Introduction
The surgical and endovascular treatments of
aneurysms and neurological intensive care have greatly improved.
Therefore, the possibility of a hemorrhage following an aneurysm
re-rupture is well-controlled. However, cerebral vasospasm (CVS)
continues to be the primary reason for mortality and disability in
patients with subarachnoid hemorrhage (SAH) (1). The pathogenesis of CVS remains to be
fully elucidated. CVS may be due to various factors, including the
increase of cerebral levels of NO, oxidative injury, platelet
activation and aggregation, that enhance cerebral vasoconstriction
and reduce cerebral vasodilation (2–5).
Matrix metalloproteinase 9 (MMP-9) is part of the
gelatinase subfamily of MMPs and is expressed in various cells in
the brain. Activated MMP-9 is responsible for the degradation of
several proteins which constitute the extracellular matrix,
including collagen types IV, V, VII and X, elastin, fibrillin,
osteonectin and laminin. Therefore, MMP-9 is important for the
pathogenesis of intracranial aneurysms (6–8). Our
previous study determined that CVS may develop in rats following
SAH (9). Additionally, MMP-9
expression levels in basilar arteries were upregulated with a time
course similar to that of the development of CVS. This suggests
that MMP-9 may be involved in the pathogenesis of CVS (9).
To the best of our knowledge, this is the first
study that has aimed to examine the association between CVS and
MMP-9. Therefore, the present study aimed to determine the
underlying molecular mechanisms of CVS pathogenesis. The importance
of MMP-9 for the contraction of cerebrovascular smooth muscle cells
was also investigated.
Materials and methods
Isolation and culture of
cerebrovascular smooth muscle cells
Four adult male Sprague-Dawley rats weighing 300–350
g were sacrificed by decapitation in each treatment group. A total
of 12 rats were used in the present study, purchased from the
Animal Center of Chinese Academy of Sciences (Shanghai, China). All
animals were housed at a constant temperature of 22°C, under a 12-h
light/dark cycle (lights switched on at 6:00 a.m.) with free access
to food and water. All rats were placed under general anesthesia
prior to fixation-perfusion and euthanasia procedures. All
procedures were approved by the Institutional Animal Care Committee
of the Zhangjiagang Hospital of Traditional Chinese Medicine
(Suzhou, China and were performed in accordance with the guidelines
of the National Institutes of Health on the care and use of
animals. Whole brains were dissected under sterile conditions and
immediately placed in a culture dish with Dulbecco's modified
Eagles medium supplemented with Gibco® nutrient mixture
F-12 (DMEM/F12), 100,000 U/l penicillin and 100,000 U/l
streptomycin (Thermo Fisher Scientific, Inc. Waltham, MA, USA).
Basilar arteries were dissected out in a laminar flow hood and
rinsed repeatedly with DMEM/F12 medium supplemented with 100,000
U/l penicillin and 100,000 U/l streptomycin. (Thermo Fisher
Scientific, Inc.). Following excision of the adventitial layer, the
vessels were sectioned into small segments of length ~0.2 mm. The
tissue was digested in 1 ml 0.1% collagenase I solution
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) at 37°C with
5% CO2 for 30 min until the tissue appeared swollen. In
order to digest the tissue further, 1 ml 0.125% trypsin solution
(Gibco; Thermo Fisher Scientific, Inc.) was added for an additional
10 min. The dispersed cells were collected and transferred to a
centrifuge tube, and DMEM/F12 medium supplemented with 20% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) was added
to terminate digestion. The cell suspension was subsequently
centrifuged at 200 × g for 5 min, and the supernatant
discarded. The cells were resuspended in DMEM/F12 medium with 20%
FBS, seeded into a 60 mm culture dish and incubated at 37°C with 5%
CO2. The medium was changed every 3 days. After 10 days,
the culture had reached 80–90% confluence, following trypsin
digestion the third passage was used for further experiments.
Preparation of hemolysate
Hemolysate was prepared using a freeze-thaw method
previously described (10),
although with several modifications, to lyse red blood cells. A
heparinized sterile syringe was used to collect 1 ml blood from the
rat tail artery. The blood was transferred to a sterile centrifuge
tube, and then centrifuged at 2,500 × g for 15 min at 4°C.
The serum was discarded, and the red blood cells were resuspended
in sterile distilled water. The cells were frozen at −20°C for 20
min and then immediately transferred to a 39°C water bath. After
the cells had completely thawed, they were centrifuged at 12,000 ×
g for 30 min at 4°C. The supernatant containing the
hemolysate was collected. Hemolysate concentration was calculated
as follows: Concentration = (hemolysate mass - distilled water
mass) / hemolysate volume. All procedures were performed under
sterile conditions.
Treatment of cells
Certain of these experiments included
2-[(4-phenoxyphenylsulfonyl)methyl]-thiirane (SB-3CT), a selective
MMP-9 inhibitor purchased from Sigma-Aldrich; Merck Millipore. As
shown in Fig. 1A, the cells were
randomly assigned into three groups: i) The control group, where
cerebrovascular smooth muscle cells were cultured under normal
conditions; ii) the hemolysate treatment group, where the cells
were treated with 1 mg/ml hemolysate for 24 h; and iii) the SB-3CT
treatment group, where cells were pretreated with 1 µM SB-3CT for 6
h, and subsequently treated with 1 mg/ml hemolysate for a further
24 h.
