Introduction
Peripheral nerve injuries are considered one of the
most challenging and difficult problems to treat with
reconstructive surgery (1).
Fractures, hematomas, contusions and compressions may induce
peripheral nerve injury, which is characterized by the disruption
of myelin sheaths and axons (2,3).
Inflammation has been shown to have an important role in the
pathogenesis of several neurodegenerative diseases, including
Parkinson's disease, Alzheimer's disease, multiple sclerosis and
amyotrophic lateral sclerosis (4–6). The
ability of the mammalian peripheral nervous system (PNS) to
regenerate axons following injury is well documented (7). Schwann cells have an important role
in axon regeneration post-injury (8,9).
Therefore, the identification of an effective
anti-neuroinflammatory and neuroprotective agent, which is able to
accelerate the proliferation of Schwann cells, thus maintaining the
Schwann cell phenotype, is of great importance.
In traditional Chinese medicine, Andrographis
paniculata is a traditional herb, which possesses
immunological, antibacterial, antiviral, anti-inflammatory,
antithrombotic, and lung and hepato-protective properties (10–13).
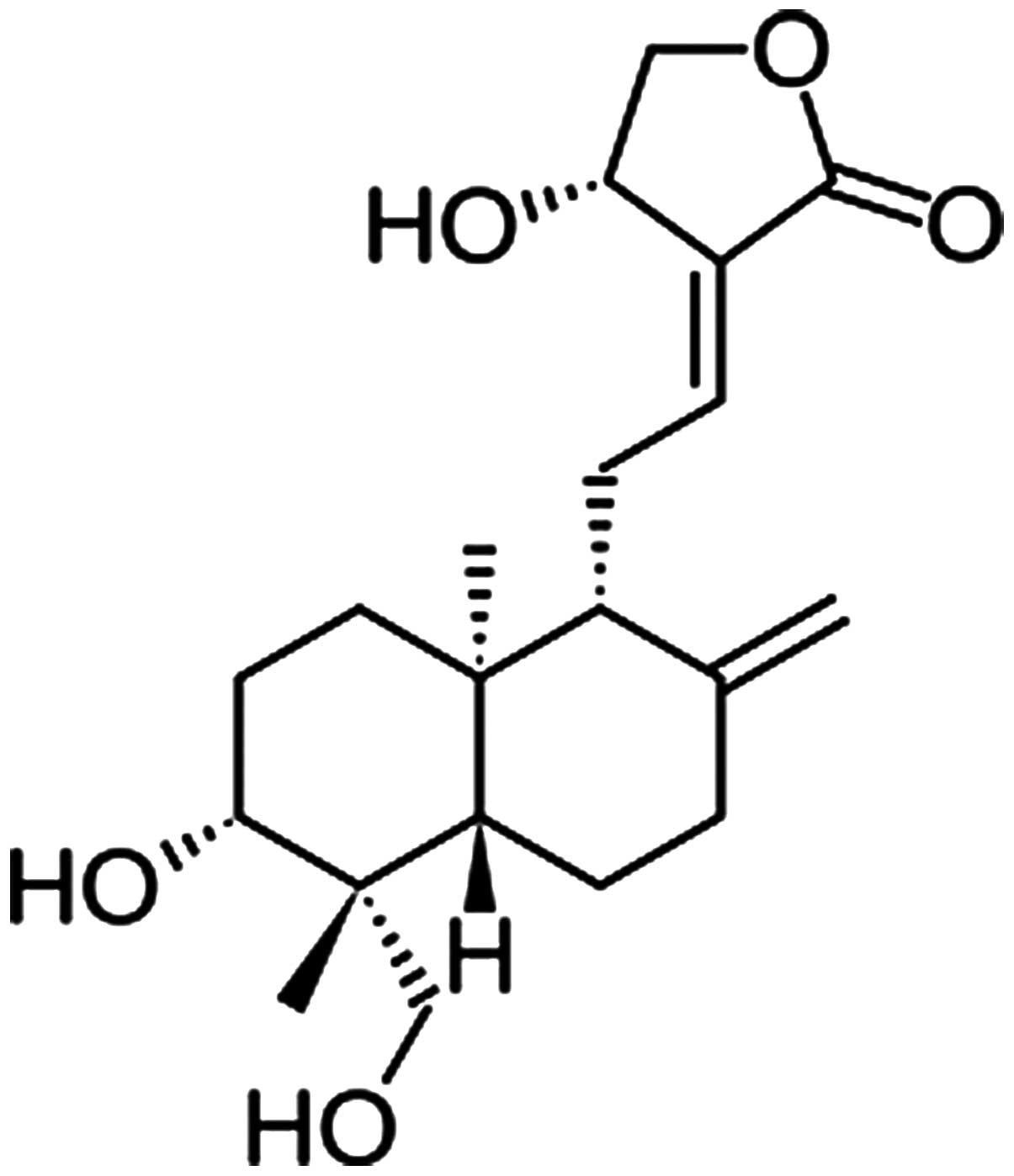
Andrographolide (Andro; Fig. 1) is
the primary active component of A. paniculata, which is
widely used in South Asia and China for the treatment of
inflammation-related diseases, due to its potent anti-inflammatory
and antiviral properties (14–16).
Andro and its derivatives, a group of diterpenes, have been
reported to exert a protective effect against
lipopolysaccharide-induced dopaminergic neurodegeneration in
mesencephalic neuron-glia cultures (17). The anti-inflammatory role of Andro
has been well-documented in several studies (18,19).
Furthermore, Andro exerts proapoptotic effects on tumor cells
(20,21). It has also been reported that Andro
facilitates cell differentiation (22). These findings suggested that Andro
may exert anti-neuroinflammatory and neuroprotective effects during
peripheral nerve regeneration, which is a vital long-term strategy
in the treatment of peripheral nerve injury.
As myelin-forming cells in the PNS, Schwann cells
have a crucial role in peripheral nerve regeneration (23). Schwann cells have been shown to be
able to provide bioactive substrates for axonal migration, and
release molecules that regulate axonal outgrowth (24). In addition, Schwann cells activate
nonresident macrophages to the site of injury, in order to complete
myelin phagocytosis, release cytokines, and secrete neurotrophic
factors that guide the resultant regeneration (25,26).
Schwann cells provide trophic support to axons via the expression
of several neurotrophic factors, including brain-derived
neurotrophic factor (BDNF), glial cell line-derived neurotrophic
factor (GDNF) and ciliary neurotrophic factor (CNTF), particularly
following nerve injury (27).
These findings indicate the specific nature of the relationship
between Schwann cells and axons, and thus confirm our
hypothesis.
Based on the hypothesis that Andro may be used as a
potential anti-inflammatory agent to relieve the destruction and
accelerate the proliferation of Schwann cells following peripheral
nerve injury, the present study investigated its effects on the
growth and phenotypic maintenance of RSC96 cells in vitro.
Examination of cell proliferation, morphology, viability, and
RSC96-specific gene expression was performed. The results suggested
that Andro may exert effects on RSC96 cell attachment, survival and
proliferation, and on the release of neurotrophic factors. The
present study may provide evidence for the application of Andro in
the clinical treatment of peripheral nerve injury.
