to chemotherapeutic treatment in the xenografted
QBC939 CCA tumors. As shown in Fig.
4A, the rate of tumor growth inhibition in xenografted
pBoBi-RARβ tumors following treatment with 5-FU, was significantly
higher than in control pBoBi-Ctrl tumors. A significant difference
in tumor growth inhibition was apparent from day 20 and increased
further during late tumor growth (Fig.
4B). Moreover, analysis of apoptosis in xenografted CCA tumor
tissues using the TUNEL assay was consistent with the results of
the in vitro assays, and indicated that the number of
apoptotic cells induced by 5-FU in xenografted pBoBi-RARβ tumors
was significantly higher than control pBoBi-Ctrl tumors (P=0.0035;
Fig. 4C). These in vivo
findings provide additional evidence that RARβ upregulation
enhances the sensitivity of CCA cells to chemotherapeutic
agents.
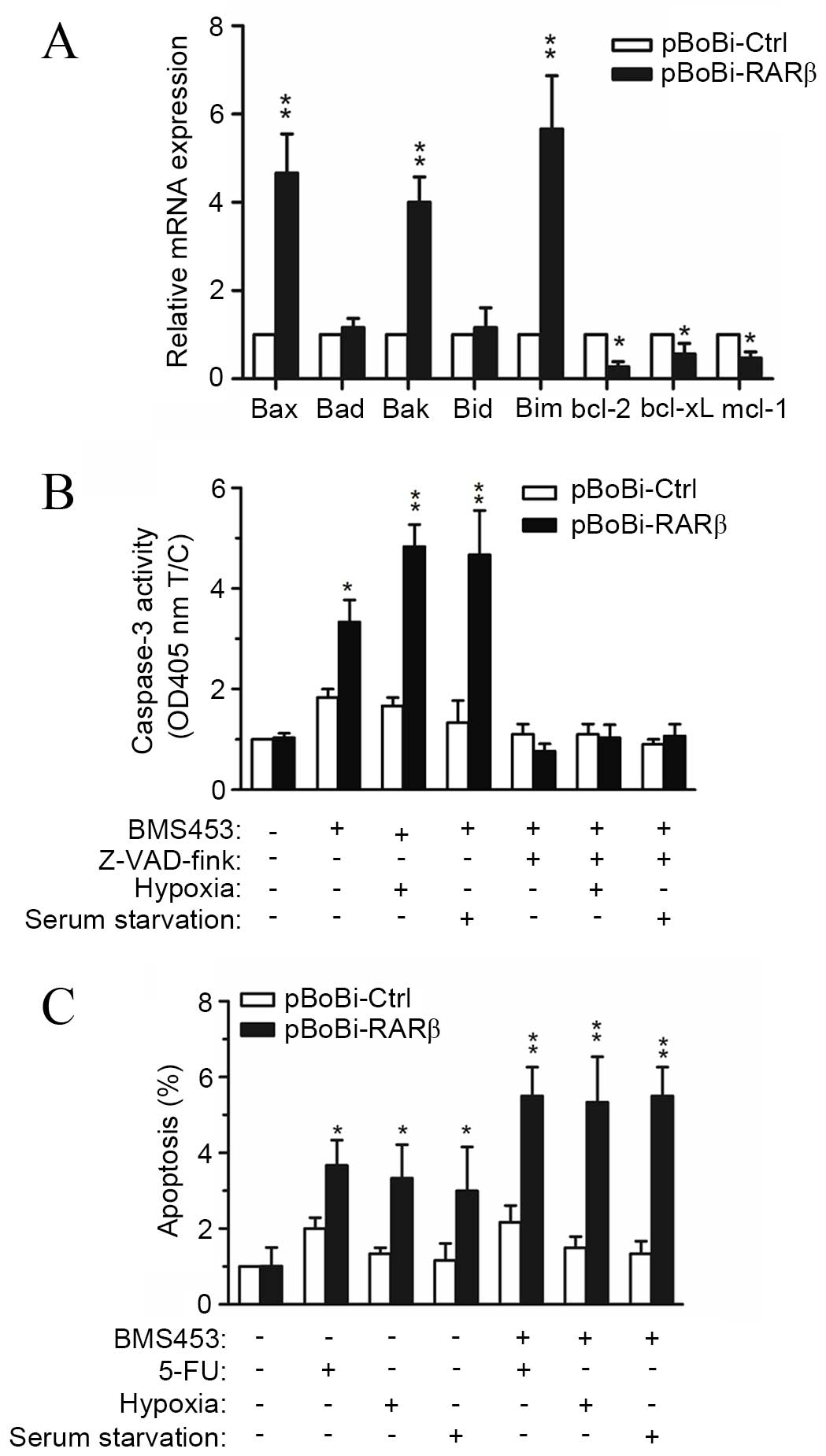
High RARβ expression renders CCA cells
more susceptible to caspase-dependent apoptosis
Taking the above results into consideration, the
observed increase in the sensitivity of CCA cells to
chemotherapeutic agents following RARβ upregulation may render CCA
cells more susceptible to apoptosis. Hence, the expression levels
of genes associated with apoptosis following RARβ upregulation were
investigated. Results from RT-qPCR assays showed that the
expression of proapoptotic genes, including bax, bak and bim, were
increased >4 fold in pBoBi-RARβ QBC939 cells, while the
expression of antiapoptotic genes, including bcl-2, bcl-xL and
mcl-1, were all significantly decreased compared with pBoBi-Ctrl
cells (Fig. 5A). To investigate
these findings further, the association between RARβ expression and
caspase-3 activity was explored. Treatment of pBoBi-RARβ cells with
the RARβ-activator, BMS453, resulted in a 3.5-fold increase in
caspase-3 activity compared with pBoBi-Ctrl cells, which exhibited
a 1.8-fold increase. The observed increase in caspase-3 activity
was further enhanced when cells were cultured under hypoxic
conditions or following serum starvation. By contrast, the activity
of caspase-3 was significantly decreased in both pBoBi-RARβ and
pBoBi-Ctrl cells after treatment with the caspase-3 inhibitor
z-VAD-fmk, even when cells were cultured under hypoxic conditions
or following serum starvation (Fig.
5B). Furthermore, apoptosis induced by 5-FU, hypoxia or serum
starvation was enhanced upon upregulation or activation of RARβ
(Fig. 5C). These results indicate
that the susceptibility of CCA cells to caspase-dependent apoptosis
may be enhanced by the upregulation of RARβ.