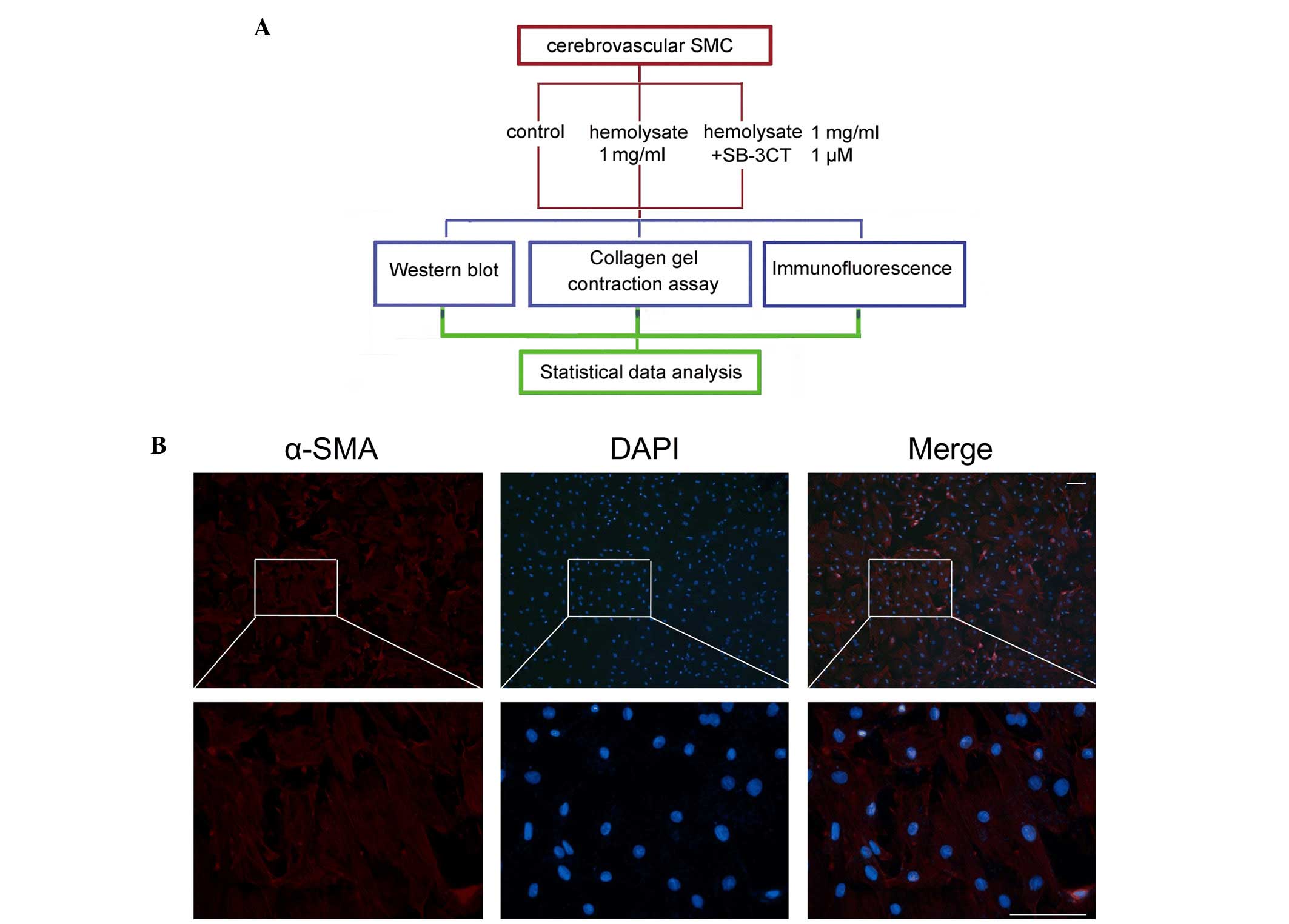 | Figure 1.Experimental design and identification
of cerebrovascular SMC. (A) Experiments were designed to detect
changes in expression levels of MMP-9, collagen IV and collagen V
in the cerebrovascular SMC following hemolysate treatment. (B)
Identification of cerebrovascular SMC. Red fluorescence indicates
staining for α-SMA, which is a specific marker for cerebrovascular
SMC. Blue fluorescence indicates nuclei stained with DAPI.