Materials and methods
Reagents and instruments
Trypsin and antibiotics (100 U/ml penicillin and 100
U/ml streptomycin) were purchased from Beijing Solarbio Science
& Technology Co., Ltd. (Beijing, China); 6-well and 96-well
cell culture plates were purchased from Costar (Corning
Incorporated, Corning, NY, USA). Anti-S100β (S100β; cat. no. BA120;
1:200) antibody and the 3,3-diaminobenzidine tetrahydrochloride
(DAB) kit were obtained from Wuhan Boster Biological Technology,
Ltd. (Wuhan, China), Dulbecco's modified Eagle's medium/F-12
supplement (DMEM/F-12), fetal bovine serum (FBS) and 3–(4,5)-dimethylthiahiazol(-z-y1)-3,5-di-phenyltetrazo-lium-bromide
(MTT) were purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Dimethyl sulfoxide (DMSO), Hoechst 33258 and
proteinase K were purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany). Multiskan GO Microplate Spectrophotometer was
obtained from Thermo Fisher Scientific, Inc. Other reagents and
instruments used in the present study were purchased from the
following companies: Hematoxylin-eosin (HE) kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China); RNeasy RNA extraction
kit (Tiangen Biotech Co., Ltd., Beijing, China); reverse
transcription (RT) kit (Fermentas; Thermo Fisher Scientific, Inc.);
Fast-Start Universal SYBR Green Master Mix (Roche Diagnostics GmbH,
Mannheim, Germany); quantitative polymerase chain reaction (qPCR)
detection system (RealPlex4; Eppendorf, Hauppauge, NY, USA);
LIVE/DEAD viability assay kit (Invitrogen; Thermo Fisher
Scientific, Inc.); laser scanning confocal microscope (Nikon
Corporation, Tokyo, Japan); and upright microscope (Olympus
Corporation, Tokyo, Japan).
Cells culture
The RSC96 cell line consists of spontaneously
immortalized rat Schwann cells, which are derived from the
long-term culture of rat primary Schwann cells. RSC96 cells were
purchased from the China Center for Type Culture Collection (Wuhan,
China), and were cultured in DMEM/F-12 supplemented with 10% (v/v)
FBS and 1% (v/v) antibiotics in a humidified atmosphere containing
5% CO2 and 95% air at 37°C. The culture medium was
replaced every 3 days after plating. RSC96 cells were passaged with
0.25% trypsin when cell confluence reached 80–90%. Confluent RSC96
cells were subsequently treated at the indicated times with the
indicated concentrations of Andro.
Chemicals
Andro was purchased from Chengdu Must Bio-technology
Co., Ltd. (Chengdu, China). Prior to experimentation, Andro was
dissolved in DMSO in order to generate a 100 mM stock solution, and
was stored at −4°C. The Andro stock solution was diluted with
cultured medium to provided various concentrations and added to the
cell culture for subsequent experiments. Prior to use, the culture
medium contained 1.5625, 3.125 and 6.25 µM Andro was
filtered using 0.22 µm filters for sterilization.
Cell cytotoxicity assay
Cell viability was estimated using a colorimetric
assay based on the conversion of MTT into a blue formazan product.
The cells were plated at 800 cells/well in 96-well cell culture
plates and were pretreated with various concentrations of Andro
(0–50 µM) for 3 days in a 5% CO2 humidified
incubator at 37°C. MTT (5 mg/ml) was then added to each well and
the plates were incubated in the dark at 37°C for 4 h.
Subsequently, culture medium was removed and the cells were treated
with 150 µl DMSO to dissolve the formazan product. The cells
were incubated in DMSO with agitation for 10 min. Optical density
of each sample was measured using a Multiskan GO Microplate
Spectrophotometer at 570 nm. Five individual cultures were used for
each test. The experiments were carried out in quintuplicate.
Cell proliferation analysis
Based on the results of the cytotoxicity assay,
three doses of Andro, which exhibited a positive effect, were
selected (1.5625, 3.125 and 6.25 µM), alongside a control
group (0 µM Andro) for cell proliferation analysis. RSC96
cells in the various groups were cultured for 2, 4 and 6 days in a
5% CO2 humidified incubator at 37°C prior to subsequent
experiments. Cells were digested with 0.25% trypsin and were
resuspended in phosphate-buffered saline (PBS) containing 60
µg/ml proteinase K for 6 h at 60°C. After dyeing with
Hoechst 33258, cell proliferation was determined by detecting DNA
production using an ultraviolet spectrofluorometer; calf thymus DNA
was used as a standard. The excitation wavelength was 346 nm and
the emission wavelength was 460 nm. The experiments were carried
out in quintuplicate.
Morphological examination
Cells were cultured for 2, 4 and 6 days, and were
fixed in 4% paraformaldehyde for 40 min at room temperature for
subsequent HE staining. Cells were incubated with a nuclear dye for
3 min, followed by a 10 sec incubation with HE. Subsequently, the
cells were rinsed with PBS, naturally dried and sealed with neutral
gum. Cells were then examined, and images were captured under an
upright microscope.
Cell viability assay
Cell viability was determined using the LIVE/DEAD
viability assay kit. Briefly, cells on coverslips were rinsed
quickly with PBS (0.01 mol/l, pH 7.4) to remove the medium.
Subsequently, 1 µM calcein-acetoxym-ethyl (calcein-AM) and 1
µM propidium iodide (PI) were added to the cell cultures and
were incubated in the dark for 5 min at 37°C. Images were captured
using a laser scanning confocal microscope.
Immunohistochemical staining
S100β protein expression was detected by
immunohistochemical staining using anti-S100 (S100β), according to
the manufacturer's protocol. Briefly, cells on coverslips were
rinsed quickly with PBS (0.01 mol/l, pH 7.4) to remove the medium.
Subsequently, the cells were fixed in 4% paraformaldehyde at room
temperature for 40 min. After washing three times with PBS and
permeabilizing with 3% Triton X-100 for 5 min, cells were incubated
with 3% H2O2 for 10 min at room temperature,
in order to suppress endogenous peroxidase activity. The cells were
then treated with goat serum for 10 min at room temperature to
block nonspecific staining. Subsequently, the cells were incubated
with rat monoclonal anti-S100 antibody (S100β; 1:150 dilution)
overnight at 4°C in a humidified chamber. After washing three times
with PBS, secondary antibodies (cat. no. SP-9000; 1:50; OriGene
Technologies, Inc., Beijing, China) and biotin-labeled horseradish
peroxidase (OriGene Technologies, Inc.) were successively added for
15 and 10 min at room temperature. The chromogenic reaction of S100
was visualized using a DAB kit, and the slides were counterstained
with hematoxylin. Finally, cells were gradually dehydrated, sealed
with neutral gum, observed, and images were captured under an
upright microscope.