Discussion
The results of the present study indicate the role
of RARβ upregulation on the sensitivity of CCA cells to
chemotherapeutic agents. Of note, RARβ upregulation was found to
increase the sensitivity of CCA cells and tumors to various
chemotherapeutic agents currently used for the treatment of CCA. In
addition, RARβ upregulation was observed to be associated with an
increase in the Bax/Bcl-2 ratio and caspase-3 activity, and
rendered CCA cells and xenograft tumors more susceptible to
apoptosis. These findings provide evidence to suggest that the
upregulation of RARβ may represent a beneficial adjunct therapeutic
strategy for the treatment of CCA.
Previous studies have shown that loss of RARβ is a
common event in the development of malignant tumors, and the
induction of RARβ expression suppresses cancer development
(2–7,17).
Increased expression of RARβ has been found to correlate with the
clinical response to various retinoids (1,18);
however, the molecular mechanism of RARβ in regulating sensitivity
to chemotherapeutic agents is little known. A major problem in the
treatment of human cancer is the lack of response of many tumors to
chemotherapeutic agents. Following exposure to anti-cancer agents,
tumor cells can alter the expression profile of a specific set of
genes to activate and/or suppress signaling networks, which
de-sensitizes cells to drug-induced death signals and confers cell
survival and chemoresistance (19,20).
Similar to other types of human carcinoma, the
results of the present study indicate that CCA, a chemoresistant
bile duct carcinoma with a poor prognosis (21), demonstrates loss of RARβ
expression. Upregulation of RARβ within the drug-resistant CCA cell
line QBC939, which exhibits loss of RARβ expression, was observed
to increase the susceptibility of cells to apoptosis induced by
chemotherapeutic agents in vitro and in vivo. Hypoxia
and nutrient deficiency are common adverse microenvironments
encountered by tumor cells. Based on the presence of specific
intracellular signaling pathways, tumor cells can either become
more aggressive or undergo apoptosis in response to these
microenvironmental stress conditions. Differentially expressed
genes, including tumor suppressor genes and oncogenes, are involved
in the processes of adaptation. Once upregulated, tumor suppressor
genes can initiate apoptosis (22). Consistent with these observations,
upregulation of RARβ in the present study was found to induce CCA
cells to undergo apoptosis in response to hypoxic and starvation
conditions.