Magnification of the upper panels, ×100; that of the lower panels,
×400. Scale bar, 50 µm. SMC, smooth muscle cells; SB-3CT,
2-[(4-phenoxyphenylsulfonyl)methyl]thiirane; α-SMA, α-smooth muscle
actin; DAPI, 4′,6-diamidino-2-phenylindole; MMP-9, matrix
metalloproteinase 9. |
Western blot analysis
Following treatment, the culture medium was
discarded and cells were rinsed three times with phosphate-buffered
saline (PBS). Cell lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) was added and the cells were scraped
off the dishes and maintained on ice. Cell lysates were placed on
ice for 30 min prior to centrifugation at 12,000 × g for 10
min at 4°C. Protein concentration was determined using the
bicinchoninic acid assay (BCA) method. The cell lysates were
transferred to a 96-well plate, and 200 µl BCA working solution was
added to each well. After a 30 min incubation at 37°C, the
absorbance at 562 nm was determined using a microplate reader. A
standard curve was generated and used to determine the
concentrations of the samples. The samples were then separated
using sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(10% gels), and transferred to a nitrocellulose membrane. The
membranes were blocked with 1X PBS-Tween 20 (PBST) solution with 5%
bovine serum albumin (BSA; Beyotime Institute of Biotechnology) for
1 h at room temperature. The membranes were then incubated with
primary antibodies against MMP-9 (cat. no. ab119906), type IV
collagen (cat. no. ab19808), type V collagen (cat. no. ab114072)
and β-actin (cat. no. ab8227; all at a dilution of 1:1,000, and
obtained from Abcam, Cambridge, MA, USA) overnight at 4°C. The
membranes were rinsed three times with PBST (5 min per rinse) and
then incubated with a horseradish peroxidase-conjugated secondary
antibodies (cat. nos. ab6789; ab 6721) at 1:5,000 dilution for 2 h
at room temperature. Finally, the membranes were rinsed with PBST
three times (5 min per rinse) and visualized using Pierce ECL
Western Blotting substrate (cat. no. 32106; Thermo Fisher
Scientific, Inc.).
Immunofluorescence staining
Cover slides were placed in 12-well plates, coated
with 0.1% poly-lysine (Sigma-Aldrich, Merck Millipore) overnight
and rinsed three times with sterile distilled water. Cells were
seeded into 12-well plates at a density of 1.0×105
cells/well, cultured until they reached 70% confluence, and then
treated with hemolysate and SB-3CT, as described above. Following
the treatment, the medium was removed and the cells were rinsed
three times with PBS and fixed in 4% paraformaldehyde for 20 min.
The cells were rinsed with PBS and blocked with PBS with 5% BSA for
30 min at room temperature. Following blocking, the cells were
incubated with a 1:1,000 dilution of primary antibody against
α-smooth muscle actin (α-SMA; a marker for cerebrovascular smooth
muscle cells; cat. no. ab7817; Abcam), MMP-9, type IV and V
collagen (Abcam) overnight at 4°C. The cells were then washed three
times with PBS and incubated with fluorescent, labeled secondary
antibodies (cat nos. ab150077; ab150115; Abcam) at room temperature
for 30 min. Following three additional washes using PBS, the cell
nuclei were stained using 4′,6-diamidino-2-phenylindole. The slides
were then sealed and observed under a BX50/BX-FLA/DP70 fluorescence
microscope (Olympus Corporation, Tokyo, Japan).
Collagen gel contraction assay
Cell contraction assay kits were purchased from Cell
Biolabs, Inc. (San Diego, CA, USA) and used according to the
manufacturer's protocol. Following treatment (Fig. 1), cells were trypsinized and
suspended, rinsed three times with PBS, centrifuged at 1,500 ×
g for 3 min and resuspended in phenol red-free DMEM (Thermo
Fisher Scientific, Inc.) at a final concentration of 5.0×106
cells/ml. Rat tail collagen solution (Gibco; Thermo Fisher
Scientific, Inc.) was diluted to a final concentration of 3 mg/ml
with 0.3 ml phenol red-free DMEM (pH 7.3–7.4). Collagen solution,
cell suspension and FBS were then combined at a ratio of 8:1:1 to
yield a final concentration of 5.0×105 cells/ml. Aliquots (1
ml/well) of the collagen-cell mixture were added to a 24-well plate
and the plates were incubated for 30 min at 37°C with 5% CO2 to
promote polymerization. Following the solidification of the gel,
the edges of the gel attached to the wells were scored using
pipette tips and an appropriate quantity of phenol red-free DMEM
was added. Gels were incubated for an additional 24 h prior to
image capture. Cell contractility was calculated using the
following formula: Contraction index = (well area - gel area) /
well area × 100%.
Statistical analysis
The western blotting and immunofluorescent staining
were analyzed using ImageJ version 1.46 software (National
Institutes of Health, Bethesda, MD, USA. The mean grayscale value
of the control group was normalized to 1, and the ratios of the
grayscale values of the experimental groups to the control group
were calculated and analyzed. Statistical analysis was performed
using GraphPad Prism version 5.0 software (GraphPad Software, Inc.,
La Jolla, CA, USA). Data are presented as the mean ± standard
error. One-way analysis of variance and Fisher's least significant
difference test was used for pairwise comparison of groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Isolation and culture of
cerebrovascular smooth muscle cells
Immunofluorescence staining was performed to
determine the phenotype of the adherent cells. As presented in
Fig. 1B, the rat cerebrovascular
smooth muscle cells had a polygonal or long, spindle-like
morphology and expanded into a monolayer when observed under a
microscope. It was demonstrated that the majority of isolated cells
stained positively for α-SMA, a specific marker of cerebrovascular
smooth muscle cells. This indicated that the cerebrovascular smooth
muscle cells had been successfully isolated (Fig. 1B).