RT-qPCR analysis
To further explore the effects of Andro on the
expression of Schwann cell-specific genes, BDNF, GDNF and CNTF mRNA
expression was analyzed by RT-qPCR. Total RNA was extracted from
RSC96 cells using an RNeasy RNA extraction kit, according to the
manufacturer's protocol. Reverse transcription of RNA was performed
at 25°C for 5 min, 42°C 60 min and then 72°C for 5 min using a
reverse transcription kit (Fermentas; Thermo Fisher Scientific,
Inc.). The RT-qPCR reactions were performed using a qPCR detection
system with a FastStart Universal SYBR Green Master Mix under the
following conditions: 10 min at 95°C, 15 sec at 95°C and 1 min at
60°C for 35 cycles. The primer sequences (BGI, Shenzhen, China) for
BDNF, GDNF, CNTF and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH; internal control) are listed in Table I. The melting curve data were
collected to verify PCR specificity. Each gene was analyzed in
triplicate to diminish operation errors. Relative gene expression
levels were calculated using the 2−ΔΔCq method (28), and were normalized to GAPDH gene
expression. Each gene was analyzed in quintuplicate to reduce
randomization error.
 | Table IPrimer sequences used in quantitative
polymerase chain reaction. |
Table I
Primer sequences used in quantitative
polymerase chain reaction.
| Gene | Primer sequence (5′
to 3′) | Length (bp) | Amplicon size
(bp) |
|---|
| GDNF | F:
AGACCGGATCCGAGGTGC | 18 | 129 |
| R:
TCGAGAAGCCTCTTACCGGC | 20 | |
| BDNF | F:
TACCTGGATGCCGCAAACAT | 20 | 182 |
| R:
TGGCCTTTTGATACCGGGAC | 20 | |
| CNTF | F:
ATGGCTTTCGCAGAGCAAAC | 20 | 191 |
| R:
CAACGATCAGTGCTTGCCAC | 20 | |
| GAPDH | F:
GTCATCATCTCAGCCCCCTC | 20 | 99 |
| R:
GGATGCGTTGCTGACAATCT | 20 | |
Statistical analysis
Statistical analyses were conducted using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. Statistical
significance was determined using one-way analysis of variance
followed by Dunnett's post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
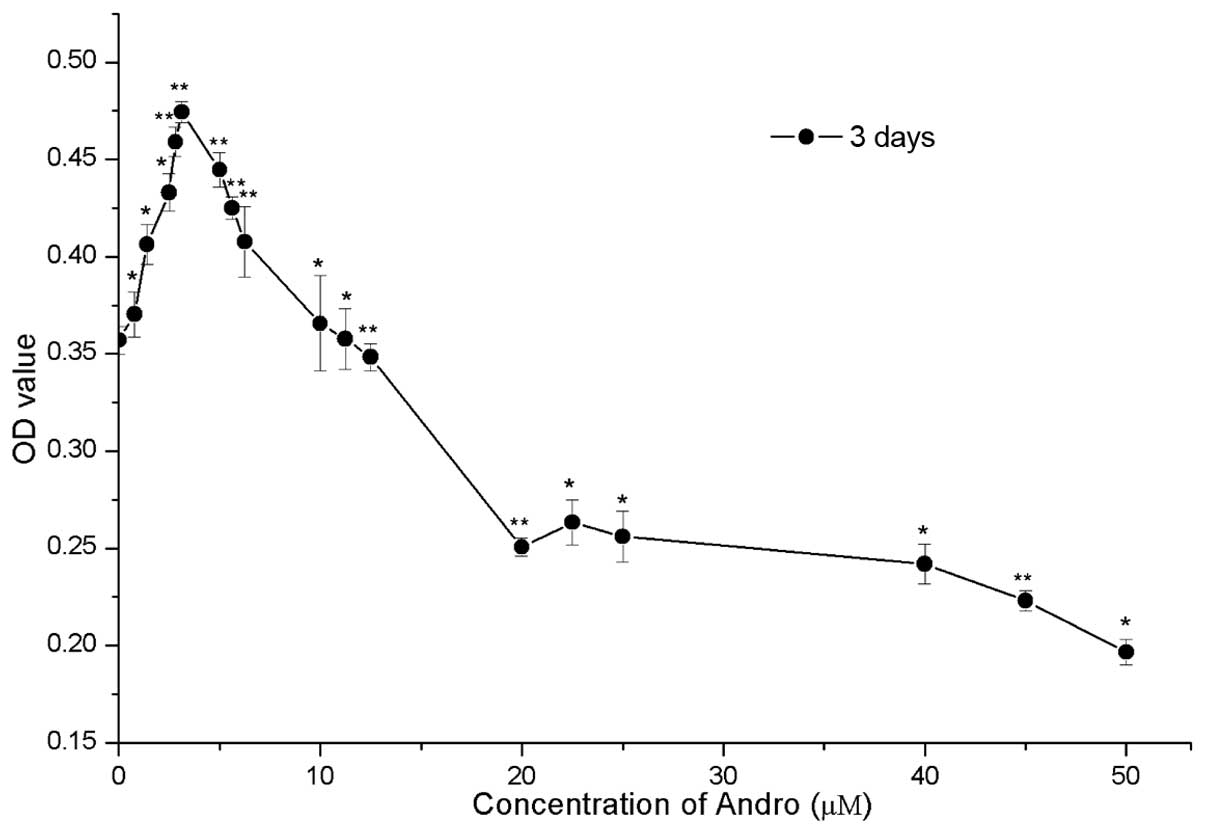
Cytotoxicity assay
The present study examined the cytotoxicity of
various concentrations of Andro on RSC96 cells using the MTT assay.
Cells were treated with increasing concentrations of Andro (0–50
µM). As shown in Fig. 2,
compared with the control group (0 µM), treatment with Andro
between 0.78 and 12.5 µM exhibited low cytotoxicity. In
addition, 0.78–12.5 µM Andro significantly accelerated cell
growth (P<0.05) with the most obvious effect being observed when
used at 3.125 µM (P<0.05). However, Andro exhibited a
suppressive effect on RSC96 cells in vitro when used between
12.5 and 50 µM, as compared with the control group.