It is possible that RARβ upregulation may initiate
the process of apoptosis. It is known that the Bcl-2 family of
proteins regulates the apoptotic pathway by balancing the
expression of proapoptotic (Bax) and antiapoptotic (Bcl-2) factors.
In this regard, the Bax/Bcl-2 ratio is suggested to be a useful
predictor of apoptotic cell death (23). In the present study, RARβ
upregulation was found to induce changes in the expression of genes
belonging to the Bcl-2 family, which resulted in an increase in the
Bax/Bcl-2 ratio. Caspase-3 functions as an executor of apoptosis by
activating DNA fragmentation (24). During the apoptotic processes,
cytochrome c is released from the mitochondria and into the
cytosol. Cytoplasmic cytochrome c then activates caspase-9, which
in turn triggers the activation of caspase-3 and leads to cell
death. Increased caspase-3 activity is associated with an increase
in the Bax/Bcl-2 ratio (25).
Despite the fact that the expression of cytochrome c and caspase-9
was not investigated in the present study, apoptosis induced by
RARβ upregulation was observed to be associated with the activation
of caspase-3, which led to an increase in the Bax/Bcl-2 ratio.
In conclusion, the data presented in the present
study demonstrate a clear functional role for RARβ in sensitizing
CCA cells to chemotherapy-induced cell death. Taken in conjunction
with previous studies, it is proposed that the use of adjunct
therapies that confer RARβ upregulation should be explored for the
treatment of chemoresistant tumors, such as CCA, with the aim of
overcoming drug resistance and/or increasing the susceptibility of
tumor cells to initiate intrinsic cell death pathways.
Acknowledgments
The present study was supported by grants from
National Nature Science Foundation of China (grant nos. 81572394
and 81201892) and the Project from Science and Technology Bureau of
Xiamen, China (grant no. 3502Z20144002).
References
|
1
|
Bialešová L, Brtko J, Lenko V and Macejová
D: Nuclear receptors-target molecules for isoflavones in cancer
chemoprevention. Gen Physiol Biophys. 32:467–478. 2013. View Article : Google Scholar
|
|
2
|
Brtko J: Role of retinoids and their
cognate nuclear receptors in breast cancer chemoprevention. Cent
Eur J Public Health. 15:3–6. 2007.PubMed/NCBI
|
|
3
|
Hua F, Fang N, Li X, Zhu S, Zhang W and Gu
J: A meta-analysis of the relationship between RARβ gene promoter
methylation and non-small cell lung cancer. PloS One. 9:e961632014.
View Article : Google Scholar
|
|
4
|
Liu Z, Zhang L, Ding F, Li J, Guo M, Li W,
Wang Y, Yu Z, Zhan Q, Wu M and Liu Z: 5-Aza-2′-deoxycytidine
induces retinoic acid receptor-beta(2) demethylation and growth
inhibition in esophageal squamous carcinoma cells. Cancer Lett.
230:271–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pérez RJ, Benoit YD and Gudas LJ: Deletion
of retinoic acid receptor β (RARβ) impairs pancreatic endocrine
differentiation. Exp Cell Res. 319:2196–2204. 2013. View Article : Google Scholar
|
|
6
|
Terra AP, Murta EF, Maluf PJ, Caballero
OL, Brait M and Adad SJ: Aberrant promoter methylation can be
useful as a marker of recurrent disease in patients with cervical
intraepithelial neoplasia grade III. Tumori. 93:572–579. 2007.
|
|
7
|
Moison C, Assemat F, Daunay A, Tost J,
Guieysse-Peugeot AL and Arimondo PB: Synergistic chromatin
repression of the tumor suppressor gene RARB in human prostate
cancers. Epigenetics. 9:477–482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alvarez S, Germain P, Alvarez R,
Rodríguez-Barrios F, Gronemeyer H and de Lera AR: Structure,
function and modulation of retinoic acid receptor beta, a tumor
suppressor. Int J Biochem Cell Biol. 39:1406–1415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng X, Green A, Shilkaitis A, Zhu Y,
Bratescu L and Christov K: Early in vitro passages of breast cancer
cells are differentially susceptible to retinoids and
differentially express RARβ isoforms. Int J Oncol. 39:577–583.