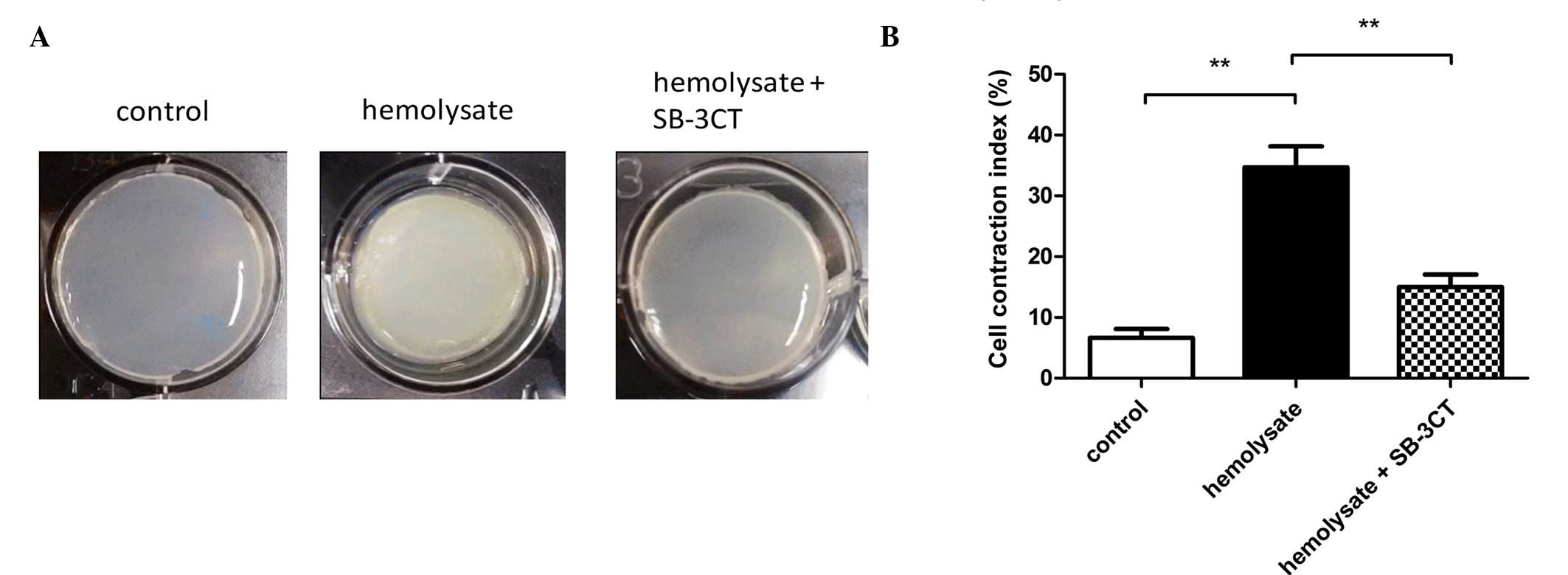
Hemolysate enhances the contractility
of cerebrovascular smooth muscle cells
As presented in Fig.
2, the contractility of cerebrovascular smooth muscle cells was
determined using a collagen gel contraction assay. Significant gel
contraction was observed in the hemolysate treatment group when
compared with the control group (P<0.01; Fig 2B). However, pretreatment with SB-3CT
significantly inhibited the hemolysate-induced gel contraction when
compared with the hemolysate treatment group (P<0.01; Fig. 2B).