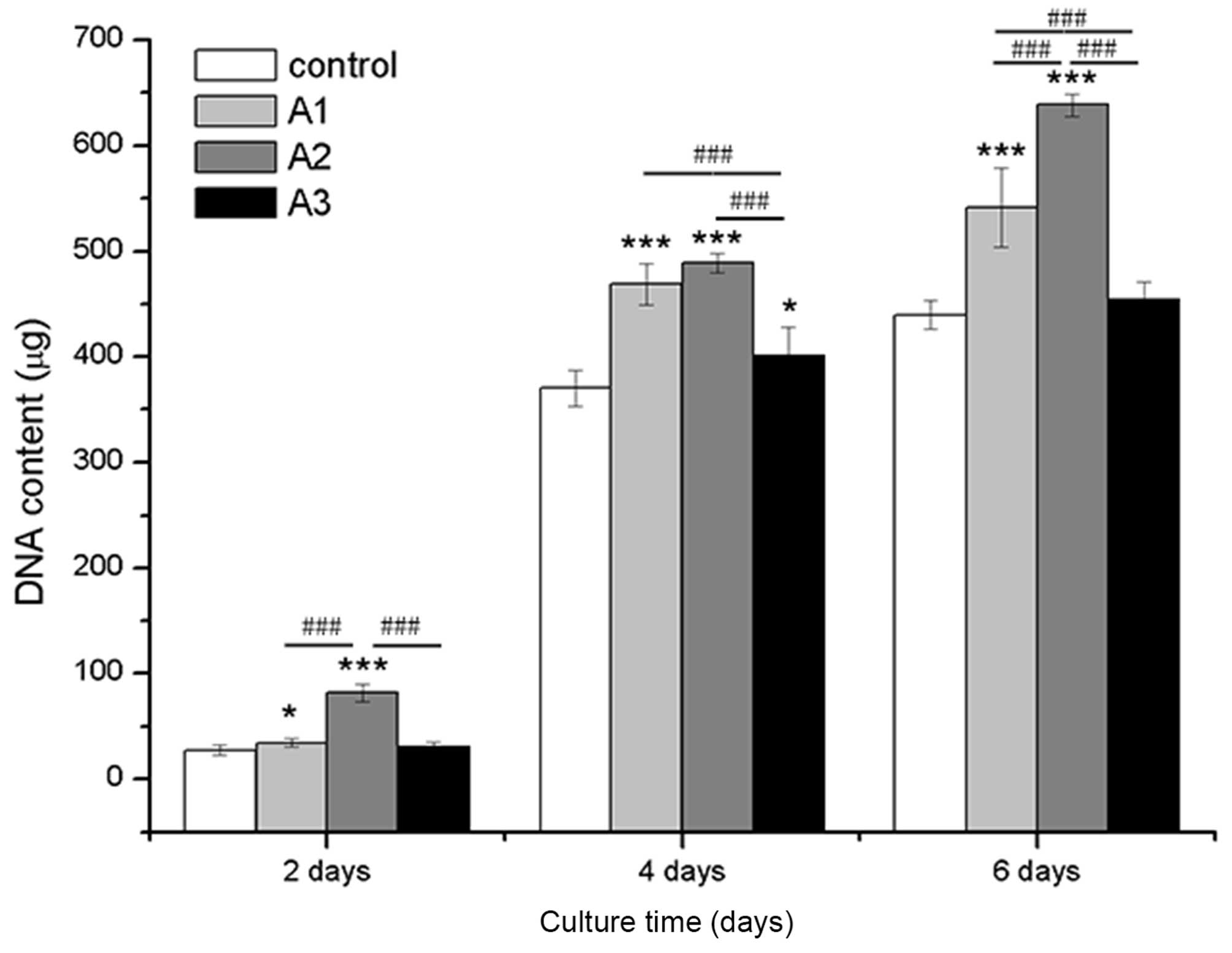
Cell proliferation
As presented in Fig.
3, RSC96 cells treated with 1.5625, 3.125 and 6.25 µM
Andro exhibited increased proliferation compared with the control
group (0 µM Andro). Proliferation was determined according
to DNA content (P<0.05), which was markedly higher in the Andro
groups compared with in the control group after the same culture
period. Among the three concentrations, 3.125 µM Andro
exhibited the strongest effect on cell growth at all time
points.
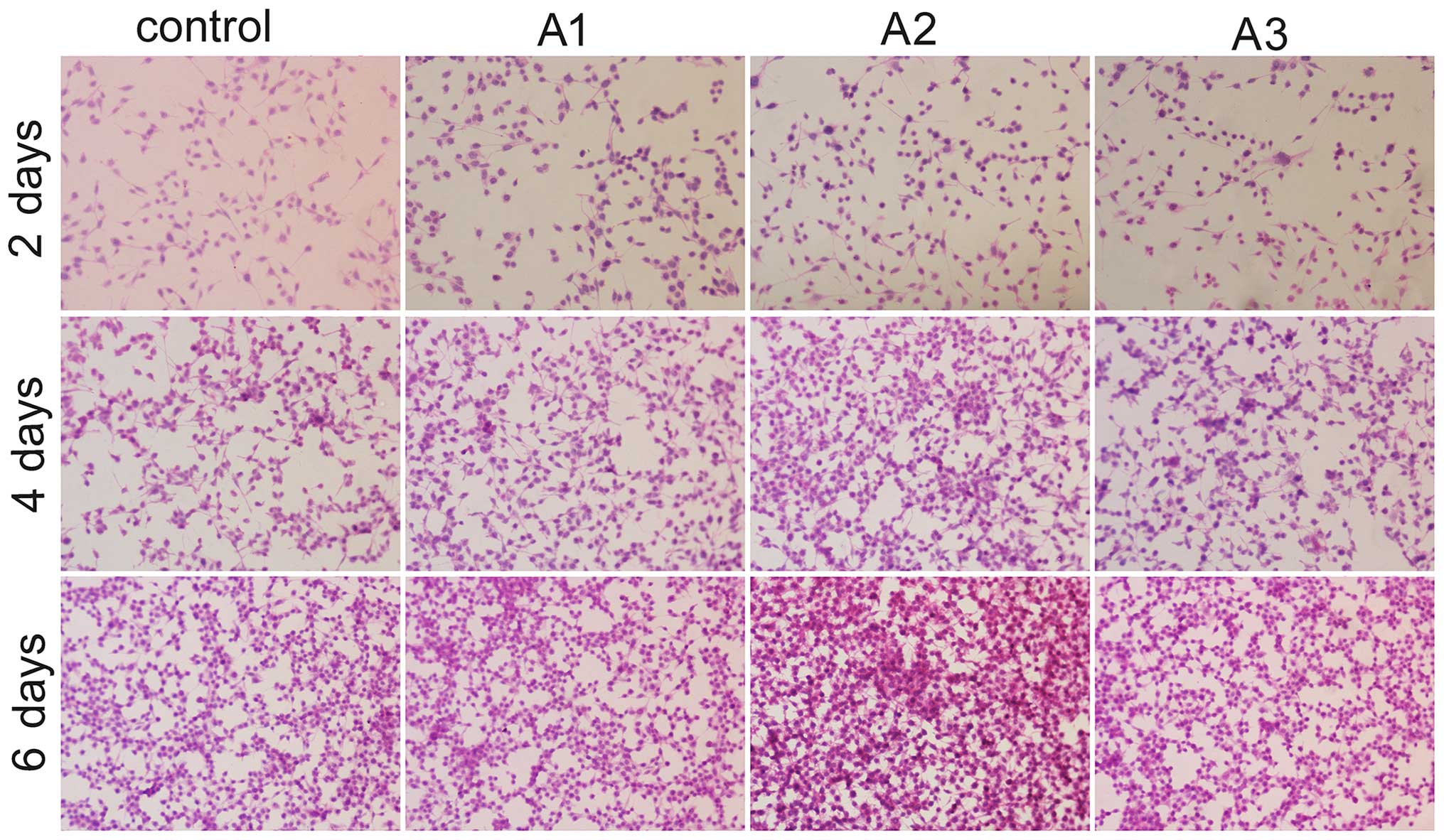
Cell morphology
HE staining was conducted using an upright
microscope to assess the morphology of RSC96 cells. The images
indicated that the Andro groups exhibited increased cell growth
compared with the control group at the same time point (Fig. 4). There were no marked differences
in Schwann cell morphology between the groups after 6 days of
culture. Compared with the control group, RSC96 cells in the
presence of Andro grew better and had a distinctive proliferative
tendency that gradually increased with time. In addition, when used
at 3.125 µM, Andro was able to enhance the proliferation of
RSC96 cells compared with the other two concentrations in
vitro.