2011.PubMed/NCBI
|
|
10
|
Fernández-Martínez AB and Lucio Cazaña FJ:
Epidermal growth factor receptor transactivation by intracellular
prostaglandin E2-activated prostaglandin E2 receptors. Role in
retinoic acid receptor-β up-regulation. Biochim Biophys Acta.
1833:2029–2038. 2013. View Article : Google Scholar
|
|
11
|
Bu P and Wan YJ: Fenretinide-induced
apoptosis of Huh-7 hepatocellular carcinoma is retinoic acid
receptor beta dependent. BMC Cancer. 7:2362007. View Article : Google Scholar
|
|
12
|
Zhang W and Yan LN: Perihilar
cholangiocarcinoma: Current therapy. World J Gastrointest
Pathophysiol. 5:344–354. 2014.PubMed/NCBI
|
|
13
|
Unno M: Review of surgical treatment of
perihilar cholangiocarcinoma: Proper patient selection for combined
vascular resection and reconstruction. Nihon Geka Gakkai zasshi.
115:181–184. 2014.In Japanese. PubMed/NCBI
|
|
14
|
Demonceau J, Ruppar T, Kristanto P, Hughes
DA, Fargher E, Kardas P, De Geest S, Dobbels F, Lewek P, Urquhart
J, et al: Identification and assessment of adherence-enhancing
interventions in studies assessing medication adherence through
electronically compiled drug dosing histories: A systematic
literature review and meta-analysis. Drugs. 73:545–562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang GL, Luo Q, Rui G, Zhang W, Zhang QY,
Chen QX and Shen DY: Oncogenic activity of retinoic acid receptor γ
is exhibited through activation of the Akt/NF-κB and Wnt/β-catenin
pathways in cholangiocarcinoma. Mol Cell Biol. 33:3416–3425. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen DY, Fang ZX, You P, Liu PG, Wang F,
Huang CL, Yao XB, Chen ZX and Zhang ZY: Clinical significance and
expression of cyclin kinase subunits 1 and 2 in hepatocellular
carcinoma. Liver Int. 30:119–125. 2010. View Article : Google Scholar
|
|
17
|
Xu XC: Tumor-suppressive activity of
retinoic acid receptor-beta in cancer. Cancer Lett. 253:14–24.
2007. View Article : Google Scholar
|
|
18
|
Wan H, Oridate N, Lotan D, Hong WK and
Lotan R: Overexpression of retinoic acid receptor beta in head and
neck squamous cell carcinoma cells increases their sensitivity to
retinoid-induced suppression of squamous differentiation by
retinoids. Cancer Res. 59:3518–3526. 1999.PubMed/NCBI
|
|
19
|
Itamochi H, Kigawa J and Terakawa N:
Mechanisms of chemoresistance and poor prognosis in ovarian clear
cell carcinoma. Cancer Sci. 99:653–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar
|
|
21
|
Morita SY and Terada T: Molecular
mechanisms for biliary phospholipid and drug efflux mediated by
ABCB4 and bile salts. Biomed Res Int. 2014:9547812014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
DeClerck K and Elble RC: The role of
hypoxia and acidosis in promoting metastasis and resistance to
chemotherapy. Front Biosci (Landmark Ed). 15:213–225. 2010.
View Article : Google Scholar
|
|
23
|
Moldoveanu T, Follis AV, Kriwacki RW and
Green DR: Many players in BCL-2 family affairs. Trends Biochem Sci.
39:101–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Snigdha S, Smith ED, Prieto GA and Cotman
CW: Caspase-3 activation as a bifurcation point between plasticity
and cell death. Neurosci Bull. 28:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi M: The anti-apoptotic effect of
regucalcin is mediated through multisignaling pathways. Apoptosis.
18:1145–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|