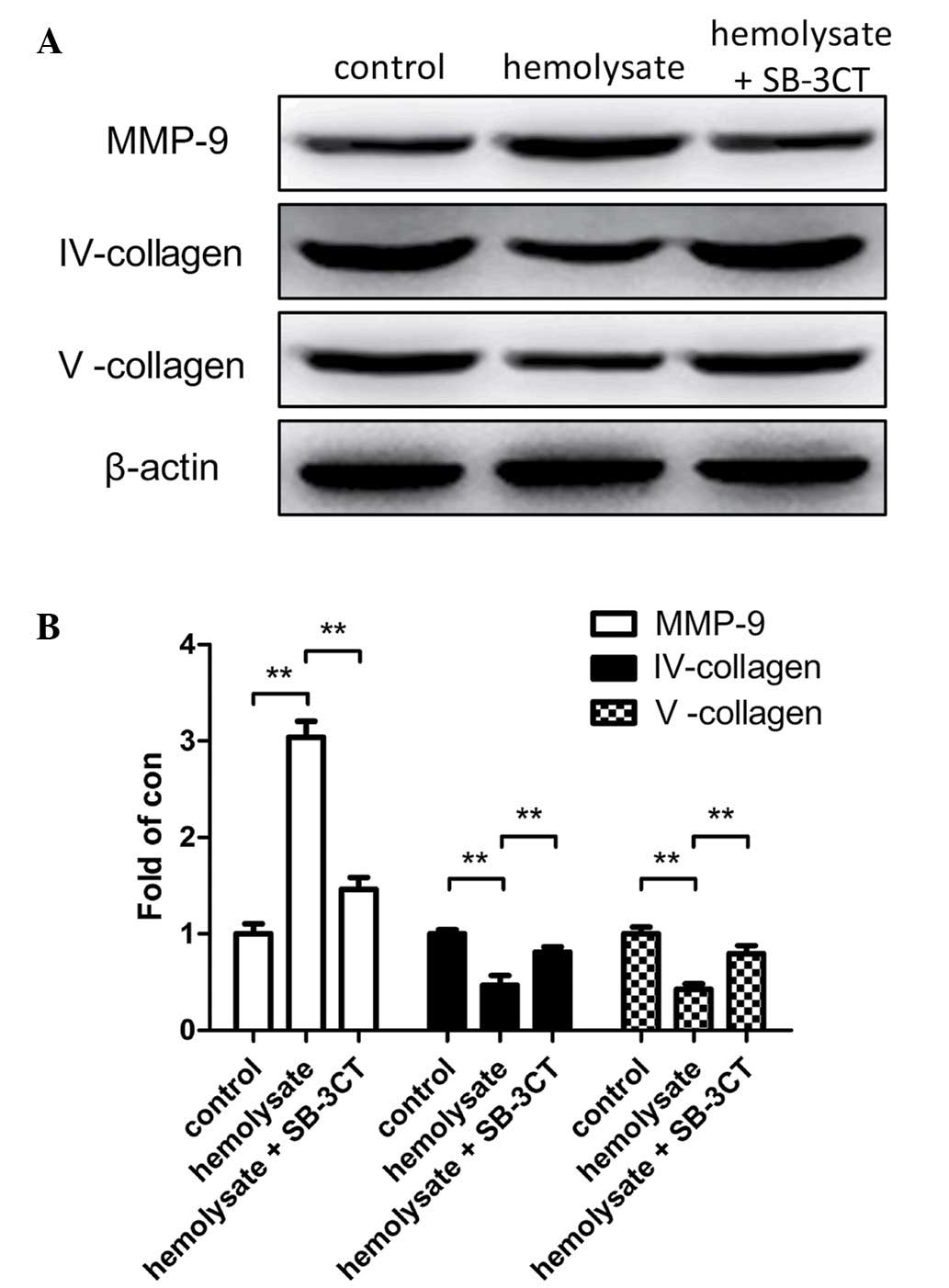
Hemolysate increases MMP-9 protein
expression levels in cerebrovascular smooth muscle cells
Western blot analysis was used to demonstrate that
hemolysate stimulation significantly increased MMP-9 protein
expression levels (P<0.01; Fig.
3) and reduced collagen IV and V protein expression levels
(P<0.01; Fig. 3B) when the
hemolysate treatment group was compared with the control. However,
pretreatment of cerebrovascular smooth muscle cells with SB-3CT
significantly reduced the expression levels of MMP-9 when compared
with the hemolysate treatment group (P<0.01; Fig. 3B). Conversely, collagen IV and V
protein expression levels were significantly greater in the SB-3CT
pretreatment group compared with the hemolysate group (P<0.01;
Fig. 3B).
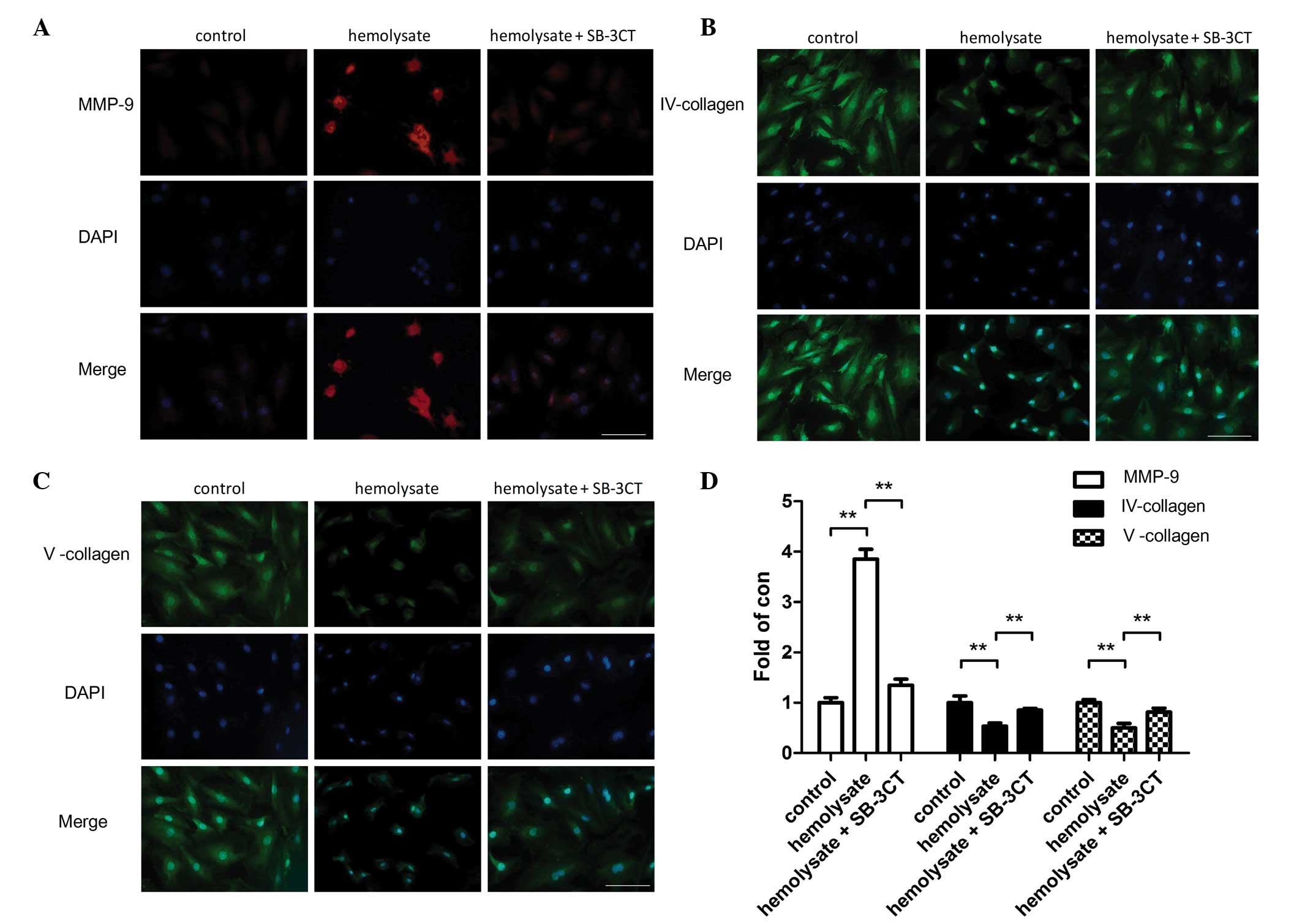
Immunofluorescence staining also demonstrated that
hemolysate treatment significantly increased the protein expression
levels of MMP-9 (P<0.01; Fig.
4) and reduced the protein expression levels of collagens IV
and V (P<0.01; Fig. 4) in
cerebrovascular smooth muscle cells compared with the control
group. However, pretreatment with SB-3CT significantly reduced the
MMP-9 expression levels (P<0.01; Fig. 4D). Collagen IV and V protein
expression levels were significantly increased in the SB-3CT
pretreatment group compared with the hemolysate group (P<0.01;
Fig. 4).
Discussion
Intracranial aneurysm is a common cerebrovascular
disease. Aneurysm re-rupture and CVS are two major complications
that may occur following a ruptured aneurysm. CVS occurs at a rate
of 30–70%, with ~30% of these patients presenting symptoms of
cerebral ischemia or severe cerebral infarctions (1). As the pathogenesis of CVS remains to
be fully elucidated, the treatment options are currently limited.
Therefore, prevention of CVS is crucial for the reduction of
morbidity and mortality rates following intracranial aneurysm
surgery.
Previous studies have determined that CVS may be due