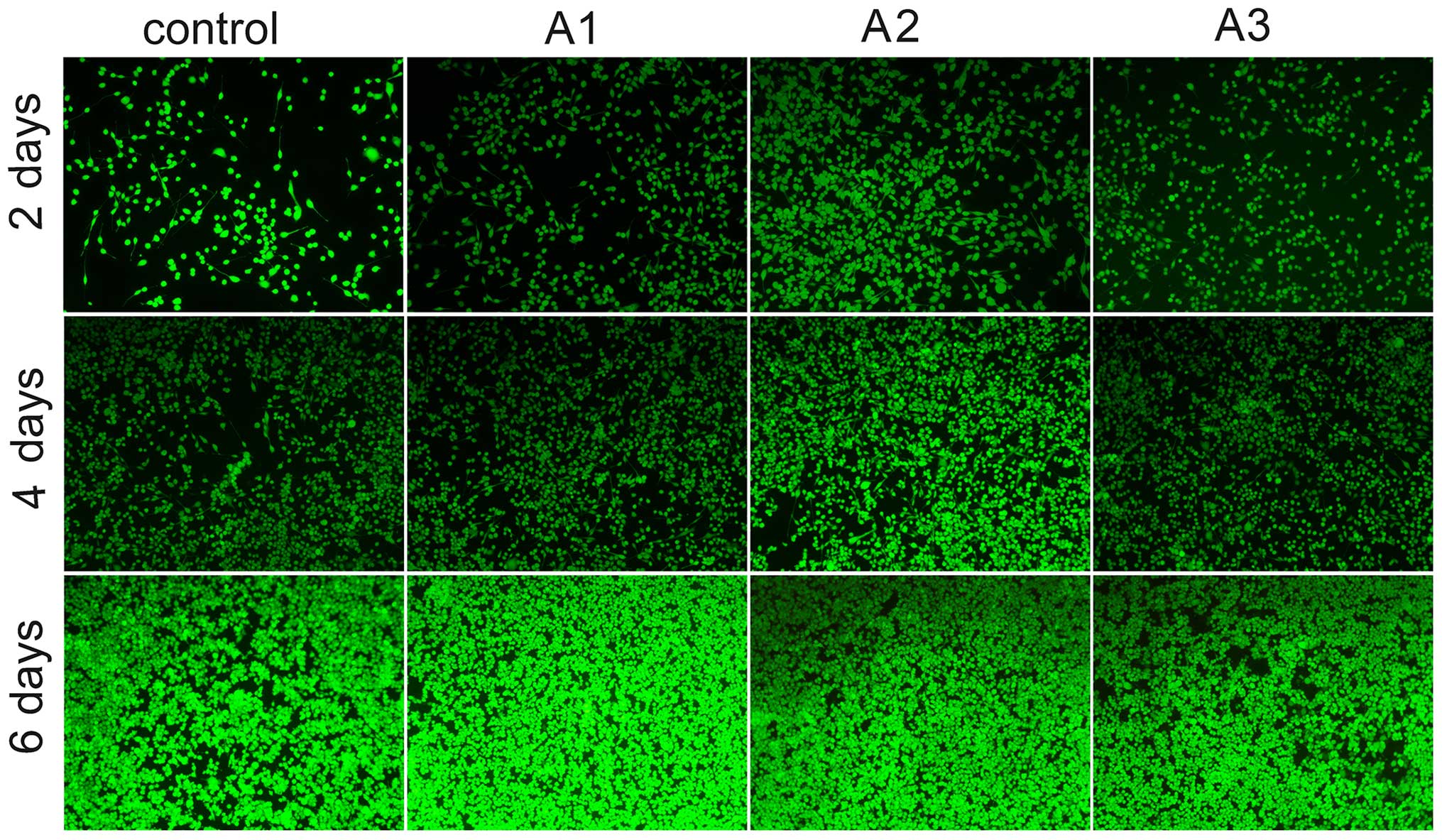
Cell viability assay
As presented in Fig.
5 viable cells and dead cells were stained with calcein-AM/PI.
The results demonstrated that Andro exerted positive effects on
survival. Images of calcein-AM/PI staining demonstrated that the
survival of cells in the Andro groups was increased compared with
in the control group. Consistent with the results of a cell
proliferation assay (Fig. 4), more
viable cells than dead cells were detected in the Andro groups,
thus implying that Andro was able to better support cell growth
compared with the control group. Among the Andro groups, treatment
with 3.125 µM exhibited the best effects, as evidenced by an
increase in the number of viable cells.