to multiple factors that enhance cerebral vasoconstriction
(2,4) and diminish cerebral vasodilation
(3,5,11).
Humphrey et al (11)
proposed a theoretical biomechanical framework of SAH vasospasm
(11), which noted that, following
SAH, extracellular clot stimulation of blood vessel walls led to
increased levels of NO scavengers, such as reactive oxygen species
and oxyhemoglobin, including higher levels of serotonin,
thromboxane A2, angiotensin-1 and thrombin. This, in turn, may lead
to increased vascular thickness and hardness, stenosis and
vasoconstriction. The vessel may return to normal when the clot
dissolves. Different treatment strategies have been evaluated based
on these mechanisms. Previous studies have determined the effects
of several antagonists on the contraction of cerebral smooth
muscle, including calcium channel blockers such as papaverine
(12) and nicardipine (13). Fenpropathrin povidone (14) and endothelin antagonists (15) were also revealed to have a
therapeutic effect. However, the overall effectiveness of these
treatments was unsatisfactory as of the exact pathogenesis of
remains to be elucidated.
Previous studies have determined that MMP-9 may be
associated with cerebrovascular disease. MMP-9 may degrade the
extracellular matrix (16–21). Additionally, it may be involved in
various pathological processes, including the degradation of the
connective tissue, inflammation, ischemia and hypoxia. Therefore,
MMP-9 is important for pathological processes associated with
cerebral hemorrhage. Compared with previous studies on cerebral
hemorrhage, the effects of MMP-9 on aneurysmal SAH remain to be
fully elucidated. However, the current study identified MMP-9 as a
potential biomarker for CVS following an aneurysm (16). Previous studies have determined
that, following aneurysmal SAH, the expression levels of MMP-9 are
increased in brain tissue, cerebrospinal fluid and peripheral blood
(17–19). Therefore, MMP-9 is important in the
pathological processes of aneurysmal SAH (17,20,21).
Our previous study on a rat model revealed that CVS
may occurs following SAH (9).
Additionally, protein expression levels of MMP-9 in the basilar
artery walls increased following SAH with a parallel time course to
development of CVS. This suggested that MMP-9 may be involved in
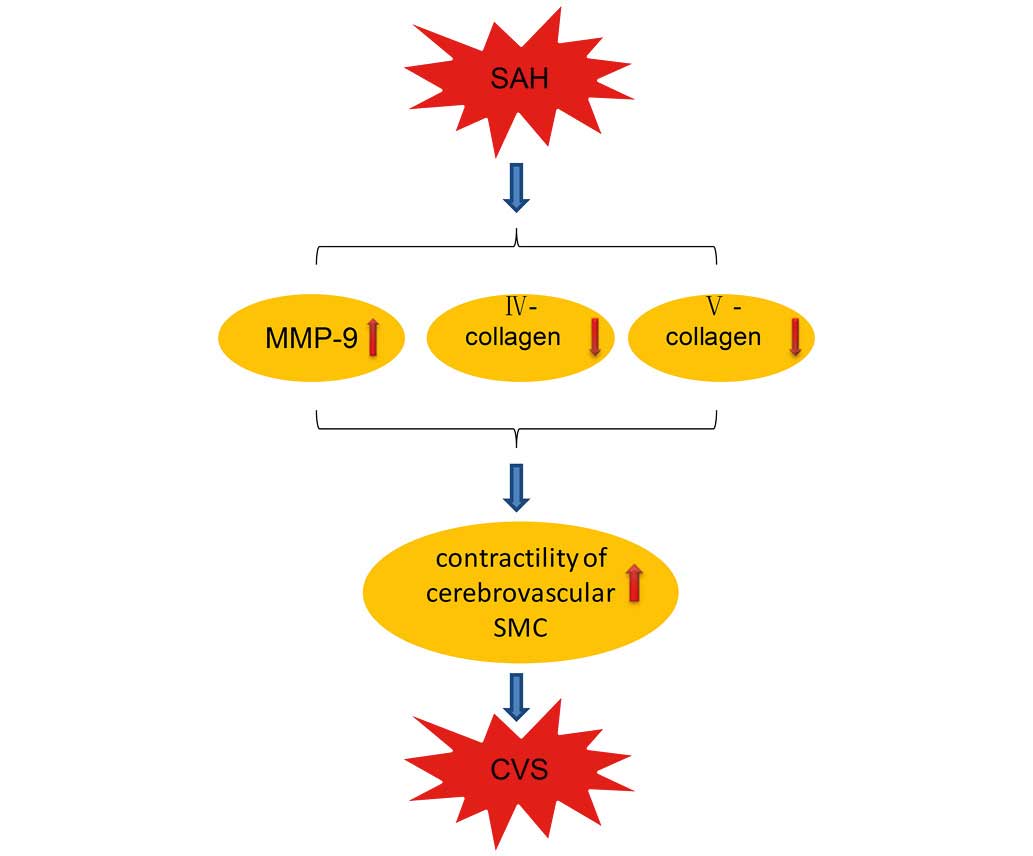
the pathological processes of CVS (9). CVS primarily occurs due to enhanced
contractility of cerebrovascular smooth muscle cells. The aim of
the present study was to determine whether MMP-9 may be involved in
this process (Fig. 5).
Cerebrovascular smooth muscle cells were treated with hemolysate as
an in vitro model of SAH. Collagen gel contraction
experiments revealed that hemolysate treatment induced a
significant contractile response in cerebrovascular smooth muscle
cells. In addition, pretreatment with SB-3CT (a selective inhibitor
of MMP-9) reduced these contractile responses. Immunofluorescence
staining and western blotting confirmed that hemolysate stimulation
increased MMP-9 protein expression levels in cerebrovascular smooth
muscle cells. In contrast, protein expression levels of collagen IV
and V were significantly decreased. This suggests that MMP-9
contributed to protease activity, catalyzed the degradation of
collagen IV and V and was important for the contractile response of
cerebrovascular smooth muscle cells. Additionally, pretreatment
with SB-3CT inhibited the protease activity of MMP-9 and the
contractile responses of the cerebrovascular smooth muscle
cells.
In conclusion, the present study used an in
vitro model of SAH to investigate changes in MMP-9 expression
levels in cerebrovascular smooth muscle cells, and its involvement
in their contractile response. Therefore, the current study
provided novel insights into the pathogenesis of CVS following SAH.
Further research is necessary to elucidate the specific molecular
mechanisms that mediate the effects of MMP-9 on the contraction of
cerebrovascular smooth muscle cells, and whether MMP-9 is involved
in cerebral inflammation following SAH.
Acknowledgements
The present study was supported by grants from the
Suzhou Government (grant nos. KJXW2014042 and ZKS1419).