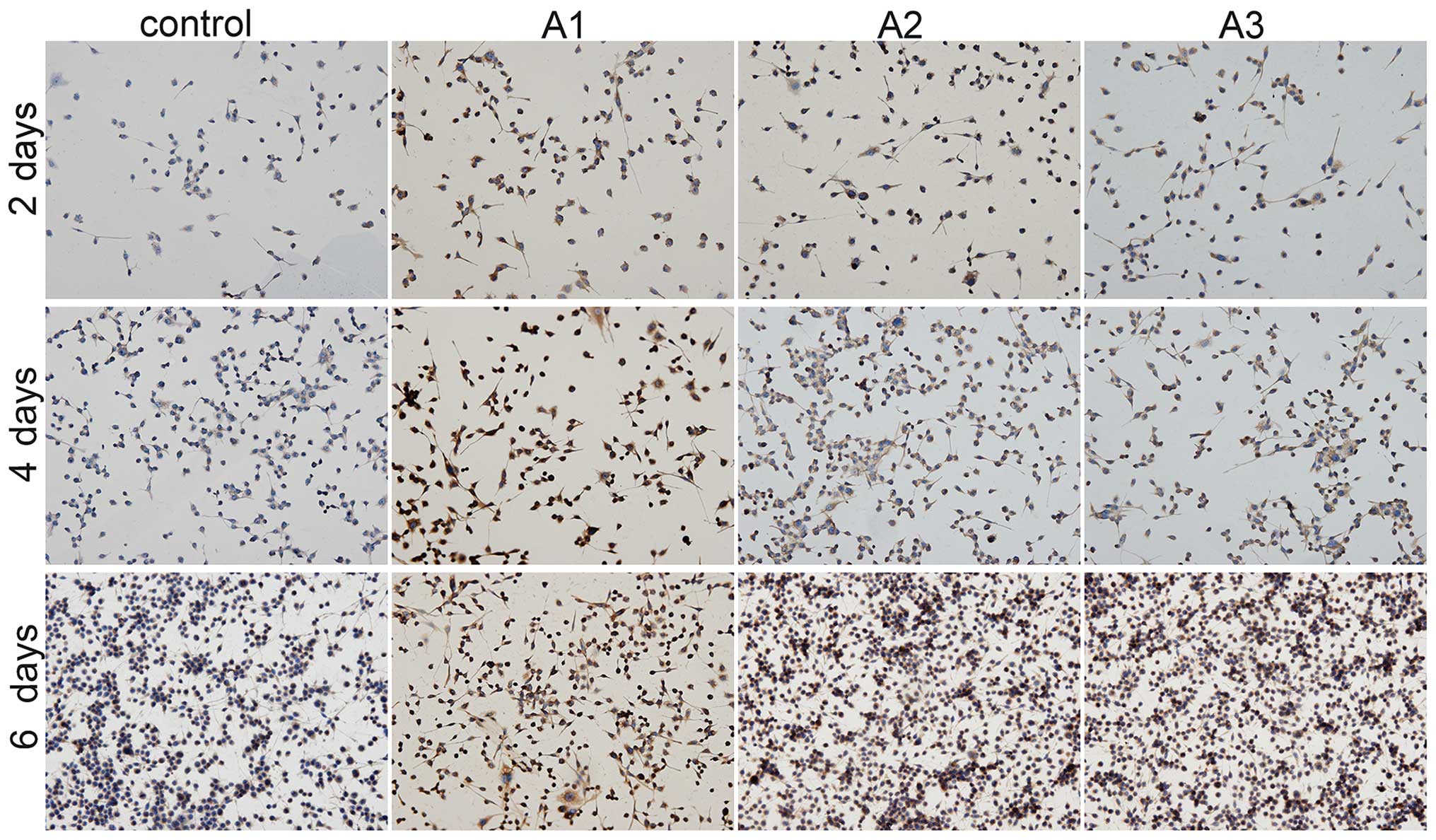
S100β secretion
The present study detected Schwann cell-specific
protein S100β expression using immunohistochemical staining
(Fig. 6). Positive S100β staining
was increased in the Andro groups compared with the control group
at the same time points. Among the three doses of Andro tested,
3.125 µM was superior compared with the others in terms of
phenotypic maintenance of Schwann cells.
Gene expression
The mRNA expression levels of RSC96 cell-specific
genes were determined by RT-qPCR analysis. Nerve growth factor
(NGF) and several neurotrophic factors, including BDNF, GDNF and
CNTF, have key roles in Schwann cells and the regeneration of
peripheral nerves. The mRNA expression levels of BDNF, GDNF and
CNTF were significantly increased in the Andro-treated groups
compared with the control group (Fig.
7) except for BDNF levels at 6.25 µM concentratio.
Furthermore, among all of the groups, 3.125 µM Andro
exhibited the best effect on upregulation of BDNF, GDNF and
CNTF.
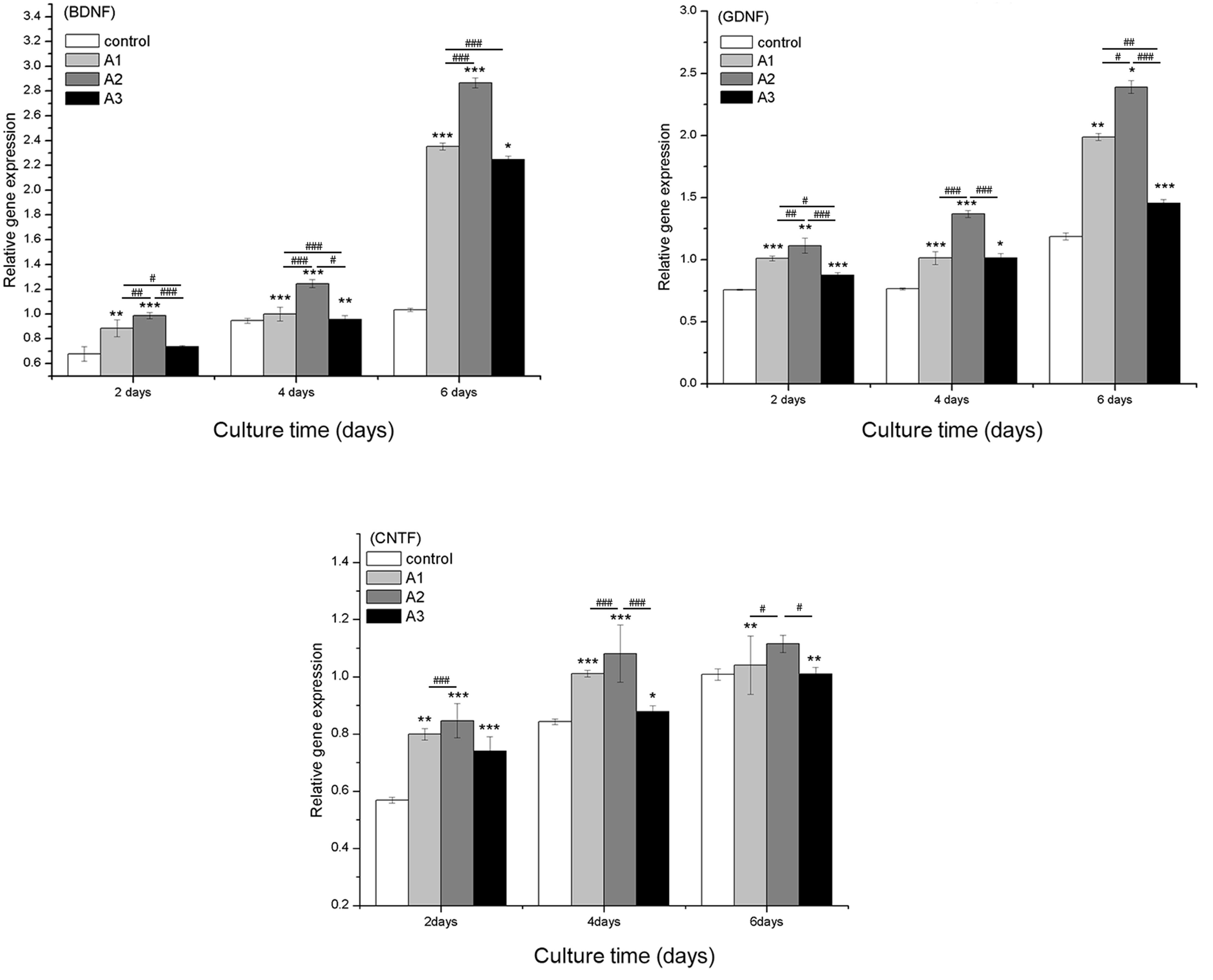 | Figure 7Quantitative comparison of
neurotrophic-related gene expression by reverse
transcription-quantitative polymerase chain reaction. The RSC96
Schwann cells were cultured with 0 µM (control), 1.5625
µM (A1), 3.125 µM (A2) and 6.25 µM (A3)
andrographolide (Andro) for 2, 4 and 6 days. The gene expression
levels in Andro-treated cells were compared with the control group
using the 2−ΔΔCq method. Glyceraldehyde 3-phosphate
dehydrogenase was used as an internal control. Data are presented
as the mean ± standard deviation of five independent experiments.