Glossary
Abbreviations
Abbreviations:
|
CVS
|
cerebral vasospasm
|
|
MMP-9
|
matrix metalloproteinase 9
|
|
DMEM/F12
|
Dulbecco's modified eagle
medium/Nutrient Mixture F-12
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Baggott CD and Aagaard-Kienitz B: Cerebral
vasospasm. Neurosurg Clin N Am. 25:497–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishizawa S and Laher I: Signaling
mechanisms in cerebral vasospasm. Trends Cardiovasc Med. 15:24–34.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pluta RM: Delayed cerebral vasospasm and
nitric oxide: Review, new hypothesis and proposed treatment.
Pharmacol Ther. 105:23–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sehba FA and Bederson JB: Mechanisms of
acute brain injury after subarachnoid hemorrhage. Neurol Res.
28:381–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suhardja A: Mechanisms of disease: Roles
of nitric oxide and endothelin-1 in delayed cerebral vasospasm
produced by aneurysmal subarachnoid hemorrhage. Nat Clin Pract
Cardiovasc Med. 1:110–116; quiz 2 p following 116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cunningham LA, Wetzel M and Rosenberg GA:
Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia.
50:329–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fatar M, Stroick M, Griebe M and Hennerici
M: Matrix metalloproteinases in cerebrovascular diseases.
Cerebrovasc Dis. 20:141–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenberg GA: Matrix metalloproteinases in
neuroinflammation. Glia. 39:279–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Fang Q, Dang BQ, Shen XM, Shu Z,
Zuo G, He WC and Chen G: Potential contribution of matrix
metalloproteinase-9 (mmp-9) to cerebral vasospasm after
experimental subarachnoid hemorrhage in rats. Ann Clin Lab Sci.
42:14–20. 2012.PubMed/NCBI
|
|
10
|
Choudhri TF, Hoh BL, Solomon RA, Connolly
ES Jr and Pinsky DJ: Use of a spectrophotometric hemoglobin assay
to objectively quantify intracerebral hemorrhage in mice. Stroke.
28:2296–2302. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Humphrey JD, Baek S and Niklason LE:
Biochemomechanics of cerebral vasospasm and its resolution: I. A
new hypothesis and theoretical framework. Ann Biomed Eng.
35:1485–1497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JH, Yi HJ, Ko Y, Kim YS, Kim DW and
Kim JM: Effectiveness of papaverine cisternal irrigation for
cerebral vasospasm after aneurysmal subarachnoid hemorrhage and
measurement of biomarkers. Neurol Sci. 35:715–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kasuya H, Onda H, Takeshita M, Okada Y and
Hori T: Efficacy and safety of nicardipine prolonged-release
implants for preventing vasospasm in humans. Stroke. 33:1011–1015.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishiguchi M, Ono S, Iseda K, Manabe H,
Hishikawa T and Date I: Effect of vasodilation by milrinone, a
phosphodiesterase III inhibitor, on vasospastic arteries after a
subarachnoid hemorrhage in vitro and in vivo: Effectiveness of
cisternal injection of milrinone. Neurosurgery. 66:158–164;
discussion 164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Macdonald RL, Higashida RT, Keller E,
Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A,
et al: Randomized trial of clazosentan in patients with aneurysmal
subarachnoid hemorrhage undergoing endovascular coiling. Stroke.
43:1463–1469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Clark JF, Pyne-Geithman G and
Caruso J: Metallomics study in CSF for putative biomarkers to
predict cerebral vasospasm. Metallomics. 2:628–637. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horstmann S, Su Y, Koziol J,
Meyding-Lamadé U, Nagel S and Wagner S: MMP-2 and MMP-9 levels in
peripheral blood after subarachnoid hemorrhage. J Neurol Sci.
251:82–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosell A, Ortega-Aznar A, Alvarez-Sabín J,
Fernández-Cadenas I, Ribó M, Molina CA, Lo EH and Montaner J:
Increased brain expression of matrix metalloproteinase-9 after
ischemic and hemorrhagic human stroke. Stroke. 37:1399–1406. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sarrafzadeh A, Copin JC, Bengualid DJ,
Turck N, Vajkoczy P, Bijlenga P, Schaller K and Gasche Y: Matrix
metalloproteinase-9 concentration in the cerebral extracellular
fluid of patients during the acute phase of aneurysmal subarachnoid
hemorrhage. Neurol Res. 34:455–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chou SH, Feske SK, Simmons SL, Konigsberg
RG, Orzell SC, Marckmann A, Bourget G, Bauer DJ, De Jager PL, Du R,
et al: Elevated peripheral neutrophils and matrix metalloproteinase
9 as biomarkers of functional outcome following subarachnoid
hemorrhage. Transl Stroke Res. 2:600–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McGirt MJ, Lynch JR, Blessing R, Warner
DS, Friedman AH and Laskowitz DT: Serum von Willebrand factor,
matrix metalloproteinase-9, and vascular endothelial growth factor
levels predict the onset of cerebral vasospasm after aneurysmal
subarachnoid hemorrhage. Neurosurgery. 51:1128–1134; discussion
1134–1135. 2002. View Article : Google Scholar : PubMed/NCBI
|