*P<0.05,
**P<0.01,***P<0.001 vs. control;
#P<0.05, ##P<0.01,
###P<0.001 vs. A1, A2 and A3. BDNF, brain-derived
neurotrophic factor; GDNF, glial cell-derived neuroptrophic factor;
CTNF, ciliary neurotrophic factor. |
Discussion
Andro is a diterpenoid lactone predominantly
extracted from Andrographis paniculata, which is widely used
in China and other regions of Asia for the treatment of
inflammation-associated diseases. In addition, Andro has been
reported to have neuroprotective properties (29–31).
Previous studies demonstrated that Andro reduced
inflammation-mediated dopaminergic neurodegeneration in
mesencephalic neuron-glia cultures by inhibiting microglial
activation, thus indicating that Andro may have clinical use for
the treatment of Parkinson's disease (17,32).
The present study suggested that Andro enhanced neuroprotection and
regeneration of peripheral nerves following injury, via its effect
on the growth and phenotypic maintenance of RSC96 cells in
vitro. The results indicated that Andro was able to promote
RSC96 cell growth compared with the control group (Fig. 2). In addition, Andro markedly
enhanced DNA synthesis and accelerated the proliferation of RSC96
cells (Figs. 3Figure 4–5).
Consistent with the increased synthesis of DNA in
RSC96 cells, Andro was able to upregulate the mRNA expression
levels of BDNF, GDNF and CNTF (Fig.
7). NGF and several neurotrophic factors, including BDNF, GDNF
and CNTF, have been reported to exert stimulatory effects on
specific neuronal populations (33,34).
Neurotrophic factor-based molecular therapies have potential for
enhancing functional recovery, as well as for increasing nerve
regeneration (35), since they
affect several important aspects of regeneration, including axon
growth, Schwann cell function and myelination (36). In addition, previous studies
regarding molecular therapeutics have concentrated primarily on the
creation of neurotrophin factor mimetics, particularly NGF,
neurotrophin-3 and BDNF mimetics (37–39).
It was reported that the secretion of neurotrophic factors by
Schwann cells which were notably increased by Andro in the present
study is necessary to promote axon growth and prevent neurons from
initiating apoptosis (23,40). Therefore, the probable underlying
mechanism is that Andro promoted RSC96 cell growth and neurotrophic
factor secretion, thus inducing phenotypic maintenance via
modulation of BDNF, GDNF and CNTF expression.
In the present study, the PCR, biochemical and
immunohistochemical analyses demonstrated that S100β, a specific
protein of Schwann cells, was effectively increased in Andro groups
(Figs. 6 and 7). The S100β family, which consists of
specific Schwann cell markers, is a family of low molecular weight
proteins characterized by two calcium-binding sites, which is
highly conserved among vertebrates (41). Furthermore, S100β, from the S100
protein family, has been identified as a potential important factor
that contributes to neuronal development and differentiation
(42,43). In addition, S100A4 is capable of
stimulating neuronal differentiation in cultures of rat hippocampal
neurons (44). In the present
study, S100β protein expression was elevated in the Andro-treated
cells. The modulation of S100β expression following treatment with
Andro suggested that Andro may increase proliferation of RSC96
cells and maintain their phenotype.
The present study is a preliminary exploration
regarding the effects of Andro on the proliferation and phenotype
maintenance of RSC96 cells. Following treatment with the
recommended concentrations of Andro (0.78–12.5 µM), the
proliferation of RSC96 cells was accelerated in vitro.
However, we cannot confirm whether Andro is suitable for the
treatment of Schwann cells from other species, including humans.
Further studies are required to elucidate the underlying mechanisms
of the effects of Andro on Schwann cells, with the aim of
identifying a promising anti-neuroinflammatory and neuroprotective
agent. Furthermore, the application of Andro on peripheral nerve
injury should be investigated.
In conclusion, Andro, which is the primary active
component isolated from A. paniculata, exerted positive
effects on the proliferation and phenotypic maintenance of RSC96
cells in vitro. These results suggested that Andro may serve
as a promising therapeutic agent for peripheral nerve regeneration
and neural tissue engineering. The present study may provide
evidence for the clinical application of Andro.
Acknowledgments
The present study was financially supported by the
Innovation Project of Guangxi Graduate Education of China (grant
no. YCSZ2015124) and the National Natural Science Foundation of
China (grant no. 81160221). The study was supported by the Research
Center for Regenerative Medicine and Collaborative Innovation
Center of Guangxi Biological Medicine.
References
|
1
|
Lundborg G: A 25-year perspective of
peripheral nerve surgery: Evolving neuroscientific concepts and
clinical significance. J Hand Surg Am. 25:391–414. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robinson LR: Traumatic injury to
peripheral nerves. Suppl Clin Neurophysiol. 57:173–186. 2004.
View Article : Google Scholar
|
|
3
|
Evans GR: Peripheral nerve injury: A
review and approach to tissue engineered constructs. Anat Rec.
263:396–404. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raine CS: Multiple sclerosis: Immune
system molecule expression in the central nervous system. J
Neuropathol Exp Neurol. 53:328–337. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rogers J and Shen Y: A perspective on
inflammation in Alzheimer's disease. Ann N Y Acad Sci. 924:132–135.
2000. View Article : Google Scholar
|
|
6
|
Qin L, Liu Y, Wang T, Wei SJ, Block ML,
Wilson B, Liu B and Hong JS: NADPH oxidase mediates
lipopolysaccharide-induced neurotoxicity and proinflammatory gene
expression in activated microglia. J Biol Chem. 279:1415–1421.
2004. View Article : Google Scholar
|
|
7
|
Zochodne DW: The microenvironment of
injured and regenerating peripheral nerves. Muscle Nerve Suppl.
9:S33–S38. 2000. View Article : Google Scholar
|
|
8
|
Dezawa M: Central and peripheral nerve
regeneration by transplantation of Schwann cells and
transdifferentiated bone marrow stromal cells. Anat Sci Int.
77:12–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Sanford MT, Xin Z, Lin G and Lue
TF: Role of Schwann cells in the regeneration of penile and
peripheral nerves. Asian J Androl. 17:776–782. 2015.PubMed/NCBI
|
|
10
|
Puri A, Saxena R, Saxena RP, Saxena KC,
Srivastava V and Tandon JS: Immunostimulant agents from
Andrographis paniculata. J Nat Prod. 56:995–999. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang XF and Tan BK: Antihyperglycaemic
and anti-oxidant properties of Andrographis paniculata in normal
and diabetic rats. Clin Exp Pharmacol Physiol. 27:358–363. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen YC, Chen CF and Chiou WF:
Andrographolide prevents oxygen radical production by human
neutrophils: Possible mechanism(s) involved in its
anti-inflammatory effect. Br J Pharmacol. 135:399–406. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu T, Wang DX, Zhang W, Liao XQ, Guan X,
Bo H, Sun JY, Huang NW, He J, Zhang YK, et al: Andrographolide
protects against LPS-induced acute lung injury by inactivation of
NF-κB. PLoS One. 8:e564072013. View Article : Google Scholar
|
|
14
|
Bao Z, Guan S, Cheng C, Wu S, Wong SH,
Kemeny DM, Leung BP and Wong WS: A novel antiinflammatory role for
andrographolide in asthma via inhibition of the nuclear
factor-kappaB pathway. Am J Respir Crit Care Med. 179:657–665.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JX, Xue HJ, Ye WC, Fang BH, Liu YH,
Yuan SH, Yu P and Wang YQ: Activity of andrographolide and its
derivatives against influenza virus in vivo and in vitro. Biol
Pharm Bull. 32:1385–1391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Luo L, Wang X, Liao B and Li G:
Inhibition of NF-kappaB expression and allergen-induced airway
inflammation in a mouse allergic asthma model by andrographolide.
Cell Mol Immunol. 6:381–385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang T, Liu B, Zhang W, Wilson B and Hong
JS: Andrographolide reduces inflammation-mediated dopaminergic
neurodegeneration in mesencephalic neuron-glia cultures by
inhibiting microglial activation. J Pharmacol Exp Ther.
308:975–983. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wen L, Xia N, Chen X, Li Y, Hong Y and Liu
Y, Wang Z and Liu Y: Activity of antibacterial, antiviral,
anti-inflammatory in compounds andrographolide salt. Eur J
Pharmacol. 740:421–427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ku CM and Lin JY: Anti-inflammatory
effects of 27 selected terpenoid compounds tested through
modulating Th1/Th2 cytokine secretion profiles using murine primary
splenocytes. Food Chem. 141:1104–1113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen YY, Hsu MJ, Sheu JR, Lee LW and Hsieh
CY: Andrographolide, a novel NF- κB inhibitor, induces vascular
smooth muscle cell apoptosis via a Ceramide-p47phox-ROS signaling
cascade. Evid Based Complement Alternat Med. 2013:8218132013.
|
|
21
|
Talei D, Valdiani A, Maziah M, Sagineedu
SR and Saad MS: Analysis of the anticancer phytochemicals in
andrographis paniculata Nees. Under salinity stress. Biomed Res
Int. 2013:3190472013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manikam SD and Stanslas J: Andrographolide
inhibits growth of acute promyelocytic leukaemia cells by inducing
retinoic acid receptor-independent cell differentiation and
apoptosis. J Pharm Pharmacol. 61:69–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lehmann HC and Höke A: Schwann cells as a
therapeutic target for peripheral neuropathies. CNS Neurol Disord
Drug Targets. 9:801–806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang J, Hu X, Lu L, Ye Z, Zhang Q and Luo
Z: Electrical regulation of Schwann cells using conductive
polypyrrole/chitosan polymers. J Biomed Mater Res A. 93:164–174.
2010.
|
|
25
|
Rotshenker S: Wallerian degeneration: The
innate-immune response to traumatic nerve injury. J
Neuroinflammation. 8:1092011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bosse F: Extrinsic cellular and molecular
mediators of peripheral axonal regeneration. Cell Tissue Res.
349:5–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan H, Zhang J, Liu H and Li Z: The
protective effects of resveratrol on Schwann cells with toxicity
induced by ethanol in vitro. Neurochem Int. 63:146–153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Chan SJ, Wong WS, Wong PT and Bian JS:
Neuroprotective effects of andrographolide in a rat model of
permanent cerebral ischaemia. Br J Pharmacol. 161:668–679. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaitone SA, Abo-Elmatty DM and Shaalan AA:
Acetyl-L-carnitine and α-lipoic acid affect rotenone-induced damage
in nigral dopaminergic neurons of rat brain, implication for
Parkinson's disease therapy. Pharmacol Biochem Behav. 100:347–360.
2012. View Article : Google Scholar
|
|
31
|
Jalali-Nadoushan M and Roghani M:
Alpha-lipoic acid protects against 6-hydroxydopamine-induced
neurotoxicity in a rat model of hemi-parkinsonism. Brain Res.
1505:68–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Z, Lai D, Wang L, Yu P, Zhu L, Guo
B, Xu L, Zhou L, Sun Y, Lee SM and Wang Y: Neuroprotective effects
of the andrographolide analogue AL-1 in the
MPP+/MPTP-induced Parkinson's disease model in vitro and
in mice. Pharmacol Biochem Behav. 122:191–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aloe L, Rocco ML, Bianchi P and Manni L:
Nerve growth factor: From the early discoveries to the potential
clinical use. J Transl Med. 10:2392012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bothwell M: NGF, BDNF, NT3, and NT4. Handb
Exp Pharmacol. 220:3–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Daly W, Yao L, Zeugolis D, Windebank A and
Pandit A: A biomaterials approach to peripheral nerve regeneration:
Bridging the peripheral nerve gap and enhancing functional
recovery. J R Soc Interface. 9:202–221. 2012. View Article : Google Scholar
|
|
36
|
Klimaschewski L, Hausott B and Angelov DN:
The pros and cons of growth factors and cytokines in peripheral
axon regeneration. Int Rev Neurobiol. 108:137–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie Y and Longo FM: Neurotrophin
small-molecule mimetics. Prog Brain Res. 128:333–347. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peleshok J and Saragovi HU: Functional
mimetics of neurotrophins and their receptors. Biochem Soc Trans.
34:612–617. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Colangelo AM, Bianco MR, Vitagliano L,
Cavaliere C, Cirillo G, De Gioia L, Diana D, Colombo D, Redaelli C,
Zaccaro L, et al: A new nerve growth factor-mimetic peptide active
on neuropathic pain in rats. J Neurosci. 28:2698–2709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gordon T: The role of neurotrophic factors
in nerve regeneration. Neurosurg Focus. 26:E32009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu H, Wang WJ, Ding WL, Li F and He J:
Effect of panaxydol on hypoxia-induced cell death and expression
and secretion of neurotrophic factors (NTFs) in hypoxic primary
cultured Schwann cells. Chem Biol Interact. 174:44–50. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Donato R: S100: A multigenic family of
calcium-modulated proteins of the EF-hand type with intracellular
and extracellular functional roles. Int J Biochem Cell Biol.
33:637–668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Donato R: Intracellular and extracellular
roles of S100 proteins. Microsc Res Tech. 60:540–551. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Novitskaya V, Grigorian M, Kriajevska M,
Tarabykina S, Bronstein I, Berezin V, Bock E and Lukanidin E:
Oligomeric forms of the metastasis-related Mts1 (S100A4) protein
stimulate neuronal differentiation in cultures of rat hippocampal
neurons. J Biol Chem. 275:41278–41286. 2000. View Article : Google Scholar : PubMed/NCBI
|